Abstract
T helper 17 (Th17) cells have both regulatory and protective roles in physiological conditions. The Th17 subset and the cytokine interleukin-17A (IL-17A) have been implicated in the pathogenesis of certain autoimmune diseases, several types of cancer and allograft rejection. However, the role of Th17 cells at the maternal/fetal interface remains unknown. Here, we demonstrate that Th17 cells are present in decidua and are increased in the peripheral blood of 10 clinically normal pregnancies based on intracellular cytokine analysis. Our results suggest a potential role of Th17 cells in sustaining pregnancy in humans. Furthermore, we demonstrate that decidual stromal cells (DSCs) but not trophoblast cells recruit peripheral Th17 cells into the decidua by secreting CCL2. The recruited Th17 cells promote proliferation and invasion and inhibit the apoptosis of human trophoblast cells by secreting IL-17 during the first trimester of pregnancy. These findings indicate a novel role for Th17 cells in controlling the maternal–fetal relationship and placenta development.
Keywords: decidual stromal cells, invasion, proliferation, Th17 cells, trophoblast cells
Introduction
The maternal deciduas and the extravillious trophoblast cells have intimate contact at the maternal/fetal interface. It is well established that leukocytes begin migrating into the maternal endometrium during the luteal phase and increase gradually following pregnancy.1,2 Recent data from both mice and humans have demonstrated that the maternal/fetal interface exhibits a TH2 bias during pregnancy.3 A failure to establish such an immune milieu or exhibiting a TH1 bias, such as the overexpression of interferon (IFN)-γ or tumor-necrosis factor-α, is associated with recurrent spontaneous abortion in humans.4,5 It also has been proven that regulatory T (Treg) cells increase during the first and second trimesters and peak in the second trimester in human decidua and peripheral blood.6 These changes at the beginning of pregnancy suggest that T cells may play an important role in the establishment of fetal tolerance during early pregnancy.
Interleukin 17 (IL-17)-producing T cells, termed T helper 17 (Th17) cells, have been characterized as a distinct lineage of CD4+ T cells in mice and differentiate from naive T-cell precursors by the polarizing cytokines transforming growth factor-β and IL-6, under the control of the transcription factors RORγt and RORα.7,8,9 As a key cytokine produced by Th17 cells, IL-17 and other cytokines are involved in certain inflammatory and autoimmune diseases, including acute coronary syndrome,10 multiple sclerosis,11 inflammatory bowel diseases12 and psoriasis.13 However, Th17 cells might also have regulatory and protective roles in the gut and liver.14,15 In addition, IL-17 has been shown to induce the proliferation of human bone marrow-derived mesenchymal stem cells16 and promotes the invasion of JEG-3 human choriocarcinoma cells.17 These results suggest that Th17 cells may be involved in the development of placental trophoblasts. Although IL-17-producing T cells have been found in healthy human peripheral blood mononuclear cell (PBMC)18 and at the healthy human maternal/fetal interface,19 it is unclear whether Th17 cells play a regulatory role in human trophoblast behavior. This study was conducted to clarify how Th17 cells are recruited into decidua and whether Th17 cells play a beneficial role in human early pregnancy.
Materials and methods
Human subjects
The first-trimester human villous and decidual tissues were obtained from 10 clinically normal pregnancies (age: 28.30±2.4 years, gestational age: 8.5±1.3 weeks) that were terminated for non-medical reasons. We also collected peripheral blood from 10 first, secondary and third trimester pregnancies. As a control, we obtained whole-blood and endometrium from 10 healthy non-pregnant women of secretory phase from the Obstetrics and Gynecology Hospital of Fudan University. Prior to initiating the study, written informed consent was obtained from every subject.
Isolation and primary culture of human decidual stromal cells (DSCs)
Decidual tissues were carefully dissected and incubated with RPMI 1640 containing 1.0 mg/ml collagenase type IV (CLS-1; Worthington Biomedical, USA) and 1% fetal bovine serum (FBS; Gibco, USA) at 37 °C for 90 min with regular gentle shaking. The cells were then passed through a 32-µm sterile stainless steel mesh to remove any non-digested tissue. The cell suspension was carefully layered over a discontinuous Percoll gradient and then centrifuged. The upper layer (density of 1.042–1.062 g/ml) was recovered and washed with RPMI 1640, and the isolated cells were cultured in 5% CO2 at 37 °C for 30 min to remove the macrophage and fibroblasts. The non-adherent cells were recovered and cultured in Dulbecco's Modified Eagle Medium (DMEM)-F12 containing 2 mM L-glutamine, penicillin (100 IU/ml) and streptomycin (100 µg/ml) supplemented with 10% FBS. After two passages, the purity of DSCs was characterized and was determined to be more than 99%.
Primary culture of the first-trimester human trophoblast cells and coculture of human trophoblast cells and DSCs
The trophoblast cells were isolated from the first-trimester placentas as described previously.20 Briefly, the obtained placenta tissue was digested and gently agitated with 0.25% trypsin and 1500 IU/ml DNase type I (AppliChem GmbH, Germany) at 37 °C for 10 min. The filtrated cell suspensions were carefully layered on a discontinuous Percoll gradient (65%–20%, in 5% step) and centrifuged. The cells between densities of 1.042–1.068 g/m were collected and washed in DMEM-high glucose medium. These cells were adjusted to a density of 1×106 cells/ml and maintained in DMEM-high glucose complete medium (2 mM glutamine, 25 Mm HEPES, 100 U/ml penicillin and 100 µg/ml streptomycin), supplemented with 20% FBS and incubated in 5% CO2 at 37 °C. The purity of trophoblast cells was characterized and was found to be more than 95%. The DSCs were cultured (1×105 cells/ml) for 12 h and then directly cocultured with the isolated trophoblasts (1×105 cells/ml) for 24 h.
Isolation of CD4+ T cells
The decidual mononuclear cells at density of 1.062–1.077 g/ml were recovered and washed with RPMI 1640; they were then plated into flasks in RPMI 1640 containing 2 mM L-glutamine, penicillin (100 IU/ml) and streptomycin (100 µg/ml) supplemented with 10% FBS and incubated in 5% CO2 at 37 °C for 2 h. The suspended cells were mainly composed of T and NK cells. PBMC were isolated by Ficoll-Hypaque density gradient centrifugation. Decidual and peripheral CD4+ T cells were sorted by magnetic affinity cell sorting using the CD4+ bead kit (Miltenyi Biotec, Germany) according to the manufacturer's instructions. The sorted CD4+ T cells were investigated by flow cytometry, and the purity was >90% (data not shown).
Th17 cells differentiation and culture
Decidual CD4+ T cells were isolated using the CD4 multisort kit (Miltenyi Biotec) according to the manufacturer's instructions. The CD45RA+ T cells were sorted from the CD4+ T cells by CD45RA expression with a CD45RA bead kit (Miltenyi Biotec). CD4+CD45RA+ naive T cells were isolated at more than 90% purity (data not shown). The naïve CD4 cells (4×104 cells/200 µl/well) were primed with plate-bound CD3-specific antibody (10 µg/ml) and CD28-sepcific antibody (5 µg/ml) in U-bottomed 96-well plates under Th17-polarizing conditions (with 10 ng/ml IL-1β, 50 ng/ml IL-6, 10 µg/ml anti-IFN-γ antibody and 10 µg/ml anti-IL-4 antibody). After 6 days of culture, the differentiated Th17 cells were collected and washed twice to remove soluble molecules. The cells were restimulated (5×105 cells/ml/well) for 48 h with plate-bound anti-CD3 (10 µg/ml) and soluble anti-CD28 (2 ng/ml) before the culture supernatant was collected.
Intracellular cytokine analysis
CD4+ T cells or PBMCs were adjusted to 1×106 cells/ml and stimulated for 5 h with PMA (25 ng/ml; Sigma, USA) and ionomycin (1 µg/ml; Sigma) in the presence of Golgistop brefeldin A (10 µg/ml; Sigma). Standard intracellular cytokine staining was performed as previously described.21 The cells were first stained with FITC-conjugated monoclonal antibody against human CD4, γδTCR, CCR7, CD161 and PE-CY5-conjugated anti-human CD45RO, αβTCR or PE-CY7-conjugated anti-human CCR6. The cells were then fixed and permeabilized with Fix & Perm solution (CALTAG Laboratories, USA) and were stained intracellularly with PE-conjugated anti-human IL-17A. The sample data were acquired using a FACS Calibur (BD, USA), and the data were analyzed using the CellQuestPro software (BD).
Immunocytochemistry
After 48 h of culture, trophoblasts, DSCs and cocultured trophoblasts and DSCs were fixed in 4% paraformaldehyde for 20 min at room temperature. The cells were then washed in phosphate buffer saline and permeabilized for 10 min in 0.5% Triton X-100-phosphate buffer saline. The cells were blocked with 3% H2O2 and incubated with 10% bovine serum. The cells were then incubated with anti-human cytokeratin-7 monoclonal antibody (Santa Cruz Biotechnology, USA), anti-human HLA-G monoclonal antibody (Affinity BioReagents, USA), anti-human vimentin monoclonal antibody (Santa Cruz Biotechnology, USA), anti-human IL-17R monoclonal antibody (R&D, USA) or anti-human CCL20 polyclonal antibody (Abcam, United Kingdom) overnight at 4 °C. The stained cells were then incubated with appropriate horseradish peroxidase-labeled secondary antibody for 30 min at room temperature. The slides were stained with 3,3′-diaminobenzidine and counterstained with hematoxylin. The experiments were repeated five times. For the double staining of the cocultured trophoblast cells and DSCs, the slides were incubated with anti-human HLA-G overnight (brown) at 4 °C, followed by anti-vimentin staining (purple).
Immunohistochemistry
The villous and decidual tissues were fixed with 10% neutral formalin for 6 h at room temperature, embedded in paraffin and sectioned at a thickness of 4 µm. The sections were deparaffinized in xylene and then rehydrated in a series of ethanol incubations. Antigen retrieval was performed by heat treatment and the slides were then blocked with methanol containing 3% H2O2, followed by 10% bovine serum. The slides were then incubated with anti-IL-17R antibody or anti-CCL20 antibody overnight at 4 °C. The sections were treated with horseradish peroxidase-labeled secondary antibody and stained with 3,3′-diaminobenzidine before counterstaining with hematoxylin. The immunohistochemical results were evaluated by a pathologist. The experiments were repeated five times.
ELISA
The secreted CCL2 and CCL20 levels in the supernatants were quantified using the Quantikine ELISA kit according to the manufacturer's protocol (Duoset; R&D Systems, USA). In this study, the minimum detection limits for CCL2 and CCL20 in the supernatants were 8 pg/ml.
Th17 cells chemotaxis
The chemotaxis lower chambers were filled with 800 µl supernatants from trophoblasts, DSCs or their coculture, or supernatants from DSC with or without neutralizing antibody to CCL20 or CCL2. The freshly isolated peripheral blood CD4+ T cells were adjusted to 1×107 cells/ml and 100 µl of these cell suspensions was placed in the upper chamber of a 0.6 µm transwell plate. The cultures were incubated at 37 °C for 4 h. The cells were collected from the lower chamber, and the numbers of Th17 cells were analyzed by intracellular cytokine assays. The results were expressed as the ratio of the migrated Th17 cells to the input Th17 cells.
Matrigel invasion assay
The Matrigel invasion assay was performed as previously described (Saito et al., 2002). We used transwell plates (6.5 mm in diameter) (Corning, Corning, NY, USA) containing polycarbonate filters with a pore size of 8.0 µm. The upper surface of the filter was coated with 10 µl Matrigel and dried aseptically. Before use, the Matrigel was rehydrated with 100 µl warm DMEM for 2 h. The isolated trophoblast cells (1×105 in 200 µl DMEM with 1% FBS) were seeded in the upper chamber. rhIL-17A at final concentrations of 0, 5, 10, 50, 100 and 200 ng/ml or Th17 cell supernatants with or without neutralizing antibody to IL-17A (5 µg/ml) were added. The lower chamber was filled with 800 µl DMEM with 1% FBS. The cells were allowed to invade for 48 h in 5% CO2 at 37 °C. The attached upper surface cells were removed. The remaining cells on the lower surface were fixed in methanol for 10 min at room temperature and stained with hematoxylin. The cells that had migrated to the lower surface were counted under a light microscope in five fields at a magnification of ×200. The assay was repeated three times. The results are expressed as the ratio of the mean number of invasive cells with treatment relative to the controls.
Cell proliferation assay
5-bromodeoxyuridine (BrdU) incorporation was used to measure the effects of rhIL-17A and the Th17 supernatants on trophoblast proliferation with or without neutralizing IL-17A antibody. The isolated trophoblast cells were resuspended in DMEM with 20% FBS and seeded at a density of 4×104 cells/200 µl/well in a 96-well flat-bottom plate that had been pre-coated with collagen type IV (Sigma). After 24 h of culture, the cells were treated with DMEM containing 1% FBS for 12 h. The recovered cells were treated with recombinant human IL-17A (1, 10, 50, 200, 400 ng/ml) or supernatants from Th17 cells with or without neutralizing IL-17A antibody (10 µg/ml) at 37 °C for 48 h. After 24 h of culture, BrdU was added to each well for 24 h. The proliferation of trophoblast cells was analyzed using a BrdU cell proliferation kit (Chemicon, USA). Briefly, the cells were fixed and incubated with anti-BrdU monoclonal antibody for 1 h at room temperature and then incubated with horseradish peroxidase-labeled goat anti-mouse IgG for 30 min at room temperature. The peroxidase substrate 3,3′,5,5′-tetramethylbenzidine was added for 30 min. The color reaction was stopped by the addition of acid stop solution. The plated cells were analyzed immediately using a spectrophotometer microplate reader at dual wavelengths of 450 and 595 nm. The results are expressed as the ratio of the optical density of cells with treatment to that without treatment. The experiment was repeated three times.
Apoptosis assay
The isolated trophoblast cells were resuspended in DMEM with 20% FBS and seeded at a density of 1×105 cells/500 µl/well in a 24-well plate that had been pre-coated with collagen type IV (Sigma). After 24 h of culture, the cells were then treated with DMEM containing 1% FBS for 12 h. The cells were removed and treated with recombinant human IL-17A (50 ng/ml) or supernatants from Th17 cells with or without neutralizing IL-17A antibody (10 µg/ml) at 37 °C for 48 h. After 48 h of culture, the apoptosis of trophoblast cells was analyzed by using an Annexin V/PI cell apoptosis kit (Invitrogen, USA).
Statistical analysis
Statistical analysis was performed using the SPSS statistical software package (SPSS 11.5; SPSS Inc., Chicago, IL, USA). Statistical evaluations of the Th17 cell percentage and cell proliferation were performed using one-way analysis of variance. All error bars in the figures indicate standard error of the mean (s.e.m.). A value of P≤0.05 was considered statistically significant.
Results
The increased Th17 cells are effector memory T cells in decidua and peripheral blood in human first-trimester pregnancy
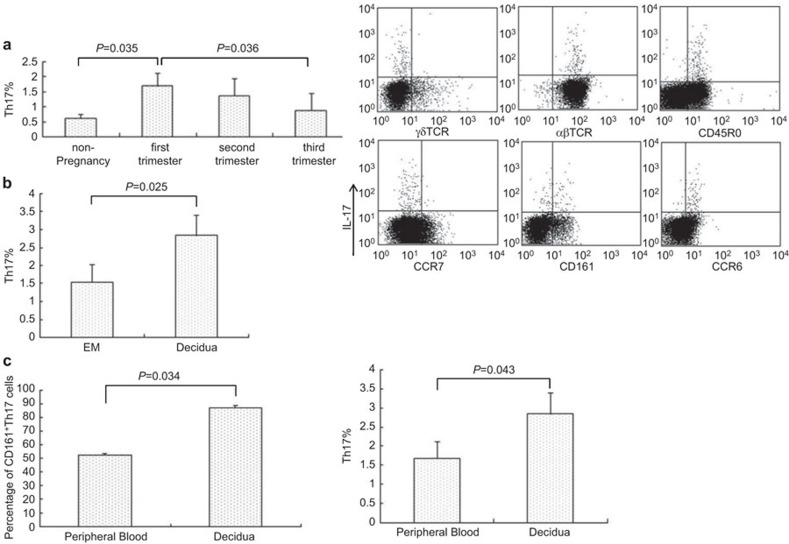
We confirmed that the Th17 cells were present in healthy PBMC of non-pregnant and pregnant women. The percentage of Th17 cells was significantly higher in the first-trimester pregnancy than in the controls (1.68%±0.44% vs. 0.61%±0.14%, P=0.036). The levels of Th17 cells gradually decreased during the second and third trimesters (1.35%±0.57% and 0.86%±0.59%). The levels of Th17 cells in the third trimester were similar to those of the controls (Figure 1a).
Figure 1.
Th17 cells are increased in human pregnancy. The percentages of IL-17-expressing CD4+ T cells were analyzed by intracellular cytokine assays. The results were gated on CD4 and MACS-sorted peripheral blood and decidual CD4+ T cells were stimulated with PMA, ionomycin and BFA for 4 hours. The surface marker of Th17 cells were analyzed by FACS. (a) The proportion of Th17 cells in PBMC during human pregnancy is compared with that in non-pregnancy (n=10, left); the phenotype of first-trimester human peripheral blood Th17 cells (right). (b) The proportion of Th17 cells in human first-trimester decidua is compared with non-pregnant endometrium of secretory phase (n=10, left); the phenotype of first-trimester human decidual Th17 cells (right). (c) Comparison of CD161 on Th17 cells between PBMC and deciduae. (d) Comparison of Th17 cells between PBMC and decidua in first-trimester human pregnancy. Error bars indicate the s.e.m. The FACS picture represents an individual sample. BFA, brefeldin A; FACS, Fluorescence-activated cell sorting; MACS, magnetic affinity cell sorting; PBMC, peripheral blood mononuclear cell; PMA, phorbol myristate acetate; s.e.m., standard error of the mean; Th17, T helper 17.
We found that Th17 cells were significantly elevated in the first-trimester decidua compared to the endometrium of the secretory phase (2.84%±0.57% vs. 1.53%±0.50%, P=0.025, Figure 1b). We also found that the relative numbers of Th17 cells were markedly increased in the decidua compared to the peripheral blood (P=0.043, Figure 1c).
We then investigated the surface phenotype of both peripheral and decidual Th17 cells and found that decidual Th17 cells were αβTCR+CD45RO+CCR7−CD161+ T cells (Figure 1b and c). Only a fraction of the peripheral Th17 cells (53.4%±1.2%) expressed CD161, which was different from the decidual Th17 cells (Figure 1a and c). However, both peripheral and decidual Th17 cells were effector memory T cells.
DSCs recruit peripheral Th17 cells into decidua via secreting CCL2
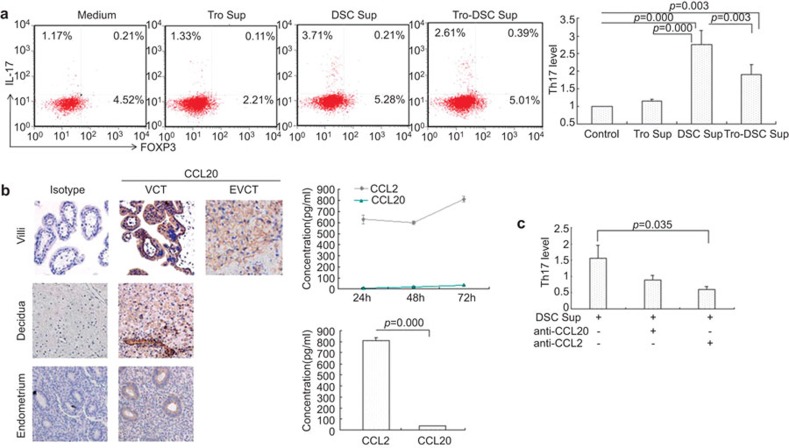
We established a coculture of trophoblast and DSCs (Supplementary Figure 1). The freshly isolated peripheral CD4+ T cells were chemotactic upon exposure to supernatants from trophoblasts, DSCs or the coculture of trophoblasts with DSCs using a chemotaxis assay. We found that DSC supernatant caused a 2.7-fold increase in the number of the recruited Th17 cells and that supernatants from the coculture of DSCs and trophoblasts induced a 1.8-fold increase compared to the control. However, trophoblast supernatant had no effect on the migration of Th17 cells. Our data show that DSCs other than trophoblasts recruit peripheral Th17 cells into decidua (P=0.0001, Figure 2a).
Figure 2.
DSCs recruit peripheral Th17 cells into decidua via secreting CCL2. (a) One case of chemotaxis for Th17 cells (left); fold increase in Th17 cells after treatment with different supernatants (right). (b) Specific brown-colored staining for CCL20 occurs in the membrane of villous cytotrophoblasts, syncytiotrophoblasts and invasive trophoblast cells in decidua. DSCs also express CCL20 moderately; glandular epithelium and ESCs of endometrium are weakly positive for CCL20. No background staining was observed in the isotype control. These results were highly reproducible in five independent experiments (including five placental samples), and the picture represents one sample (×200, left). Accumulated concentration of CCL2 and CCL20 in supernatants of primary DSCs was examined by ELISA (right upper). The level of CCL2 was higher than CCL20 from primary DSCs after 72 h of culture (right lower). (c) Fold change in Th17 cells after treatment with DSC supernatant with or without neutralizing antibody to CCL20 or CCL2. *P<0.05, **P<0.01; error bars: s.e.m. DSC, decidual stromal cell; ESC, endometrial stromal cell; DSC sup, DSCs supernatant; s.e.m., standard error of the mean; Th17, T helper 17; Tro-DSC sup, supernatant from the coculture of trophoblasts with DSCs; Tro. sup, trophoblasts supernatant.
Immunohistochemistry and ELISA results demonstrated that DSCs express and secrete the CCR6 ligand CCL20 (0.13±0.007 and 36.6±1.2, Figure 2b). Our data indicate that DSCs released higher levels of CCL2 compared to CCL20 (811.1±26.5 vs. 36.6±1.2, P=0.001, Figure 2b). We compared the effect of DSC supernatants with or without neutralizing antibody to the effect of CCL20 or CCL2 on the migration of Th17 cells. Our data show that anti-CCL2 neutralizing antibody abolished the migration of Th17 cells induced by DSC supernatants (0.6±0.08 vs. 1.56±0.39, P=0.035). However, there was no significant difference in the Th17 cell migration induced by DSC supernatants with an anti-CCL20 neutralizing antibody compared to DSC supernatants without an anti-CCL20 neutralizing antibody (0.89±0.16 vs. 1.56±0.39, P=0.056, Figure 2c).
Th17 cells promote invasion of human first-trimester trophoblasts by secreting IL-17
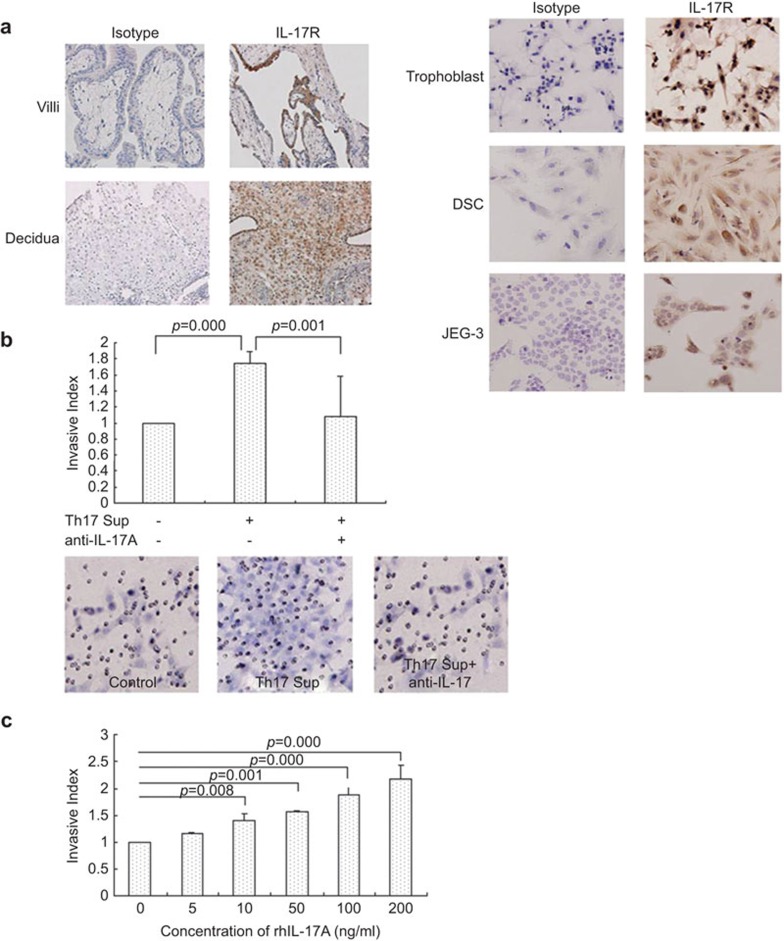
We found that trophoblasts (including villous cytotrophoblast and extravillous cytotrophoblasts) and DSCs express IL-17 receptor. Additionally, extravillous cytotrophoblasts expressed higher levels of IL-17 receptor than did DSCs (Figure 3a), which suggests that Th17 cells may affect the functions of trophoblasts. We investigated the invasion of trophoblasts after treatment with supernatants from the activated Th17 cells by Matrigel invasion assay. The results indicate that Th17 cell-derived supernatants promote trophoblast invasion and that anti-IL-17 neutralizing antibody abolishes the Th17 cell-induced trophoblast invasion increase (1.74±0.15 vs. 1.09±0.49, P=0.001, Figure 3b). Moreover, rhIL-17 also promotes trophoblast invasion in a dose-dependent manner (Figure 3c).
Figure 3.
Th17 cells promote invasion of first-trimester human trophoblasts through secreting IL-17. (a) Left: Expression of IL-17R on human first-trimester villi and decidua (left); expression of IL-17R on primary trophoblast, DSCs and human choriocarcinoma JEG-3. (×200, right). (b) Effect of Th17 cell-derived supernatants on the invasive capacity of trophoblasts with or without neutralizing antibody to IL-17. Upper: The neutralizing antibody to IL-17 completely inhibits trophoblast invasion increase induced by Th17 cell supernatants. Lower: Representative of three experiments (×200). (c) Effect of rhIL-17A on the invasive capacity of trophoblasts; error bars: s.e.m. Th17 sup, Th17 cell supernatants. DSC, decidual stromal cell; EVCT, extravillous cytotrophoblast; IL-17R, interleukin-17 receptor; s.e.m., standard error of the mean; Th17, T helper 17.
Th17 cells promote growth of human first-trimester trophoblasts by secreting IL-17
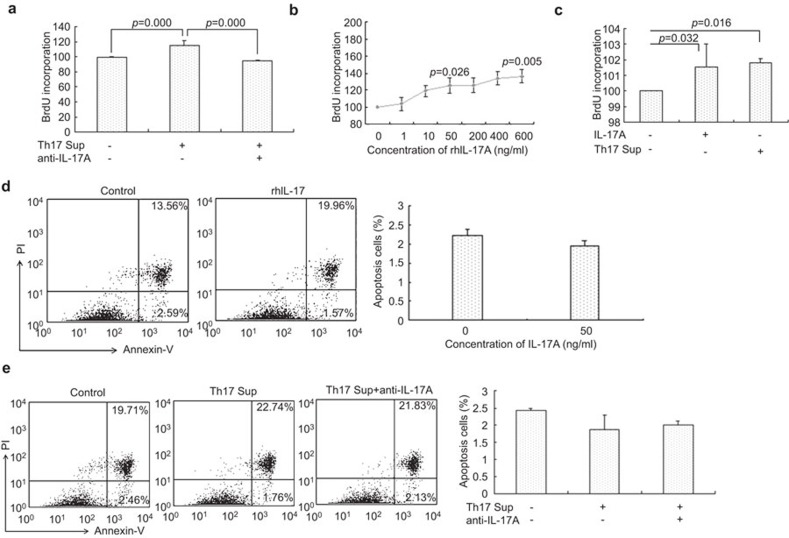
We found that the Th17 cell supernatants promoted trophoblast proliferation and that anti-IL-17 neutralizing antibody abolished the Th17 cell-induced trophoblast proliferation (101.8±0.27 vs. 99.08±0.39, P=0.0003, Figure 4a). Moreover, rhIL-17 also promoted trophoblast proliferation in a dose-dependent manner (Figure 4b). There was no significant difference in trophoblast proliferation between treatment with the rhIL-17 and the Th17 cell-derived supernatants (101.54±1.47 vs. 101.8±0.27, P=0.063, Figure 4c).
Figure 4.
Th17 cells promote growth of first-trimester human trophoblasts by secreting IL-17. (a) Effect of Th17 cells-derived supernatants on the proliferation of trophoblasts with or without neutralizing antibody to IL-17. (b) The effect of different concentrations of rhIL-17A on the proliferation of trophoblasts. (c) Comparison of trophoblast proliferation induced by rhIL-17A or Th17 cell-derived supernatants. (d) Effect of rhIL-17 on the apoptosis of trophoblasts. (e) Effect of Th17 cell-derived supernatants on trophoblast apotopsis. Flow cytometry plot is a representative of three experiments. *P<0.05; **P<0.01; error bars: s.e.m. s.e.m., standard error of the mean; Th17, T helper 17; Th17 sup, Th17 cell-derived supernatant.
After treatment with rhIL-17 (50 ng/ml), the percentage of apoptotic trophoblasts decreased to 1.94%±0.12% compared to the control (2.23%±0.15%, P=0.066, Figure 4d). The percentage of apoptotic trophoblast decreased to 1.88%±0.42% when treated with supernatants from the activated Th17 cells. The anti-IL-17 neutralizing antibody inhibited the Th17 cell-induced anti-apoptotic action on trophoblasts (Figure 4e).
Discussion
Here, we demonstrated that Th17 cells are present in human first-trimester decidua and peripheral blood. The prevalence of Th17 cells in decidual tissue suggests that Th17 cells are involved in sustaining normal pregnancy in humans. The change in Th17 cells is similar to that observed in natural Treg cells during human pregnancy,6,22,23 and there is a reciprocal interaction between Treg cells and Th17 cells.9 Therefore, we hypothesized that Th17 cells may act in concert with Treg cells to maintain maternal–fetal tolerance. Th17 cells impair Th1 response in the gut,15 and whether Th17 cells impair Th1 response in coordination with Treg cells at the maternal/fetal interface remains to be elucidated. Recent data show the Th17 prevalence is similar in healthy pregnancy to healthy non-pregnant controls.24 The difference from our study is the previous data focused on the second and third trimesters, while our studies examined the first trimester.
It is well known that chemokines and their receptors act together with adhesion molecules to control the migration of lymphocytes to lymphoid and non-lymphoid tissues. For example, CCR9 selectively attracts specific subsets of gut-homing lymphocytes, whereas CCR4 and CCR10 direct skin-trophic T-cell trafficking.25 Similar selective mechanisms that targets NK cells to the decidua have also been demonstrated.26 It has been reported that Th17 cells are characterized by the combined expression of CCR4 and CCR627 or by the expression of CCR2 and lack of CCR5.28 CCR6 is essential for the migration of Th17 cells.29,30 Human placental cells express a series of functional chemokines,31 and we speculate that placental cells may attract peripheral Th17 cells into decidua. It has been demonstrated that DSCs other than trophoblasts recruit peripheral Th17 cell into decidua. We also verify that the CCR2–CCL2 axis in the decidua has an important role in migration of Th17 cells into maternal/fetal interface. However, the CCR6–CCL20 axis has little effect on the recruitment of Th17 cells. Our results are different from data reported in other studies.29,30 The difference may be due to the different expression spectrum of chemokines in decidua and chemokine receptors of the decidual Th17 cells. We have also found that trophoblast cells inhibit the migration of Th17 cells caused by DSCs. We propose that trophoblast cells may downregulate the expression of chemokines in the decidua that are specific to the migration of Th17 cells or downregulate the expression of chemokine receptors on Th17 cells by producing regulatory molecules. In summary, DSCs cooperate with trophoblasts to sustain the homeostasis of Th17 cells in decidua.
CD161 is a C-type lectin-like receptor that is expressed not only in almost all NK cells, but also in a subset of T cells.32 One ligand for human CD161 is lectin-like transcript 1, which belongs to the C-type lectin domain family 2.33 Th17 cells are attracted into skin in pathological skin diseases such as contact dermatitis, psoriasis and atopic dermatitis34 via C-type lectin domain family 2A that is expressed in the skin.35 It has been reported that gut-resident Th17 cells express CD161.36 Our present study shows most decidual Th17 cells express CD161. These findings support the possibility that this molecule plays a role in favoring transendothelial migration of Th17 cells into the maternal/fetal interface and in selecting decidual Th17 cells.
During normal placenta development, the proliferation and invasion of trophoblasts are strictly controlled. Various factors such as adhesion molecules37 and cytokines38 are involved in these processes. The defect of trophoblast invasion is related to human pregnancy complications such as pre-eclampsia and placenta increta. Extravillous trophoblast cells migrate and invade into the deciduas. Thus, we examined the invasiveness of the isolated first-trimester trophoblasts using a Matrigel invasion assay. Th17 cells induce the invasion of trophoblast cells by secreting IL-17, and Th17 cells exhibit a significant stimulatory effect on the proliferation of trophoblast that is similar to rhIL-17A. The Th17 cell-derived supernatant promotes trophoblast proliferation by secreting IL-17. It has been shown that Th17 cells can promote tumor growth through an IL-6/STAT3 signaling pathway.39 It is unclear whether Th17 cells promote trophoblast proliferation through the STAT3 signaling pathway.
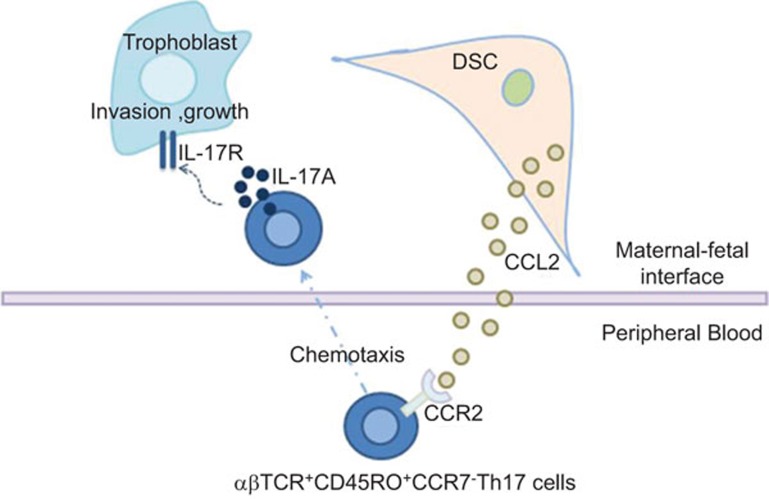
Apoptosis is an active process by which dysfunctional cells are eliminated to maintain normal tissue stability. Apoptosis plays an important role in normal placental development. It has been demonstrated that trophoblast apoptosis occurs in normal pregnancy and that the apoptotic trophoblast cells increase as gestation proceeds.40,41 It is also known that abnormal trophoblast apoptosis is involved in human pregnancy complications such as preeclampsia or fetal growth restriction. Little is known about the role of Th17 cells in trophoblast apoptosis. Here, we show that Th17 cells inhibit trophoblast apoptosis mainly by secreting IL-17, but it cannot be excluded that other cytokines produced by Th17 cells are also involved in the regulation of trophoblast apoptosis. It has been reported that Th1 cytokines such as tumor-necrosis factor-α and IFN-γ induce trophoblast apoptosis, but the Th2 cytokine IL-10 antagonizes the pro-apoptotic effect of tumor-necrosis factor-α and IFN-γ. These results suggest that Th17 cells may have a similar function in the modulation of trophoblast apoptosis. Our study has shown that Th17 cells are involved in first-trimester placentation by regulating proliferation, invasion and apoptosis of trophoblasts (Figure 5).
Figure 5.
Roles of Th17 cells at the maternal/fetal interface. Th17 cells are recruited by DSC-secreted CCL2 into decidua and improve the growth and invasiveness of trophoblast cells through secreting IL-17 during the first trimester of human pregnancy. DSC, decidual stromal cell; Th17, T helper 17.
We have found that Th17 cells are significantly elevated in the first-trimester deciduae compared to non-pregnant endometrium. Recent data have shown that Th17 cells are increased in decidua from spontaneous abortion42,43 and preeclampsia44 patients. Thus, further studies are required to clarify which subset of Th17 cells is involved in miscarriage, preecelampsia or normal pregnancy. The limitation of this study is its low sample size and the lack of data on the balance between Th17 cells and Treg cells in the first trimester.
Author contributions
HXW conducted all experiments, prepared the figures and wrote the manuscript. LPJ assisted with the FCM analysis. BX and SSL examined patients, obtained specimens and generated clinical data. DJL initiated and supervised the research and edited the manuscript.
Acknowledgments
This work is supported by the National Basic Research Program of China (2006CB944007), Major International Joint Research Project of NSFC 30910103909, National Natural Science Foundation of China 31270969, National and Shanghai Leading Academic Discipline Project (211XK22), Program for Outstanding Medical Academic Leader (all to DJL) and Research Fund for Doctoral Program from Education Ministry of China 200802461019 (to LPJ).
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991;6:791–798. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- Strominger JL. Human decidual lymphocytes and the immunobiology of pregnancy. J Reprod Immunol. 2004;62:17–18. doi: 10.1016/j.jri.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Piao HLL, Tao Y, Zhu R, Wang SC, Tang CL, Fu Q, et al. The CXCL12/CXCR4 axis is involved in the maintenance of Th2 bias at the maternal/fetal interface in early human pregnancy. Cell Mol Immunol. 2012;9:423–430. doi: 10.1038/cmi.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michimata T, Tsuda H, Sakai M, Fujimura M, Nagata K, Nakamura M, et al. Accumulation of CRTH2-positive T-helper 2 and T-cytotoxic 2 cells at implantation sites of human decidua in a prostaglandin D2-mediated manner. Mol. Hum Reprod. 2002;8:181–187. doi: 10.1093/molehr/8.2.181. [DOI] [PubMed] [Google Scholar]
- Zhu XY, Zhou YH, Wang MY, Jin LP, Yuan MM, Li DJ. Blockade of CD86 signaling facilitates a Th2 bias at the maternal–fetal interface and expands peripheral CD4+CD25+ regulatory T cells to rescue abortion-prone fetus. Biol Reprod. 2005;72:338–345. doi: 10.1095/biolreprod.104.034108. [DOI] [PubMed] [Google Scholar]
- Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-β induces development of the Th17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Huang GH, Wang YY, Chi HB. Regulation of TH17 cell differentiation by innate immune signals. Cell Mol Immunol. 2012;9:287–295. doi: 10.1038/cmi.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Giovannini G, de Luca A, D'Angelo C, Casagrande A, Iannitti RG, et al. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis Cell Mol Immunol . 2012;9:276–286. doi: 10.1038/cmi.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Ma YL, Du YY, Liao M, Li H, Liang W, et al. The altered expression of inflammation-related microRNAs with microRNA-155 expression correlates with Th17 differentiation in patients with acute coronary syndrome. Cell Mol Immunol. 2011;8:486–495. doi: 10.1038/cmi.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schad E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OConnor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Kim HJ, Chang EJ, Lee ZH, Hwang SJ, Kim HM, et al. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 2009;16:1332–1343. doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- Pongcharoen S, Niumsup P, Sanguansermsri D, Supalap K, Butkhamchot P. The effect of interleukin-17 on the proliferation and invasion of JEG-3 human choriocarcinoma cells. Am J Reprod Immunol. 2006;55:291–300. doi: 10.1111/j.1600-0897.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- Amadi-Obi A, Yu CR, Liu XB, Mahdi RM, Clarke GL, Nussenblatt RB, et al. Egwuagu. Th17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Ito M, Yoneda S, Shiozaki A, Hidaka T, Saito S. Circulating and decidual Th17 cell levels in healthy pregnancy. Am J Reprod Immunol. 2010;63:104–109. doi: 10.1111/j.1600-0897.2009.00771.x. [DOI] [PubMed] [Google Scholar]
- Wu X, Li DJ, Yuan MM, Zhu Y, Wang MY. The expression of CXCR4/CXCL12 in first-trimester human trophoblast cells. Biol Reprod. 2004;70:1877–1885. doi: 10.1095/biolreprod.103.024729. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Yang H, Qiu L, Chen G, Ye Z, Lv C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2008;89:656–661. doi: 10.1016/j.fertnstert.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot-Swings GM, et al. Differential distribution of CD4+CD25bright and CD8+CD28− T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27:S47–S53. doi: 10.1016/j.placenta.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Toldi G, Molvarec A, Stenczer B, Müller V, Eszes N, Bohács A, et al. Peripheral Th1/Th2/Th17/regulatory T-cell balance in asthmatic pregnancy. Int Immunol. 2011;23:669–677. doi: 10.1093/intimm/dxr074. [DOI] [PubMed] [Google Scholar]
- Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ. Human first-trimester trophoblast cells recruit CD56brightCD16− NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol. 2005;1:61–68. doi: 10.4049/jimmunol.175.1.61. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Sato W, Aranami T, Yamamura T. Cutting edge: human Th17 cells are identified as bearing CCR2+CCR5− phenotype. J Immunol. 2007;178:7525–7529. doi: 10.4049/jimmunol.178.12.7525. [DOI] [PubMed] [Google Scholar]
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6- expressing Th17 cells to inflamed joints via CCL-20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohannl D, Benvenuto F, Bottinelli1 D, Lira S, et al. C–C chemokine receptor 6-regulated entry of Th17 cells into the CNS through the chorioid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- Ishii M, Hayakawa S, Suzuki MK, Yoshino N, Honda M, Nishinarita S, et al. Expression of functional chemokine receptors of human placental cells. Am J Reprod Immunol. 2000;44:365–373. doi: 10.1111/j.8755-8920.2000.440608.x. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Chang C, Philips JH. Human NKR-P1A. A disulphide- linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol. 1994;153:2417–2428. [PubMed] [Google Scholar]
- Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol. 2005;175:7796–7799. doi: 10.4049/jimmunol.175.12.7796. [DOI] [PubMed] [Google Scholar]
- van Beelen AJ, Teunissen MB, Kapsenberg ML, de Jong CE. Interleukin-17 in inflammatory skin disorders. Curr Opin Allergy Clin Immunol. 2007;7:374–381. doi: 10.1097/ACI.0b013e3282ef869e. [DOI] [PubMed] [Google Scholar]
- Spreu J, Kienle EC, Schrage B, Steinle A. CLEC2A: a novel, alternatively spliced and skin-associated member of the NKC encoded AICL-CD69-LLT1 family. Immunogenetics. 2007;59:903–912. doi: 10.1007/s00251-007-0263-1. [DOI] [PubMed] [Google Scholar]
- Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, et al. Circulating and gut-resident human Th17 cell express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin JD. Adhesion molecules in implantation. Rev Reprod. 1997;2:84–93. doi: 10.1530/ror.0.0020084. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Chamley L, Mitchell MD, Keelan JA. Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta. 2002;23:239–256. doi: 10.1053/plac.2001.0781. [DOI] [PubMed] [Google Scholar]
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng DF, Yu H. IL-17 can promote tumor growth through an IL-6–Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SC, Baker PN, Symonds EM. Placental apoptosis in normal human pregnancy. Am J Obstet Gynecol. 1997;177:57–65. doi: 10.1016/s0002-9378(97)70438-1. [DOI] [PubMed] [Google Scholar]
- Smith SC, Leung TN, To KF, Baker PN. Apoptosis is a rare event in first-trimester placental tissue. Am J Obstet Gynecol. 2000;183:697–699. doi: 10.1067/mob.2000.106555. [DOI] [PubMed] [Google Scholar]
- Liu YS, Wu L, Tong XH, Wu LM, He GP, Zhou GX, et al. Study on the relationship between Th17 cells and unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2011;65:503–511. doi: 10.1111/j.1600-0897.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Ito M, Shima T, Bac ND, Hidaka T, Saito S. Accumulation of IL-17-positive cells in decidua of inevitable abortion cases. Am J Reprod Immunol. 2010;64:4–11. doi: 10.1111/j.1600-0897.2010.00812.x. [DOI] [PubMed] [Google Scholar]
- Toldi G, Rigó J, Jr, Stenczer B, Vásárhelyi B, Molvarec A. Increased prevalence of IL-17-producing peripheral blood lymphocytes in pre-eclampsia. Am J Reprod Immunol. 2011;66:223–229. doi: 10.1111/j.1600-0897.2011.00987.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.