Abstract
Fucoidan can cure both antimony-sensitive and antimony-resistant visceral leishmaniasis through immune activation. However, the signaling events underlying this cellular response remain uncharacterized. The present study reveals that fucoidan induces activation of p38 and ERK1/2 and NF-κB DNA binding in both normal and Leishmania donovani-infected macrophages, as revealed by western blotting and electrophoretic mobility shift assay (EMSA), respectively. Pharmacological inhibition of p38, ERK1/2 or the NF-κB pathway markedly attenuated fucoidan-induced pro-inflammatory cytokine synthesis and inducible nitric oxide synthase (iNOS) gene transcription, resulting in a reduction of parasite clearance. To decipher the underlying mechanism of fucoidan-mediated parasite suppression, the expression and functionality of various protein kinase C (PKC) isoforms were evaluated by immunoblotting and enzyme activity assay. Fucoidan elicited an increase in expression and activity of PKC-α, -βI and -βII isoforms in infected macrophages. Functional knockdown of PKC-α and -β resulted in downregulation of p38 and ERK1/2, along with a marked reduction of IL-12 and TNF-α production in fucoidan-treated infected macrophages. Collectively, these results suggest that the curative effect of fucoidan is mediated by PKC-dependent activation of the mitogen-activated protein kinase (MAPK)/NF-κB pathway, which ultimately results in the production of nitric oxide (NO) and disease-resolving pro-inflammatory cytokines.
Keywords: fucoidan, MAPK, NF-κB, PKC, visceral leishmaniasis
Introduction
Visceral leishmaniasis (VL) is a progressive fatal infection caused by the protozoan parasite Leishmania donovani. These parasites survive the hostile environment of the macrophage by hindering their microbicidal mechanisms, such as the generation of nitric oxide (NO) and reactive oxygen species (ROS).1,2 Multiple intracellular signaling pathways stringently regulate macrophage effector responses. The protein kinase C (PKC) signaling pathway is one of the most ancient and conserved pathways and plays an important role in the generation of ROS and NO.2,3,4 One of the major tactics used by Leishmania to promote its survival within the host is the dysregulation of PKC signaling. Lipophosphoglycan, a leishmanial surface molecule, negatively regulates typical PKC isoforms by inhibiting their membrane insertion.4 Moreover, the expression of atypical PKC isoforms (PKC-ε and -ζ) increases, whereas Ca2+-dependent PKC-β expression decreases during infection.5 Deactivation of PKC-β is correlated with increased IL-10 production, a hallmark of the Th2 cytokine response associated with disease progression. Additionally, PKC signaling activates NF-κB, a major transcription factor involved in enhancing the expression of molecules that may assist in host defense.6 The importance of NF-κB in host defense against leishmaniasis was shown by gene deletion studies, as both c-Rel and p52 null mice were unable to effectively clear the infection due to impaired immune responses.7,8 Leishmania parasites were able to inhibit induction of the MAPK pathway, which is a major factor in the control of infection in response to a variety of agonists, thus helping the parasite to grow and survive within the host.9,10 Studies revealed that ERK1/2 and p38 MAPK play a key role in the regulation of inducible nitric oxide synthase (iNOS).11 Activation of p38 by anisomycin enhanced macrophage-dependent leishmanicidal effects, whereas its inhibition by Leishmania correlates well with impaired production of iNOS and NO.12 Activation of MAPK signaling pathways by various stimuli induced NF-κB activation either through the phosphorylation of its inhibitor IκB or by direct post-transcriptional modification of its p65 subunit.13 Thus, Leishmania successfully evades the macrophage microbicidal machinery and thrives in these cells through deregulating the production of NO and ROS. Establishment of an effective immune response therefore may depend on stimulation of PKC signaling as well as MAPK-mediated activation of NF-κB. Knowledge in this area could improve leishmaniasis therapy.
Fucoidan, an immunomodulatory sulfated polysaccharide from the marine brown algae Fucus vesiculosus, has been used extensively in traditional medicine. Accumulating evidence suggests that fucoidan exhibits a variety of pharmacological effects including anti-thrombotic, anti-malarial and immunomodulatory activities.14,15,16 The immunomodulatory effects of fucoidan may be associated with the expression of pro-inflammatory cytokines along with the generation of NO and ROS.17,18 Our previous study has established fucoidan as a novel antileishmanial agent.19 The antileishmanial effect of fucoidan was associated with a shift from disease-promoting Th2 to disease-resolving Th1 cytokine response in conjunction with the generation of NO and ROS. However, the intricate signaling events associated with this response are not known. The present investigation was aimed toward exploring the cellular mechanisms underlying the antileishmanial effect of fucoidan in in vitro macrophage culture and an in vivo animal model of visceral leishmaniasis.
Materials and methods
Ethics statement
The present study was carried out in agreement with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol used for animal experiments was approved by the Committee on the Ethics of Animal Experiments of the Indian Institute of Chemical Biology. All experiments conformed to the National Regulatory Guidelines issued by Committee for the Purpose of Supervision of Experiments on Animals, Ministry of Environment and Forest, Government of India.
Chemicals
All antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA). BAY 11–7085, BAY 11–7082, PKC-β inhibitor and 25Ser-substituted peptide from the pseudosubstrate region of PKC were acquired from Calbiochem (Cambridge, MA, USA). Fucoidan from F. vesiculosus was purchased from Sigma (St Louis, MO, USA) and consists primarily of a polymer of α-(1→3) linked fucose with sulfate groups substituted at the C-4 position (Figure 1a and b).20,21 The pNF-κB-luciferase plasmid containing five copies of NF-κB consensus sequences (pNF-κB Luc) was obtained from Stratagene (La Jolla, CA, USA). AP-1 and the pCMV-β-galactosidase reporter vector were purchased from Promega (Madison, WI, USA). All other chemicals were obtained from Sigma, unless otherwise indicated.
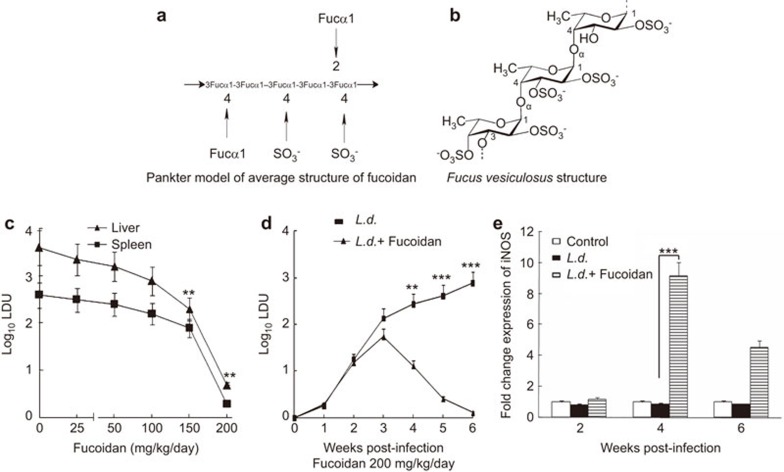
Figure 1.
Structure of fucoidan and effect of fucoidan therapy on the modulation of iNOS and parasite burden in BALB/c mice. (a) Average structure of fucoidan found in brown seaweed. (b) Monomer of fucoidan from F. vesiculosus. (c) L. donovani–infected mice were given various doses of fucoidan 14 days post-infection, as described in the section on ‘Materials and methods'. Liver and splenic parasite burden was measured 6 weeks after infection. (d) Splenic parasite burden of infected and treated mice as in (c) with 200 mg/kg/day of fucoidan was measured for the indicated time points (1–6 weeks) after infection. (e) Splenocytes from L. donovani-infected and fucoidan-treated mice were isolated at various time points; iNOS mRNA expression was evaluated by real-time PCR and expressed as a fold change compared with control. In vivo experiments were conducted with six animals per group. Results are representative of three independent experiments and are expressed as means±s.d., n=3: **P<0.001, ***P<0.0001; Student's t-test. iNOS, inducible nitric oxide synthase.
Cell culture and infections
The RAW 264.7 murine macrophage cell line was maintained in RPMI 1640 supplemented with 10% fetal bovine serum with 100 µg/ml streptomycin and 100 units/ml penicillin (Invitrogen, Carlsbad, CA, USA). Bone marrow-derived macrophages (BMMs) were cultured as described previously.5 L. donovani promastigotes (MHOM/IN/1983/AG83) were grown as described previously.22 For in vitro infections, macrophages were infected with stationary phase L. donovani promastigotes at a 10∶1 parasite/macrophage ratio. Infection was allowed to progress for 4 h, after which unphagocytized parasites were removed by washing the plates three times with medium, and the cells were allowed to grow for 20 h. Soluble leishmanial antigen was prepared by freeze–thawing the cells as described previously.23 For in vivo experiments, 4- to 6-week-old female BALB/c mice were injected with 107 L. donovani promastigotes through the tail vein. Fucoidan (25–200 mg/kg/day, given three times weekly) was given orally for a period of 4 weeks starting 14 days after infection. Infection was quantified by removing the spleen and liver from infected mice at 6 weeks, and parasite burden was calculated as Leishman–Donovan unit (LDU) after staining with Giemsa.22
Immunoblot analysis and ELISA
Immunoblot analysis was performed as described previously.22 Densitometric analyses for all experiments were carried out using QUANTITY ONE software from Bio-Rad (Hercules, CA, USA). Band intensities on the immunoblots were normalized to β-actin and expressed in arbitrary units. The ratios of the optical densities of particular bands/endogenous control are indicated as bar graphs adjacent to the figures. Levels of IL-12 and TNF-α in culture supernatants obtained from BMMs, splenocytes or RAW 264.7 cells were measured using the Quantikine mouse sandwich ELISA kit from R&D Systems (Minneapolis, MN, USA), as described previously.5 Spleen cells were stimulated with 20 µg/ml soluble leishmanial antigen for 48 h before analysis. The detection limit of these assays was >5.1 and >2.5 pg/ml for TNF-α and IL-12 p70, respectively.
Real-time PCR
Total RNA was isolated from BMMs, RAW 264.7 cells or splenocytes using the RNeasy Mini kit from Qiagen (Valencia, CA, USA), and real-time PCR was performed as described previously.5
PKC activity assay
RAW 264.7 cells were lysed in HEPES buffer (1 ml), pH 7.5, containing 5 mM EDTA, 0.25 M sucrose, 10 mM mercaptoethanol, 2 mM phenylmethylsulfonyl fluoride, 1 mM leupeptin and 0.2% Triton X-100, sonicated, kept on ice for 20 min and centrifuged at 100 000 g for 30 min. The supernatant consisted of both membrane and cytosolic fractions. PKC isoforms (α, βI and βII) were immunoprecipitated (2 µg antibody/150 µg total protein in a total volume of 300 µl) with their respective PKC antibodies (Santa Cruz Biotechnology) and captured by adding 75 µl of protein A-sepharose, incubating the sample for 2 h at 4 °C and pelleting. Beads were washed twice with radioimmunoprecipitation assay buffer (10 mM Tris-HCl, 150 mM NaCl, 1% Triton-X 100, 2 mM EDTA, 1% deoxycholate, 1 mM EGTA, 1 mM sodium orthovanadate and protease inhibitors). Beads were then resuspended in reaction buffer consisting of 100 µM ATP, 40 µg/ml of L-α phosphatidylserine and 33 µM of 1,2-sn-dioleoylglycerol. The kinase reaction was performed by adding 15 µl of buffer containing 5 µCi γ32 ATP and synthetic peptide MBP from Upstate Biotechnology (Lake Placid, NY, USA). The samples were then spotted on a phosphocellulose filter (Whatman 3MM) and washed with a solution containing 5% TCA and 10 mM sodium pyrophosphatase. A liquid scintillation counter was then used to count the radioactivity.
Transient transfection and reporter assay
RAW 264.7 cells (2×106 cells) were transfected with either AP-1 or NF-κB constructs along with the β-galactosidase expression vector in serum-free medium using Lipofectamine (Invitrogen) as described previously.24 Three hours after transfection, cells were washed and the medium was replaced with RPMI medium containing 10% FBS. NF-κB or AP-1 luciferase activity was measured using a luminometer. Cells were harvested using reporter lysis buffer (Promega), and luciferase activity was normalized with co-transfected β-galactosidase expression vector. For siRNA-mediated inhibition, the cells were transfected with 1 µg of the appropriate siRNA construct (Santa Cruz Biotechnology) according to the manufacturer's protocol and then stimulated with fucoidan 24 hours later for the indicated time periods.
Electrophoretic mobility shift assay (EMSA)
RAW 264.7 or spleen cells were resuspended in hypotonic buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.2 mM phenylmethylsulfonyl fluoride and 0.5 mM DTT) and incubated on ice for 10 min and then treated with 1% NP-40. A dounce homogenizer was used to homogenize cells, and the nuclei were separated by centrifugation at 3300g for 5 min at 4 °C. The pellet was resuspended in nuclear extraction buffer (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 0.5 mM phenylmethylsulfonyl fluoride and 0.5 mM DTT) and pelleted by centrifugation at 12 000 g for 30 min. The supernatant containing the nuclear extract was collected for EMSA. The NF-κB standard consensus sequence oligonucleotide (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) was used for the preparation of radiolabeled probes. A 100-fold molar excess of unlabeled competitor oligonucleotide was added as a control. An NF-κB supershift assay was performed as described previously.24 Antibodies against individual components of the NF-κB complex were purchased from Santa Cruz Biotechnology.
Statistical Analysis
Experiments were performed in triplicate. Triplicate macrophage cultures were used, and 5–6 mice per group were used for animal experiments. Student's t-test was performed to evaluate the statistical significance between pairs of data sets. P<0.05 was considered significant.
Results
Therapeutic effects of fucoidan
We have previously demonstrated that the curative effect of fucoidan from F. vesiculosus on experimental visceral leishmaniasis was associated with favorable host T-cell responses and NO production.19 To evaluate the potential contribution of pro-inflammatory cytokines and NO in fucoidan-mediated parasite suppression, a 200 mg/kg/day oral dose of fucoidan alone or in combination with 200 µg of anti-IFN-γ, anti-TNF-α, anti-IL-12 or control IgG (given i.p., three times weekly) was administered to L. donovani-infected mice 14 days post-infection. In another set of experiments, mice were infected and treated with fucoidan as stated above along with 5 mg/kg/day of 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine (AMT). Splenic parasite burden was then determined 6 weeks after infection. During the experiment, no marked difference in body weight was noted in any of the experimental groups. In fucoidan-treated infected mice, a dose-dependent inhibition of parasite burden was observed in both the liver and the spleen. Near-complete inhibition was observed (>99% parasite suppression) in both the spleen and the liver at a dose of 200 mg/kg/day (Figure 1c). Additionally, a time course analysis showed that parasite burden in the spleen was markedly attenuated at 4 weeks post-infection and was almost completely inhibited at 6 weeks (Figure 1d). Anticytokine monoclonal antibodies reactive against IFN-γ, TNF-α or IL-12 greatly reduced fucoidan-mediated protection (only 57%, 52% and 63% parasite suppression was observed in anti-IFN-γ, anti-TNF-α and anti-IL-12 antibody-treated mice, respectively, compared to >99% suppression in fucoidan-treated mice; see Table 1). No significant difference was found between the parasite loads in mice treated with fucoidan plus control antibody or with fucoidan alone (Table 1). Moreover, mice treated with AMT also showed much less parasite suppression (53%) compared to >99% in fucoidan-treated mice (Table 1). Consistent with these results, the mRNA levels of iNOS, the primary enzyme that facilitates the formation of NO, were upregulated in fucoidan-treated infected mice (9.15-fold induction) at 4 weeks post-infection and remained elevated even at 6 weeks post-infection (Figure 1d). These data collectively suggest that fucoidan not only confers protection against leishmaniasis, but also helps in the activation of a favorable host immune response.
Table 1. Effect of anticytokine mAbs and AMT on parasite suppression in infected micea.
| Treatment group | Splenic parasite burden (LDU) | % Parasite suppression | P value |
|---|---|---|---|
| L.d.+PBS | 380±41.2 | 0 | |
| L.d.+fucoidan | 3±0.3 | 99 | <0.0001 |
| L.d.+fucoidan+anti-IFN-γ | 163±17.2 | 57 | <0.001 |
| L.d.+fucoidan+anti-IL-12 | 141±15.6 | 63 | <0.001 |
| L.d.+fucoidan+anti-TNF-α | 198±20.2 | 52 | <0.05 |
| L.d.+fucoidan+AMT | 176±18.7 | 53 | <0.05 |
| L.d.+fucoidan+control IgG | 18±1.4 | 95 | <0.001 |
Abbreviations: AMT, 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine; LDU, Leishman–Donovan unit; mAb, monoclonal antibody.
L. donovani-infected mice were given various doses of fucoidan (200 mg/kg/day) orally (three times weekly) after 14 days of infection for 4 weeks. On day 14, mice were treated with 200 µg of TNF-α, IFN-γ, IL-12 or control IgG mAbs. In another set of experiments, AMT (5 mg/kg/day) was administered along with fucoidan three times weekly. Splenic parasite burden was evaluated 6 weeks after infection. The results are representative of three independent experiments performed in triplicate±s.d., n=3.
Involvement of MAPK pathway in the fucoidan-mediated immune response
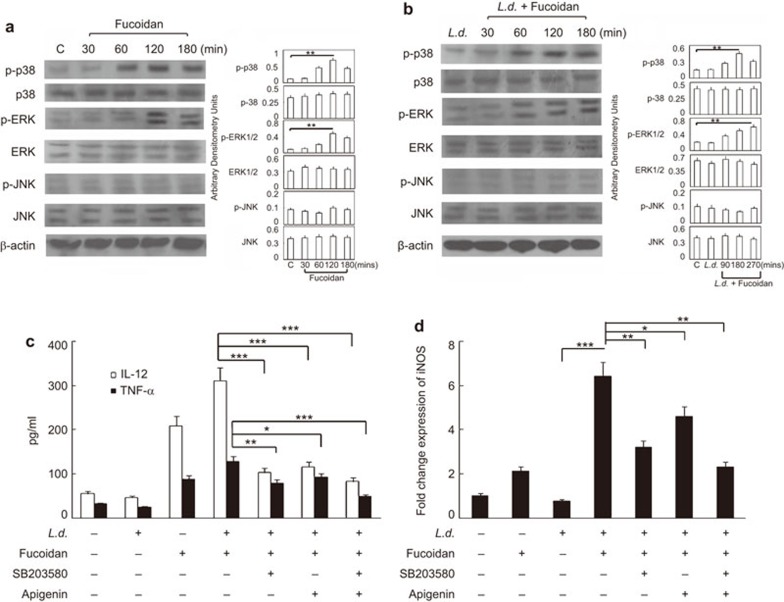
To ascertain the molecular mechanisms underlying the leishmanicidal effects of fucoidan, we examined the induction of the MAPK pathway in response to fucoidan treatment (50 µg/ml). A time course analysis (0–3 h) demonstrated a time-dependent increase in the phosphorylation status of p38 and ERK1/2 in BMMs by western blot, with a maximum induction of 5.8- and 4.7-fold at 2 h post-treatment compared to untreated control (Figure 2a). However, no significant induction was observed for p46 and p54 JNK (Figure 2a). Kinetic analysis (0–3 h) in L. donovani-infected BMMs following stimulation with fucoidan also revealed the time-dependent phosphorylation of p38 and ERK1/2 with less induction and differential kinetics (3.0- and 2.8-fold for p-p38 and p-ERK1/2, respectively, at 2 h and 3 h post-treatment) (Figure 2b). The role of the MAPK pathway in the activation of pro-inflammatory cytokines following fucoidan treatment was assessed by pre-incubating infected cells with the ERK1/2 inhibitor apigenin, the p38 inhibitor SB203580 or both for 1 h prior to stimulation with 50 µg/ml of fucoidan for 24 h. These treatments resulted in a significant decrease in both IL-12 and TNF-α levels (Figure 2c). However, the decrease in the level of TNF-α was minimal upon ERK1/2 inhibition. A comparative cytokine profile showed that IL-12 and TNF-α levels decreased by 68.9% and 41.7%, respectively, in cells treated with apigenin, whereas their levels decreased by 75.1% and 67.9% in cells treated with SB203580 (Figure 2c). Moreover, co-treatment with apigenin and SB203580 resulted in a marked decrease of 88.9% and 85.8% for IL-12 and TNF-α, respectively, in fucoidan-treated infected macrophages (Figure 2c). Increases in iNOS levels are intimately associated with the induction of the MAPK signaling cascade and confer a protective immune response in experimental visceral leishmaniasis. We therefore investigated whether fucoidan-induced expression of iNOS is under the control of the MAPK pathway. Thus, the effect of ERK1/2 and p38 inhibitors on iNOS mRNA levels was evaluated. Similar to pro-inflammatory cytokines, pre-incubation of infected BMMs with either SB203580 or apigenin for 1 h followed by stimulation with fucoidan for 24 h caused a decrease (58.6% and 33.9% decrease for SB203580 and apigenin treated cells, respectively) (Figure 2d) in iNOS mRNA expression levels. Additionally, cotreatment with ERK1/2 and p38 inhibitors resulted in a 75.3% decrease of iNOS transcript levels, implicating the involvement of p38 and, to a lesser extent, ERK1/2 in the protective immune response associated with fucoidan.
Figure 2.
Involvement of MAPKs in fucoidan-dependent pro-inflammatory cytokine response and iNOS expression in BMM. BMMs were left uninfected or infected with L. donovani for 4 h. Unphagocytized promastigotes were removed by washing, and cells were further cultured for 20 h. Normal or infected macrophages were then treated with 50 µg/ml fucoidan for different time periods. (a and b) Activation of MAPKs in fucoidan treated uninfected (a) and infected macrophages (b) was detected using western blotting. (c, d) L. donovani-infected BMMs were incubated with SB203580 (30 µM), apigenin (40 µM) or both for 1 h, followed by treatment with 50 µg/ml fucoidan for 24 h. Levels of IL-12 and TNF-α (c) in culture supernatants by ELISA and iNOS expression and (d) by real-time PCR were measured. Densitometries are shown as bar graphs on the right-hand side of each panel. The data shown are means±s.d., n=3. The results are representative of three separate experiments, *P<0.05, **P<0.001, ***P<0.0001; Student's t-test. BMM, bone marrow-derived macrophage; iNOS, inducible nitric oxide synthase; MAPK, mitogen-activated protein kinase.
Involvement of distinct PKC isoforms in the fucoidan-mediated immune response
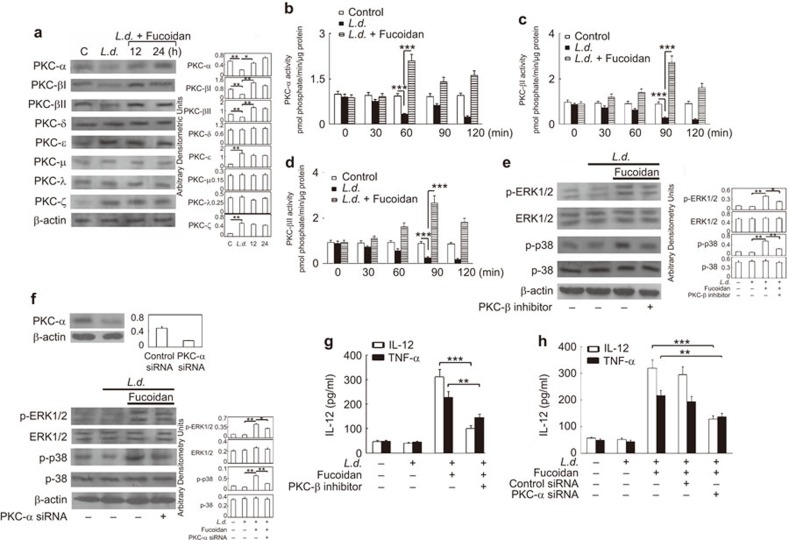
One of the vital strategies applied by Leishmania to survive in the hostile environment of the macrophage is the differential modulation of PKC isoforms, which also regulate the activity of MAPK.5,25,26 We therefore investigated the specific PKC isoforms that could be affected by infection and whether fucoidan has any role in modulating the expression and activity of these PKCs within macrophages. Because not all PKC isoforms are present in BMMs, we used the murine macrophage cell line RAW 264.7 for our subsequent experiments. Immunoblot analysis of all eight PKC isoforms (α, βI, βII, δ, ε, μ, λ and ζ) showed that PKC-ε and -ζ expression was upregulated in infected macrophages (4.5- and 4.4-fold increase as compared to control macrophages, respectively), whereas levels of PKC-α, -βI and -βII were markedly attenuated (59.6%, 54.9% and 42.6% decrease, respectively, as compared to uninfected control) (Figure 3a). No appreciable change was observed in expression levels of PKC-δ, -ε, -μ, -λ and -ζ (Figure 3a). However, treatment with fucoidan caused a marked upregulation of PKC isoforms α, βI and βII (2.2-, 3.8- and 3.4-fold, respectively) only at 12 h post-treatment (Figure 3a) without altering the expression of the other PKC isoforms. To further ascertain the role of PKC-α, -βI and -βII, immunocomplex kinase assays were performed to measure the activity of these isoforms. Interestingly, treatment of infected macrophages with fucoidan induced a time-dependent increase in the kinase activities of PKC-α, -βI and -βII with distinct activation kinetics (the maximum activity of PKC-α was 2.1-fold at 60 min post-treatment, whereas that of PKC-βI and -βII was found to be 2.7- and 2.67-fold at 90 min post-treatment, respectively, as compared to untreated control) (Figure 3b–d). Next, PKC-α and -β inhibition was used to elucidate the functional significance of the induction of PKC-α and -β expression on the phosphorylation of p38 and ERK1/2 in response to fucoidan. Pre-incubation of a PKC-β-specific inhibitor peptide effectively blocked p38 phosphorylation (67.5% reduction at 3 h post-treatment) and, to a lesser extent, ERK1/2 phosphorylation (42.5% decrease at 3 h post-treatment) (Figure 3e). Similarly, inhibition of PKC-α by siRNA resulted in a 66.2% decrease in the phosphorylation levels of p38, along with a 47.5% decrease in that of p-ERK1/2 (Figure 3F). Transfection of PKC-α-specific siRNA caused a significant reduction in PKC-α protein levels (65.9% decrease) (inset of Figure 3f). We next sought to determine whether PKC signaling plays a role in the fucoidan-mediated macrophage inflammatory response. As depicted in Figure 3g, pre-treatment of RAW 264.7 cells with a PKC-β-specific inhibitor markedly attenuated fucoidan-induced IL-12 synthesis (79.5% decrease as compared to infected and fucoidan-treated cells) and, to a lesser extent, TNF-α synthesis (46.1% decrease), whereas treatment with PKC-α siRNA caused a decrease of 72.5% and 47.6% for IL-12 and TNF-α, respectively (Figure 3h). The specificity of siRNA targeted against PKC-α was measured using western blotting. Taken together, these results show a functional correlation between differential regulation of PKC isoforms, coupled with increased p38 and ERK1/2 phosphorylation, thereby facilitating increased expression of disease-resolving pro-inflammatory cytokines in response to fucoidan treatment.
Figure 3.
Effect of fucoidan on differential induction of PKC isoforms, MAPK activation and cytokine synthesis. (a) RAW 264.7 cells were infected with L. donovani for 4 h. Unphagocytized promastigotes were removed by washing, and cells were allowed to grow for another 12 h. Macrophages were then treated with 50 µg/ml fucoidan for the indicated time periods, and immunoblot analysis was performed for different PKC isoforms. Macrophages were infected as stated above, treated with 50 µg/ml fucoidan for the indicated time periods and lysed. Immunoprecipitates were obtained using the respective PKC antibodies, and the activity of PKC-α (b), PKC-βI (c) and PKC-βII (d) was measured as stated in the section on ‘Materials and methods'. (e–h) RAW 264.7 cells were infected with L. donovani as described in (a), treated with PKC-β inhibitor for 2 h (e and g) or PKC-α siRNA for 24 h (f and h) and then treated with 50 µg/ml fucoidan. Phosphorylation levels of p38 and ERK1/2 were measured after 3 h (e and f), and levels of IL-12 and TNF-α were measured 24 h after treatment with fucoidan (g and h) (inset of (f): PKC-α siRNA specificity was determined in RAW 264.7 cell lysates expressing either PKC-α or control/scrambled siRNA by western blotting). Densitometries are shown as bar graphs. The error bar represents means±s.d., n=3. The data shown are representative of three independent experiments. **P<0.001, ***P<0.0001; Student's t-test. MAPK, mitogen-activated protein kinase; PKC, protein kinase C.
The role of NF-κB and AP-1 modulation in fucoidan-induced protective immunity
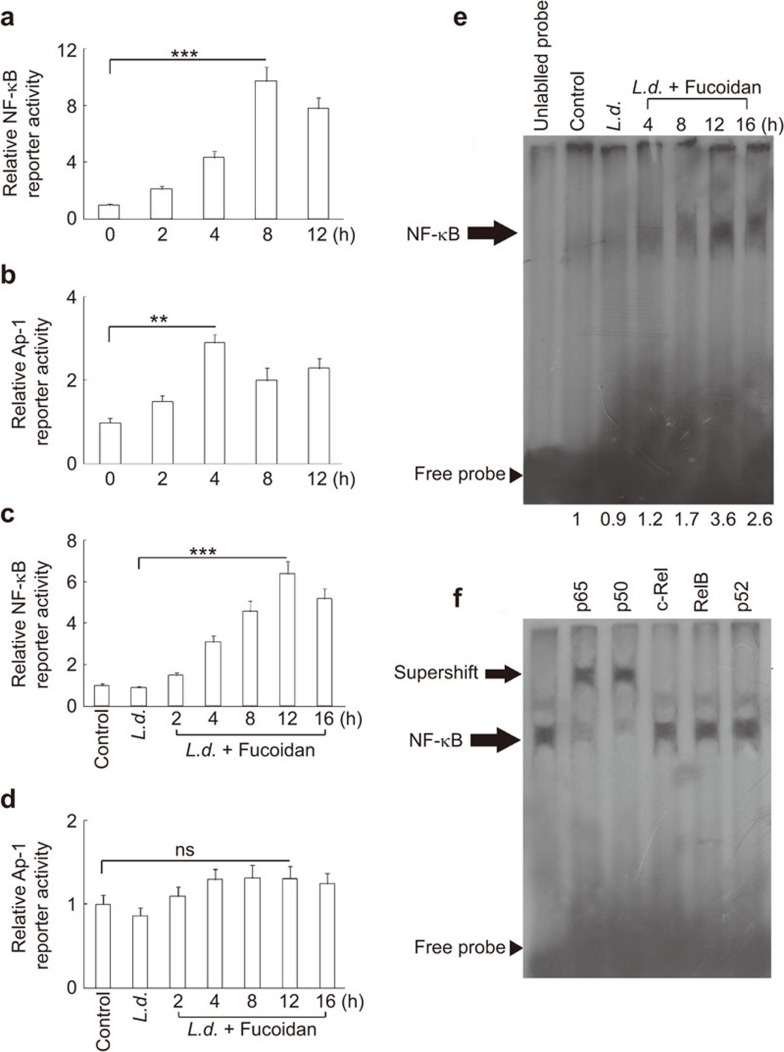
Because several pro-inflammatory genes, as well as iNOS expression, are regulated by the transcription factors NF-κB and AP-1,27 we employed a luciferase reporter assay to determine the effect of fucoidan on the activation of these transcription factors. RAW 264.7 cells were transiently transfected with a plasmid containing either an NF-κB or an AP-1-binding site, and their luciferase activities were measured. Administration of fucoidan alone at 50 µg/ml significantly increased NF-κB-dependent luciferase activity in a time-dependent manner with a maximum induction of 9.7-fold at 8 h post-treatment (Figure 4a). Fucoidan also induced the luciferase activity of AP-1 (2.8-fold as compared to untreated controls at 4 h post-treatment) (Figure 4b). We then checked whether fucoidan treatment could also modulate the luciferase activity of NF-κB and AP-1 in infected macrophages. Fucoidan elicited a time-dependent increase in NF-κB-dependent luciferase activity with a maximum induction of 6.4-fold at 12 h post-treatment in L. donovani-infected macrophages (Figure 4c). However, no significant change in AP-1-dependent luciferase activity was observed in infected macrophages (1.4-fold as compared to untreated control at 12 h) (Figure 4d). Moreover, EMSA analysis revealed that, similar to the reporter assay, fucoidan treatment elicited a marked induction in NF-κB binding (3.6-fold at 8 h post-treatment) as compared to infected control (Figure 4e). To further characterize the subunits of NF-κB that may be involved in fucoidan-mediated NF-κB activation, a supershift assay was performed using specific antibodies against p50, p52, p65, c-Rel and Rel B. Whereas anti-c-Rel, anti-p52 and anti-Rel B antibodies had no effect on the migration of the complex, anti-p65 and anti-p50 antibodies caused a marked shift of the entire signal (Figure 4f), thereby strongly indicating that fucoidan-mediated induction of NF-κB is regulated through increased nuclear translocation and DNA binding of p50 and p65 subunits.
Figure 4.
Effect of fucoidan on the modulation of NF-κB and AP-1. (a and b) RAW 264.7 cells were transfected with 1 µg of NF-κB or AP-1 luciferase reporter vector along with 0.5 µg pCMV-β-galactosidase. After 24 h, cells were treated with 50 µg/ml of fucoidan for different time periods (0–12 h) and lysed. The luciferase activity of NF-κB (a) and AP-1 (b) was measured. (c and d) Infected RAW 264.7 cells (24 h) were transfected with NF-κB or AP-1 luciferase reporter vector along with pCMV-β-galactosidase, followed by treatment with fucoidan for the indicated time periods. Luciferase activities of NF-κB (c) and AP-1 (d) were measured. (e and f) RAW 264.7 cells were infected with L. donovani promastigotes as described in Figure 3a and treated with fucoidan for the indicated time periods. (e) EMSA was performed with nuclear extracts after incubation with labeled NF-κB probe, and fold changes are indicated at the bottom. (f) The supershift assay was performed as described in the section on ‘Materials and methods' with antibodies against individual components of NF-κB complex. Statistical evaluations show means±s.d., n=3. The results are representative of three individual experiments. ** P<0.001, *** P<0.0001; Student's t-test. EMSA, electrophoretic mobility shift assay.
Involvement of NF-κB in the fucoidan-mediated regulation of iNOS and pro-inflammatory cytokines
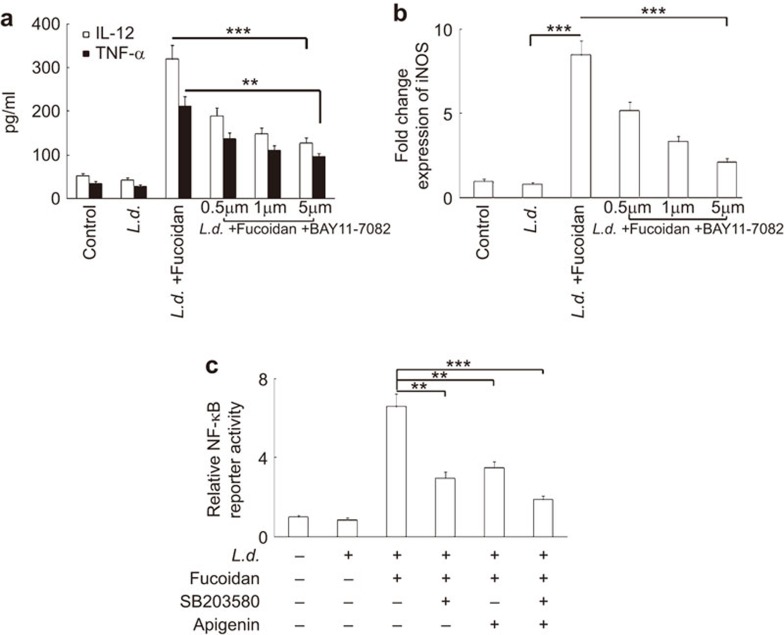
To further evaluate the role of NF-κB in the regulation of fucoidan-induced cytokine balance and iNOS expression, we used BAY 11-7082, a pharmacological inhibitor of NF-κB. To this end, infected cells were incubated with increasing concentrations of BAY 11-7082 (0.5–5 µM), followed by treatment with fucoidan for 24 h. Levels of IL-12 and TNF-α were then assessed by ELISA, and these studies revealed that BAY 11-7082 markedly reduced the levels of IL-12 and TNF-α in a concentration-dependent manner. At higher doses (5 µM), levels of IL-12 and TNF-α were suppressed by 71.6% and 65.6%, respectively (Figure 5a). Consistent with our ELISA studies, BAY 11-7082 treatment significantly decreased the level of iNOS transcript with increasing doses of the inhibitor, and a maximum inhibition of 85.2% was observed at a dose of 5 µM (Figure 5b). As the induction of NF-κB requires increased MAPK activity, we thought it worthwhile to investigate the role of p38 and ERK1/2 MAPK in this context. Pre-incubation of p38 inhibitor SB203580 (30 µM) resulted in a 64.8% decrease in fucoidan-induced NF-κB luciferase reporter activity, whereas a 55.5% decrease was observed when cells were pre-incubated with 40 µM apigenin (Figure 5c). However, a combination of SB203580 and apigenin markedly inhibited NF-κB luciferase activation (84.2%), suggesting that both p38 and ERK1/2 MAPK are required to maintain the fucoidan-mediated NF-κB activation. Taken together, these results suggest a functional correlation between p38 and ERK1/2 MAPK activation with the induction of NF-κB, which in turn facilitates iNOS and Th1 cytokine expression in a fucoidan-mediated leishmanicidal response.
Figure 5.
Effect of fucoidan on the modulation of iNOS and pro-inflammatory cytokines by NF-κB. Infected RAW 264.7 cells (24 h) were treated for 1 h with BAY 11-7082 (0.5–5 µM) and then treated with 50 µg/ml of fucoidan for 24 h. (a) IL-12 and TNF-α levels were measured by ELISA. L. donovani-infected RAW 264.7 cells were incubated with SB203580 (30 mM), apigenin (40 mM) or both for 1 h, followed by treatment with 50 mg/ml fucoidan for 24 h. (b) iNOS mRNA expression was evaluated using Taqman probes and expressed as fold change compared with control. (c) NF-κB reporter assay was performed as described in the legend of Figure 4c. The results shown are representative of three individual experiments. The data represent means±s.d., n=3. **P<0.001, ***P<0.0001; Student's t-test. iNOS, inducible nitric oxide synthase.
Effect of fucoidan on the modulation of NF-κB in vivo
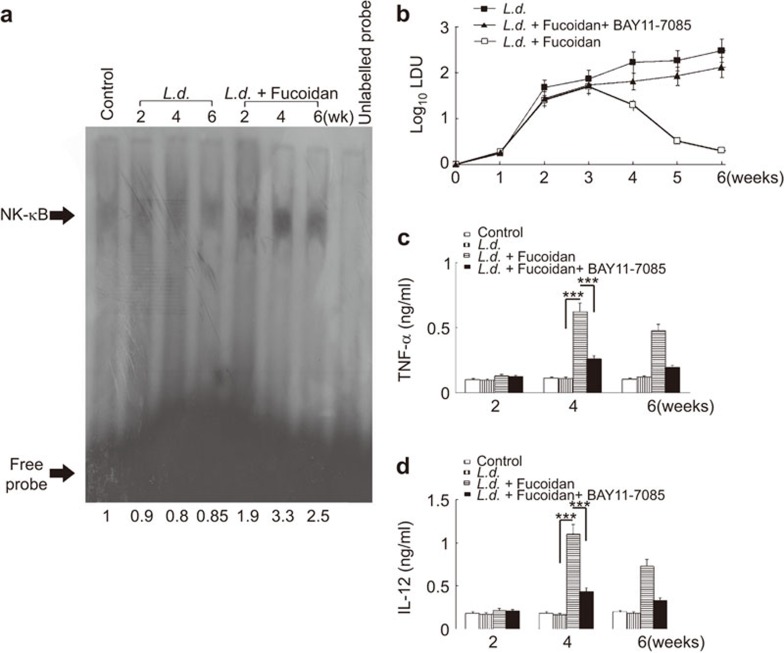
To ascertain the role of fucoidan on the modulation of the NF-κB pathway in vivo, fucoidan (200 mg/kg/day) was administered orally to L. donovani-infected BALB/c mice three times weekly for 4 weeks starting at 14 days post-infection, and splenocytes were isolated for subsequent analysis. The effect of fucoidan on NF-κB binding activity was examined by EMSA. Consistent with our in vitro finding, fucoidan treatment significantly increased the NF-κB binding in infected mice as compared to untreated controls and was found to be at its maximum at 4 weeks post-infection (3.3-fold) (Figure 6a). To further substantiate the effect of NF-κB pathways in the fucoidan-mediated curative response, the NF-κB inhibitor BAY 11-7085 (5 mg/kg/day, given thrice weekly) was administered to separate groups of infected mice simultaneously treated with fucoidan over a 4-week period. Splenic parasite burden and levels of IL-12 and TNF-α were measured in all groups (infected, infected fucoidan-treated and infected fucoidan-treated+BAY 11-7085-treated). No marked effect on body weight was noted in any of the experimental groups during the experiment. Complete inhibition of splenic parasite burden was observed in fucoidan-treated animals at 6 weeks (Figure 6b). Similar to the in vitro findings, fucoidan treatment elicited a robust increase in both IL-12 and TNF-α synthesis (6.1- and 5.5-fold, respectively) in infected mice at 4 weeks, which remained significantly elevated even after 6 weeks (Figure 6c and d). Interestingly, in vivo pharmacological inhibition of NF-κB by BAY 11-7085 markedly attenuated the fucoidan-induced TNF-α and IL-12 cytokine production (71.6% and 73.2% decrease, respectively, at 4 weeks post-infection) (Figure 6c and d). This decrease in pro-inflammatory cytokine synthesis has further been reflected in in vivo parasitemia. Fucoidan-mediated suppression of splenic parasite burden was significantly reversed in the NF-κB inhibitor treated mice (67.3% reduction at 6 weeks post-treatment) (Figure 6b). Collectively, these results suggest that NF-κB activation plays an important role in the fucoidan-mediated antileishmanial response and is vital for the generation of protective immunity in vivo.
Figure 6.
Effect of fucoidan on NF-κB mediated anti-leishmanial response. (a) Splenocytes from infected and infected treated mice were isolated at the indicated time points, as described in the legend of Figure 1b. EMSA for NF-κB was performed with nuclear extracts of splenocytes after incubation with NF-κB labeled probe. Bands were analyzed by densitometry, and fold changes are indicated at the bottom. (b–d) In another set of experiments, mice were infected with L. donovani and treated with fucoidan alone or fucoidan along with BAY11-7085 (5 mg/kg/day). Splenic parasite burden (b) was determined by Giemsa staining and levels of TNF-α (c) and IL-12 (d) were determined by ELISA. The animal experiments were carried out using five mice per group. The results are representative of three individual experiments and the error bars represent mean±s.d., n=3. ***P<0.0001; Student's t-test. EMSA, electrophoretic mobility shift assay.
Discussion
The occurrence of Leishmania infection depends on successful suppression of the Th1 response along with induction of the Th2 response.28,29 Control of visceral leishmaniasis depends on stimulating the host signaling cascades that lead to the activation of phagocytic cells, which in turn successfully control and largely determine the clearance of parasitemia.28 In this context, natural dietary components are gaining immense importance as potent immunomodulators with multifarious roles and targets, as they not only boost the immune system to produce microbicidal molecules such as NO and ROS, but also modulate the Th1/Th2 cytokine balance in favor of the host.30,31 Although the mouse model of VL suffers from several limitations as it differs from symptomatic human VL, it remains a good experimental model for early infection of L. donovani. The present study has exploited the use of a mouse model of VL up to 6 weeks, a time frame for which it closely mimics human VL, and can be used for further development of fucoidan as a therapeutic target against L. donovani. Fucoidan from F. vesiculosus is a homofucan that has a simple chemical composition, mainly fucose and sulfate.32 Carbohydrate analysis of fucoidan from F. vesiculosus predicted that it contains 97% fucose with only trace amounts of galactose, xylose or uronic acid.33 Moreover, the molecular weight of fucoidan from F. vesiculosus ranges from 100 to 180 kDa, implying that fucoidan from F. vesiculosus has a simple structure and only contains sulfate groups.34 In macrophages, fucoidan has been known to induce the expression and activity of PKC, along with NF-κB-mediated induction of iNOS.17,35 Our previous study also revealed the role of fucoidan as a potent antileishmanial agent against antimony-sensitive and antimony-resistant L. donovani, both in vitro and in vivo, through the upregulation of NO and ROS as well as the induction of pro-inflammatory cytokines.19 The present study demonstrated that fucoidan-mediated induction of iNOS and pro-inflammatory cytokines was dependent on the activation of p38 and ERK1/2 MAPK, which in turn activated NF-κB and the antileishmanial response associated with it. Interestingly, further investigations illuminated the functional involvement of distinct PKC isoforms in fucoidan-mediated MAPK activation. Moreover, the present investigation provides the first in vivo evidence that activation of NF-κB by fucoidan may be a prerequisite for shifting the cytokine balance toward the disease-resolving Th1 mode for complete elimination of organ parasite burden.
Leishmania infection is associated with a significant downregulation of the MAPK/NF-κB pathway, signifying a parasite survival mechanism by which they dampen the anti-parasitic inflammatory response of the host.8,9 A growing body of evidence suggests that production of leishmanicidal molecules such as ROS and NO by many drugs, including sodium antimony gluconate, might result from activation of the host MAPK pathway.3 The present study also indicated that the fucoidan-mediated increase in iNOS levels is primarily dependent upon p38 and ERK1/2 activation. Interestingly, IL-12 levels were found to be primarily controlled by p38, whereas those of TNF-α were controlled by ERK1/2. However, the combination of p38 and ERK1/2 inhibitors was much more effective than either used alone, indicating that both p38 and ERK1/2 are involved in the pro-inflammatory response induced by fucoidan. The microbicidal activity of macrophages is dependent upon normal PKC signaling, as pathogenic microbes tend to dysregulate the PKC pathway to avoid host-protective immune responses in macrophages.2,4 Leishmania parasites differentially regulate PKC-dependent signaling to survive in the hostile macrophage environment.5,25 The inhibition of Ca2+-dependent PKC isoforms, in particular PKC-β, has already been established; however, accumulating evidence also indicates a role for the induction of Ca2+-independent PKC isoforms such as PKC-ε and -ζ.5,25 The present study demonstrates that a significant increase in protein levels of host-protective PKC-α, -βI and -II isoforms occurs following fucoidan treatment. Our study reveals a discrepancy in the timing of the expression of PKC isoforms (at 12 h post-treatment) and the phosphorylation of MAPK (at 2 h post-treatment), two intimately associated events. However, further exploration of PKC activity, confirmed by in vitro kinase activities, correlates well with increased MAPK activation. Moreover, functional inhibition of PKC-α and -β markedly abrogated fucoidan-induced p38 phosphorylation and, to a lesser extent, that of ERK1/2, along with the induction of IL-12 and TNF-α, which suggests that the fucoidan-mediated leishmanicidal effect may be dependent upon differential regulation of PKC isoforms, in agreement with similar observations.36
Recent evidence suggests a crucial role for the NF-κB family of transcription factors in expressing pro-inflammatory genes and NO, which are pivotal for controlling Leishmania infection.8 Some studies suggest that the AP-1 transcription factor may also be involved in NO production.27 We found a significant upregulation of NF-κB-dependent luciferase activity in infected macrophages, but the same was not true for AP-1. The most prominent NF-κB complex present is the p50/p65 heterodimer, which plays an essential role in activation of the classical pathway, and many anti-leishmanial agents are reported to act through the induction of the p50/p65 complex.22,24 Our studies suggest that the p65 and p50 subunits of NF-κB are mainly responsible for fucoidan-mediated activation of NF-κB DNA binding using a supershift assay, which may contribute to the induction of host-protective inflammatory gene expression. Recent evidence suggests a direct correlation between MAPK activation and NF-κB induction leading to upregulation of the disease-resolving Th1 response along with NO generation. Our results further indicate that fucoidan-mediated induction of NF-κB, as well as the cellular and immunological response is dependent on both p38 and ERK1/2, as cotreatment with fucoidan and p38 and ERK inhibitors resulted in a much more significant decrease in NF-κB-dependent luciferase activity as compared to either p38 or ERK inhibitor alone. Mitogen- and stress-activated protein kinase 1, a downstream kinase of both p38 and ERK1/2, activates NF-κB through phosphorylation of its p65 subunit, and inhibition of p38 or ERK1/2 or both was able to significantly reduce NF-κB DNA binding activity, suggesting a possible role of mitogen- and stress-activated protein kinase 1 in this context. Using a pharmacological inhibitor of NF-κB, we demonstrated that it is essential for the generation of IL-12 and TNF-α culminating in iNOS induction both in vitro and in vivo. Fucoidan-mediated suppression of splenic parasite burden was significantly attenuated by inhibiting NF-κB, which suggests that NF-κB has an important role in the suppression of parasitemia. In line with our previous observation, which suggested that fucoidan-mediated induction of pro-inflammatory cytokines as well as generation of NO is the highest at 4 weeks post-infection,19 we found a time-dependent increase in the DNA binding activity of NF-κB in vivo, which also peaked at 4 weeks. However, clearance of the organ parasite burden was found to be highest at 6 weeks after fucoidan treatment, which seemed relevant to the culmination of effector responses of the cellular defense machinery.
Overall, the results of the present study have shed light on the immunomodulatory and leishmanicidal effect of fucoidan, which is mediated through differential contribution of PKC isoforms. Furthermore, sequential activation of p38, ERK1/2 and modulation of its target transcription factor NF-κB promoted signal transduction, resulting in activation of macrophage immune functions during the fucoidan-mediated leishmanicidal effect. However, further in-depth study is required for a better understanding of the role of fucoidan, not only for the treatment of non-healing leishmaniasis, but also for other chronic infections.
Acknowledgments
This work was supported by the Network Project (BSC 0206), a Supra Institutional Project (BSC 0114) grant of the Council of Scientific and Industrial Research and the J C Bose Fellowship (DST), Government of India.
References
- Gantt KR, Goldman TL, McCormick ML, Miller MA, Jeronimo SM, Nascimento ET, et al. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J Immunol. 2001;167:893–901. doi: 10.4049/jimmunol.167.2.893. [DOI] [PubMed] [Google Scholar]
- Olivier M, Brownsey RW, Reiner NE. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc Natl Acad Sci USA. 1992;89:7481–7485. doi: 10.1073/pnas.89.16.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookerjee Basu J, Mookerjee A, Sen P, Bhaumik S, Sen P, Banerjee S, Naskar K, et al. Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob Agents Chemother. 2006;50:1788–1797. doi: 10.1128/AAC.50.5.1788-1797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoteaux A, Matlashewski G, Turco SJ. Inhibition of macrophage protein kinase C-mediated protein phosphorylation by Leishmania donovani lipophosphoglycan. J Immunol. 1992;149:3008–3015. [PubMed] [Google Scholar]
- Kar S, Ukil A, Sharma G, Das PK. MAPK-directed phosphatases preferentially regulate pro- and anti-inflammatory cytokines in experimental visceral leishmaniasis: involvement of distinct protein kinase C isoforms. J Leukoc Biol. 2010;88:9–20. doi: 10.1189/jlb.0909644. [DOI] [PubMed] [Google Scholar]
- Chen BC, Lin WW. PKC- and ERK-dependent activation of I kappa B kinase by lipopolysaccharide in macrophages: enhancement by P2Y receptor-mediated CaMK activation. Br J Pharmacol. 2001;134:1055–1065. doi: 10.1038/sj.bjp.0704334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis G, Zhan Y, Grumont RJ, Metcalf D, Handman E, Cheers C, et al. The Rel subunit of NF-kappaB-like transcription factors is a positive and negative regulator of macrophage gene expression: distinct roles for Rel in different macrophage populations. EMBO J. 1996;15:7099–7107. [PMC free article] [PubMed] [Google Scholar]
- Speirs K, Caamano J, Goldschmidt MH, Hunter CA, Scott P. NF-kappa B2 is required for optimal CD40-induced IL-12 production but dispensable for Th1 cell differentiation. J Immunol. 2002;168:4406–4413. doi: 10.4049/jimmunol.168.9.4406. [DOI] [PubMed] [Google Scholar]
- Martiny A, Meyer-Fernandes JR, de Souza W, Vannier-Santos MA. Altered tyrosine phosphorylation of ERK1 MAP kinase and other macrophage molecules caused by Leishmania amastigotes. Mol Biochem Parasitol. 1999;102:1–12. doi: 10.1016/s0166-6851(99)00067-5. [DOI] [PubMed] [Google Scholar]
- Nandan D, Lo R, Reiner NE. Activation of phosphotyrosine phosphatase activity attenuates mitogen-activated protein kinase signaling and inhibits c-FOS and nitric oxide synthase expression in macrophages infected with Leishmania donovani. Infect Immun. 1999;67:4055–4063. doi: 10.1128/iai.67.8.4055-4063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajizian SJ, English BK, Meals EA. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J Infect Dis. 1999;179:939–944. doi: 10.1086/314659. [DOI] [PubMed] [Google Scholar]
- Junghae M, Raynes JG. Activation of p38 mitogen-activated protein kinase attenuates Leishmania donovani infection in macrophages. Infect Immun. 2002;70:5026–5035. doi: 10.1128/IAI.70.9.5026-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebeler M, Gillitzer R, Kilian K, Utzel K, Bröcker EB, Rapp UR, et al. Multiple signaling pathways regulate NF-kappaB-dependent transcription of the monocyte chemoattractant protein-1 gene in primary endothelial cells. Blood. 2001;97:46–55. doi: 10.1182/blood.v97.1.46. [DOI] [PubMed] [Google Scholar]
- Barroso EM, Costa LS, Medeiros VP, Cordeiro SL, Costa MS, Franco CR, et al. A non-anticoagulant heterofucan has antithrombotic activity in vivo. Planta Med. 2008;74:712–718. doi: 10.1055/s-2008-1074522. [DOI] [PubMed] [Google Scholar]
- Chen JH, Lim JD, Sohn EH, Choi YS, Han ET. Growth-inhibitory effect of a fucoidan from brown seaweed Undaria pinnatifida on Plasmodium parasites. Parasitol Res. 2009;104:245–250. doi: 10.1007/s00436-008-1182-2. [DOI] [PubMed] [Google Scholar]
- Kim MH, Joo HG. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett. 2008;115:138–143. doi: 10.1016/j.imlet.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Suzuki H, Wada Y, Kodama T, Doi T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-kappaB-dependent signaling pathways through macrophage scavenger receptors. Biochem Biophys Res Commun. 2006;343:286–294. doi: 10.1016/j.bbrc.2006.02.146. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Teruya K, Eto H, Shirahata S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS ONE. 2011;6:e27441. doi: 10.1371/journal.pone.0027441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S, Sharma G, Das PK. Fucoidan cures infection with both antimony-susceptible and -resistant strains of Leishmania donovani through Th1 response and macrophage-derived oxidants. J Antimicrob Chemother. 2011;66:618–625. doi: 10.1093/jac/dkq502. [DOI] [PubMed] [Google Scholar]
- Li B, Lu F, Wei X, Zhao R. Fucoidan: structure and bioactivity. Molecules. 2008;13:1671–1695. doi: 10.3390/molecules13081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkamp AD, Kelly S, Ulber R, Lang S. Fucoidans and fucoidanases—focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl Microbiol Biotechnol. 2009;82:1–11. doi: 10.1007/s00253-008-1790-x. [DOI] [PubMed] [Google Scholar]
- Ukil A, Biswas A, Das T, Das T. 18 Beta-glycyrrhetinic acid triggers curative Th1 response and nitric oxide up-regulation in experimental visceral leishmaniasis associated with the activation of NF-kappa B. J Immunol. 2005;175:1161–1169. doi: 10.4049/jimmunol.175.2.1161. [DOI] [PubMed] [Google Scholar]
- Das L, Datta N, Bandyopadhyay S, Das PK. Successful therapy of lethal murine visceral leishmaniasis with cystatin involves up-regulation of nitric oxide and a favorable T cell response. J Immunol. 2001;166:4020–4028. doi: 10.4049/jimmunol.166.6.4020. [DOI] [PubMed] [Google Scholar]
- Kar S, Ukil A, Das PK. Signaling events leading to the curative effect of cystatin on experimental visceral leishmaniasis: involvement of ERK1/2, NF-kappaB and JAK/STAT pathways. Eur J Immunol. 2009;39:741–751. doi: 10.1002/eji.200838465. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Ghosh S, Sen P, Roy S, Majumdar S. Selective impairment of protein kinase C isotypes in murine macrophage by Leishmania donovani. Mol Cell Biochem. 2001;216:47–57. doi: 10.1023/a:1011048210691. [DOI] [PubMed] [Google Scholar]
- Giorgione JR, Turco SJ, Epand RM. Transbilayer inhibition of protein kinase C by the lipophosphoglycan from Leishmania donovani. Proc Natl Acad Sci USA. 1996;93:11634–11639. doi: 10.1073/pnas.93.21.11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Bhattacharyya S, Sirkar M, Sa GS, Das T, Majumdar D, et al. Leishmania donovani suppresses activated protein 1 and NF-kappaB activation in host macrophages via ceramide generation: involvement of extracellular signal-regulated kinase. Infect Immun. 2002;70:6828–6838. doi: 10.1128/IAI.70.12.6828-6838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A, Mathur RK, Saha B. Immune response to Leishmania infection. Indian J Med Res. 2004;119:238–258. [PubMed] [Google Scholar]
- Rogers KA, DeKrey GK, Mbow ML, Gillespie RD, Brodskyn CI, Titus RG. Type 1 and type 2 responses to Leishmania major. FEMS Microbiol Lett. 2002;209:1–7. doi: 10.1111/j.1574-6968.2002.tb11101.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Pando R, Aguilar-Leon D, Orozco H, Serrano A, Ahlem C, Trauger R, et al. 16alpha-Bromoepiandrosterone restores T helper cell type 1 activity and accelerates chemotherapy-induced bacterial clearance in a model of progressive pulmonary tuberculosis. J Infect Dis. 2005;191:299–306. doi: 10.1086/426453. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S, Gupta G, Bhattacharya P, Mukherjee A, Mujumdar SB, Pal P, et al. Quassin alters the immunological patterns of murine macrophages through generation of nitric oxide to exert antileishmanial activity. J Antimicrob Chemother. 2009;63:317–324. doi: 10.1093/jac/dkn479. [DOI] [PubMed] [Google Scholar]
- Queiroz KC, Medeiros VP, Queiroz LS, Abreu LR, Rocha HA, Ferreira CV, et al. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed Pharmacother. 2008;62:303–307. doi: 10.1016/j.biopha.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Patankar MS, Oehninger S, Barnett T, Williams RL, Clark GF. A revised structure for fucoidan may explain some of its biological activities. J Biol Chem. 1993;268:21770–21776. [PubMed] [Google Scholar]
- Suppiramaniam V, Vaithianathan T, Manivannan K, Dhanasekaran M, Parameshwaran K, Bahr BA. Modulatory effects of dextran sulfate and fucoidan on binding and channel properties of AMPA receptors isolated from rat brain. Synapse. 2006;60:456–464. doi: 10.1002/syn.20319. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Chiu SL, Wen MH, Chen KY, Hua KF. Ligands of macrophage scavenger receptor induce cytokine expression via differential modulation of protein kinase signaling pathways. J Biol Chem. 2001;276:28719–28730. doi: 10.1074/jbc.M011117200. [DOI] [PubMed] [Google Scholar]
- Sudan R, Srivastava N, Pandey SP, Majumdar S, Saha B. Reciprocal regulation of protein kinase C isoforms results in differential cellular responsiveness. J Immunol. 2012;188:2328–2337. doi: 10.4049/jimmunol.1101678. [DOI] [PubMed] [Google Scholar]