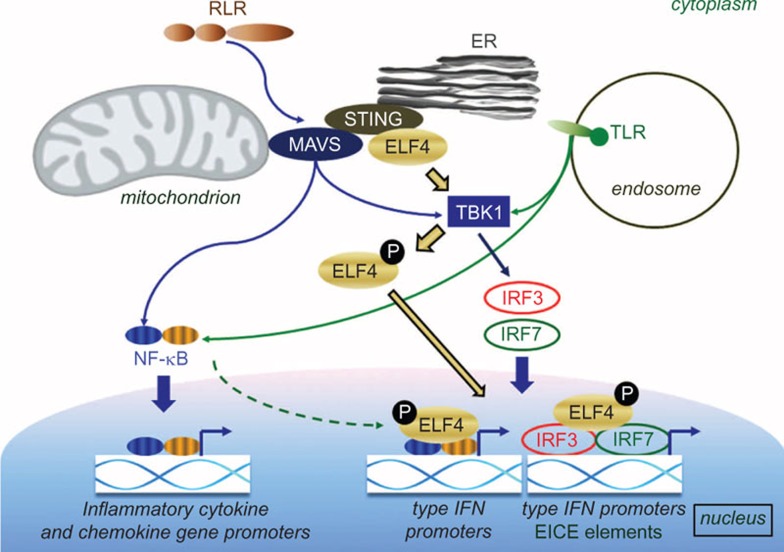
Type I interferon (IFN) production is a key event during innate immune responses. This early and prompt mechanism is mediated by multiple factors that involve various pattern recognition receptors (PRRs), adaptor proteins, kinases and transcription factors. Stimulator of IFN genes (STING) is an important transmembrane adaptor protein that plays a role in the activation of downstream transcription factors, such as IFN regulatory factor 3 (IRF3) and signal transducer and activator of transcription 6, via TANK-binding kinase 1 (TBK1), which has been considered the main innate immune defense weaponry against viruses and intracellular pathogens. In a current issue of Nature Immunology, You et al. showed that, after viral infection, the ETS-related transcription factor ELF4 (previously known as MEF) interacts with STING and is consequently activated by TBK1. This process leads to the nuclear translocation of ELF4 and its binding to IFN promoters (Figure 1). The group also demonstrated that ELF4 acts as an IFN transcription factor, as it has the potential to increase the binding affinity of IRF3 and IRF7 through cooperative binding to newly identified enhancer elements (EICE) in the IFN promoters.1 These new findings not only extend our knowledge about the details of the fine-tuning of the innate IFN responses, but also represent a new milestone by describing the role of EICE elements in this process.
Figure 1.
The role of ELF4 in type I IFN signaling. Cellular signals induced by the specific activation of TLRs or RLRs are transmitted through the MAVS/STING pathway to NF-κB and TBK1. The subsequent phosphorylation of NF-κB leads to its nuclear translocation and the expressional control of inflammatory and chemokine genes. TBK1 is an essential cytoplasmic kinase, which upon PRR activation phosphorylates the transcription factors IRF3 and IRF7. Activated IRFs move to the nucleus and induce type I IFN production. The PRR-induced interaction of ELF4 with STING results in its phosphorylation by TBK1. Activated ELF4 is then translocated to the nucleus where it increases the binding affinity of IRF3, IRF7 and the NF-κB subunit p65 to type I IFN promoters through binding of IRF3/7 to the EICE elements. IFN, interferon; IRF, interferon regulatory factor; PRR, pattern recognition receptor; RLR, RIG-I-like receptor; STING, stimulator of interferon gene; TBK, TANK-binding kinase; TLR, Toll-like receptors.
Innate immune responses are initiated by invading pathogens through the detection of their evolutionally conserved pathogen-associated molecular patterns. Detection of viral pathogen-associated molecular patterns is carried out by intracellular Toll-like receptors (TLRs), RIG-I-like receptors (RLRs) and several other nucleic acid sensors, such as AIM2-like receptors.2 As a result of pathogen-associated molecular pattern-induced activation, PRRs trigger NF-κB-dependent inflammatory cytokine and chemokine responses and the production of type I IFNs through the activation of IRF3 and IRF7.3 Type I IFNs are critical components in the establishment of the antiviral state and the resistance of host cells to viral replication. PRRs use different adaptor proteins to link the downstream signals to the NF-κB and IRF transcription factors. A crucial element in these pathways is mitochondrial MAVS (also known as VISA, Cardif or IPS-1), which is an ancient adaptor protein that couples RLRs to NF-κB and promotes TBK1 and IRF3 signaling (Figure 1). Previous studies have revealed that the ETS family transcription factor ELF4 takes part in various cellular processes, such as tumorigenesis,4 the DNA damage response5 and cell cycle regulation.6 Despite the growing body of research in this field, the exact physiological role of ELF4 remained unknown.
In their article, You et al. demonstrate that ELF4 is a critical factor in the regulation of type I IFN responses and plays a significant role in the antiviral host defense. ELF4 was shown to interact with STING, as demonstrated by coimmunoprecipitation analysis in HeLa cells. The type I IFN-inducing effect of ELF4 was also tested at both the mRNA and protein levels, and it was shown that the overexpression of this ETS protein was able to induce type I IFN secretion in 293T cells. Using another reporter assay, the group also showed that the ability of ELF4 to induce type I IFN production depended on the C-terminal region of the protein, which is responsible for its interaction with STING. Owing to the ability of ELF4 to induce IFN responses, the group then examined the response of ELF4 to viral challenges. These results showed that the expression of the Elf4 gene was increased by both viral challenge (SeV or VSV) and IFN-β stimulation. Furthermore, overexpression of the ELF4 protein led to the inhibition of viral replication, as was shown by immunoblot and plaque-forming assays. These results confirmed that the ETS domain of ELF4 was essential in the establishment of this antiviral activity. To demonstrate the in vivo significance of ELF4 in the antiviral host defense, the sensitivity of Elf4−/− mice to lethal WNV infection was compared to that of wild-type mice. The results showed that the ELF4-deficient mice were significantly more susceptible to virus exposure than their wild-type counterparts. The type I IFN production of macrophages isolated from Elf4−/− mice was also impaired, showing that ELF4 was involved in the induction of IFN production and antiviral immunity against WNV. These findings are consistent with previous reports that showed the importance of ELF4 in antiviral cellular immune responses.6,7 Interestingly, the group also found that ELF4 controls viral replication in vivo by directly regulating the type I IFN response, not through the contribution of natural killer and natural killer T cells or cytotoxic T lymphocytes.
One of the most interesting aspects of this study is the demonstration that ELF4 also participates in TLR, RLR and dsDNA receptor-mediated signaling. Using various human and mouse cell types and a wide range of experimental approaches, You et al. showed that ELF4 is essential for TLR3/4/7/9- and RLR-induced signal transduction and acts downstream of MyD88, TRIF, STING and MAVS. Furthermore, using an elegant experimental set-up, they also demonstrated that, upon activation, ELF4 becomes phosphorylated by TBK1 and is translocated into the nucleus in a STING- and MAVS-dependent manner. When the two main downstream pathways of PRR activation were investigated, the group found that ELF4 is crucial for the efficient binding of NF-κB, IRF3 and IRF7 to the various IFN promoters (Figure 1). The chromatin immunoprecipitation and quantitative PCR results showed that the VSV-induced translocation of IRF3 and p65 to the Ifnb1 gene and the translocation of IRF7 to the Ifna4 promoters were dramatically reduced in the Elf4−/− macrophages. The EICE element in the Ifnb1 promoter appeared to be a critical component that supported the cooperative interaction of ELF4, IRF3 and p65. Thus, this group demonstrated the importance of ELF4 in the binding of the downstream elements IRF3, IRF7 and p65 to the IFN promoters (Figure 1). Similar to IRF7, the baseline expression of ELF4 was shown to be low in many tissues, which may be a built-in mode in resting cells to prevent unwanted inflammatory reactions in the absence of viral stimuli.
Identifying ELF4 as a key element of the intracellular nucleic acid sensing PRR-mediated signaling pathways may open up novel ways to manipulate the antiviral and innate immune responses. Consistent with its primary role in the rapid upregulation of type I IFN secretion upon viral infection, which results in the effective clearance of intracellular pathogens along with the induction of IFN-stimulated genes, the manipulation of IFN responses through ELF4 may open up new avenues in the treatment of infectious diseases. These avenues involve the targeted stimulation of innate immune receptors and/or type I IFN-producing immune cell subsets.8 However, misguided IFN responses targeting the host tissues may cause severe symptoms and subsequently lead to the development of adaptive immune responses against self structures.9 In contrast, the targeted inhibition of ELF4 may result in type I IFN-dependent immune suppression, which may present new strategies for drug design against autoimmune diseases. Because type I IFNs have recently emerged as potent anticancer agents,10 these findings might also be of particular importance in future anticancer therapies. It has been observed that some cancer patients exhibit adaptive immune responses against the tumor tissues, and these responses are mediated by activated and infiltrating effector T cells, as well as by the production of cancer-specific antibodies, which exhibit an extreme prognostic value at the early stages of the disease. Recent studies also demonstrated that immune responses elicited against certain types of tumors exhibited a characteristic type I IFN signature, which has been shown to support the development of host CTL responses against the tumor tissue (reviewed by Gajewski et al.).11 The requirement of dendritic cells in the production of type I IFN and in the initiation of this anticancer response suggests the existence of a PRR-based innate immune mechanism that may involve ELF4 activity. ELF4 may also play an important role in various autoimmune pathologies. In multiple sclerosis and rheumatoid arthritis, local IFNβ secretion induces the IFN-stimulated genes, as well as IL-10 and IL-1β secretion, which result in the suppression of autoimmunity. In contrast, the increased levels of IFN-α that are observed in systemic lupus erythematosus result in inflammation and autoimmunity, and mice deficient in type I IFN do not develop lupus. In conclusion, the orchestration of the IFN responses by ELF4 offers new means for controlling antiviral and anticancer states, as well as controlling the outcome of IFN-induced autoimmune disorders.
The authors state no conflicting interests
References
- You F, Wang P, Yang L, Yang G, Zhao YO, Qian F, et al. ELF4 is critical for induction of type I interferon and the host antiviral response. Nat Immunol. 2013;14:1237–1246. doi: 10.1038/ni.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic acid recognition by the innate immune system. Annu Rev Immunol. 2011;29:185–214. doi: 10.1146/annurev-immunol-031210-101340. [DOI] [PubMed] [Google Scholar]
- Szabo A, Rajnavolgyi E. Collaboration of Toll-like and RIG-I-like receptors in human dendritic cells: tRIGgering antiviral innate immune responses. Am J Clin Exp Immunol. 2013;2:195–207. [PMC free article] [PubMed] [Google Scholar]
- Sashida G, Liu Y, Elf S, Miyata Y, Ohyashiki K, Izumi M, et al. ELF4/MEF activates MDM2 expression and blocks oncogene-induced p16 activation to promote transformation. Mol Cell Biol. 2009;29:3687–3699. doi: 10.1128/MCB.01551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashida G, Bae N, Di Giandomenico S, Asai T, Gurvich N, Bazzoli E, et al. The mef/elf4 transcription factor fine tunes the DNA damage response. Cancer Res. 2011;71:4857–4865. doi: 10.1158/0008-5472.CAN-11-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Park CS, Mamonkin M, Lacorazza HD. Transcription factor ELF4 controls the proliferation and homing of CD8+ T cells via the Krüppel-like factors KLF4 and KLF2. Nat Immunol. 2009;10:618–626. doi: 10.1038/ni.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacorazza HD, Miyazaki Y, Di Cristofano A, Deblasio A, Hedvat C, Zhang J, et al. The ETS protein MEF plays a critical role in perforin gene expression and the development of natural killer and NK-T cells. Immunity. 2002;17:437–449. doi: 10.1016/s1074-7613(02)00422-3. [DOI] [PubMed] [Google Scholar]
- Szabo A, Bene K, Gogolák P, Réthi B, Lányi Á, Jankovich I, et al. RLR-mediated production of interferon-β by a human dendritic cell subset and its role in virus-specific immunity. J Leukoc Biol. 2012;92:159–169. doi: 10.1189/jlb.0711360. [DOI] [PubMed] [Google Scholar]
- Hall JC, Rosen A. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat Rev Rheumatol. 2010;6:40–49. doi: 10.1038/nrrheum.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013;34:67–73. doi: 10.1016/j.it.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Fuertes MB, Woo SR. Innate immune sensing of cancer: clues from an identified role for type I IFNs. Cancer Immunol Immunother. 2012;61:1343–1347. doi: 10.1007/s00262-012-1305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]