Abstract
Liver disease encompasses a wide variety of liver conditions, including liver failure, liver cirrhosis and a spectrum of acute and chronic hepatitis, such as alcoholic, fatty, drug, viral and chronic hepatitis. Liver injury is a primary causative factor in liver disease; generally, these factors include direct liver damage and immune-mediated liver injury. Neutrophils (also known as neutrophilic granulocytes or polymorphonuclear leukocytes (PMNs)) are the most abundant circulating white blood cell type in humans, and PMNs are a major innate immune cell subset. Inappropriate activation and homing of neutrophils to the microvasculature contributes to the pathological manifestations of many types of liver disease. This review summarizes novel concepts of neutrophil-mediated liver injury that are based on current clinical and animal model studies.
Keywords: liver disease, liver injury, neutrophil
Introduction
The liver is strategically positioned between the intestine and the circulatory system and is continuously exposed to bacterial products, toxins and food-derived antigens. Hepatocytes comprise 60%–80% of all liver cells, and they conduct the metabolic, biosynthetic, detoxification and biliary secretory functions of the liver. Many diseases can affect the liver, including alcoholic, fatty, drug-induced and viral hepatitis. Liver inflammation and necrosis can generally cause liver diseases. The innate immune system is the first line of defense against initial environmental challenges and injury and is activated much more rapidly than the adaptive immune system. In the liver, the innate immune system is driven by a complex set of leukocytes and anti-microbial proteins, including natural killer cells (NKs), natural killer T cells (NKTs), dendritic cells (DCs), neutrophils, eosinophils and complement components. Of these cell subsets, neutrophils have an especially crucial role in the defense against infections. However, overwhelming activation of neutrophils is known to induce liver damage. Therefore, liver neutrophil activation is considered to be a double-edged sword. Herein, we review the evidence from investigative patient and animal model studies that implicate aberrant neutrophil activity as the cause of liver disease.
Neutrophils in the defense against infection
The initiation of infection- and sterile-based types of inflammation results in the trafficking and localization of neutrophils to the injured sites. The profound susceptibility to bacterial and fungal infections that results from neutropenia or defects in neutrophil trafficking demonstrates the essential role of neutrophils in host defense.1 Neutrophils are derived from the bone marrow and circulate in the peripheral blood. Generally, neutrophils develop over a 14-day period in the bone marrow. They subsequently survive for a short period of time, with a 12- to 18-h half-life in the peripheral blood. However, under some conditions, the half-life of circulating neutrophils has been demonstrated to be much longer (approximately 3.75 days), and the half-life of tissue neutrophils has been estimated to be 6–15 times longer than that of circulating neutrophils.2 Granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor-CSF are cytokines that possess the potential to release neutrophils from the bone marrow. Both cytokines increase the number of circulating neutrophils, promote the maturation and activation of neutrophils and extend the lifespan of neutrophils. In mice, it has been observed that a single injection of G-CSF leads to a fivefold increase in circulating neutrophils.3 However, aberrant activation and an extended lifespan of tissue-infiltrating neutrophils can increase the probability of extracellular damage.
High levels of circulating bacterial lipopolysaccharide, which occurs during Gram-negative sepsis or endotoxemia, were found to stimulate Kupffer cells and other resident leukocytes to produce large amounts of interleukin-10 (IL-10). IL-10 can cause the downregulation of neutrophil Mac-1 surface expression. This effect results in neutrophil recruitment to the liver microvasculature and is primarily dependent on CD44 binding to endothelial hyaluronan. The removal of hyaluronan from the sinusoidal endothelium or the blockage of the interaction of hyaluronan with its principal receptor (CD44) significantly reduced neutrophil recruitment to the liver.4 In sterile tissue injury, neutrophils do not function as antimicrobial effectors; instead, they clear debris and initiate the wound-healing process.5 Tissue damage and necrotic cell death often result in the release of damage-associated molecular patterns, which stimulate local intravascular sentinel cells (Kupffer cells) to produce IL-1β, leading to intercellular adhesion molecular-1 (ICAM-1) upregulation on sinusoidal endothelial cells. Neutrophils are then recruited to endothelial ICAM-1 via a β2 integrin (Mac-1)-dependent adhesion mechanism. Cytokines, such as tumor-necrosis factor-α, activate complement factors and, to a lesser degree, CXC chemokines are potent activators of neutrophils, triggering their accumulation in the sinusoids.6
During a bacterial infection, neutrophils rapidly traffic from an axial stream to a bordering group of cells that are primed for the elimination of pathogenic bacteria. Neutrophils exist in three states: resting (unstimulated), primed (following an encounter with an inflammatory agonist or microbial-derived product that has lowered the threshold stimulus needed for activation) and activated (having undertaken a defined function). The transition of neutrophils from a resting state (in circulation) to an activated state (at an infection site) is triggered by an ordered sequence of signals from the priming stimuli—e.g. C5a, LPS or cytokines.7
In addition to their ability to clear pathogens, neutrophils also have the potential to regulate the immune response. Recently, a new neutrophil subpopulation (CD11cbright/CD62Ldim/CD11bbright/CD16bright) was identified, and this population has the capacity to inhibit the T-cell response. The release of hydrogen peroxide from neutrophils into the neutrophil–T-cell immunological synapse suppressed T-cell proliferation.8 Furthermore, a recent study revealed an intricate relationship between neutrophils and marginal zone B cells.9 Another report confirmed that neutrophils likely play a role in the regulation of the terminal differentiation and functional responsiveness of NKs. This effect was demonstrated through the colocalization and direct physical interaction between these two cell types.10
Precise control of neutrophil death programs provides a balance between defense functions and safe clearance. The removal of neutrophils by apoptosis is a homoeostatic mechanism that prevents damage to healthy tissues. Accumulating evidence indicates that outside-in signaling through the β2 integrin Mac-1 protein can generate contrasting cues in neutrophils, leading to increased survival or apoptosis. The binding of Mac-1 to its ligand, ICAM-1, suppresses apoptosis, whereas Mac-1-mediated bacterial phagocytosis induces apoptotic cell death.11 Neutrophils that are undergoing apoptosis are accumulated by local phagocytes, thereby preventing the onset of tissue damage. During liver injury, excessive neutrophil activation has been implicated in the pathogenesis of organ damage (Figure 1). Therefore, neutrophils might be critical inducers of liver damage.
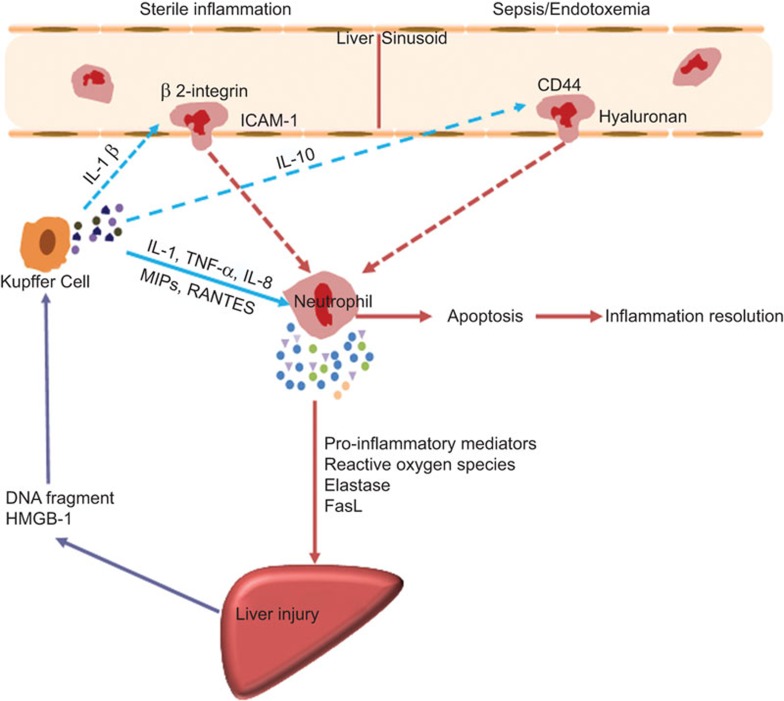
Figure 1.
Mechanisms of neutrophil-mediated liver injury. Different adhesion molecules mediate neutrophil recruitment to the liver during sterile inflammation and sepsis/endotoxemia. After migrating to the site of inflammation, neutrophil mediated hepatocyte injury through production of pro-inflammatory mediators, reactive oxygen species, elastase, etc. Once inflammation is cleared, neutrophils die by apoptosis and trigger an active program to resolve inflammation. FasL, Fas ligand; HMGB-1, high mobility group box protein 1; ICAM-1, intercellular adhesion molecular-1; MIP, membrane intrinsic protein.
Neutrophils and hepatic ischemia/reperfusion (I/R) injury
Hepatic I/R injury is a pathophysiological process that occurs when blood flow and oxygen delivery are restored after hypoxia, leading to increased organ damage.12 Hepatocyte damage can occur during both the ischemic and reperfusion phases. The end result is cellular death through a combination of apoptosis and necrosis. It has been established that the length and method of ischemia applied to the liver and the background liver condition determine the degree of I/R injury that is sustained.13
Generally, liver I/R injury is characterized by neutrophil recruitment and infiltration into the post-ischemic tissue.14 The acute inflammatory response consists of two phases. During the Kupffer cell-mediated phase (0–6 h of reperfusion), the generation of reactive oxygen species aggravates organ damage. Activated Kupffer cells and infiltrating lymphocytes produce cytokines that further promote the inflammatory response. In the second phase (6–24 h of reperfusion), neutrophils become fully activated and express several types of mediators that dominate the liver injury process. These mediators include reactive oxygen species, complement components, proteases, CXCL-1 and CXCL-2.15 IL-17A has been shown to be a key regulator in the initiation of neutrophil-induced inflammatory responses in the subacute phase.16 In IL-1R1 and IL-17A deficient mice, hepatic I/R injury and neutrophil recruitment were attenuated.17
A study of human liver damage identified a neutrophil elastase inhibitor that had therapeutic potential, and this inhibitor was associated with a reduced release of high mobility group box protein 1 and reduced IL-6 levels.18 Additionally, an MMP-9 knockout model of liver I/R demonstrated reduced liver damage, and this reduction was associated with decreased neutrophil transmigration through the liver sinusoids.19 Neutrophil transmigration across endothelial and extracellular matrix barriers is a complex process in liver I/R injury. The expression of L-selectin and β2 integrin (CD11b/CD18) on neutrophils has been shown to be important for adherence to the surface of sinusoidal endothelial cells and hepatocytes.20 Furthermore, CD44 plays a pivotal role in neutrophil infiltration in murine hepatic I/R injury. Liver histology confirmed that the administration of an anti-CD44 antibody decreased the number of infiltrating neutrophils and improved sinusoidal congestion and hepatocellular necrosis.21 However, despite very strong preclinical data, clinical trials for anti-adhesion therapy in I/R injury failed to show a significant benefit.22
Neutrophils and viral hepatitis
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are the most common causes of liver disease in the world. Effective anti-viral immunity is believed to be important for the clearance of HBV and HCV, while the innate immune response, particularly neutrophil accumulation in the liver, and acute inflammation often cause collateral damage to the host liver tissue. Neutrophils can be induced to express a number of mediators that can influence inflammatory and immune responses. In transgenic mice infected with HBV, inhibiting neutrophil elastase was associated with the improvement of liver injury.23 In the clinic, a reduction of neutrophils during the treatment of hepatitis C with Peginterferon was associated with a sustained virological response.24
Passively transferred HBV-specific cytotoxic T cells recruit antigen-nonspecific lymphomononuclear and polymorphonuclear inflammatory cells that contribute to the pathogenesis of liver disease. However, the depletion of neutrophils was associated with diminished recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of HBV-specific cytotoxic T cells.25 This finding may be significant for the development of immunotherapeutic approaches for the treatment of chronic HBV infection.
Neutrophils and non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease, one of the most common causes of chronic liver disease in all regions of the world with modern industrialized economies, represents several overlapping clinical pathological states, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH). Although dysregulated lipid accumulation occurs across the non-alcoholic fatty liver disease spectrum, the features of liver cell injury, such as hepatocyte ballooning, cytoskeletal changes (Mallory–Denk bodies) and hepatocyte apoptosis, predominantly occur in NASH and distinguish NASH from simple steatosis.26
A prominent feature of the inflammation observed in NASH is neutrophil accumulation. Neutrophil-derived MPO can enhance macrophage cytotoxicity and induce neutrophil activation in a NASH mouse model.27 In the foz/foz NASH metabolic syndrome model, a reduction of hepatic cholesterol stores was associated with ameliorated liver injury, and apoptosis and macrophage and neutrophil accumulation.28 Additionally, dietary reversion suppressed uncoupling protein 2 expression and increased hepatic ATP levels, conditions that might favor apoptosis rather than reactive oxygen species-mediated hepatocyte necrosis.29 Neutrophil dysfunction was also associated with liver fibrosis and cirrhosis in NASH. Indeed, human neutrophil peptides have the ability to enhance hepatic fibrosis in fatty liver diseases by inducing hepatic stellate cell proliferation.30 Furthermore, the neutrophil-to-lymphocyte ratio (NLR) was higher in patients with NASH and advanced fibrosis, and this ratio can be used as a novel, non-invasive marker to predict advanced disease.31
Neutrophils and alcoholic liver disease
Alcoholic liver disease (ALD) describes liver damage due to alcohol abuse. The clinical spectrum of ALD includes alcoholic fatty liver, alcoholic steatohepatitis with or without fibrosis, cirrhosis and hepatocellular cancer. The presence of neutrophils within the liver during alcoholic steatohepatitis has been previously described. Alcohol-induced hepatotoxicity and oxidative stress are important mechanisms that contribute to ALD pathogenesis.32 In particular, ethanol has a dose-dependent inhibitory effect on several key neutrophil functions, such as oxidative burst, β2 integrin adhesion molecule expression and chemotaxis. The production of key neutrophil immune mediators was also altered by ethanol. For example, acute ethanol exposure in vitro can inhibit the production of pro-inflammatory cytokines IL-8, tumor-necrosis factor-α and HGF.33
The migration of sinusoid neutrophils through the endothelium and into the liver parenchyma is essential for the development of alcohol-induced hepatic inflammation. The process of neutrophil adherence to the endothelium requires the interaction of CD11b/CD18 integrin (on the neutrophil surface) with ICAM-1 (on the endothelial cell surface).34 This notion is supported by a study that showed that an ICAM-1 deficiency significantly reduced hepatic injury and neutrophil infiltration in a continuous enteral alcohol feeding mouse model.35 Recently, the expression of hepatic E-selectin was also found to be pivotal for neutrophil infiltration into the liver, and contributed to the pathogesis of early stages of human alcoholic liver disease.36
The initial innate immune response that leads to alcoholic hepatitis may be triggered by alcohol in the liver and through increased translocation of intestinal LPS, which activates hepatic Kupffer cells and recruits dysfunctional neutrophils to the liver. It is believed that activated Kupffer cells can produce a variety of cytokines and chemokines, including IL-8, RANTES, MIPs, IL-17 and others, which subsequently recruit neutrophils into the liver.37 Previous studies have also shown that neutrophils from patients with alcoholic liver cirrhosis have a significantly higher resting activation threshold than that in patients with cirrhosis or in healthy subjects. This increased activation threshold is dependent on high level of endotoxin. Serum LPS-binding protein may serve as a marker of inflammation in alcoholics.38 In addition to endotoxin, CCL2 is involved in ALD pathogenesis through neutrophil recruitment. Expression levels of CCL2 in the serum and liver was increased in alcoholic hepatitis patients, and this increase was pronounced in severe forms of the disease.39 Furthermore, Ziol and colleagues40 analyzed 35 alcoholic hepatitis patients and found that the hepatocyte apoptotic index, but not the ballooning hepatocyte index, was strongly correlated with the neutrophil infiltration index. Therefore, understanding of the role of the innate immune system, particularly the role of neutrophils, in alcoholic liver disease pathogenesis may help to identify novel therapeutic targets for this disease.
Neutrophils and liver fibrosis/cirrhosis
Hepatic fibrosis represents a ubiquitous liver response to acute or chronic injury. The hepatic fibrosis process is driven primarily by inflammation in response to parenchymal injury. An improved understanding of the processes that govern inflammation and fibrosis has made it clear that both the adaptive and innate immune systems are involved in the regulation of fibrosis.
In liver cirrhosis patients, the intestinal barrier is altered by increased inflammatory cytokine production and systemic inflammation. The increased number of neutrophils and lower lymphocyte count reflects the level of inflammation in patients. The NLR was shown to predict mortality in liver cirrhosis patients independent of CTP and MELD scores. More importantly, the NLR could predict mortality in patients with low MELD and/or CTP scores.41 Furthermore, neutrophil gelatinase-associated lipocalin was found to be a predictor of mortality in cirrhosis patients with hepatorenal syndrome.42 Anti-neutrophil cytoplasmic antibodies are a non-uniform family of antibodies that recognizes diverse neutrophil components. Enhanced ANCA IgA formation is a feature of cirrhosis regardless of its etiology, and the presence of ANCA IgA has been found to be significantly higher in cirrhosis patients (in alcoholics and non-alcoholics) than in either chronic HCV patients or healthy controls. Levels of ANCA IgA increase with disease progression.43
Recently, there has been evidence that a functional defect in neutrophils occurs even in stable cirrhosis, which is characterized by the release of inflammatory mediators into inflamed peripheral tissues.44 A previous study of alcoholic patients with cirrhosis showed that neutrophils with an increased oxidative burst and reduced phagocytic capacity were associated with infection, organ failure and mortality. However, the neutrophil dysfunction was reversible using endotoxin-removal strategies.45 These data demonstrated that neutrophil dysfunction was associated with poor liver cirrhosis outcomes.
Neutrophils and liver failure
Liver failure is a life-threatening condition. The specific mechanisms governing neutrophil-induced acute liver injury have recently been summarized.46 Inflammatory mediators, such as tumor-necrosis factor-α, IL-1, platelet-activating factor, IL-8, high mobility group box protein 1 and lipid peroxidation products released from dying or dead hepatocytes as well as CXC chemokines, are very potent promoters of neutrophil extravasation into the hepatic parenchyma.47,48 This response triggers complete neutrophil activation, including prolonged adherence-dependent oxidative stress and degranulation. Moreover, oxidants diffuse into hepatocytes and trigger intracellular oxidative stress.49 Alternatively, neutrophils can express Fas ligand and kill hepatocytes through an apoptosis-induced mechanism.50
Acetaminophen (APAP)-induced liver failure is known to activate neutrophils, which leads to neutrophil accumulation in the hepatic vasculature. Following APAP administration, a significant number of neutrophils are recruited into the liver, which results in the development of cellular injury between 4 and 24 h after drug treatment. Neutrophils are guided to the sites of liver necrosis by CXC chemokine receptor 2 (CXCR2) and formyl peptide receptor 1, and the blockage of neutrophil infiltration by anti-granulocyte receptor 1 or combined CXCR2–formyl peptide receptor 1 antagonism has been shown to significantly reduce hepatotoxicity in mice.51 However, the role of neutrophils in the pathophysiology of APAP hepatotoxicity is still controversial. For example, previous studies have demonstrated that pretreatment with a neutropenia-inducing anti-Gr-1 monoclonal antibody for 24 h attenuated hepatic neutrophil accumulation and liver injury.52 However, inducing neutropenia with the same antibody treatment following APAP bioactivation did not protect against liver injury.53 Furthermore, several antibodies against neutrophil β2 integrins have not been demonstrated to be protective in APAP-induced liver injury.54 Additionally, APAP-induced hepatic neutrophil accumulation and inflammatory liver injury still occurred in CD18-deficient mice.55 It was hypothesized that IL-1α and IL-1β were critical mediators of APAP hepatotoxicity. However, a massive overdose of IL-1β administered after APAP recruited more neutrophils into the liver but did not enhance APAP-induced liver injury.56
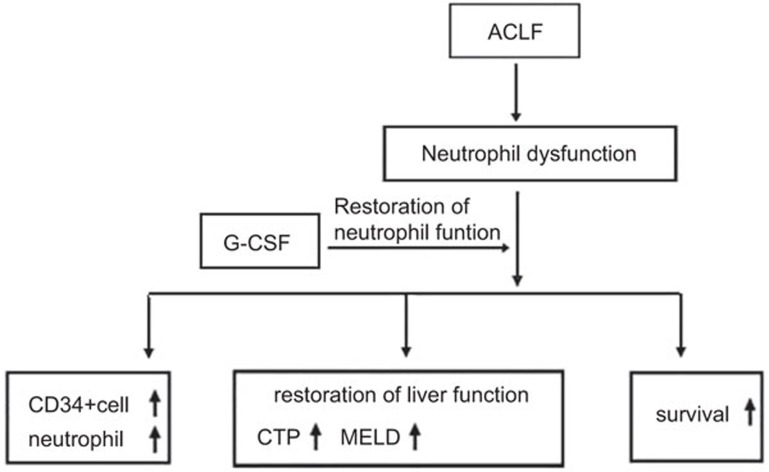
Clinically, circulating neutrophil function indices are important biomarkers for acute liver failure. Neutrophils may be involved in the development of organ dysfunction and increased susceptibility to sepsis.57 When used in the clinic, G-CSF provided improved survival benefits for acute-on chronic liver failure patients. G-CSF therapy was found to significantly increase the frequency of CD34+ cells and neutrophils in the peripheral blood and to reduce patient CTP, MELD and SOFA scores. Additionally, this therapy was demonstrated to prevent the development of sepsis, hepatorenal syndrome and hepatic encephalopathy (Figure 2). Those data showed that therapeutically reversing defective neutrophil functions may be directly associated with the effects of acute-on chronic liver failure treatment. However, further large studies are required to evaluate this possibility.
Figure 2.
Role of G-CSF therapy in ACLF. ACLF, acute-on chronic liver failure; CTP, Child–Turcotte–Pugh; G-CSF, granulocyte colony-stimulating factor; MELD, model for end-stage liver disease.
Neutrophils and hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is one of the most common malignant tumor types worldwide, and its incidence is increasing. It is not known what exactly causes liver cancer; however, neutrophils are thought to play a key role in both HCC formation and progression. Increased neutrophil levels have been observed in several types of tumors, but depending on the microenvironment, tumor-infiltrating neutrophils are capable of being pro- or anti-tumorigenic.
Recently, some studies have demonstrated that increased numbers of neutrophils may provide a sufficient environment for tumor growth and tumor metastasis through angiogenesis. In these studies, neutrophils were present primarily in the peritumoral stroma, and this localization enhanced tumor invasion through a mechanism involving the regulation of a paracrine-mediated hepatocyte growth factor.58 Furthermore, functional IL-17 positive cells in the peritumoral stroma were shown to stimulate CXC chemokine production from epithelial cells, resulting in increased neutrophil trafficking to tumors.59 Accumulated neutrophils in the peritumoral stroma have been shown to be a major source of MMP-9, which can trigger an angiogenic switch at the adjacent invading edge.60 Circulating vascular endothelial growth factor, a key pro-angiogenic factor, is also produced by neutrophils. Neutrophils can contribute to cancer metastasis through the promotion of cancer cell motility and adhesion to hepatic sinusoids.61 Therefore, these data provide direct evidence that neutrophils play an important role in tumor progression by serving as a link between the pro-inflammatory response and angiogenesis in the tumor milieu.
Inflammatory cells and mediators are key components of the tumor microenvironment.62 Various markers, including cytokines, C-reactive protein and the absolute blood neutrophil or lymphocyte count and ratio, have been investigated for their diagnostic roles in liver cancer.63 Patients with a decreased NLR were found to have better survival outcomes than those with an increased NLR. For those HCC patients who underwent liver transplantation, NLR was a reliable independent predictor of overall survival and recurrence-free survival. NLR measurements have been demonstrated to be a useful and easily obtained secondary test, which can be paired with end-stage liver disease score and Milan criteria to evaluate which patients will gain the most survival benefits from transplantation. Recent reports have shown that human HCC samples expressed higher CXCR6 levels and contained an increased number of CD66b+ neutrophils. Additionally, the combination of CXCR6 and neutrophil measurements was a better predictor for recurrence and survival in HCC patients than other methods.64 In addition to CXCR6, CXCL5 also has the ability to promote HCC cell proliferation, invasion and intratumoral neutrophil infiltration. CXCL5 overexpression, alone or in combination with intratumoral neutrophils, was an important HCC prognostic predictor.65
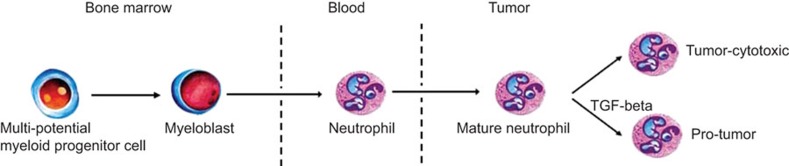
The tumor microenvironment polarizes tumor-associated macrophages toward a pro-tumor (M2) versus an anti-tumor (M1) phenotype.66 Like tumor-associated macrophages, tumor-associated neutrophils also have differential states of activation/differentiation, suggesting a tumor-associated neutrophil classification scheme that is similar to that of the tumor-associated macrophages. Tumor-associated neutrophils can thus take on an anti-tumorigenic or a pro-tumorigenic phenotype (Figure 3). Recently, three distinct neutrophil subsets were identified in mice, and each subset had a different reaction to methicillin-resistant Staphylococcus aureus infection. The neutrophil subsets included freshly isolated neutrophils from normal mice (PMN-N), neutrophils that produced IL-12 and CCL3 (PMN-I) and neutrophils that produced IL-10 and CCL2 (PMN-II).67 In humans, several reports have demonstrated that neutrophils are heterogeneous with regard to their locomotion, phagocytosis capacity, density, membrane depolarization and protein synthesis. Neutrophils from HCC patients are known to produce high levels of CCL2 and CCL3. However, only CCL2-producing neutrophils were found to be significantly increased in proportion to tumor load. These results suggest that distinct neutrophil subsets may exist in the peripheral blood of HCC patients.68 Studying the mechanisms that can selectively modulate neutrophil function might provide novel strategies for anticancer therapies.
Figure 3.
The origin and differentiation of neutrophil. Neutrophils originate from pluripotent stem cell. In tumor, neutrophils can polarize to either anti-tumor phenotype or pro-tumor phenotype. TGF-β is an important effector in the polarization. TGF, transforming growth factor.
Conclusions
Neutrophils play a critical role in liver disease. This review has described liver neutrophil dysfunction in relation to several liver diseases, including hepatic I/R injury, viral hepatitis, non-alcoholic fatty liver disease, alcoholic liver disease, liver cirrhosis, liver failure and hepatocellular carcinoma. Great progress has been made in the mechanistic understanding of neutrophil dysfunctions; therefore, potential therapeutic approaches that aim to improve neutrophil function should also be developed.
Acknowledgments
This work was supported by grants from the National Grand Program on Key Infectious Disease (No. 2012ZX10002-007-002) and the National Science Fund for Outstanding Young Scholars (No. 81222024).
The authors declare no financial or commercial conflicts of interest.
References
- Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Cheretakis C, Leung R, Sun CX, Dror Y, Glogauer M. Timing of neutrophil tissue repopulation predicts restoration of innate immune protection in amurine bone marrow transplantation model. Blood. 2006;108:2821–2826. doi: 10.1182/blood-2006-04-018184. [DOI] [PubMed] [Google Scholar]
- Ulich TR, del Castillo J, Souza L. Kinetics and mechanisms of recombinant human granulocyte-colony stimulating factor-induced neutrophilia. Am J Pathol. 1988;133:630–638. [PMC free article] [PubMed] [Google Scholar]
- McDonald B, McAvoy EF, Lam F, Gill V, de la Motte C, Savani RC, et al. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liversinusoids. J Exp Med. 2008;205:915–927. doi: 10.1084/jem.20071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Jaeschke H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am J Physiol Gastrointest Liver Physiol. 2001;281:1188–1195. doi: 10.1152/ajpgi.2001.281.5.G1188. [DOI] [PubMed] [Google Scholar]
- Guo RF, Ward PA. Mediators and regulation of neutrophil accumulation in inflammatory responses in lung: insights from the IgG immune complex model. Free Radic Biol Med. 2002;33:303–310. doi: 10.1016/s0891-5849(02)00823-7. [DOI] [PubMed] [Google Scholar]
- Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2011;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger BN, Donadieu J, Cognet C, Bernat C, Ordoñez-Rueda D, Barlogis V, et al. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J Exp Med. 2012;209:565–580. doi: 10.1084/jem.20111908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kebir D, Filep JG. Modulation of neutrophil apoptosis and the resolution of inflammation through β2 integrins. Front Immunol. 2013;4:60. doi: 10.3389/fimmu.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–936. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol. 2007;35:757–766. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir Bras. 2005;20:336–343. doi: 10.1590/s0102-86502005000400013. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia–reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- Kono H, Fujii H, Ogiku M, Hosomura N, Amemiya H, Tsuchiya M, et al. Role of IL-17A in neutrophil recruitment and hepatic injury after warm ischemia–reperfusion mice. J Immunol. 2011;187:4818–4825. doi: 10.4049/jimmunol.1100490. [DOI] [PubMed] [Google Scholar]
- Tan Z, Jiang R, Wang X, Wang Y, Lu L, Liu Q, et al. RORγt+IL-17+ neutrophils play a critical role in hepatic ischemia–reperfusion injury. J Mol Cell Biol. 2013;5:143–146. doi: 10.1093/jmcb/mjs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Amara M, Yang SY, Tapuria N, Fuller B, Davidson B, Seifalian A. Liver ischemia/reperfusion injury: processes in inflammatory networks—a review. Liver Transpl. 2010;16:1016–1032. doi: 10.1002/lt.22117. [DOI] [PubMed] [Google Scholar]
- Kim MS, Lee KH, Lee WM, Jun JH, Kim DH. CD44 disruption attenuates murine hepatic ischemia/reperfusion injury. J Korean Med Sci. 2011;26:919–926. doi: 10.3346/jkms.2011.26.7.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Freitas MC, Zhao D, Busuttil RW, Kupiec-Weglinski JW. The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury. Transplantation. 2010;89:1050–1056. doi: 10.1097/TP.0b013e3181d45a98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta G, Fuller BJ, Davidson BR. Molecular mechanisms of liver ischemia reperfusion injury: insights from transgenic knockout models. World J Gastroenterol. 2013;19:1683–1698. doi: 10.3748/wjg.v19.i11.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan JM, Winn RK. Leukocyte–endothelial interactions: clinical trials of anti-adhesion therapy. Crit Care Med. 2002;30:S214–S219. doi: 10.1097/00003246-200205001-00007. [DOI] [PubMed] [Google Scholar]
- Takai S, Kimura K, Nagaki M, Satake S, Kakimi K, Moriwaki H. Blockade of neutrophil elastase attenuates severe liver injury in hepatitis B transgenic mice. J Virol. 2005;79:15142–15150. doi: 10.1128/JVI.79.24.15142-15150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Uria G, Day JN, Nasir AJ, Russell SK, Vilar FJ. Reduction in neutrophil count during hepatitis C treatment: drug toxicity or predictor of good response. Dig Dis Sci. 2010;55:2058–2062. doi: 10.1007/s10620-009-0969-z. [DOI] [PubMed] [Google Scholar]
- Sitia G, Isogawa M, Kakimi K, Wieland SF, Chisari FV, Guidotti LG. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 2002;99:13717–13722. doi: 10.1073/pnas.172521999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SH. Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis. Clin Mol Hepatol. 2013;19:210–215. doi: 10.3350/cmh.2013.19.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensen SS, Bieghs V, Xanthoulea S, Arfianti E, Bakker JA, Shiri-Sverdlov R, et al. Neutrophil-derived myeloperoxidase aggravates non-alcoholic steatohepatitis in low-density lipoprotein receptor-deficient mice. PLoS ONE. 2012;7:e52411. doi: 10.1371/journal.pone.0052411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larter CZ, Yeh MM, Haigh WG, van Rooyen DM, Brooling J, Heydet D, et al. Dietary modification dampens liver inflammation and fibrosis in obesity-related fatty liver disease. Obesity. 2013;21:1189–1199. doi: 10.1002/oby.20123. [DOI] [PubMed] [Google Scholar]
- Inzaugarat ME, Ferreyra Solari NE, Billordo LA, Abecasis R, Gadano AC, Cherñavsky AC. Altered phenotype and functionality of circulating immune cells characterize adult patients with nonalcoholic steatohepatitis. J Clin Immunol. 2011;31:1120–1130. doi: 10.1007/s10875-011-9571-1. [DOI] [PubMed] [Google Scholar]
- Ibusuki R, Uto H, Arima S, Mawatari S, Setoguchi Y, Iwashita Y, et al. Transgenic expression of human neutrophil peptide-1 enhances hepatic fibrosis in mice fed a choline-deficient, L-amino acid-defined diet. Liver Int. 2013;33:1547–1556. doi: 10.1111/liv.12203. [DOI] [PubMed] [Google Scholar]
- Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TA, Yerian L, et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:297–302. doi: 10.1111/j.1478-3231.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taïeb J, Delarche C, Ethuin F, Selloum S, Poynard T, Gougerot-Pocidalo MA, et al. Ethanol-induced inhibition of cytokine release and protein degranulation in human neutrophils. J Leukoc Biol. 2002;72:1142–1147. [PubMed] [Google Scholar]
- Woodfin A, Voisin MB, Nourshargh S. Recent developments and complexities in neutrophil transmigration. Curr Opin Hematol. 2010;17:9–17. doi: 10.1097/MOH.0b013e3283333930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Uesugi T, Froh M, Rusyn I, Bradford BU, Thurman RG. ICAM-1 is involved in the mechanism of alcohol-induced liver injury: studies with knockout mice. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1289–G1295. doi: 10.1152/ajpgi.2001.280.6.G1289. [DOI] [PubMed] [Google Scholar]
- Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology. 2013;58:1814–1823. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, Nagy L, et al. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516–G525. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24:48S–54S. [PubMed] [Google Scholar]
- Degré D, Lemmers A, Gustot T, Ouziel R, Trépo E, Demetter P, et al. Hepatic expression of CCL2 in alcoholic liver disease is associated with disease severity and neutrophil infiltrates. Clin Exp Immunol. 2012;169:302–310. doi: 10.1111/j.1365-2249.2012.04609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziol M, Tepper M, Lohez M, Arcangeli G, Ganne N, Christidis C, et al. Clinical and biological relevance of hepatocyte apoptosis in alcoholic hepatitis. J Hepatol. 2001;34:254–260. doi: 10.1016/s0168-8278(00)00047-7. [DOI] [PubMed] [Google Scholar]
- Biyik M, Ucar R, Solak Y, Gungor G, Polat I, Gaipov A, et al. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2013;25:435–441. doi: 10.1097/MEG.0b013e32835c2af3. [DOI] [PubMed] [Google Scholar]
- Papp M, Sipeki N, Vitalis Z, Tornai T, Altorjay I, Tornai I, et al. High prevalence of IgA class anti-neutrophil cytoplasmic antibodies (ANCA) is associated with increased risk of bacterial infection in patients with cirrhosis. J Hepatol. 2013;59:457–466. doi: 10.1016/j.jhep.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Gungor G, Ataseven H, Demir A, Solak Y, Gaipov A, Biyik M, et al. Neutrophil gelatinase-associated lipocalin in prediction of mortality in patients with hepatorenal syndrome: a prospective observational study. Liver Int. 2013;34:49–57. doi: 10.1111/liv.12232. [DOI] [PubMed] [Google Scholar]
- Tritto G, Bechlis Z, Stadlbauer V, Davies N, Francés R, Shah N, et al. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol. 2011;55:574–581. doi: 10.1016/j.jhep.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831–840. doi: 10.1002/hep.21737. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Li B, Xu D, Zhang Z, Zhao JM, Zhou G, et al. Imbalanced intrahepatic cytokine expression of interferon-gamma, tumor necrosis factoralpha, and interleukin-10 in patients with acuteon-chronic liver failure associated with hepatitis B virus infection. J Clin Gastroenterol. 2009;43:182–190. doi: 10.1097/MCG.0b013e3181624464. [DOI] [PubMed] [Google Scholar]
- Antoniades CG, Berry PA, Wendon JA, Vergani D. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49:845–861. doi: 10.1016/j.jhep.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Ho YS, Fisher MA, Lawson JA, Farhood A. Glutathione peroxidase-deficient mice are more susceptible to neutrophil-mediated hepatic parenchymal cell injury during endotoxemia: importance of an intracellular oxidant stress. Hepatology. 1999;29:443–450. doi: 10.1002/hep.510290222. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2006;2:493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BH, et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology. 2012;56:1971–1982. doi: 10.1002/hep.25801. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Liu J. Neutrophil depletion protects against murine acetaminophen hepatotoxicity: another perspective. Hepatology. 2007;45:1588–1589. doi: 10.1002/hep.21549. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010;30:1280–1292. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Farhood A, Jaeschke H. Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflammation and liver injury. Toxicol Appl Pharmacol. 2010;247:169–178. doi: 10.1016/j.taap.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NJ, Nishtala A, Manakkat Vijay GK, Abeles RD, Auzinger G, Bernal W, et al. Circulating neutrophil dysfunction in acute liver failure. Hepatology. 2013;57:1142–1152. doi: 10.1002/hep.26102. [DOI] [PubMed] [Google Scholar]
- Wislez M, Rabbe N, Marchal J, Milleron B, Crestani B, Mayaud C, et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003;63:1405–1412. [PubMed] [Google Scholar]
- Tanaka S, Yoshimoto T, Naka T, Nakae S, Iwakura Y, Cua D, et al. Natural occurring IL-17 producing T cells regulate the initial phase of neutrophil mediated airway responses. J Immunol. 2009;183:7523–7530. doi: 10.4049/jimmunol.0803828. [DOI] [PubMed] [Google Scholar]
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2009;125:1298–1305. doi: 10.1002/ijc.24409. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Dan J, Zhang Y, Peng Z, Huang J, Gao H, Xu L, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS ONE. 2013;8:e58184. doi: 10.1371/journal.pone.0058184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH, Sun J, et al. CXCR6 upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma. Cancer Res. 2012;72:3546–3556. doi: 10.1158/0008-5472.CAN-11-4032. [DOI] [PubMed] [Google Scholar]
- Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, et al. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellularcarcinoma. Hepatology. 2012;56:2242–2254. doi: 10.1002/hep.25907. [DOI] [PubMed] [Google Scholar]
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21:215–226. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Fukui H, Asai A, Fukunishi S, Miyaji K, Fujiwara S, et al. An immunosuppressive subtype of neutrophils identified in patients with hepatocellular carcinoma. J Clin Biochem Nutr. 2012;51:204–212. doi: 10.3164/jcbn.12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]