Abstract
The interplay between the CD4-lineage transcription factor ThPok and the CD8-lineage transcription factor, runt-related transcription factor 3 (Runx3), in T-cell development has been extensively documented. However, little is known about the roles of these transcription factors in invariant natural killer T (iNKT) cell development. CD1d-restricted iNKT cells are committed to the CD4+CD8− and CD4−CD8− sublineages, which respond to antigen stimulation with rapid and potent release of T helper (Th) 1 and Th2 cytokines. However, previous reports have demonstrated a new population of CD8+ NKT cells in ThPok-deficient mice. In the current study, we sought to determine whether Runx3 was involved in the re-expression of CD8 and function of iNKT cells in the absence of ThPok. We used mice lacking Runx3, ThPok or both and verified that Runx3 was partially responsible for the appearance of CD8+ iNKT cells in ThPok knockout mice. Additionally, Runx3 participated in the immune response mediated by iNKT cells in a model of α-galactosylceramide-induced acute hepatitis. These results indicate that Runx3 is crucial for the phenotypic and functional changes observed in ThPok-deficient iNKT cells.
Keywords: α-galactosylceramide, iNKT cells, liver, Runx3, ThPok
Introduction
Natural killer T (NKT) cells are an innate-like T-cell lineage possessing both NK cell surface markers (NK1.1 in B6 mice) and the canonical T-cell receptor α (TCRα) chain (Vα14–Jα18 in mice and V24–J18 in humans) coupled to specific Vβ chains (Vβ8, Vβ7 and Vβ2 in mice and Vβ11 in humans). These receptors are responsible for the interaction of NKT cells with CD1d molecules expressed on CD4+CD8+ double-positive (DP) thymocytes.1 NKT cells are classified into type I (defined as invariant (iNKT) or classical NKT cells) and type II based on the antigens recognized by each and their TCR repertoires.2 Our study focused on type I NKT cells (hereafter referred to as iNKT cells). iNKT cells recognize the glycosphingolipid antigen α-galactosylceramide (α-GalCer) and α-GalCer loading with the tetrameric form of CD1d is often used to define the iNKT cell subset because of the high affinity of CD1d for the iNKT cell TCR.3 Although iNKT cells originate from CD4+CD8+ (DP) thymocytes4 and are selected by MHC class I-like CD1d molecules, they comprise functionally distinct CD4+ and CD4−CD8− double-negative (DN) subsets, unlike conventional T cells. Although iNKT cells lack CD8 expression in mice,5,6 several studies have documented the presence of CD8+ NKT cells in humans,7,8,9 and this population has a distinct cytokine profile, exhibits cytotoxicity against tumors and influences immune balance.9,10,11 It has been demonstrated that activated iNKT cells are capable of rapidly releasing high amounts of T helper 1 (Th1) cytokines, such as IFN-γ, TNF-α and IL-6, and Th2 cytokines, such as IL-4, when stimulated by antigen. Thus, iNKT cells act as a bridge between innate and adaptive immune responses. In addition, a population of IL-17-producing NKT cells has been identified; these cells have been found to produce IL-17 upon activation, thereby promoting autoimmune disease.12,13,14 More recently, several investigators have proposed three iNKT sublineages based on thymic development and distinct functions.14,15,16,17,18 These sublineages include NKT1 cells, which have high expression of the T-box transcription factor T-bet (Th1-like iNKT cells) and secrete large amounts of IFN-γ upon stimulation; NKT2 cells highly express GATA-binding protein 3 (GATA-3) (Th2-like iNKT cells) and produce IL-4 upon stimulation, whereas NKT-17 cells (Th17-like iNKT cells) express high levels of retinoic acid receptor-related orphan receptor-γt (RORγt) and produce IL-17 under activated conditions.17 The above description raises the possibility that the function of specific iNKT cell sublineages may be determined by transcriptional programs.19 During iNKT cell development, several transcription factors are involved in regulating phenotype and function, including a BTB-ZF promyelocyticzinc finger transcription regulator, which is specifically expressed in iNKT cells. Promyelocyticzinc finger transcription regulator may control the development of iNKT cells, as mice lacking it exhibit an ∼90% decrease in the number of thymic iNKT cells, and the iNKT cells presents how a defect in secretion of Th1 and Th2 cytokines upon stimulation.20,21 Studies have demonstrated that the zinc-finger transcription factor, ThPok (also called ZBTB7B or cKrox), drives CD4 expression on MHC class II-restricted T cells22,23by inhibiting the Runx1- and Runx3-mediated downregulation of CD4 in αβ T cells.24 Interestingly, subsequent research revealed that ThPok-deficient iNKT cells were unable to express CD4, and a subset of CD8+ NKT cells was instead detected.25,26 Apart from its influence on phenotype, ThPok is also required for the effector function of iNKT cells, as ThPok deficiency markedly diminished IL-4 levels and to a lesser extent, IFN-γ levels, upon exposure to αGalCer26 compared with wild-type (WT) mice. In addition, ThPok negatively regulates NKT-17 cells by repressing the Th17 signature regulator, RORγt, and the subsequent secretion of IL-17.27 Studies have indicated that ThPok prevents Runx3-mediated CD8+ lineage differentiation in conventional T cells.28 Reciprocally, Runx1 and Runx3 are associated with the development and maturation of CD8+ thymocytes, as they suppress CD4 expression in T cells at different stages.29,30 Several studies have revealed that Runx3 is required for the expression of CD8 in mature CD8+ T cells by silencing CD4 expression because mice with conditional knockout of Runx3 have a significant reduction in CD8+ cells.31,32 Furthermore, Runx3 functions to skew naive CD4+ T cells toward the Th1 lineage, and Runx3 and T-bet interact to enhance the expression of IFN-γ and silence IL-4 in Th1-type cells.33 Moreover, Runx1 and Runx3 are both involved in IL-17 production by T regulatory cells.34 Studies in mice have confirmed that in the absence of ThPok, CD4+ NKT cells completely disappear and a new population of CD8+ NKT cells emerges; thus, we sought to determine whether Runx1 and Runx3 are involved in the development of CD8+ iNKT cells in ThPok-deficient mice and whether they play a part in the response of iNKT cells to antigen stimulation. Because iNKT cells are almost completely abolished in the absence of Runx1,4 and little is known about the role of Runx3 in iNKT cells, we hypothesized that Runx3 might be responsible for the re-expression of CD8 on ThPok-deficient iNKT cells. To address this hypothesis, we investigated the phenotype and function of iNKT cells in mice lacking Runx3 or both ThPok and Runx3.
Materials and methods
Ethics statement
The animal experiments were conducted in accordance with Zhejiang University institutional guidelines, and the study was approved by the Ethics Committee of Zhejiang University. Euthanasia of mice was performed by carbon dioxide inhalation, which limits fear, anxiety and pain.
Mice
CD4-cre transgenic and ThPokF/F mice were generously provided by Dr Rémy Bosselut (National Institutes of Health, Bethesda, MD), and Runx3F/F mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Runx3-ThPok double knockout (DKO) mice were generated by backcrossing for more than nine generations onto the B6 background. Genotyping of tail-biopsy DNA by PCR and phenotyping by flow cytometry of blood samples were then performed to identify the correct genotype. All mice used in the study were between 6 and 8 weeks of age and were maintained in the Experimental Animal Center of Zhejiang University under specific pathogen-free conditions.
Flow cytometry
αGalCer (KRN7000) was provided by Funakoshi Co., Ltd, Japan, and a PBS57-loaded and unloaded (control) mouse PE-conjugated CD1d tetramer and isotype control were obtained from the NIH Tetramer Core Facility, USA. Single-cell suspensions of mouse thymus and spleen were prepared, washed with PBS and stained with fluorescently labeled antibodies. Hepatic lymphocytes were isolated using a previously described protocol with minor modifications.35 Briefly, mice were euthanized by intraperitoneal injection with pentobarbital sodium, and the liver was perfused with PBS via the portal vein until it became pale. The liver was then removed and gently pressed through a 200-gauge stainless steel mesh into PBS containing 2% FCS (Gibco, Carlsbad, CA, USA). After the cell suspension was washed in RPMI 1640 medium (31800; Gibco) and centrifuged at 800g for 5 min, the cell pellet was resuspended and overlaid with 15 ml of a 33% isotonic Percoll solution (17-0891-01; GE Healthcare, Pittsburgh, PA, USA) followed by centrifugation for 30 min at 800g at room temperature. After treatment with erythrocyte lysis buffer for 5 min at room temperature, liver mononuclear cells (MNCs) were counted and analyzed by flow cytometry on a FACSAria (BD Biosciences, San Jose, CA, USA). The antibodies used for staining included the following: anti-TCR (clone H57-597), anti-NK1.1 (clone PK136), anti-CD4 (clone RM4-5), anti-CD8 (clone 53-6.7), anti-CD8β (clone H35-17.2), anti-CD44 (clone IM7), anti-CD69 (clone H1.2F3), anti-CD122 (clone TM-b1), anti-CD62L (cloneMEL-14) and anti-CD16/32 (clone93) (eBioscience, San Diego, CA, USA). Anti-Ly6C (clone HK1.4) was from Biolegend, San Diego, CA, USA (USA). All antibodies were used according to the manufacturer's instructions. Data were analyzed using FlowJo software (Treestar, San Diego, CA, USA).
iNKT cell sorting
To sort iNKT cells, mouse thymocytes were labeled with the PE-conjugated CD1d-PBS57 tetramer and then enriched using anti-PE microbeads with LS columns following the manufacturer's protocol (Miltenyi Biotec, Auburn, CA, USA). Enriched NKT cells from 5–8 mice were pooled and further sorted on a FACSAria (BD Biosciences, San Jose, CA, USA). A purity >95% was obtained.
Quantitative RT-PCR
After sorting iNKT cells, total RNA was extracted using the Trizol reagent (Cat. No. 15596026; Invitrogen, Carlsbad, CA, USA), cDNA was synthesized from 500 ng of total RNA using the Prime Script reverse transcriptase (Cat. No. DRR063A; Takara, SHG, Japan) and PCR was performed on an ABI Prism 7500 analyzer (Applied Biosystems, Carlsbad, CA, USA) using SYBR Premix ExTaq (Cat. No. DRR041A; Takara). All gene expression levels were normalized to mouse β-actin expression. The sequences of the primers were as follows:
ThPok: 5′-GTCTGCGGCGTCCGCTTC-3′ (forward) and 5′-CGGTCCCCGGTGTGCAG-3′ (reverse); Runx1: 5′-GAGCGGCTCAGTGAATTGGA-3′ (forward) and 5′-GAAATGGGTGTCGCTGGGTG-3′ (reverse); Runx3: 5′-TTCCTCTGCTCCGTGCTGC-3′ (forward) and 5′-TGACAGCGGAAGCGTTGCG-3′ (reverse); GATA-3: 5′-CCTACCGGGTTCGGATGTAA-3′ (forward) and 5′-AGCCTTCGCTTGGGCTTGAT-3′ (reverse); β-actin: 5′-CGTTGACATCCGTAAAGACC-3′ (forward) and 5′-AACAGTCCGCCTAGAAGCAC-3′ (reverse).
Model of acute hepatitis and serum alanine amino transferase (ALT) assay
To induce iNKT cell-driven acute liver injury, mice were administered a single-tail vein injection of αGalCer (100 µg/kg body weight dissolved in 5.6% sucrose, 0.75% L-histidine and 0.5% Tween-20) or ConA (10 mg/kg body weight diluted in sterile PBS, approximately 200 µl/mouse). Control mice were administered an equivalent volume of vehicle containing pyrogen-free PBS. Twenty-four hours after challenge, mice were euthanized and blood samples were collected by cardiac puncture. Sera were obtained by centrifugation at 800g for 10 min and ALT (the gold standard for evaluation of liver damage36,37) levels were detected using an ALT kit from Shanghai Rongsheng Biotech Co. (Shanghai, China). The ALT values are presented as IU/l.
Histological analysis
To determine the degree of injury 24 h after αGalCer challenge, the liver was fixed in 4% paraformaldehyde and embedded in paraffin. Sections (4-µm) were cut and stained with hematoxylin and eosin.
ELISA
For in vivo cytokine detection, serum IFN-γ, TNF-α, IL-4, IL-6 and IL-17 were measured by ELISA using Ready-Set-Gokits (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions. For in vitro assays, iNKT cells sorted from thymocytes were stimulated overnight with plate-bound anti-CD3 (1 µg/ml) in 96-well plates (1×105 cells/well) with DMEM (C0006; Gibco, Carlsbad, CA, USA) supplemented with 2 mM glutamine, 100 units/ml penicillin, 100 mg/ml streptomycin sulfate and 10% heat-inactivated FBS (Gibco, Carlsbad, CA, USA) at 37 °C under 5% CO2. The supernatants were then collected for cytokine determination at the indicated time points. All assays were performed in triplicate.
Construction of the ThPok-overexpressing adenovirus vector and rescue experiments
A recombinant vector encoding Mus musculus ThPok was generated using PCR and subcloned into the PCDNA3 vector with bilaterally inserted restriction endonucleases (EcoRI and NotI). The clonal product was identified by sequencing and sent to Shanghai Bioeasy Biotechnology Co., Ltd (Shanghai, China) for packaging into adenoviruses using the pAd-Easy1 system. Following the manufacturer's protocol, titers of the ThPok adenovirus (Ad-ThPok) reached 7.68×1011 infectious units (IFU)/ml. The GFP control adenovirus (Ad-GFP) had a titer of 1.2×1012 IFU)/ml. In rescue experiments, ThPok-deficient mice were challenged with Ad-ThPok or Ad-GFP 3 days prior to αGalCer injection, and blood samples were collected for ALT determination.
Cell stimulation in vitro and intracellular staining of IL-17A
Liver mononuclear cells were prepared as previously described. For stimulation experiments, cell suspensions at 3×106 cells/ml were stimulated with 100 nM PMA, 100 nM ionomycin and Golgi-Stop (BD Biosciences, San Jose, CA, USA) and cultured at 37 °C for 3 h in RPMI 1640 medium supplemented with 10% FBS and 50 μM β-mercaptoethanol. After stimulation, the cells were washed, stained for surface markers and then fixed and stained intracellularly with an Clone eBio17B7 (eBioscience, San Diego, CA, USA) or clone eBM2a antibody.
Statistical analysis
All statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA) and SPSS 20, and data were expressed as the mean±s.e.m. Comparisons between groups were performed using Student's t-test or the Mann–Whitney test, and comparisons among more than two groups were conducted using the Kruskal–Wallis method. Significant differences were considered at P<0.05.
Results
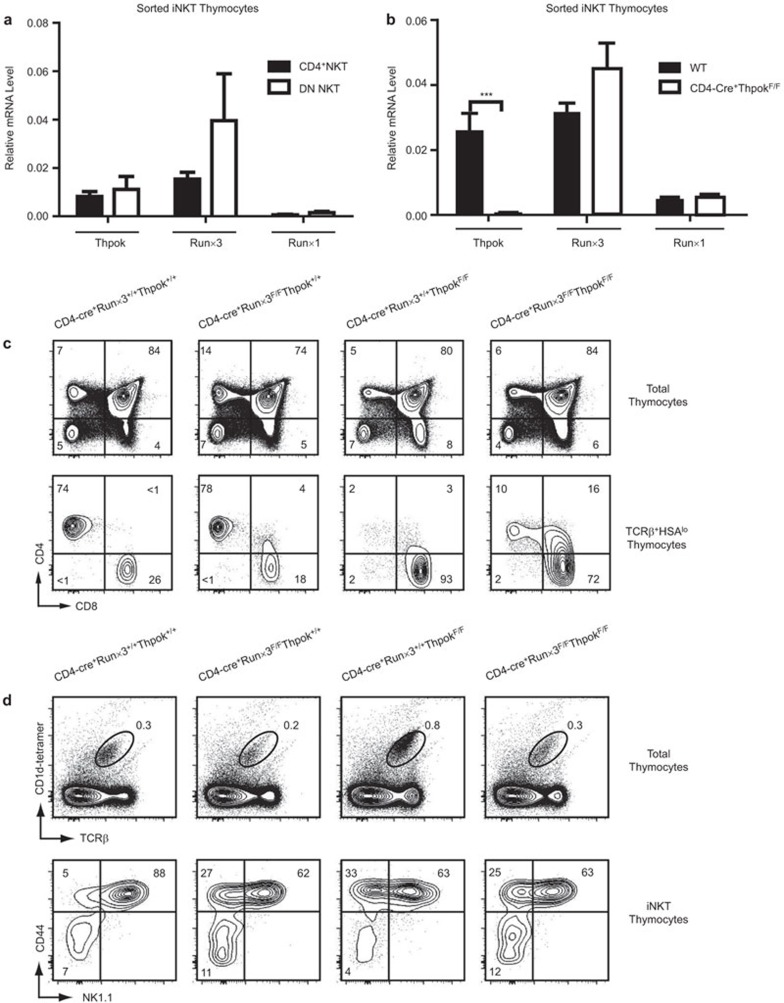
Altered T-cell phenotype in CD4-cre+Runx3F/F, CD4-cre+ThPokF/F and CD4-cre+Runx3F/FThPokF/F mice
The conventional T-cell fate model has indicated that ThPok is barely detectable in CD8+ T cells,22,38 and Runx3 expression in turn is decreased in CD4+ T cells.39,40 However, our real-time PCR analyses revealed that Runx3 and ThPok were both expressed in the CD4+ and DN subsets of iNKT cells, whereas a low abundance of Runx1 mRNA was detected (Figure 1a). These results are consistent with previous studies.25,41,42 Although published results have shown a subset of CD8+ NKT cells arising in ThPok-deficient mice,25 results of real-time PCR analyses in the current study showed no notable increase in Runx1 orRunx3 levels in iNKT cells in the absence of ThPok (Figure 1b). This finding prompted us to consider the role of Runx3 in iNKT cells, as several studies have noted that Runx3 activates CD8 expression by binding to the Cd4 silencer and the Cd8 locus, whereas ThPok functions to keep the loci apart.43,44 To further determine whether the induction of CD8 requires Runx3 as noted previously,45 we generated mice lacking both Runx3 and ThPok (hereafter referred to as DKO mice) or mice lacking each gene separately using CD4-cre transgenic mice. First, we characterized the T-cell phenotype of these mice. Knockout of Runx3 alone resulted in fewer CD8+ T cells in the thymus and a small cluster of CD4+CD8+ DP thymocytes (Figure 1c). In contrast, in ThPok-deficient mice, CD4+ T cells were almost completely absent in TCRβ+HSAlow thymocytes, and instead, as previously reported, CD8+ T cells were mainly observed.46 Conditional inactivation of both Runx3 and ThPok in DKO mice resulted in the appearance of a population of mature CD4+ T cells and CD4+CD8+ DP T cells in the thymus, similar to results obtained with Lck-cre+CbfβF/FThPokF/F mice.28 In the periphery, similar alterations in mature T-cell subsets in the absence of Runx3 or ThPok or both were observed, and the alterations were more pronounced than those observed in the thymus (Supplementary Figure 1a). Due to these changes in T-cell development, we further explored whether the iNKT cells in these mice were also affected. We first assessed the percentages and absolute numbers of iNKT cells in the thymus, spleen and liver, and found that the Runx3-deficient mice had basal percentages and absolute numbers of iNKT cells almost identical to littermate controls (Supplementary Figure 1b). In ThPok-deficient mice, we found a higher percentage of αGalCer+TCR+ iNKT cells as previously reported41 (Figure 1d), whereas the absolute numbers of iNKT cells did not differ between these mice (Supplementary Figure 1b). Assessment of NKT cell development revealed that in all three knockout strains, fewer of the most mature stage 3 NKT cells (CD44+NK1.1+) were detected, mainly owing to reduced NK1.1 expression (Figure 1d). These results indicate that the survival and development of thymus-derived NKT cells were not greatly affected by the absence of Runx3 or ThPok or both.
Figure 1.
Altered T-cell phenotype in CD4-cre+Runx3F/F, CD4-cre+ThPokF/F and CD4-cre+Runx3F/FThPokF/F mice. (a) We sorted thymic iNKT cells from WT mice into CD4+ and DN subsets and extracted total RNA to measure the expression levels of ThPok, Runx3 and Runx1 by quantitative real-time PCR. Transcript levels were normalized to the transcript levels of β-actin in each sample. Results are shown as the mean±standard deviation, and data are representative of at least three independent experiments. (b) Determination of mRNA levels of ThPok, Runx3 and Runx1 in sorted thymic iNKT cells from ThPok-deficient and WT mice by flow cytometry and RT-PCR. Results are shown as the mean±standard deviation, and data are representative of three independent experiments. ***P<0.001. (c) Thymocytes from Runx3-deficient, ThPok-deficient, DKO and WT mice were prepared as previously described and analyzed by multicolor flow cytometry. The WT mice are shown as the control; the top row indicates the thymic T-cell phenotype by CD4 versus CD8; in the second row, cells were gated on TCRβ+HSAlow T cells, indicating a population of mature T cells in the thymus. Numbers indicate the percentage of the indicated cell population. Data are representative of more than five independent experiments. (d) Flow cytometric analysis of thymocytes from Runx3-deficient, Thpok-deficient, DKO and age-matched litter mate control mice. The first line shows iNKT cells defined as the αGalCer-CD1d tetramer+TCRβ+ population. Numbers represent the percentage of iNKT cells. The second line depicts the staining of CD1d tetramer+TCRβ+ iNKT cells for NK1.1 and CD44. The latter markers were used to determine the three maturation stages of iNKT cells in the thymus. Data are representative of at least eight independent experiments. αGalCer, α-galactosylceramide; DKO, double knockout; DN, double-negative; iNKT, invariant natural killer T; Runx3, runt-related transcription factor 3; TCR, T-cell receptor; WT, wild-type.
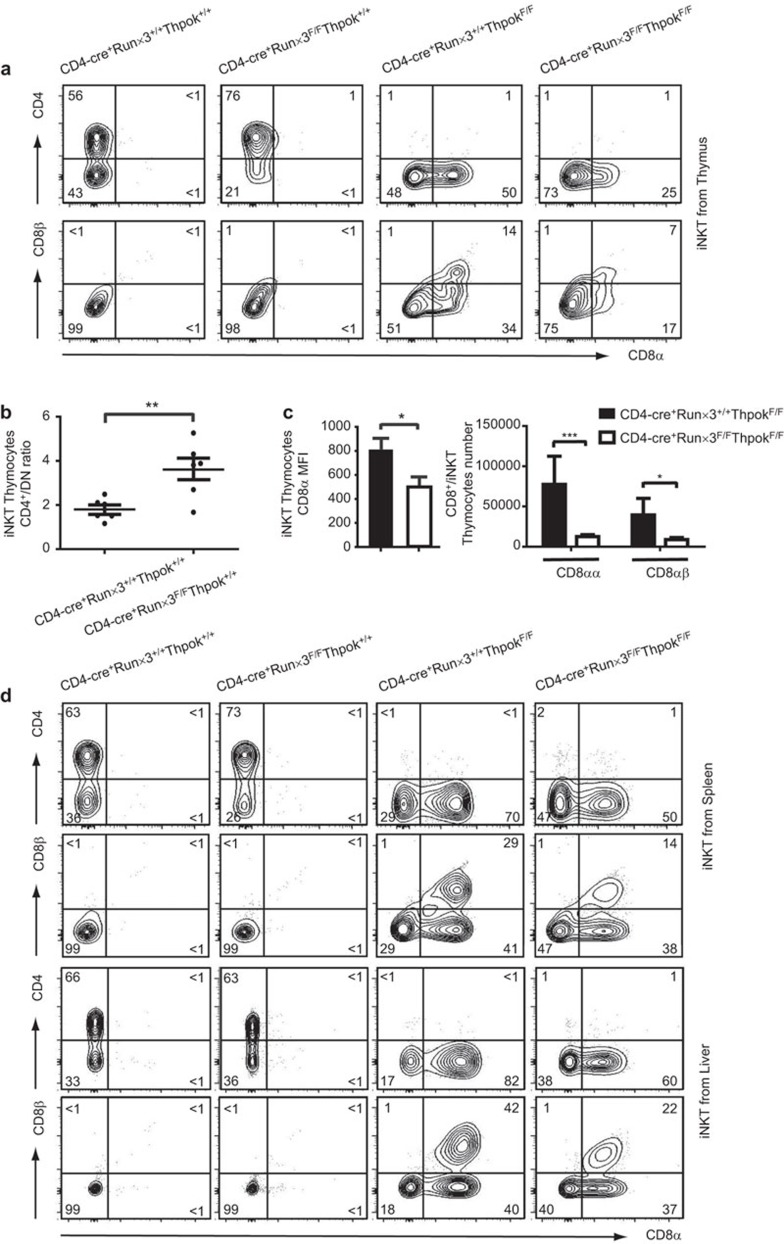
Characterization of iNKT cells in Runx3-deficient, ThPok-deficient and DKO mice
Our results confirmed that the thymic iNKT cells re-expressed CD8 in the absence of ThPok as previously reported,25 whereas in the Runx3-deficient mice, the phenotype of thymic iNKT cells was almost identical to WT controls except for an increased ratio of CD4+/DN cells (Figure 2a and b). As expected, we found that the numbers of CD8-expressing iNKT cells from DKO mice diminished by almost half compared with those in ThPok-deficient mice. The analysis also showed that numbers of both CD8αα and CD8αβ iNKT cells declined by the same amount (Figure 2a and c). In addition, mean fluorescence intensity (MFI) results showed that the decrease in CD8+ iNKT cells was mainly a result of the downregulation of CD8α (Figure 2c) in the DKO mice. We subsequently explored the phenotype of peripheral iNKT cells (spleen and liver) and found that it resembled the phenotype of iNKT cells in the thymus; the percentages and absolute numbers were equivalent among the mice (data not shown). Examination of the iNKT cell phenotype in the Runx3-deficient mice showed no disparity in the ratio of CD4+/DN cells with that of WT mice, unlike that found in the thymus. Compared with the ThPok-deficient iNKT cells, the numbers of CD8+ NKT cells from both the spleen and liver were consistently decreased by ∼20% in DKO mice (Figure 2d). In addition, analysis of absolute number and MFI showed that in the liver, the decrease in numbers of CD8+ iNKT cells was mainly due to the CD8αβ iNKT cells (Supplementary Figure 2). However, although there was a prominent reduction in CD8 expression, the DKO mice still displayed a proportion of CD8+ NKT cells. The latter result may be due to the functional redundancy of Runx1 analogous to that in conventional T cells.32,47 Thus, the data indicate that Runx3 is partially responsible for the expression of CD8 in ThPok-defective iNKT cells.
Figure 2.
Characterization of iNKT cells in Runx3-deficient, ThPok-deficient and DKO mice. (a) αGalCer CD1d+TCRβ+ iNKT cells from the indicated mice were identified from the total thymocyte population and then stained with CD4 versus CD8 to differentiate subsets as shown in the first panel. iNKT cells shown in the bottom panel were further stained with CD8α and CD8β for a more detailed analysis. (b) The ratios of CD4+/DN iNKT cells in the thymi of Runx3-deficient and WT mice were calculated from six separate experiments. Each plot represents an individual mouse. P values were determined by a paired, two-tailed Student's t-test, and statistically significant differences are marked (*P<0.05, **P<0.01, ***P<0.001). (c) The bar graphs show the MFI of CD8α molecules (left) in ThPok-deficient and DKO mice. The absolute numbers of CD8αα+ or CD8αβ+ iNKT cells from ThPok-deficient (filled bars) and DKO (open bars) mice (n=8) are shown in succession (right). Error bars indicate s.e.m. and significance is indicated (*P<0.05, **P<0.01, ***P<0.001). Results are representative of eight independent experiments. (d) Flow cytometric analysis of peripheral iNKT cells in the indicated mice. The upper panels display CD4 and CD8 expression in iNKT cells gated on αGalCer-loaded CD1d+TCRβ+ splenocytes of the indicated mice. The splenic iNKT cells were further stained with CD8α and CD8β (second panel). Liver MNCs were isolated and stained with αGalCer-loaded CD1d tetramer, anti-TCRβ, anti-CD4 and anti-CD8 (third panel). These cells were assessed for CD8α and CD8β expression for further analysis (bottom panel). Numbers in each quadrant show the percentage of each subpopulation of iNKT cell. αGalCer, α-galactosylceramide; DKO, double knockout; iNKT, invariant natural killer T; MFI, mean fluorescence intensity; MNC, mononuclear cell; Runx3, runt-related transcription factor 3; TCR, T-cell receptor; WT, wild-type.
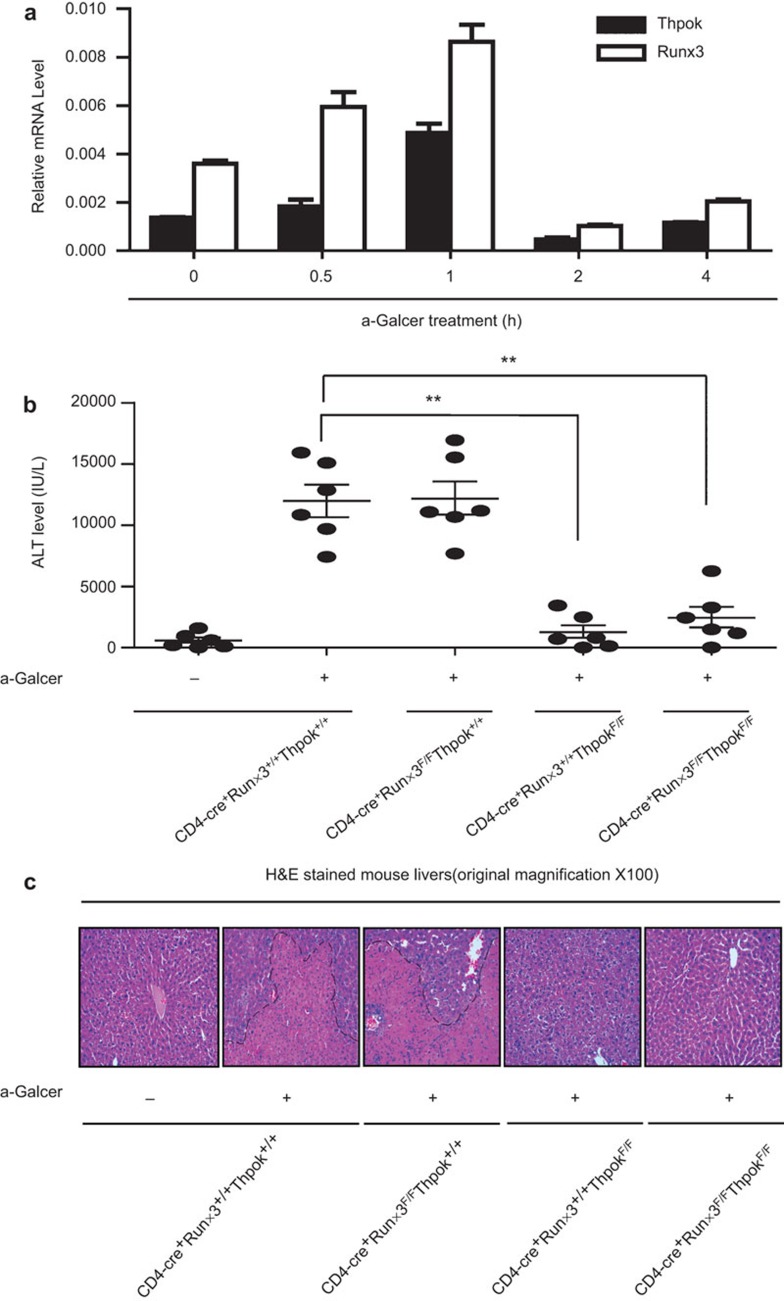
Assessment of hepatic injury in Runx3-deficient, ThPok-deficient and DKO mice
To determine whether Runx3 and ThPok expression was affected by NKT cell activation, we used the classical acute hepatic injury model. To induce hepatic injury, C57BL/6 mice were given an intravenous injection of 40 µg/kg αGalCer (a highly active agonist that specifically activates NKT cells5,48). We found that the mRNA expression of both Runx3 and ThPok mRNA was increased in the liver 1 h after αGalCer injection (Figure 3a). Twenty-four hours after the αGalCer injection, WT and Runx3-deficient mice exhibited a significant increase in ALT, whereas ThPok-deficient and DKO mice showed little ALT increase, suggesting protection from hepatic injury (Figure 3b). Consistent with the ALT results, analysis of liver histology showed severe hepatic necrosis in Runx3-deficient and WT mice 24 h after αGalCer administration, whereas the livers of ThPok-deficient and DKO mice had little damage (Figure 3c). Further, we used a ConA-induced liver injury model and obtained the same results (Supplementary Figure 3a). Thus, Runx3 does not contribute to the liver injury induced by αGalCer and ConA, as liver damage was barely detectable in mice lacking ThPok or both ThPok and Runx3.
Figure 3.
Assessment of hepatic injury in Runx3-deficient, ThPok-deficient and DKO mice. (a) mRNA expression of Runx3, ThPok and Runx1 after αGalCer treatment at the indicated time points in WT mice. Mice were injected with 40 µg/kg of αGalCer, and liver MNCs and total cellular RNA were prepared at different time points. Runx3, ThPok and Runx1 levels were assayed by RT-PCR. Expression of each gene was normalized to β-actin expression. Data are representative of more than three independent experiments. (b) Serum ALT levels in mice lacking Runx3 or ThPok or both and littermate controls were measured 24 h after αGalCer treatment (or no treatment) and presented as the mean±s.e.m.. Each plot represents an individual mouse (n=6); ***P<0.001. (c) Runx3-deficient, ThPok-deficient, DKO and WT mice were challenged with αGalCer or vehicle for 24 h. Livers were then removed, sectioned and stained with H&E and evaluated by microscopy. The photomicrographs display the liver damage (original magnification: ×100) and are representative of at least three groups of separate experiments. αGalCer, α-galactosylceramide; ALT, aminotransferase; DKO, double knockout; H&E, hematoxylin and eosin; MNC, mononuclear cell; Runx3, runt-related transcription factor 3; WT, wild-type.
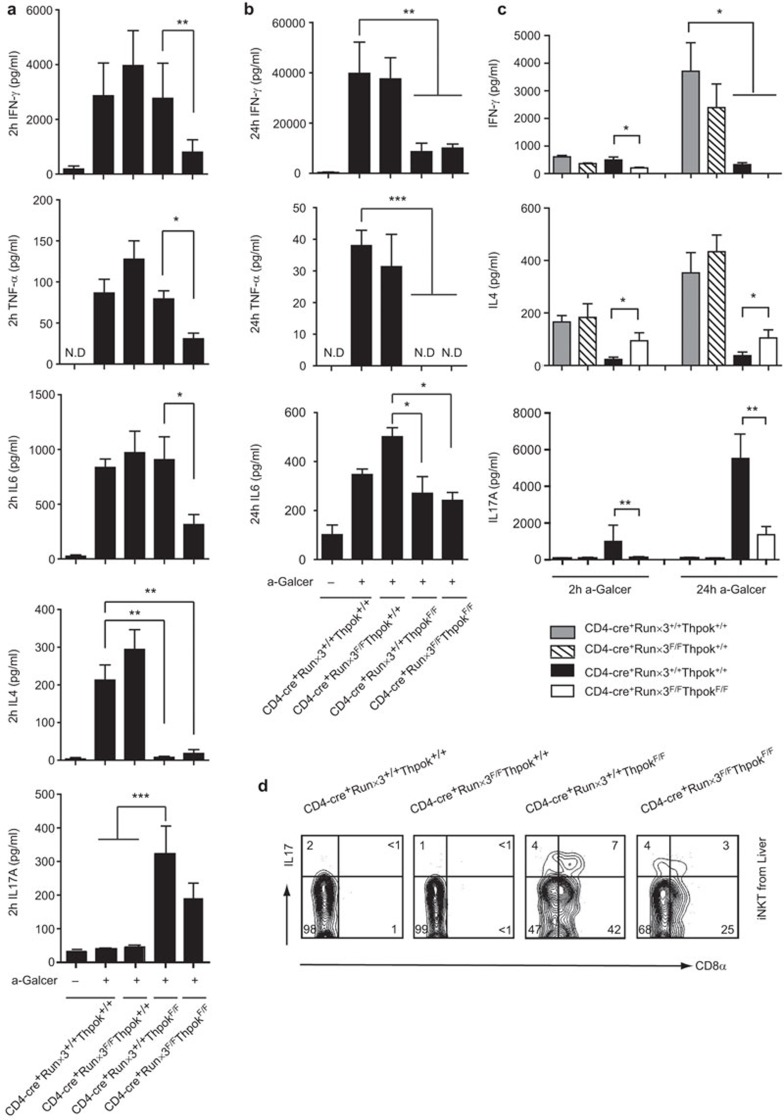
Cytokine defects in DKO mice in vivo and in vitro
Liver injury is exacerbated by high levels of Th1 (IFN-γ) and Th2 (IL-4) cytokines, as well as IL-6, IL-17 and TNF-α secreted by iNKT cells after immunization with αGalCer. We therefore examined the expression levels of several cytokines by ELISA in blood samples to determine whether the absence of Runx3 or ThPok or both affected the cytokine profile of iNKT cells. The results showed that after in vivo αGalCer stimulation for 2 h, the induction of IFN-γ, TNF-α and IL-6 was comparable among Runx3-deficient, Thpok-deficient and WT mice. However, DKO mice exhibited a notable reduction in cytokine levels, indicating that the iNKT secretion of IFN-γ, TNF-α and IL-6 during the early stage of in vivo αGalCer activation is mediated by both ThPok and Runx3 (Figure 4a). We found that the Runx3-deficient mice displayed elevated IL-4 levels similar to WT mice, but in ThPok-deficient mice, there was a dramatic reduction, which was expected based on an earlier report;26 reduced IL-4 was also found in DKO mice. Conversely, as ThPok negatively regulates NKT-17 cells,27 IL-17A levels in ThPok-deficient mice was significantly higher than those observed in Runx3-deficient and WT mice. iNKT cells from DKO mice also secreted substantial amounts of IL-17A, but the levels were less than those secreted by iNKT cells from ThPok-deficient mice (Figure 4a). In experiments assessing intracellular levels of IL-17A in liver MNCs, both the DN and CD8+ subpopulations of iNKT cells in ThPok-deficient mice were able to produce IL-17A upon stimulation, and these subsets were also able to produce IL-17A in DKO mice, indicating that IL-17A expression was not affected in the CD8+ subpopulation of iNKT cells (Figure 4d). Of note, we then measured serum cytokines 24 h after intravenous αGalCer injection, as elevation of the Th1 cytokine IFN-γ is delayed after αGalCer treatment. We found that IFN-γ levels were similar between Runx3-deficient and WT mice and were higher than those observed at 2 h post-injection. However, the ThPok-deficient and DKO mice had much lower IFN-γ levels than the other two groups (Figure 4b). These mice also demonstrated reduced TNF-α and IL-6 production compared with Runx3-deficient and WT mice, whereas IL-4 and IL-17A were undetectable (data not shown). To directly and specifically explore the ability of iNKT cells to produce cytokines in Runx3-deficient, Thpok-deficient and DKO mice, and to rule out the possibility of defects in the microenvironment of iNKT cells, thymic iNKT cells were purified from 5–7 mice/group and the sorted iNKT cells were activated with anti-CD3 for 2 or 24 h. We consistently found that after stimulation for 2 h, the expression of IFN-γ in iNKT cells from Runx3-deficient, Thpok-deficient mice did not differ from iNKT cells from WT mice, but a decrease in IFN-γ was observed in iNKT cell supernatants from DKO mice compared with iNKT cell supernatants from ThPok-deficient mice (Figure 4c). However, after stimulation for 24 h, the iNKT cells from Runx3-deficient mice secreted high amounts of IFN-γ similar to iNKT cells from WT mice, but relatively low levels were measured in iNKT cells from ThPok-deficient and DKO mice (Figure 4c). Results assessing in vitro IL-4 secretion were similar to results of the in vivo assays, namely, markedly reduced IL-4 levels were observed in iNKT cells from ThPok-deficient mice both at 2 and 24 h. However, it was noteworthy that iNKT cells from DKO mice produced more IL-4 than iNKT cells from ThPok-deficient mice, although the levels were lower than those produced by iNKT cells from Runx3-deficient and WT mice. The recovered IL-4 production in DKO mice was expected because several studies have reported an inhibitory effect of Runx3 on IL-4 expression in T cells,33,49 suggesting a similar mechanism of action of Runx3 in iNKT cells. In accord with the findings of IL-17A secretion in response to αGalCer in vivo, the in vitro experiments indicated that ThPok disruption led to significantly higher expression of IL-17A by thymus-derived iNKT cells upon stimulation compared with iNKT cells from Runx3-deficient and WT control mice, whereas the sorted iNKT cells from DKO mice secreted less IL-17A than the iNKT cells from ThPok-deficient mice (Figure 4c). The latter result could be due to the enhancing effect of Runx3 on the expression of IL-17A, similar to that observed in certain subsets of T cells.50,51 The above data confirmed that, in the absence of Runx3, cytokine production was not markedly altered in vivo and in vitro, whereas in the absence of both Runx3 and ThPok, the cytokine profile of iNKT cells was affected, indicating that Runx3 is involved in the function of iNKT cells in ThPok-deficient mice.
Figure 4.
Cytokine defects in DKO mice both in vivo and in vitro. (a) Runx3-deficient, ThPok-deficient, DKO and WT mice were injected i.p. with 40 µg/kg of αGalCer for 2 h. Protein levels of IFN-γ, TNF-α, IL-6, IL-4 and IL-17A were then detected in the serum by ELISA. Each group of experiments was performed more than five times. *P<0.05, **P<0.01, ***P<0.001. (b) Twenty-four hours after αGalCer treatment, serum was collected and used to determine IFN-γ, IL-6 and TNF-α levels. IL-4 and IL-17A were not detected. (c) Thymic iNKT cells were sorted from the indicated mice and stimulated with plate-bound anti-CD3 (1 µg/ml) for 2 or 24 h. After stimulation, the supernatant was obtained to determine the secretion of the indicated cytokines by ELISA. The results in c are representative of four to eight independent experiments. (d) Liver MNCs were isolated and activated for 3 h by PMA and ionomycin, stained for surface markers, then intracellularly stained with Abs against IL-17A or with isotype controls. All plots are gated on αGalCer+TCRβ+ iNKT cells. Data are from three independent experiments. Ab, antibody; αGalCer, α-galactosylceramide; DKO, double knockout; iNKT, invariant natural killer T; MNC, mononuclear cell; Runx3, runt-related transcription factor 3; WT, wild-type.
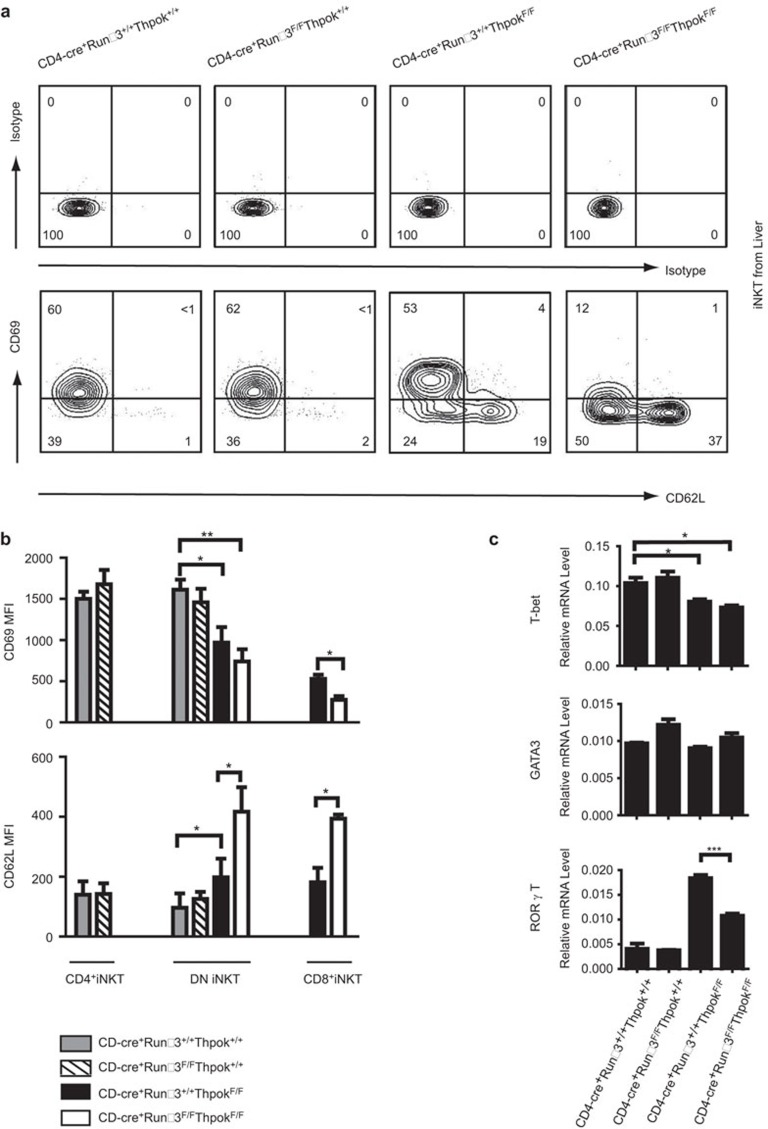
Activated and memory phenotype of iNKT cells differs among Runx3-deficient, ThPok-deficient, DKO and littermate control mice
Next, to clarify the mechanism of the functional diversity of iNKT cells among the three strains of conditional knockout mice, we first examined two crucial surface markers expressed on iNKT cell subsets: CD69, which identifies activated and memory iNKT cells, and CD62L, which identifies naive iNKT cells.35,52 The data revealed no marked difference in CD69 and CD62L expression on liver iNKT cells from Runx3-deficient and WT mice; however, iNKT cells from ThPok-deficient mice expressed a lower level of CD69 and a higher level of CD62L than control iNKT cells (Figure 5a). The iNKT cells in DKO mice appeared much more immature than iNKT cells in ThPok-deficient mice. Moreover, MFI analysis of the DN and CD8+ iNKT cell subsets showed a decrease in CD69 expression and an increase inCD62L expression in iNKT cells from DKO mice compared with iNKT cells from ThPok-deficient mice (Figure 5b). The reduced expression of the activation marker and increased expression of the naive marker in iNKT cells from DKO mice are notable, as they may account for the differential cytokine profile exhibited by iNKT cells.
Figure 5.
Phenotype and expression of transcription factors in iNKT cells from Runx3-deficient, ThPok-deficient, DKO and their littermate controls. (a) Liver MNCs were purified from the indicated mice, gated on αGalCer+TCRβ+ iNKT cells, and further assessed for CD69 and CD62L expression by flow cytometric analysis. (b) Bar graphs show the MFI of CD69 and CD62L in the indicated iNKT cell populations from liver MNCs. Data are representative of four mice analyzed in five independent experiments. Bars depict the mean value of all samples collected. *P<0.05, **P<0.01, ***P<0.001. (c) Thymic iNKT cells were sorted to detect the expression of T-bet, GATA-3 and RORγt by RT-PCR. Data are representative of five independent experiments. *P<0.05, **P<0.01, ***P<0.001. DKO, double knockout; iNKT, invariant natural killer T; MNC, mononuclear cell; RORγt, receptor-related orphan receptor-γt; Runx3, runt-related transcription factor 3.
Because the transcription factors, T-bet, GATA-3 and RORγt, are associated with the production of the cytokines described above, we further assessed whether the defective cytokine profile in DKO mice compared with ThPok-deficient mice after αGalCer challenge was related to the expression of these transcription factors. We sorted iNKT cells from the thymi of Runx3-deficient, Thpok-deficient, DKO and littermate control mice and assessed the mRNA expression of the signature transcription factors. T-bet is known to regulate the expression of the Th1 cytokine, IFN-γ, by directly binding to the promoter and enhancer regions of the gene.53,54 We found that T-bet expression in Runx3-deficient mice was similar to that of WT mice, but in Thpok-deficient and DKO mice, T-bet expression was diminished (Figure 5c). This result may explain the impaired level of IFN-γ observed in iNKT cells from ThPok-deficient and DKO mice 24 h after αGalCer injection. Moreover, because enhanced IFN-γ production in T cells requires the cooperation of T-bet and Runx3,33 the DKO mice showed decreased IFN-γ expression early after αGalCer challenge. In addition, we assessed the levels of the Th2 cytokine-regulating transcription factor, GATA-3, in purified iNKT cells and found no difference between Runx3-deficient, Thpok-deficient, DKO and WT controls (Figure 5c). This finding indicated that GATA-3 was not responsible for the absence of IL-4 production in iNKT cells in ThPok-deficient and DKO mice, suggesting that other transcription factors may be involved. We also found that the expression of the NKT17 regulator, RORγt, corresponded to the secretion of IL-17A after αGalCer challenge. That is, ThPok-deficient mice had very high levels of RORγt, and the levels were lower in DKO mice (Figure 5c). Taken together, these data indicate that in mice lacking both Runx3 and ThPok, the cytokine profile of αGalCer-challenged iNKT cells is likely associated with the expression of the transcription factors, T-bet and RORγt.
Discussion
Although a considerable number of studies have investigated the interplay between Runx3 and ThPok in T-cell lineage commitment, very little is known about their roles in iNKT cell development and function. We used Runx3-deficient and ThPok-deficient as well as Runx3/ThPok double-deficient mice to demonstrate that the phenotype of mouse iNKT cells was similar between Runx3-deficient and WT mice, and cells from these mice contained CD4 single-positive and CD4−CD8− DN sublineages. In the absence of ThPok, the mice completely lost the CD4+ iNKT cells, but harbored a group of CD8+ NKT cells as reported previously.25,41 When both Runx3 and ThPok were absent, the numbers of CD8+ iNKT cells in the thymus, spleen and liver were decreased, indicating that Runx3 plays an integral role in the appearance of CD8+ iNKT cells in ThPok-deficient mice. At the same time, a population of CD8+iNKT cells remained, probably due to compensation by Runx1 as occurs in T cells.32 Additionally, we found that Runx1 was equally expressed in ThPok-deficient and DKO mice.
Three iNKT developmental stages have been defined based on CD44 and NK1.1 expression in the thymus. These stages range from the most immature stage 1 (CD44lowNK1.1−), differentiating through stage 2 (CD44highNK1.1−) to stage 3 (CD44highNK1.1+) and representing the gradual maturation of iNKT cells.55 We found that the absence of ThPok affected the development of iNKT cells, as the expression of NK1.1 was diminished as iNKT cells gradually matured in stage 3 (CD44+NK1.1+). The Runx3-deficient mice had decreased NK1.1 levels similar to those observed in ThPok-deficient mice. Of particular interest, in the DKO mice, we found equally reduced expression of NK1.1 rather than an additive effect. This finding may be interpreted as follows: the absence of ThPok led to reduced NK1.1 expression by affecting the maturation of iNKT cells, whereas Runx3 only influenced the surface expression of NK1.1 because the phenotype and function of iNKT cells in Runx3-deficient mice were similar to those of WT mice.
Despite several studies confirming that ThPok contributes to the maintenance of CD4+ iNKT cells and that deletion of ThPok impairs the function of iNKT cells, few studies have been performed to explore the function of ThPok in liver injury. Thus, we established two widely used liver injury models in Runx3-deficient, Thpok-deficient and DKO mice. Twenty-four hours after αGalCer/ConA injection, the ThPok-deficient and DKO mice had no detectable liver injury. Combined with the results that iNKT cells from ThPok-deficient and DKO mice showed significantly increased IL-17A production after stimulation, the data likely reflect the fact that ThPok biases iNKT development towards a population of iNKT–IL-17-producing cells in the thymus.16,27 As these cells produce less IFN-γ and IL-4,17 the influence of ThPok on liver damage may be indirect, as the damage may be a result of altered iNKT cell development in the thymus. We also used the recombinant adenovirus system to overexpress ThPok in ThPok-deficient mice 3 days prior to injection of αGalCer or vehicle. Twenty-four hours after challenge, we assessed serum ALT levels and found that, compared with empty vector or mock-injected ThPok-deficient mice, the forced expression of ThPok resulted in elevated ALT levels. These results indicated that the adenovirus-mediated ectopic expression of ThPok was able to significantly rescue its function in response to αGalCer (Supplementary Figure 3b).
Experiments assessing the function of iNKT cells in ThPok-deficient and DKO mice revealed that peripheral iNKT cells from both strains acquired a naive-like phenotype: i.e., diminished CD69 and increased CD62L expression. At early times after stimulation in both in vivo and in vitro studies, the Th1-type cytokine profile of iNKT cells did not differ between ThPok-deficient and WT mice, but the iNKT cells from DKO mice had markedlyreduced expression of IFN-γ, TNF-α and IL-6, indicating that Runx3 is involved in the altered function of iNKT cells in ThPok-deficient mice. However, we found no such difference either in vivo or in vitro between ThPok-deficient and DKO mice at longer periods after stimulation. In addition, because IL-17A production is generally dependent on the transcription factor, RORγt,56 and an earlier study demonstrated increased RORγt and IL-17A expression in ThPok-deficient mice, we assessed RORγt and IL-17A expression in DKO mice. The results indicated that expression of both was lower in DKO than in ThPok-deficient mice, a finding that may be explained by the function of Runx3 on RORγt expression to a certain extent. The study of CD8+ NKT cells is also important because a population of CD8+ NKT cells exists in human. The functional potential of subsets of human NKT cells has been well studied, and they have been reported to play vital roles in autoimmune diseases, such as type 1 diabetes and several types of cancer.57,58,59 Our results provide an explanation for the emergence of CD8+ iNKT cells in ThPok-deficient mice and suggest an indirect correlation between Runx3 and the function of iNKT cells using DKO mice. However, the precise mechanisms by which Runx3 and ThPok influence the subsets and functions of iNKT cells in mice remain to be elucidated. It would be of particular interest to determine whether the other Runx family members, including Runx1, participate in the expression of CD8 on iNKT cells and their function.
Acknowledgments
We thank Dr. Rémy Bosselut for providing the CD4-cre+ThPokF/Fmice, Dr.Iain Bruce for editing the manuscript, and Yingying Huang for assistance with flow cytometry. This work was supported by grants from the National Natural Science Foundation of China (J20111945, J20121461 and C080102), the National Basic Research Program of China (973 Program and 863 Program) (2012CB966603, 2012ZX10002006 and 2012AA020901), the Zhejiang Provincial Natural Science Foundation of China (R20110298), the Doctoral Fund of the Ministry of Education of China (20110101120102) and the Fundamental Research Funds for the Central Universities
The authors declare no financial or commercial conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cellular &Molecular Immunology's website.
Supplementary Information
References
- Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- Santodomingo-Garzon T, Swain MG. Role of NKT cells in autoimmune liver disease. Autoimmun Rev. 2011;10:793–800. doi: 10.1016/j.autrev.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- Egawa T, Eberl G, Taniuchi I, Benlagha K, Geissmann F, Hennighausen L, et al. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Ho LP, Urban BC, Jones L, Ogg GS, McMichael AJ. CD4−CD8alphaalpha subset of CD1d-restricted NKT cells controls T cell expansion. J Immunol. 2004;172:7350–7358. doi: 10.4049/jimmunol.172.12.7350. [DOI] [PubMed] [Google Scholar]
- Rogers PR, Matsumoto A, Naidenko O, Kronenberg M, Mikayama T, Kato S. Expansion of human Valpha24+ NKT cells by repeated stimulation with KRN7000. J Immunol Methods. 2004;285:197–214. doi: 10.1016/j.jim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Chiba S, Nieda M, Azuma T, Ishihara S, Shibata Y, et al. Cutting edge: analysis of human V alpha 24+CD8+ NK T cells activated by alpha-galactosylceramide-pulsed monocyte-derived dendritic cells. J Immunol. 2002;168:3140–3144. doi: 10.4049/jimmunol.168.7.3140. [DOI] [PubMed] [Google Scholar]
- Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doisne JM, Becourt C, Amniai L, Duarte N, Le Luduec JB, Eberl G, et al. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor (gamma)t+ and respond preferentially under inflammatory conditions. J Immunol. 2009;183:2142–2149. doi: 10.4049/jimmunol.0901059. [DOI] [PubMed] [Google Scholar]
- Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4–NK1.1–NKT cell population. Proc Natl Acad Sci USA. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, et al. Identification of an IL-17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr Opin Immunol. 2013;25:161–167. doi: 10.1016/j.coi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, Watarai H, Inoue S, Sekine E, Nakagawa R, Hase K, et al. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J Exp Med. 2008;205:2727–2733. doi: 10.1084/jem.20080698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, et al. Development and function of invariant natural killer T cells producing Th2- and Th17-cytokines. PLoS Biol. 2012;10:e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Dave VP, Zhang Y, Hua X, Nicolas E, Xu W, et al. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- Sun G, Liu X, Mercado P, Jenkinson SR, Kypriotou M, Feigenbaum L, et al. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat Immunol. 2005;6:373–381. doi: 10.1038/ni1183. [DOI] [PubMed] [Google Scholar]
- Wildt KF, Sun G, Grueter B, Fischer M, Zamisch M, Ehlers M, et al. The transcription factor Zbtb7b promotes CD4 expression by antagonizing Runx-mediated activation of the CD4 silencer. J Immunol. 2007;179:4405–4414. doi: 10.4049/jimmunol.179.7.4405. [DOI] [PubMed] [Google Scholar]
- Wang L, Carr T, Xiong Y, Wildt KF, Zhu JF, Feigenbaum L, et al. The sequential activity of Gata3 and Thpok is required for the differentiation of CD1d-restricted CD4+ NKT cells. Eur J Immunol. 2010;40:2385–2390. doi: 10.1002/eji.201040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel I, Hammond K, Sullivan BA, He X, Taniuchi I, Kappes D, et al. Co-receptor choice by Vα14i NKT cells is driven by Th-POK expression rather than avoidance of CD8-mediated negative selection. J Exp Med. 2010;207:1015–1029. doi: 10.1084/jem.20090557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel I, Hammond K, Sullivan BA, He X, Taniuchi I, Kappes D, et al. The transcription factor Th-POK negatively regulates Th17 differentiation in Valpha14i NKT cells. Blood. 2012;120:4524–4532. doi: 10.1182/blood-2012-01-406280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa T, Littman DR. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat Immunol. 2008;9:1131–1139. doi: 10.1038/ni.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf E, Xiao C, Fainaru O, Lotem J, Rosen D, Negreanu V, et al. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci USA. 2003;100:7731–7736. doi: 10.1073/pnas.1232420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter B, Petter M, Egawa T, Laule-Kilian K, Aldrian CJ, Wuerch A, et al. Runx3 regulates integrin alpha E/CD103 and CD4 expression during development of CD4−/CD8+ T cells. J Immunol. 2005;175:1694–1705. doi: 10.4049/jimmunol.175.3.1694. [DOI] [PubMed] [Google Scholar]
- Wong WF, Kurokawa M, Satake M, Kohu K. Down-regulation of Runx1 expression by TCR signal involves an autoregulatory mechanism and contributes to IL-2 production. J Biol Chem. 2011;286:11110–11118. doi: 10.1074/jbc.M110.166694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8:145–53. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- Li L, Patsoukis N, Petkova V, Boussiotis VA. Runx1 and Runx3 are involved in the generation and function of highly suppressive IL-17-producing T regulatory cells. PLoS ONE. 2012;7:e45115. doi: 10.1371/journal.pone.0045115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protoc. 2008;3:70–78. doi: 10.1038/nprot.2007.515. [DOI] [PubMed] [Google Scholar]
- Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46:2050–2068. doi: 10.1093/clinchem/46.12.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musana KA, Yale SH, Abdulkarim AS. Tests of liver injury. Clin Med Res. 2004;2:129–131. doi: 10.3121/cmr.2.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi R, Tachibana M, Naoe Y, Muroi S, Akiyama K, Tezuka C, et al. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science. 2008;319:822–825. doi: 10.1126/science.1151844. [DOI] [PubMed] [Google Scholar]
- Taniuchi I, Osato M, Egawa T, Sunshine MJ, Bae SC, Komori T, et al. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- Ehlers M, Laule-Kilian K, Petter M, Aldrian CJ, Grueter B, Wurch A, et al. Morpholino antisense oligonucleotide-mediated gene knockdown during thymocyte development reveals role for Runx3 transcription factor in CD4 silencing during development of CD4−/CD8+ thymocytes. J Immunol. 2003;171:3594–3604. doi: 10.4049/jimmunol.171.7.3594. [DOI] [PubMed] [Google Scholar]
- Enders A, Stankovic S, Teh C, Uldrich AP, Yabas M, Juelich T, et al. ZBTB7B (Th-POK) regulates the development of IL-17-producing CD1d-restricted mouse NKT cells. J Immunol. 2012;189:5240–5249. doi: 10.4049/jimmunol.1201486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Sato T, Kohu K, Takeda K, Okumura K, Satake M, et al. Runx proteins are involved in regulation of CD122, Ly49 family and IFN-gamma expression during NK cell differentiation. Int Immunol. 2008;20:71–79. doi: 10.1093/intimm/dxm120. [DOI] [PubMed] [Google Scholar]
- Collins A, Hewitt SL, Chaumeil J, Sellars M, Micsinai M, Allinne J, et al. RUNX transcription factor-mediated association of Cd4 and Cd8 enables coordinate gene regulation. Immunity. 2011;34:303–314. doi: 10.1016/j.immuni.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui J, Liu H, Zhu X, Cui Y, Liu X. Epigenetic silencing of CD8 genes by ThPOK-mediated deacetylation during CD4 T cell differentiation. J Immunol. 2012;189:1380–1390. doi: 10.4049/jimmunol.1201077. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Godfrey DI. Directing traffic on the NKT-cell highway: a key role for ThPOK. Eur J Immunol. 2010;40:2372–2375. doi: 10.1002/eji.201040844. [DOI] [PubMed] [Google Scholar]
- Wang L, Wildt KF, Zhu J, Zhang X, Feigenbaum L, Tessarollo L, et al. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4+ T cells. Nat Immunol. 2008;9:1122–1130. doi: 10.1038/ni.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoe Y, Setoguchi R, Akiyama K, Muroi S, Kuroda M, Hatam F, et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the IL4 silencer. J Exp Med. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Jeong HM, Choi JM, Cho YC, Kim TS, Lee KY, et al. Runx3 inhibits IL-4 production in T cells via physical interaction with NFAT. Biochem Biophys Res Commun. 2009;381:214–217. doi: 10.1016/j.bbrc.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Klunker S, Chong MM, Mantel PY, Palomares O, Bassin C, Ziegler M, et al. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J Exp Med. 2009;206:2701–2715. doi: 10.1084/jem.20090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nat Immunol. 2013;14:271–280. doi: 10.1038/ni.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ueno A, Bao M, Wang Z, Im JS, Porcelli S, et al. Control of NKT cell differentiation by tissue-specific microenvironments. J Immunol. 2003;171:5913–5920. doi: 10.4049/jimmunol.171.11.5913. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Shnyreva M, Weaver WM, Blanchette M, Taylor SL, Tompa M, Fitzpatrick DR, et al. Evolutionarily conserved sequence elements that positively regulate IFN-gamma expression in T cells. Proc Natl Acad Sci USA. 2004;101:12622–12627. doi: 10.1073/pnas.0400849101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Berzins SP, Cochrane AD, Pellicci DG, Smyth MJ, Godfrey DI. Limited correlation between human thymus and blood NKT cell content revealed by an ontogeny study of paired tissue samples. Eur J Immunol. 2005;35:1399–1407. doi: 10.1002/eji.200425958. [DOI] [PubMed] [Google Scholar]
- Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet HJ, von Blomberg BM, Nishi N, Reijm M, Voskuyl AE, van Bodegraven AA, et al. Circulating V(alpha24+) Vbeta11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin Immunol. 2001;100:144–148. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.