Plasmacytoid dendritic cells (pDCs) express intracellular Toll-like receptors (TLRs) 7 and 9 and, once activated, produce large amounts of (anti-viral) type I interferons. As such, pDCs play a key role in the physiopathology of the immune response and in infection, autoimmunity and tumors. In the current issue of Nature Immunology, Agudo and colleagues report that the survival and function of pDCs are modulated by the microRNA miR-126 via the VEGFR2 pathway. These findings unveil the unknown capacity of miR-126—a microRNA that had previously been associated only with angiogenesis—to control pDCs homeostasis, and impose new considerations on the effects on innate immune responses when targeting the vascular endothelial growth factor. (VEGF) signaling pathway for therapeutic purposes.
pDCs, TLRs and innate immunity
The immune system has the ability to discriminate between intact and damaged self-components, depending on the cellular localization of the target molecule.
In innate immune cells, multiple cellular and biochemical mechanisms become activated upon engagement with pathogen-associated molecular patterns that derive from viruses, bacteria, fungi or protozoa. Pathogen-associated molecular patterns are recognized by TLRs—evolutionarily conserved receptors that are expressed in immune cells including antigen presenting cells—to provide a rapid line of host defense against microorganisms. At the molecular level, the recognition of a ligand by TLRs leads to recruitment of MyD88 (except for TLR3), whose primary effect is to activate NFκB and mitogen-activated protein kinases. Schematically, the formation of complexes of MyD88 with IRAK kinases allows NFκB to activate transcription and the release of inflammatory cytokines.1
Among TLRs, TLR7 and TLR9 can be found on endosomal membranes, where they bind nucleic acids that derive from microbes (single-stranded RNA and unmethylated CpG DNA, respectively). The subsequent secretion of pro-inflammatory cytokines—including type I interferons (IFNs)—further contributes to the fight of pathogens and the eradication of infection, as well as in the shaping of adaptive immune responses.1
Although the activation of TLRs is critical for host defense, it can also associate with the pathogenesis of inflammatory and autoimmune diseases.2 For example, the MyD88 pathway is activated in pDCs, which are immune cells that secrete large amounts of type I IFNs and thus, represent key modulators not only of infection, but also of autoimmunity and cancer.3
In recent times, a link has been reported between TLRs signaling and microRNAs (miRNAs) activity.4,5 miRNAs are small RNAs that regulate gene expression by preventing protein translation via the inhibition of the expression of mRNA transcripts. As such, miRNAs have emerged as key regulators of the immune response, leading to considerations on possibly targeting these molecules for diagnostic and/or therapeutic purposees. Moreover, miRNA dysregulation is thought to significantly impact immune responses and the development of certain diseases.6 Yet, at present, only limited data are available about the miRNA control of TLRs signaling and/or pDCs function.
In the current issue of Nature Immunology, Agudo et al.7 demonstrate the new role of miR-126 as modulator, via the VEGFR2 pathway, of pDCs homeostasis and function.7
miR-126: a role beyond angiogenesis
The authors were initially interested in studying the contribution of miR-155 to TLR stimulation and early IFN response in viral infection. To that aim, they treated miR-155-deficient (Mir155−/−) mice with TLR9 and TLR7 agonists, using as controls both wild-type mice and mice deficient in miR-126 (Mir126−/− mice) (the testing of miR-126 was chosen as control because this microRNA regulates processes that are unrelated to TLR signaling, such as angiogenesis and vasculogenesis).8 Serendipitously, the authors observed that circulating levels of IFN-α were lower in Mir126−/− mice as compared to both wild-type and Mir155−/− mice. Since TLR7 and TLR9 sense HIV infection and induce production of type I IFNs,9 the authors wanted at this point determine whether miR-126 was essential in virus sensing (and TRL activation). Therefore, they infected mice with vesicular stomatitis virus-pseudotyped, non-replicating HIV. While wild-type mice and Mir155−/− mice displayed strong immune responses to vesicular stomatitis virus-HIV, including IFN-α production, Mir126−/− inefficiently responded to infection, with very low IFN-α levels and limited immune cellularity, suggesting that in response to viral infection, miR-126 was required for type I IFN secretion via the TLR7/9 pathway.
To identify the immune cell subset that expressed miR-126 along with TLR7/9, the authors then performed miRNA profiling in subsets of T cells, dendritic cells (DCs), macrophages and B cells. They found that miR-126 was expressed only in the DC subset of pDCs, and confirmed those results in human pDCs. Importantly, they also showed that the frequency and absolute number of pDCs in Mir126−/− mice were lower than in control mice, and this aspect was specific for pDCs and not for other immune cell types, reiterating the influence of miR-126 on pDCs in relation to TLR7/9 and IFN-α production. As this finding also indicated a possible effect of miR-126 on pDCs homeostasis, experiments were done to assess whether the observed decrease in the number of pDCs in Mir126−/− mice should be ascribed to abnormalities in cell development/differentiation or to effects on cell survival. Phenotypic analyses in vitro and in vivo for markers of DCs differentiation, proliferation and death indicated that an increase in apoptosis was responsible for the observed reduction in the number of pDCs in Mir126−/− mice. These data clearly indicated that miR-126 could control pDCs survival.
However, a consideration had to be made. Although numerically reduced, pDCs were still present in Mir126−/− mice. To address whether the function of the residual pDCs was conserved or not, isolated pDCs from MiR-126−/− mice were tested in comparison with wild-type pDCs for their capacity to produce IFN-α, to become activated and for the ability to migrate to lymphoid tissues after TLR stimulation. The results showed an impaired pDCs function in Mir126−/− mice, suggesting an involvement of miR-126 in the control of a normal pDCs function.
To also explore the molecular basis of the impaired function of pDCs in Mir126−/− mice, transcriptional profiling was done. This set of investigations led to the identification of an altered expression of 253 genes in Mir126−/− pDCs as compared to wild-type pDCs. As expected, Mir126−/− mice had a lower expression of genes involved in TLR signaling, cytokine production and chemotactic responses, as well as in genes encoding viral sensors, when compared to controls. Subsequent analyses found that miR-126 decreased the expression of the protein Tsc1 (more represented in Mir126−/− pDCs than in wild-type pDCs), which was an interesting finding because Tsc1 encodes a negative regulator of the mammalian target of rapamycin (mTOR).10 Although the positive role of mTOR on pDCs survival had already been reported,11 those findings indicated for the first time that miR-126 could control the expression of a negative regulator of the mTOR pathway. The fact that an increased expression of Tsc1 could be detected in the absence of miR-126 further suggested a link between the impaired cell survival in Mir126−/− pDCs and the negative regulation of the mTOR pathway.
Additionally, in the absence of miR-126, a downregulation of the Kdr gene in pDCs was observed. Kdr encodes VEGFR2, the main receptor for the growth factor VEGF-A, and VEGF is involved in angiogenesis and vasculogenesis, having a pivotal role in the promotion of endothelial cells proliferation.12 Cell surface expression of VEGFR2 was observed on pDCs from both mouse and human tissues, with a reduced expression in Mir126−/− pDCs as compared to controls.
Considering that the lack of miR-126 in pDCs caused a reduction in VEGFR2 expression and mTOR activation, it was reasonable to assume that mTOR signaling influenced VEGFR2 expression. The positive regulation of miR-126 on VEGFR2 expression in pDCs via mTOR was confirmed by finding that inhibition of mTOR in vitro and in vivo with rapamycin led to a downregulation of the expression of VEGFR2.
Finally, to assess whether VEGF enhanced pDCs survival, and to establish a role of VEGFR2 in the process, mice with Kdr deletion specifically in DCs were generated. A ∼40% reduction in the number of pDCs was observed in those mice as compared to Mir-126−/− mice, indicating that Kdr promoted survival of pDCs, and in vivo stimulation with TLR agonists associated with impaired production of type I IFNs in pDCs from the mice lacking Kdr.
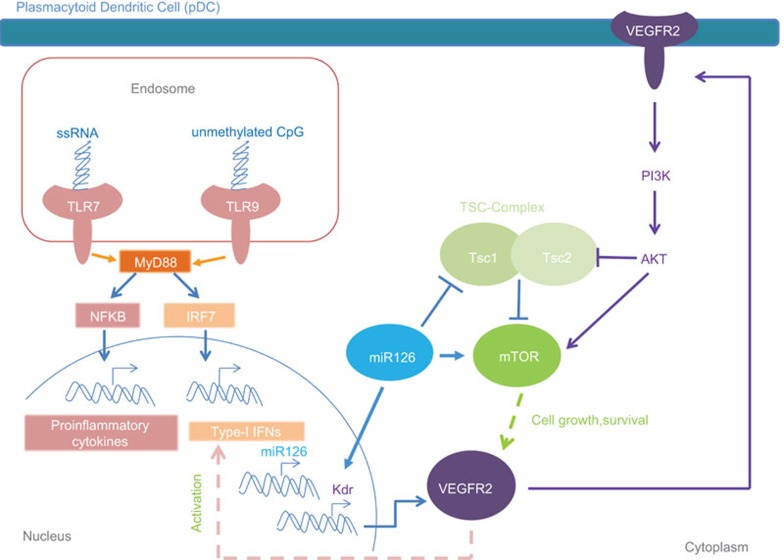
Taken together, the above data indicate an involvement of VEGF signaling in pDCs survival and function, and identify miR-126 as a positive regulator of VEGFR2 in pDCs, via the mTOR pathway (Figure 1).
Figure 1.
Schematic representation of the effects of miR-126 and VEGF signaling in pDCs. The activation of TLR7/9 induces the transcription of pro-inflammatory cytokines and type I IFNs. In physiological conditions, miR-126 ensures proper TLR7/9 responses and pDC survival (a reduction of Tsc1 promotes the activation of mTOR and subsequent VEGFR2 expression). VEGFR2, in turn, promotes production of type I IFNs and, in a positive feedback, further enhances mTOR by inhibiting Tsc2. The abundance of VEGFR2 in the presence of miR-126 preserves pDC homeostasis and function, acting on both cell growth and activation. IFN, interferon; mTOR, mammalian target of rapamycin; pDC, plasmacytoid dendritic cell; TLR, Toll-like receptor; VEGF, vascular endothelial growth factor.
Future directions
This study shows that miR-126 modulates the innate immune response in pDCs, and uncovers a new function of miR-126 as a modulator of pDCs survival and function. Until now, miR-126 was only recognized for its role on endothelial cells, tissue inflammation (as regulator of cell adhesion proteins such as vascular cell adhesion molecule-1) and tumorigenesis (where miR-126 can act as oncogene or oncosuppressor).13,14
pDCs are central components of the immune response to pathogens and play a key role in the maintenance of immune tolerance. As mentioned before, pDCs produce large amount of type I IFNs, following TLR7/9 activation, in response to pathogens. While this aspect has clear protective effects in fighting infection, an excessive secretion of IFNs can favor the development of autoimmune disease.1 Since TLR agonists can act as adjuvants in vaccination, the understanding of how pDCs respond to TLR stimulation has thus important implications for the design of approaches aimed at potentiating vaccine immunogenicity and/or anti-tumor immunotherapy.
The work by Agudo et al. implies that a reduced expression of miR-126 could favor viral infection but also tumorigenesis, since tumor-infiltrating pDCs produce reduced amounts of IFN-α upon TLR stimulation, favoring tumor escape and cancerogenesis.15 As pDCs are attracted to tumor sites by chemotactic stimuli mediated in part by adhesion to endothelial cells, miR-126 could favor recruitment of pDCs in cancer, and tissue-inflitrating pDCs would then produce low amounts of IFN-α locally.
On the other side, miR-126 upregulation could promote chronic inflammation and autoimmunity. Thus, a balanced expression of miR-126 expression should be sought, to avoid development of disparate pathologies.
The contribution of the current manuscript to the understanding of the role of miR-126 in VEGF signaling in pDCs also suggests to better ponder the use of drugs that target this pathway. VEGFR2 stimulates proliferation of endothelial cells and in cancer, the blockade of the VEFG pathway has been proposed to exert protective effects. However, since this manuscript shows that VEGFR2 is crucial to preserve pDC function, dampening VEGFR2 stimulation could compromise protective innate responses mediated by pDCs. Broader analyses on VEGF-targeted therapies, and more information on the pathways involved, are now awaited.
References
- Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of Toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol. 2011;11:163–175. doi: 10.1038/nri2957. [DOI] [PubMed] [Google Scholar]
- Nahid MA, Satoh M, Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol. 2011;8:388–403. doi: 10.1038/cmi.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottiers V, Näär AM. MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol. 2012;13:239–250. doi: 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudo J, Ruzo A, Tung N, Salmon H, Leboeuf M, Hashimoto D, et al. The miR-126–VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat Immunol. 2014;15:54–62. doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa R, Seano G, di Blasio L, Gagliardi PA, Isella C, Medico E, et al. The miR-126 regulates angiopoietin-1 signaling and vessel maturation by targeting p85β. Biochim Biophys Acta. 2012;1823:1925–1935. doi: 10.1016/j.bbamcr.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K–Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Laar L, van den Bosch A, Boonstra A, Binda RS, Buitenhuis M, Janssen HL, et al. PI3K–PKB hyperactivation augments human plasmacytoid dendritic cell development and function. Blood. 2012;120:4982–4991. doi: 10.1182/blood-2012-02-413229. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–1133. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, et al. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003;63:6478–6487. [PubMed] [Google Scholar]