CD8 T cells play critical roles in controlling intracellular pathogens and tumors. To accomplish this the small number of antigen-inexperienced naive CD8 T cells that are capable of recognizing the antigen of interest must first become primed. These initial activation events occur while the responding T cells are sequestered within lymph nodes, but many of the cells subsequently egress into the periphery and migrate into other organs where they can fulfill their principle mission of identifying and eliminating infected or malignant target cells. The antigen-driven differentiation of CD8 T cells results in the establishment of subpopulations with distinct phenotypic attributes, migratory properties and anatomic locations. Central-memory T cells preferentially reside in secondary lymphoid organs and can proliferate rapidly to boost and replenish the response upon restimulation. Effector and effector-memory populations enter and patrol tissues before recirculating, and can more promptly elicit host protective functions. A selection of activated T cells also become permanently lodged within non-lymphoid tissues and do not recirculate.1,2 The presence of these tissue-resident T cells is important as they are now immediately available within tissues and are poised to rapidly respond if they detect new or reactivating infections, and can confer superior immunological protection.3,4 What distinguishes whether a T cell will migrate to, and subsequently egress from a tissue, or instead choose to take up and maintain residency is not fully deciphered, but a recent report by Skon et al.,5 has revealed commonalities between the molecular regulators that restrict the exit of T cells from lymph nodes and those that prevent the departure of tissue-resident populations (Figure 1).
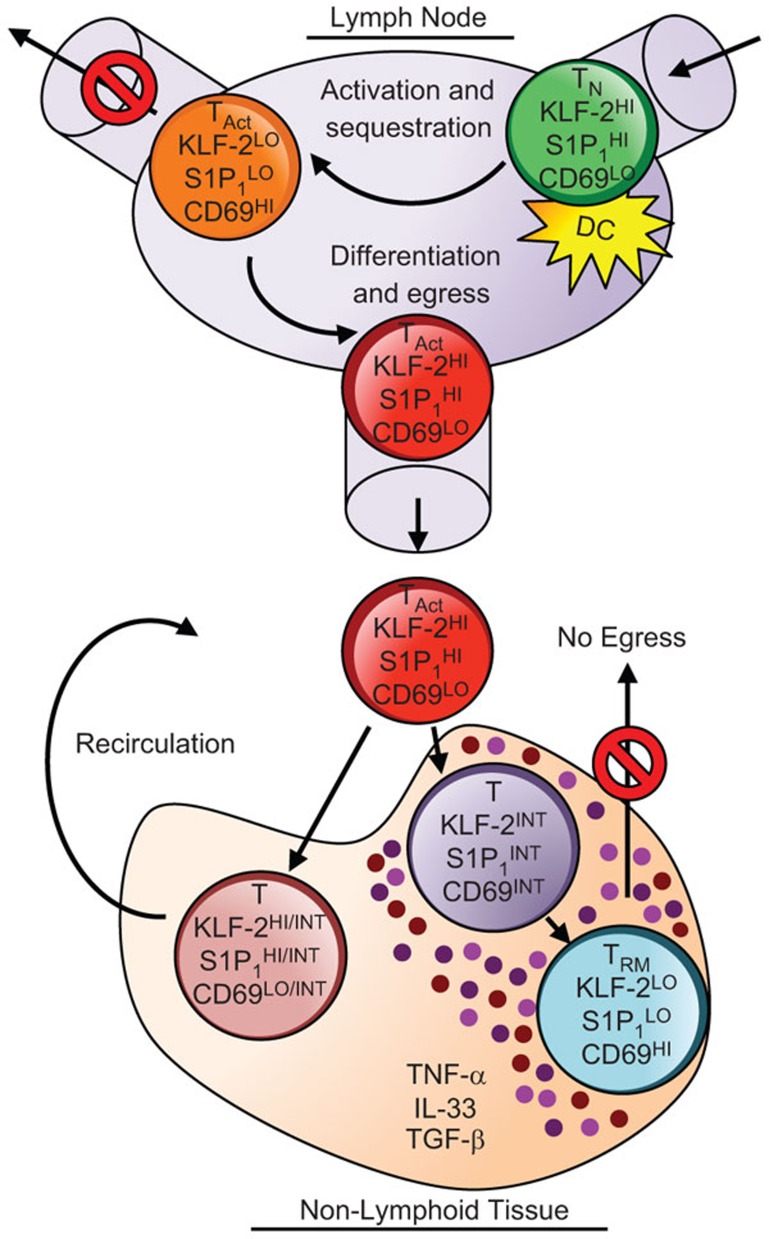
Figure 1.
Downregulation of KLF-2 and S1P1 promotes the retention of both activated CD8 T cells in lymphoid organs and nascent tissue-resident T cells in non-lymphoid organs. Naive T cells (TN) circulate through lymph nodes, but are transiently retained upon activation (TAct) due to downregulation of the transcriptional regulator KLF-2, resulting in loss of S1P1 expression and an accompanying increase the levels of CD69 at the cell surface. As the response proceeds KLF-2 becomes re-expressed, S1P1 upregulated and CD69 levels decrease. The resurrected ability to perceive the chemoattractive lipid S1-P allows the egress of the primed T cells into the periphery. These circulating cells traffic to non-lymphoid organs where local environmental factors, including the cytokine milieu, drive a decrease in KLF-2 and S1P1 expression, trapping the cells in the tissue, and promoting the formation of tissue-resident (TRM) populations. DC, dendritic cell; KLF-2, Kruppel-like factor 2; S1-P, sphingosine 1-phosphate.
Kruppel-like factor 2 (KLF-2), formerly known as lung Kruppel-like factor, is a transcriptional regulator that influences the differentiation and trafficking of T cells in part by modulating the expression of CD62L, CCR7 and S1P1.6,7 During the inception of the response, as naive T cells are stimulated for the first time with their cognate antigen, KLF-2 becomes downregulated.8 This causes the loss of S1P1 (S1pr1) expression, a receptor for the chemoattractive lipid, sphingosine 1-phosphate, and leads to an accompanying increase in the surface levels of CD69, a C-type lectin which counteracts S1P1.9,10,11 These changes nurture the retention of the responding T cells within lymph nodes and allows them to receive the differentiation signals that program the response and direct their developmental fates. Eventually, the responding cells evolve and KLF-2 is re-expressed, S1P1 upregulated, CD69 extinguished and T cells are released from their secondment and allowed to disburse into the periphery. Using reporter mice in which the expression green fluorescent protein correlates with KLF-2 levels, Skon et al.5 showed that this transcription factor is expressed by memory CD8 T-cell populations in secondary lymphoid organs (the spleen and lymph nodes), but not by putative tissue-resident T cells in non-lymphoid organs including the salivary glands, brain, kidney and small intestine. This downregulation of KLF-2 expression, which occurs shortly after effector T-cell seed non-lymphoid sites, and the associated downregulation of S1pr1, are vital steps for preventing the egress of the developing tissue-resident pool.
Inspection of the dynamics of KLF-2 expression by lymphocytic choriomeningitis virus-specific CD8 T cells revealed that the levels are highest during the early effector phase of the response, at 5 days following infection, as non-lymphoid tissues first become seeded with the anti-viral T cells. KLF-2 levels then rapidly decline as the tissue-resident population matures. Studies with parabiotic mice indicated that as homeostasis is established, a small number of memory T cells can enter non-lymphoid organs; however, these visiting T cells are unique and express intermediate levels of KLF-2 and CD69, and are distinct from both the KLF-2lo, CD69hi non-recirculating tissue-resident memory T cells and the more abundant KLF-2hi, CD69lo circulating memory CD8 T cells that traffic to and equilibrate between secondary lymphoid organs. All of this evidence supports the concept that downregulation of KLF-2 is a key molecular switch that permits the establishment of tissue-resident T cells. These findings also implicate the resulting loss of S1pr1 expression, which occurs as KLF-2 declines, as a mechanism which helps to trap the T cells by limiting their ability to perceive sphingosine 1-phosphate egress signals. The importance of S1P1 is further suggested by studies using Cd69-deficient T cells. High expression of CD69 is a property shared by both recently activated T cells in lymphoid tissues as well as by tissue-resident populations in non-lymphoid organs. CD69 associates with S1P1 to block its functions and is maintained at the cell surface if S1P1 expression is ablated.11,12 Cd69−/− T cells are not efficiently retained in lymphoid tissues and also fail to establish or sustain tissue residency.5,11,13 Thus, there are striking parallels between the regulatory pathways used to retain T cells in lymph nodes during the priming phase of the response and those that sustain tissue-resident populations (Figure 1).
To further determine the connections between S1P1 expression and the formation and maintenance of the tissue-resident T-cell population, studies were performed in which this receptor was ectopically expressed. Enforced expression of S1P1 in lymphocytic choriomeningitis virus-specific CD8 T cells did not affect the accumulation of these cells in secondary lymphoid organs, but significantly curtailed their presence in non-lymphoid sites following infection. This may be due to a failure of the S1P1-transduced T cells to seed non-lymphoid sites; however, additional experiments using skin inflammation models indicate that if S1P1 expression is sustained on T cells, then these cells do migrate to irritated skin, but their numbers then decay over time. This failure to retain the nascent tissue-resident population was also observed if the T cells were transduced to express KLF-2, the regulator of S1Pr1. Collectively, these findings suggest that constitutive expression of S1P1 or KLF-2 results in a defect in the retention of cells in non-lymphoid organs, preventing stable tissue-resident T-cell memory.
In the lymph nodes, the loss of KLF-2 is triggered by antigen-dependent T-cell receptor (TCR) signaling, but similar regulatory mechanisms may not account for the maintenance of tissue-resident populations. These resident populations are established following acute infections in which the antigen is cleared, and can also form independently of antigen at sites which have become inflamed. This suggests that sustained or recurrent TCR engagement is unlikely to be required within tissues to maintain KLF-2 downregulation and prevent the egress of resident T cells. This was further confirmed by checking the levels of Nur77, a transcriptional regulator which is transiently upregulated upon TCR triggering.14 In antigen-free recipients, the donor T cells recovered from the spleen, salivary gland, and kidneys expressed only basal levels of Nur77. T cells recovered from the small intestine lamina propria expressed modestly elevated levels of Nur77 suggesting that the resident cells in this compartment may be reacting to environmental antigens. Nevertheless, it is unlikely that strong TCR signals, due to local deposits of antigen, are essential to hold the tissue-resident T cells in place.
So what is responsible for the loss of KLF-2 expression as the tissue-resident T cells mature? Cytokines serve as pivotal extrinsic response modifiers by controlling the levels of transcriptional regulators, including KLF-2, which steer the developmental fates of the responding T cells. Skon et al., therefore, evaluated panels of cytokines which potentially dictate effector and memory CD8 T-cell differentiation, as well as push the formation of tissue-resident T cells, for their ability to regulate KLF-2. TGF-β, in conjunction with the pro-inflammatory mediator TNF-α and the alarmin IL-33, caused substantial downregulation of KLF-2. Notably, these cytokine combinations have been shown to promote the development of putative tissue-resident precursors and upregulate the expression of CD103, an integrin which permits the persistence of tissue-resident populations within several non-lymphoid organs.13,15 Thus, the cytokine milieu is likely critical for nurturing the emergence of tissue-resident populations by directing their transcriptional profiles. To further investigate the molecular pathways responsible for KLF-2 downregulation and cementing the formation of tissue-resident T cells, a series of studies were conducted in which the kinases PI(3)K and Akt were inhibited. The PI(3)K–Akt pathway was selected for investigation as it has been implicated in the cytokine driven loss of KLF-2 expression.16,17 In vitro activation in the presence of PI(3)K inhibitor LY294002 or the Akt inhibitor Akti prevented the typically observed reduction of KLF-2. Moreover, treatment of lymphocytic choriomeningitis virus-infected mice with LY294002, to block PI(3)K signaling reduced the abundance virus-specific T cells in non-lymphoid tissues, but not in the spleen, and was associated with an increase in KLF-2 levels. Thus, cytokine driven signals via the PI(3)K–Akt pathway likely play a central role in establishing tissue-resident T cells, by terminating KLF-2 expression, and ultimately halting their egress.
Loss of KLF-2 expression and downregulation of S1pr1 appears to be a shared mechanism for preventing the egress of naive T cells from secondary lymphoid organs during the priming phase of the response, as well as for retaining subsets of activated T cells in non-lymphoid tissues leading to the formation of resident populations. Tissue-resident T cells are, however, distinct from their naive counterparts, and transcriptional profiling has shown that their patterns of gene expression diverge from that of other memory T-cell subsets.13,18 This reflects their progressive maturation following a multistep process that encompasses activation, migration, retention and maintenance, which ultimately allows these to become permanently established in non-lymphoid organs. Their presence contributes to host defense at barrier sites, at which new or recurring infections may be encountered. Since the downregulation of KLF-2 and S1pr1, as well as the tethering by the integrin CD103, contributes to the formation of the tissue-resident pool, finely dissecting their temporal, relative and possible redundant requirements remains important.5,13 Moreover, how tissue-specific factors shape the establishment and properties of these immunological sentinels at local sites, and uncovering the molecular regulators responsible for their long-term maintenance, is not fully elucidated. By defining the extrinsic and intrinsic factors responsible for the development and longevity of tissue-resident populations, new strategies for cultivating these responses following vaccination or natural infections, or even possibly curtailing these T cells in pathogenic settings, may be forthcoming.
Acknowledgments
We thank Shannon Kahan and Yuan Tian for helpful comments.
References
- Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstein G, Abraham RT. Moving out: mobilizing activated T cells from lymphoid tissues. Nat Immunol. 2008;9:455–457. doi: 10.1038/ni0508-455. [DOI] [PubMed] [Google Scholar]
- Hart GT, Hogquist KA, Jameson SC. Kruppel-like factors in lymphocyte biology. J Immunol. 2012;188:521–526. doi: 10.4049/jimmunol.1101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Veselits ML, Leiden JM. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EH, Suresh M. Role of PI3K/Akt signaling in memory CD8 T cell differentiation. Front Immunol. 2013;4:20. doi: 10.3389/fimmu.2013.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol. 2012;189:3462–3471. doi: 10.4049/jimmunol.1201305. [DOI] [PMC free article] [PubMed] [Google Scholar]