Abstract
Apoptosis inhibitor of macrophages (AIMs), a homologue of human Spα, is a mouse soluble member of the scavenger receptor cysteine-rich superfamily (SRCR-SF). This family integrates a group of proteins expressed by innate and adaptive immune cells for which no unifying function has yet been described. Pleiotropic functions have been ascribed to AIM, from viability support in lymphocytes during thymic selection to lipid metabolism and anti-inflammatory effects in autoimmune pathologies. In the present report, the pathogen binding properties of AIM have been explored. By using a recombinant form of AIM (rAIM) expressed in mammalian cells, it is shown that this protein is able to bind and aggregate Gram-positive and Gram-negative bacteria, as well as pathogenic and saprophytic fungal species. Importantly, endogenous AIM from mouse serum also binds to microorganisms and secretion of AIM was rapidly induced in mouse spleen macrophages following exposure to conserved microbial cell wall components. Cytokine release induced by well-known bacterial and fungal Toll-like receptor (TLR) ligands on mouse splenocytes was also inhibited in the presence of rAIM. Furthermore, mouse models of pathogen-associated molecular patterns (PAMPs)-induced septic shock of bacterial and fungal origin showed that serum AIM levels changed in a time-dependent manner. Altogether, these data suggest that AIM plays a general homeostatic role by supporting innate humoral defense during pathogen aggression.
Keywords: innate immunity, pattern recognition receptor, scavenger receptor
Introduction
The innate immune system represents the first line of host defense for multicellular organisms to maintain homeostasis of the internal environment against foreign pathogens (microorganisms, chemicals).1 Both the humoral and the cellular arms of the innate immune system are equipped with a set of germ-line encoded receptors named pattern recognition receptors (PRRs), which recognize a relatively small spectrum of highly conserved and broadly distributed structures of protein, saccharide, lipid and nucleic acid nature, of exogenous as well as endogenous origin.2 Typically, PRRs recognize pathogen-associated molecular patterns (PAMPs), which are conserved components essential for pathogen survival and not shared by the host; examples of PAMPs include lipopolysaccharide (LPS) from Gram-negative bacteria, lipotheicoic acid (LTA) and peptydoglycan (PGN) from Gram-positive bacteria, and mannan and β-glucan from fungi. Recognition of PAMPs by PRRs usually initiates an inflammatory cascade that involves recruitment of leukocytes to the site of infection, activation of antimicrobial effector mechanisms and induction of adaptive immune responses that promote clearance of infection and long-term immune memory.2 PRRs can be either secreted molecules that circulate in blood and lymph, or cell-surface receptors present on haemopoietic, endothelial and/or epithelial cells. Examples of PRRs include C-type lectins (i.e., Dectin-1), Toll-like receptors (i.e., TLR1–11) and scavenger receptors (i.e., SR-AI/II, CD36, MARCO).
The scavenger receptor cysteine-rich superfamily (SRCR-SF) is an ancient and highly conserved group of membrane-bound and/or soluble proteins mostly reported in the animal kingdom, from low invertebrates to mammals.3,4 Members of the SRCR-SF are characterized by the presence of one or several repeats of a highly conserved cysteine-rich extracellular SRCR domain (of about 100–110 aa in size) first identified in the type A macrophage scavenger receptor 1.5 Despite the overall structure conservation of the SRCR domains across different species, no unifying biological function has been reported for them. They do not possess enzymatic activity, although some SRCR domains have been involved in protein–protein interactions, the best studied examples being those of CD6 with CD166/ALCAM,6 and of CD163 with the haptoglobin–haemoglobin complex.7 In recent years, a number of studies also support the recognition of PAMPs by some SRCR-SF members such as MARCO,8 DMBT1/SAG/gp340,9 Spα,10 CD6,11 CD5,12 S5D-SRCRB,13 SCARA514 and CD163.15
The apoptosis inhibitor of macrophages (AIMs), also known as Api6 (for apoptosis inhibitor 6) or CD5L (for CD5-like), is a mouse soluble member of the SRCR-SF which is secreted by mature tissue macrophages. AIM/Api6/CD5L was initially reported as an apoptosis inhibitor supporting the survival of macrophages and also other myeloid and lymphoid cell types against various apoptosis-inducing stimuli.16,17,18 AIM/Api6/CD5L is considered the mouse homologue of human Spα19 with which it shares an overall 73% amino acid identity.20 However, differences in the glycosylation and tissue expression patterns of these two proteins could account for functional discrepancies between them.18,20 As secreted molecules, they are readily detected (at ng µg/ml level) in human and mouse serum20,21 with Spα levels varying in different clinical situations.16,22,23,24,25 RNA analysis indicates that both molecules are expressed in lymphoid tissues (thymus, spleen fetal liver, bone marrow), but a more detailed immunohistochemical analysis on AIM/Api6/CD5L expression showed that it is restricted to only a fraction of tissue macrophages.18 Interestingly, increased AIM mRNA levels were observed in thioglycollate-induced peritoneal exudate macrophages, which could not be re-induced by treatment with PMA, LPS and cytokines like IFN-γ or interleukins.18 Taken together, all these data suggest that AIM/Api6/CD5L expression requires specific microenvironments as well as that this molecule is involved in both regulation of the inflammatory response and in the development and maintenance of the lymphoid compartment.18,19
Recent accumulating evidence has revealed the involvement of AIM/Api6/CD5L in obesity-related metabolic disorders.26 Accordingly, AIM/Api6/CD5L was found expressed in foam cells at atherosclerotic lesions, where it supports the survival of macrophages causing inflammatory responses and atherosclerosis development.27 Moreover, in adipose tissue, AIM/Api6/CD5L is endocytosed and stimulates lipolysis through inhibition of fatty acid synthase activity.28
Infections are thought to be at the basis of the atherogenesis process by promoting chronic inflammation and atherogenic lipid profiles.29 Thus, a possible role of AIM/Api6/CD5L in pathogen recognition as well as on the in vivo innate immune response against microbial aggression was investigated in the present work. Although a previous report has shown the ability of Spα to bind and aggregate bacteria,10 very little evidence if any is available for AIM/Api6/CD5L in this regard, apart from the description that it enhances the phagocytic function of macrophages in Corynebacterium parvum-induced hepatitis.30 The results herein reported demonstrate that AIM/Api6/CD5L binds to different PAMPs present not only on bacterial (either Gram-negative or Gram-positive), but also fungal (either saprophytic or pathogenic) cell surfaces and support a role for AIM/Api6/CD5L as a component of the humoral arm of the innate immune system during early phases of pathogen aggression.
Materials and methods
Ethical and biosafety approval
This study was approved by the ethical research committee of the Fundació Clínic per a la Recerca Biomèdica. In vivo studies were carried out under protocols approved by the Ethics Committee for Animal Research of the University of Barcelona. All efforts were made to minimize animal suffering.
Cell lines
The Human Embryonic Kidney cell line HEK293-EBNA, which stably expresses the EBNA-1 antigen from Epstein - Barr virus, was kindly provided by Takako Sasaki (Max-Planck-Institut für Biochemie, Martinsried, Germany). HEK293 cells stably expressing TLR2 (HEK293-TLR2) were a kind gift from Dr Golenbock (University of Massachusetts Medical School, Worcester, MA, USA). Both HEK293-EBNA and HEK293-TLR2 cells were grown in DMEM/F12 (Gibco Life Science, Grand Island, NY, USA) supplemented with antibiotics (100 U/ml penicillin, 100 µg/ml streptomycin), 10% heat-inactivated fetal calf serum (FCS) (Walkersville, Biowhittaker, MD, USA) and 250 µg/ml Geneticin (Gibco, NY, USA).
Expression of recombinant AIM/Api6/CD5L
A recombinant form of AIM/Api6/CD5L (rAIM) was expressed into HEK293-EBNA cells by using an episomal eukaryotic system (pCEP-Pu/BM40)31 in a way similar to that previously reported for Spα.21 Briefly, AIM/Api6/CD5L cDNA was obtained by reverse transcription of mouse peritoneal exudate macrophage mRNA isolated using the Ultraspec RNA isolation system (Biotecx Laboratories, Houston, TX, USA) from healthy C57Bl/6 mice and subsequent polymerase chain reaction amplification with the 5′-GCCCGGCTAGCGTCTCCAACCAAAGTGCAG-3′ (forward) and 5′-CGCGGATCCTCACACATCAAAGTCTGTGCA-3′ (reverse) AIM-specific primers, which incorporated NheI and BamHI restriction sites (underlined), respectively. The amplified polymerase chain reaction product (1013 bp) was cloned into appropriately restricted pCEP-Pu/BM-40 vector such that the AIM protein sequence starting at Ser23 (from the immature unprocessed protein) and ending at Val351 was fused in frame just after the BMP-40 signal peptide in the final construct. The resulting plasmid (20 µg) was transfected into HEK293-EBNA cells (106 cells in 10-cm culture dishes) using the calcium phosphate method. Stable transfectants were cultured in DMEM/F12 plus 10% FCS and 250 µg/ml Geneticin and selected with 1 µg/ml of puromycin (Sigma, St Louis, MO, USA) until confluence. To determine rAIM protein expression, 1 ml of serum-free culture supernatant was precipitated in 14% trichloroacetic acid and 0.14% Triton-X and then centrifuged at 13 400 g for 30 min at 4 °C. The pellet was washed once with cold acetone, resolved on SDS–PAGE under reducing conditions and stained with Coomassie blue.
When cells reached confluence, complete growth medium was replaced with serum-free DMEM/F12 plus Geneticin and puromycin; cells were then grown for a further 6–9 days, with rAIM-containing supernatant being collected every 3 days. rAIM-containing supernatant was then concentrated in Amicon Ultra-15 Centrifugal Filter Units (Millipore, MA, USA), then resolved on SDS–PAGE and stained with Coomassie blue for purity assessment and quantification by band densitometry using known amounts of bovine seroalbumin (BSA) as standard. rSpα was affinity-purified as previously described.10
Protein biotinylation was performed with EZ-Link PEO-maleimide-activated biotin (Pierce/Perbio Science, Cheshire, UK) following the manufacturer's instructions and monitored by western blotting.
Generation of anti-AIM/Api6/CD5L antibodies
A rabbit polyclonal serum against Spα was obtained in our laboratory by immunizing rabbits fortnightly with four intramuscular injections of trichloroacetic acid-precipitated serum-free Spα supernatant (50 µg total protein each) in complete (first) and incomplete (following doses) Freund's adjuvant (Life Technologies, Gaithersburg, MD, USA). Cross-reactivity of this polyclonal antiserum for AIM/Api6/CD5L was tested by western blot analysis of mouse serum (Supplementary Figure 1). To this end, diluted mouse serum pools (1∶50 and 1∶100 in phosphate-buffered saline. (PBS)) as well as rAIM and rSpα (used as positive controls) were run on SDS–PAGE in duplicate, transferred to nitrocellulose membranes and analyzed with the rabbit polyclonal antiserum diluted in blocking buffer (2% BSA in PBS, left panel) or blocking buffer plus rAIM supernatant (1∶500) (right panel), followed by horseradish peroxidase (HRP)-labelled anti-rat IgG.
Bacterial and fungal protein binding assays
Escherichia coli ATCC25922 and Staphylococcus aureus ATCC29213 were obtained from the American Type Culture Collection. Saccharomyces pombe and Saccharomyces cerevisiae were kindly provided by the Cell Biology Unit of the University of Barcelona. The rest of the bacterial and fungal strains used in this study are clinical isolates characterized by the Department of Microbiology of the Hospital Clinic of Barcelona, using standard biochemical procedures. Bacteria were grown overnight (o/n) at 37 °C in Luria Bertoni medium and fungi were grown at 28 °C in yeast peptone dextrose medium with aeration, then harvested by centrifugation at 3500 r.p.m. for 10 min and resuspended in PBS to a final density of 1010 colony forming units (cfu)/ml (for bacteria) or 108 cfu/ml (for fungi). Quantification was performed by plating bacteria and fungi dilutions on agar. For microbial binding assays, aliquots of ∼5×107 bacteria and ∼5×106 fungi were incubated o/n at 4 °C under gentle orbital rotation with different amounts of biotin-labelled rAIM in Tris-buffered saline (TBS) plus 3% BSA and 5 mM CaCl2 to a final volume of 0.5 ml, in the presence or absence of different competitors (LPS and LTA at 0, 10, 50 or 100 µg, and rSpα at 0, 0.5, 1 and 2 µg). Following incubation, microbial cells were pelleted and washed once with 0.5 ml of TBS plus BSA and Ca2+ and twice with 0.5 ml of TBS alone to remove nonspecifically bound proteins. The washed pellets were resuspended in reducing Laemmli's sample buffer for 15 min at 100 °C and separated on 8% SDS–PAGE gels, followed by electrotransfer to a nitrocellulose membrane (BioRad, CA, USA). Membranes were subjected to western blot analysis as described above, using HRP-labelled streptavidin (Roche, Basel, Switzerland) or rabbit polyclonal anti-AIM/Spα antiserum plus HRP-labelled goat anti-rabbit IgG (GE Healthcare, Buckinghamshire, UK).
For bacterial and fungal binding assays of AIM present in serum, E. coli (5×107 cfu/ml) and C. albicans (5×106 cfu/ml) were incubated in a 100 µl volume of binding buffer with 5 µl mouse serum for 1 h at 4 °C under gentle orbital rotation. Bacteria and fungi were then pelleted at 5000 r.p.m. for 5 min and the supernatant incubated again with more bacteria or fungi under the same conditions. This process was repeated for a total of eight times, with the final incubation being o/n at 4 °C. A small aliquot of supernatant was subjected to western blotting for detection of AIM as described before, using rabbit polyclonal anti-AIM/Spα antiserum.
Aggregation assays
Aggregation assays were performed by using fresh o/n cultures of E. coli, S. aureus, Candida albicans and S. cerevisiae harvested by centrifugation at 3500 r.p.m. for 10 min and then resuspended in PBS to a final density of 1010 bacteria or 108 fungi per milliltre. Fluorescein isothiocyanate (FITC) (Sigma) was dissolved in PBS to a concentration of 10 mM and then added to the bacterial/fungal suspensions to a final concentration of 1 mM for 30 min at room temperature (RT). Following removal of excess unbound FITC by extensive washing with PBS, 10 µl of FITC-labelled bacterial/fungal suspension (5×107 bacteria and 5×106 fungi, respectively) in TBS containing 5 mM CaCl2 were incubated o/n at RT with 20 µg/ml of concentrated rAIM or undiluted supernatant from AIM-producing HEK293-EBNA transfectants. Then 10 µl of the fresh samples were examined by fluorescence microscopy (OptiPhot-2; Nikon, Tokyo, Japan).
ELISA assays
Interaction of rAIM with PAMPs was analyzed by coating 96-well microtiter plates (Nunc, Roskilde, Denmark) o/n at 4 °C with 2 µg/well of purified LPS from E. coli K12 (InvivoGen, San Diego, CA, USA), PGN (cat# 77140; Sigma), LTA from S. aureus (cat# 2515; Sigma), Zymosan A (cat# z4250; Sigma), Glucan from Baker's yeast (cat# g5011; Sigma), β(1→3)-glucan (cat# 89862; Sigma), β-D-glucan (cat# g6513; Sigma) or Mannan (cat# m7504; Sigma) in coating buffer (100 mM NaHCO3, pH 9.5). Nonspecific binding to plastic wells was prevented by incubation for 1 h at RT in blocking solution (PBS plus 3% BSA). Serum-free rAIM supernatant was then added to the wells and incubated for 2 h at RT. After extensive washing, 1 µg of biotin-labelled rSpα was added for 1 h at RT followed by addition of HRP-labelled streptavidin at a 1∶1000 dilution for a further 30 min. Between each incubation step, excess unbound proteins were washed off three times with PBS 0.01% Tween 20. Colour was developed by adding 3,3′,5,5′-tetramethylbenzidine (TMB) liquid substrate (Sigma) and the absorbance was measured at 450 nm. The assay was repeated at least twice with similar results.
Cytokine release assays
Parental HEK293 cells and HEK293-TLR2 transfectants were seeded on a 96-well plate at a density of 5×104 cells per well for 24 h and then cultured in FCS-free DMEM/F12 media for a further 24 h. Cells were then pulsed with 20 µg/ml PGN for 24 h in the presence of FCS-free supernatants from HEK293-EBNA transfectants expressing rAIM, rSpα or untransfected HEK-EBNA as negative control. After this time, culture supernatant samples (25 µl) were collected and assayed for human IL-8 by ELISA (BD OptEIA, Human IL-8 ELISA Set; BD Biosciences, San Diego, CA, USA) following manufacturer's instructions.
For TNF-α release, plastic-adherent splenocytes from C57Bl/6 mice were seeded on a 48-well plate at a density of 2.5×105 cells per well, grown for 24 h and then cultured in RPMI serum-free media for a further 24 h. Cells were then pulsed with 1 µg LPS or β-𝒹-Glucan for 24 h in the presence or absence of FCS-free supernatants from HEK293-EBNA transfectants expressing rSpα or rAIM. TNF-α was then determined in culture supernatant samples (100 µL) by ELISA (BD OptEIA, Mouse TNF-α ELISA Set; BD Biosciences) following manufacturer's instructions.
Macrophage AIM/Api6/CD5L release assays
Spleen cells from C57Bl/6 mice were dissociated through a 10 µm filter and incubated in a Petri dish for 1 h at 37 °C in RPMI medium supplemented with 10% FCS, plus antibiotics. The plate was washed and the plastic-adherent cells were recovered with 10 ml of CellMates Accutase Cell Detachment Solution (Miltenyi Biotech, Cologne, Germany) for 5 min at 37 °C, and were then cultured at a density of 8×105 cells/ml in a 48-well culture plate (Nunc, Denmark, Roskilde, Denmark) with RPMI 1640 medium supplemented with 1.2 µg/ml Gentamicin (Braun, Kronbeg, Germany). The following day, paraformaldehyde-fixed whole bacterial cell suspensions (E. coli and S. aureus, 108 cfu/ml) or PAMPs (LPS or LTA, 3 µg/ml) were added to the culture, incubated at 37 °C, and the supernatants recovered at different time points. ELISA plates were coated with 100 µl of the culture supernatants o/n at 4 °C. Nonspecific binding to plastic wells was prevented by incubation for 1 h at RT in blocking solution (PBS plus 3% BSA). AIM was detected by incubation with a rabbit anti-AIM/Spα polyclonal antiserum generated in our laboratory, followed by incubation with a 1∶2500 dilution of HRP-labelled goat anti-rabbit IgG (GE Healthcare). Between each incubation step, excess unbound protein was washed off three times with PBS 0.01% Tween 20. Colour was developed by adding TMB liquid substrate and the absorbance was measured at 450 nm. The assay was repeated at least twice with similar results.
Immunofluorescence studies
The presence of AIM was studied in plastic-adherent splenocytes cultured on fibronectin-coated coverslips (50 µg/ml in cell culture media). Cells adhered to the coverslip were washed with PBS and blocked with 10% FCS in PBS for 1 h at RT. They were then incubated with a 1∶25 dilution of polyclonal anti-AIM antiserum for 1 h at RT, followed by incubation with Alexa 488-conjugated donkey anti-rabbit IgG (Invitrogen, CA, USA). Coverslips were mounted with diamidino-2-phenylindole, visualized in a fluorescence microscope, and images were analyzed by ImageJ software ImageJ.net. Approximately 100 cells were counted for each condition and the staining intensity calculated as the total intensity of the image divided by the number of cells in the field. Results are expressed as arbitrary units.
Mouse models of PAMP-induced septic shock
Two models of LPS-induced septic shock were used where the dose of LPS was considered non-lethal (1 mg/kg) as previously determined by preliminary experiments. LPS was injected i.p. to 10-week-old C57Bl/6 mice (Charles River, Barcelona, Spain) and serum was obtained 2, 6 and 24 h following LPS injection (n=3 for each time point, where three different mice were killed and bled at each time point). Zymosan-induced septic shock models were carried out in the same way, with a non-lethal (500 mg/kg) dose used, as empirically determined. Serum samples were diluted (1∶100) and boiled in reducing Laemmli's sample buffer for 15 min. They were then separated on 8% SDS–PAGE gels, followed by electrotransfer to a nitrocellulose membrane. Membranes were subjected to western blot analysis using a rabbit anti-AIM/Spα polyclonal antiserum followed by incubation with 1∶2500 diluted HRP-labelled goat anti-rabbit IgG. Bands were revealed by using Amersham ECL Prime WB Reagent (GE Healthcare).
Statistical analysis
Statistical significance of differences between result groups was determined using Student's t-test. In all experiments, differences were considered statistically significant when P was <0.05.
Results
Bacterial binding and aggregating properties of AIM/Api6/CD5L
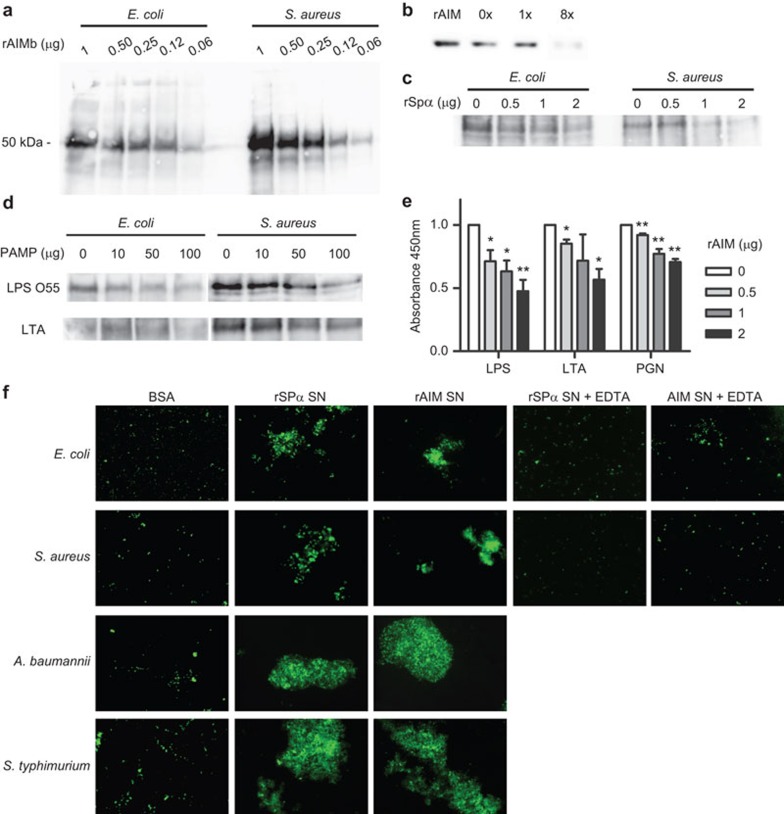
The microbial binding properties of AIM/Api6/CD5L were assessed in vitro by using a recombinant form of this receptor (rAIM) devoid of any protein tag and expressed by using a mammalian cell episomal system (pCEP-Pu/HEK293-EBNA). In these experiments, whole live bacterial cell suspensions were incubated with biotin-labelled serum-free rAIM supernatants, and the presence of cell-bound protein was further detected by western blot analysis. As shown by Figure 1a, rAIM could be detected in cell pellets from both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria in a dose-dependent manner. Importantly, serum AIM from C57Bl/6 mice was also shown to bind to whole bacterial cells. As illustrated by Figure 1b, depletion of serum AIM was achieved after several rounds (×8) of adsorption to bacterial cell pellets of Gram-negative origin (E. coli). Moreover, binding of biotin-labelled rAIM to both Gram-negative and Gram-positive bacteria was competed in a dose-dependent manner by unlabelled affinity-purified rSpα, the homologous human protein for which bacterial binding properties have been previously reported 10 (Figure 1c).
Figure 1.
rAIM binds to and aggregates whole bacteria through recognition of conserved cell wall components. (a) Increasing amounts of biotin-labelled rAIM were incubated o/n at 4 °C with whole live bacteria cell suspensions (1×1010 cfu/ml; 200 µl) in binding buffer (TBS plus 5 mM CaCl2 and 1% BSA). Extensively washed bacterial pellets were separated by SDS–PAGE for further western blot analysis using HRP-labelled streptavidin and developed by chemiluminescence. Figure shows a representative experiment out of three performed. (b) Mouse serum (10 µl) was incubated for 1 h at 4 °C with E. coli (5×107 cfu) in 100 µl binding buffer. Bacteria were then pelleted and the serum supernatant re-incubated with fresh bacteria for a new immunoprecipitation round. An aliquot of supernatant before (0×) and after ×1 and ×8 rounds was analyzed by western blot using a polyclonal anti-AIM antiserum followed by HRP-labelled anti-rabbit IgG. Figure shows a representative experiment out of two performed. (c) Bacteria (1×1010 cfu/ml; 200 µl) were incubated o/n at 4 °C with biotin-labelled rAIM (1 µg) in the presence of increasing amounts of unlabelled rSpα, and then processed as in a. Figure shows a representative experiment out of three performed. (d) Bacteria (1×1010 cfu/ml; 200 µl) were incubated o/n at 4 °C with biotin-labelled rAIM (1 µg) in the presence of the indicated increasing amounts of LPS or LTA. Washed bacterial pellets were separated by SDS–PAGE and analyzed as in a. Figure shows a representative experiment out of three performed. (e) ELISA plates coated with LPS, LTA or PGN (2 µg/well, each) were incubated with increasing amounts of unlabelled rAIM for 2 h at RT. After extensive washing, biotin-labelled rSpα (1 µg) was added for 1 h at RT followed by addition of HRP-labelled streptavidin (1∶1000 dilution) for a further 30 min. Colour was developed with TMB and absorbance read at 450 nm. Absorbance is represented relative to absorbance when no rAIM supernatant has been added. Results represent averaged values from three independent experiments. *P<0.05, **P<0.01. (f) FITC-labelled Gram-negative (A. baumannii, S. typhimurium, E. coli) and Gram-positive (S. aureus) bacteria (1×1010 cfu/ml; 10 µl) were incubated o/n at RT with rAIM (1 µg) in binding buffer in the presence (+EDTA) or absence of 5 mM EDTA. Fresh samples were then visualized in a fluorescence microscope. Magnification, ×100. Figure shows a representative experiment out of four performed. AIM, apoptosis inhibitor of macrophage; BSA, bovine seroalbumin; FITC, fluorescein isothiocyanate; HRP, horseradish peroxidase; LPS, lipopolysaccharide; LTA, lipotheicoic acid; PGN, peptydoglycan; RT, room temperature; TBS, Tris-buffered saline; TMB, 3,3′,5,5′-tetramethylbenzidine.
To better identify the precise nature of the cell wall component/s responsible for the bacterial binding ability of rAIM, further competitive bacterial binding and ELISA assays were carried out. As shown in Figure 1d, the binding of biotin-labelled rAIM supernatant to both Gram-positive and Gram-negative bacteria was competed by purified bacterial PAMPs such as LPS or LTA, which are in turn derived from Gram-negative and Gram-positive bacteria, respectively. This is in contrast with data from Spα, which show that binding of this protein to E. coli was competed by LPS but not LTA.10
In a further set of competitive ELISA assays, the ability of rAIM to interfere with the previously reported binding of Spα to conserved bacterial cell wall components was investigated. To this end, ELISA plates were coated with purified PAMPs from both Gram-negative (LPS) and Gram-positive (LTA, PGN) bacteria and then incubated with unlabelled rAIM supernatants. After extensive washing, biotin-labelled affinity purified rSpα was added to the wells. As shown by Figure 1e, unlabelled rAIM competed the binding of biotinylated rSpα to all the bacterial PAMPs tested in a dose-dependent manner. Because of the sequential addition of rAIM and rSpα, competitive effect can be ascribed to direct binding of rAIM to PAMPs as opposed to rAIM binding to rSpα.
In agreement with its pathogen-binding properties, it was also investigated whether rAIM was able to induce bacterial aggregation, a simple and effective mechanism to control microbial dissemination and infection. As shown in Figure 1f, addition of rAIM-containing supernatants induced divalent cation-dependent aggregation of different bacterial cell suspensions of both Gram-positive (S. aureus) and Gram-negative (E. coli, A. baumannii, S. typhimurium) origin, in a way similar to that previously reported for rSpα,10 here used as a positive control. No aggregation was observed following addition of an unrelated protein such as BSA (Figure 1f).
Fungal binding and aggregating properties of AIM/Api6/CD5L
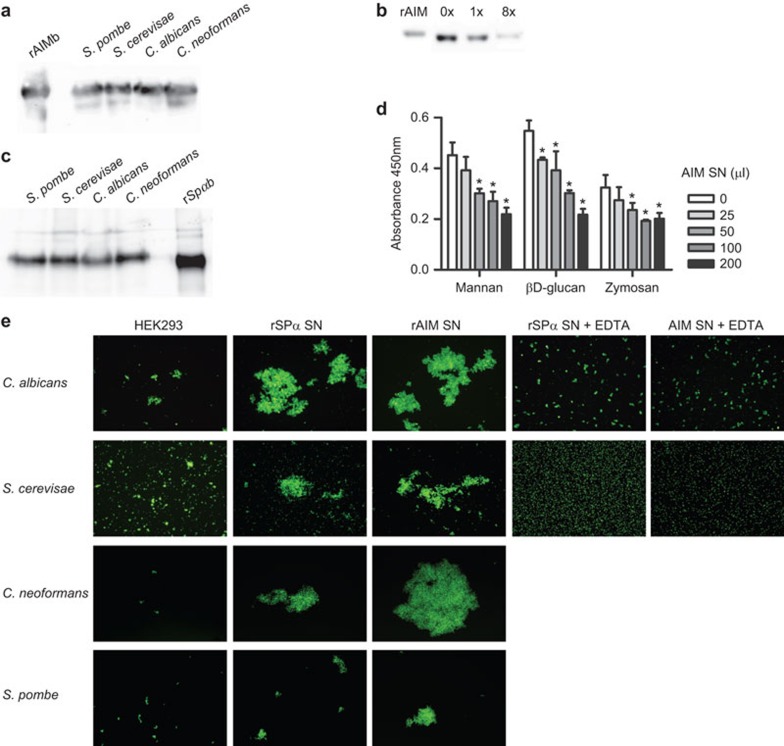
PRRs are able to recognize conserved structural components from a wide variety of pathogens, thus often being able to bind not only bacterial but also fungal, viral or parasitic cell walls. In order to test whether the microbial binding properties of AIM could be extended to fungi, protein binding experiments were carried out with whole fungal cells. As shown in Figure 2a, biotin-labelled rAIM supernatant bound to all the fungal cells analyzed, either saprophytic (S. cerevisiae, S. pombe) or pathogenic (C. albicans, C. neoformans). Again, AIM from serum was also shown to bind to whole fungal cells, as shown by progressive depletion by several rounds (×8) of adsorption to fungal cell suspensions (Figure 2b). Interestingly, the fungal binding properties were also shared by biotin-labelled rSpα (Figure 2c), a protein for which only bacterial binding properties have been reported so far.10
Figure 2.
rAIM binds to and aggregates whole live fungal cells through recognition of conserved cell wall components. (a) Biotin-labelled rAIM (1 µg) was incubated o/n at 4 °C with whole live saprophytic (S. pombe, S. cerevisiae) or pathogenic (C. albicans, C. neoformans) fungal cells (1×108 cfu/ml; 200 µl) in binding buffer (TBS plus 5 mM CaCl2 and 2% BSA). The cell pellets were extensively washed and then analyzed by western blot with HRP-labelled streptavidin and developed by chemiluminescence. Figure shows a representative experiment out of three performed. (b) Fungi (5×106 cfu/ml) were incubated with 10 µl mouse serum in 100 µl binding buffer for 1 h at 4 °C; fungal cells were then pelleted and the supernatant was re-incubated with fresh fungi for a new round. An aliquot of supernatant before (0×) and after 1× and 8× rounds was analyzed by western blot using a polyclonal anti-AIM antibody followed by anti-rabbit-HRP. Figure shows a representative experiment out of two performed. (c) The same assays as in a were performed by using biotin-labelled rSpα (1 µg). Figure shows a representative experiment out of three performed. (d) ELISA plates were coated with mannan, β-𝒹-glucan or zymosan (2 µg each/well) o/n at 4 °C, and then incubated with increasing amounts (in µl) of serum-free supernatant from rAIM-expressing HEK293-EBNA cells for 2 h at RT. This was followed by incubation with biotin-labelled rSpα (1 µg) for 1 h at RT and further streptavidin-HRP for an additional 30 min. Colour was developed with TMB and absorbance read at 450 nm. Results represent averaged absorbance values from three independent experiments. *P<0.05. (e) FITC-labelled fungal cells (1×107 cfu/ml; 50 µl) were incubated o/n at RT with serum-free supernatant from rAIM-expressing HEK293-EBNA cells (50 µl) in binding buffer in the presence (+EDTA) or absence of 5 mM EDTA. Fresh samples were then visualized in a fluorescence microscope. Magnification, ×100. Figure shows a representative experiment out of four performed. AIM, apoptosis inhibitor of macrophage; BSA, bovine seroalbumin; FITC, fluorescein isothiocyanate; HRP, horseradish peroxidase; RT, room temperature; TMB, 3,3′,5,5′-tetramethylbenzidine.
As illustrated by competitive ELISA assays shown in Figure 2d, direct binding of both rAIM and rSpα to the fungal particle Zymosan as well as to purified conserved fungal cell wall constituents such as mannan and β-𝒹-glucan was observed. The fact that the addition of rAIM and rSpα is sequential again indicates that the observed competitive effect can be ascribed to direct binding of rAIM to fungal PAMPs.
Moreover, serum-free rAIM-containing supernatants were also able to aggregate whole fungal cell suspensions in a manner dependent on the presence of divalent cations (Figure 2e). Taken together, the results indicate that AIM and its human counterpart Spα can bind to a number of different microbia, both pathogenic and saprophytic.
Inhibition of PAMP-induced cytokine release by AIM/Api6/CD5L
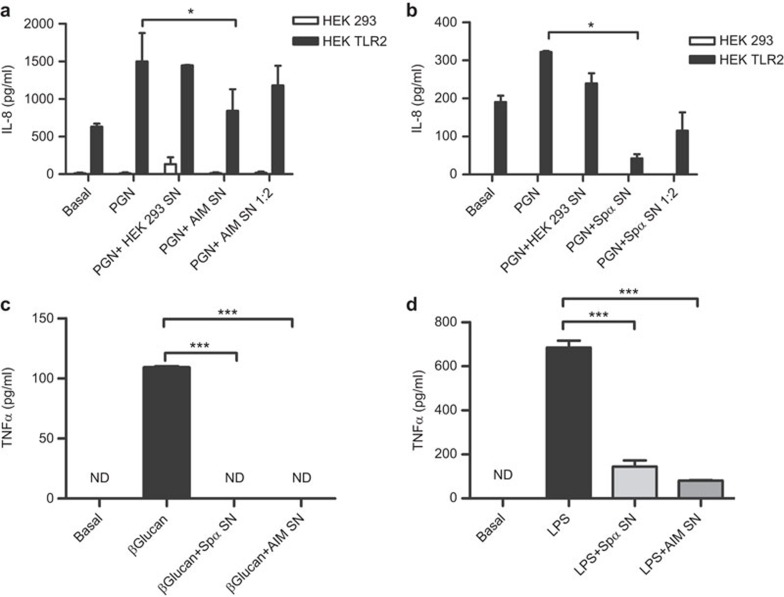
To further analyze the biological consequences of the interaction of AIM/Api6/CD5L with bacterial and fungal PAMPs, the effect of rAIM on PAMP-induced cytokine release was investigated in different cellular systems. To this end, HEK293 cells stably transfected with TLR2 32 were exposed to PGN, a well-known TLR2 ligand, in the presence or absence of serum-free rAIM-containing culture supernatants, and the IL-8 levels monitored 18 h later. As shown in Figure 3a, the PGN-induced release of IL-8 was inhibited by undiluted rAIM-containing supernatants. No inhibition was observed when using either diluted (by half) rAIM-containing supernatants or supernatants from untransfected HEK293 cells. No IL-8 release was detected following exposure of parental untransfected HEK293 cells to PGN. When the same experiments were performed in the presence or absence of serum-free rSpα-containing supernatants, similar inhibitory effects were observed (Figure 3b). Moreover, undiluted rAIM- and rSpα-containing supernatants were also able to reduce the in vitro TNF-α production by spleen macrophages in response to bacterial LPS, a well-known TLR4 ligand, as well as to fungal β-𝒹-glucan, a well-known Dectin-1 ligand (Figure 3c and d). These results indicate that by binding to bacterial and fungal ligands of TLRs and/or other PRRs, AIM (and Spα) would interfere with the subsequent cytokine-mediated inflammatory response.
Figure 3.
Immunomodulating properties of rSpα and rAIM. (a and b) IL-8 release assay. HEK293 and HEK293 TLR2 cells were seeded on a 96-well plate at a density of 5×104 cells per well, grown for 24 h and then cultured in serum-free media for a further 24 h. Cells were then pulsed with 20 µg/ml PGN for 24 h in the presence of serum-free supernatants (undiluted and diluted in half in serum-free media) from HEK293-EBNA transfectants expressing rAIM (a) or rSpα (b), or undiluted supernatant from HEK293-untransfected cells as control. IL-8 was then determined in culture supernatant samples by ELISA. Figure shows averaged results from three independent experiments. *P<0.05. (c and d) TNF-α release assay. Plastic-adherent splenocytes were seeded on a 48-well plate at a density of 2.5×105 cells per well, grown for 24 h and then cultured in serum-free media for a further 24 h. Cells were then pulsed with 1 µg β-𝒹-Glucan or LPS for 24 h in the presence or absence of supernatants from HEK293-EBNA transfectants expressing rSpα or rAIM. TNF-α was then determined in culture supernatant samples by ELISA. Figure shows averaged results from two independent experiments. ***P<0.005. AIM, apoptosis inhibitor of macrophage; ND, not detected; LPS, lipopolysaccharide; PGN, peptidoglycan; TLR, Toll-like receptor.
AIM is released by macrophages in vitro in response to PAMPs
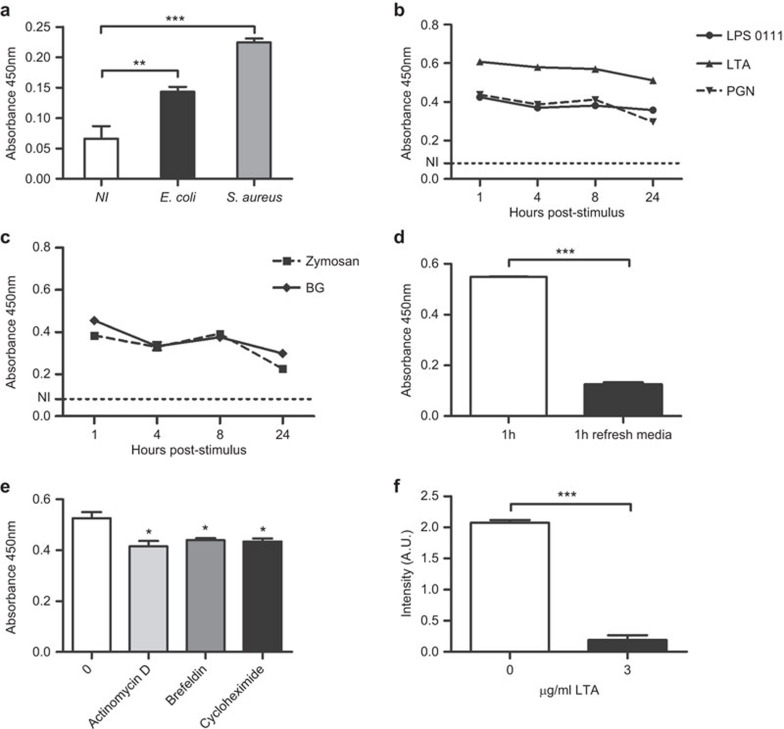
Previous reports had shown that AIM mRNA was not induced in response to LPS.18 However, given the potential PAMP-binding and anti-inflammatory properties of AIM during PAMP exposure, it was further investigated whether the presence of PAMPs would induce the release of AIM. To confirm this, plastic-adherent mouse splenocytes were exposed to paraformaldehyde-fixed whole bacteria (E. coli and S. aureus) and the levels of AIM measured in culture supernatant by direct ELISA. As shown in Figure 4a, both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria increased AIM levels in culture supernatants at 1 h post-stimulation. A similar increase in AIM levels was observed following exposure of plastic-adherent spleen cells to purified bacterial (LPS, PGN and LTA) and fungal (Zymosan and β-glucan) PAMPs (Figure 4b and c). Interestingly, the increase peaked as soon as 1 h post-stimulation and did not increase any further, suggesting a rapid release of the preformed protein from cellular deposits. Indeed, when LTA was washed out from spleen cell cultures after 1 h of stimulation and replaced with fresh media, AIM levels detected after a further 8 h incubation showed no additional increase (Figure 4d). Similar results were obtained with Spα when stimulating plastic-adherent cells derived from human PBMCs (data not shown). In a further set of experiments, pre-incubation of plastic-adherent spleen cells with inhibitors of transcription (actinomycin D), protein synthesis (cycloheximide) or protein transport into Golgi (brefeldin) before exposure to LTA for 1 h, only slightly inhibited AIM release into cell culture media (Figure 4e). Although this inhibition was significant, the low magnitude and the fact that all three different compounds induced a similar level of inhibition suggest that this was due to a general toxic effect of the compounds used.
Figure 4.
Secretion of AIM is stimulated by the presence of bacterial and fungal PAMPs. (a–c) Plastic-adherent cells (2.5×105) from C57Bl/6 mouse spleens were cultured in RPMI media and stimulated for 1 h with 108 cfu/ml E. coli or S. aureus (a) or for different time periods with 3 µg/ml of LPS O111, LTA and PGN (b), as well as Zymosan and β-𝒹-glucan (c). AIM levels were then determined in an ELISA assay, coating plates with supernatant from the splenocyte cultures and detecting the presence of AIM with an anti-AIM polyclonal antibody, followed by HRP-conjugated anti-rabbit IgG. Figure shows a representative experiment out of three performed. (d) Plastic-adherent cells (2.5×105) from C57Bl/6 mouse spleens were cultured in RPMI media and stimulated with 3 µg/ml of LTA. After a 1-h incubation, the media was removed and replaced by fresh media. AIM levels were then determined in an ELISA assay as above. Figure shows a representative experiment out of three performed. (e) Plastic-adherent splenocytes from murine spleens were cultured and stimulated with LTA as above in the presence of 1 µg/ml of brefeldin, cycloheximide or actinomycin D. AIM was measured in cell culture supernatants as previously described. Figure shows a representative experiment out of two performed. *P<0.05. (f) Plastic-adherent cells from murine spleens were grown on fibronectin-coated coverslips and stained with anti-AIM polyclonal antibody, followed by an Alexa-488-conjugated anti-rabbit IgG antibody. Cells were then photographed in a fluorescence microscope and the total fluorescence intensity divided by the number of cells in the field. At least three fields were counted for each treatment. Results are expressed as arbitrary units (A.U.). AIM, apoptosis inhibitor of macrophage; HRP, horseradish peroxidase; LPS, lipopolysaccharide; LTA, lipotheicoic acid; PAMP, pathogen-associated molecular pattern; PGN, peptydoglycan.
Support for the putative release of AIM from preformed storage was also obtained by staining permeabilized plastic-adherent spleen cells with a polyclonal anti-AIM antiserum after treatment with LTA or vehicle for 1 h at 37 °C. The fluorescence intensity for cells treated with vehicle or LTA was then determined, as the total fluorescence measured divided by the number of cells in the field. As shown in Figure 4f, fluorescence intensity for AIM staining was decreased in LTA-treated cells, confirming the release of AIM into the culture supernatant in response to LTA treatment.
In vivo PAMP challenge decreases serum AIM levels
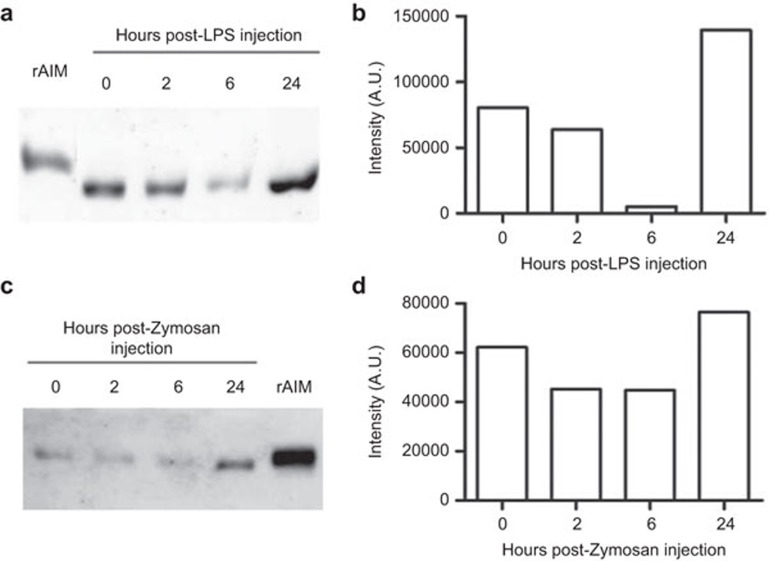
As previously mentioned, AIM is an abundant serum protein with PAMP- binding ability. Accordingly, it was further investigated whether infusion of bacterial and/or fungal PAMPs would be able to affect circulating AIM levels during in vivo challenge. To this end, the serum levels of AIM were monitored over time following i.p. administration of lethal or sublethal doses of LPS or Zymosan to C57Bl/6 mice, as empirically determined. Serum samples were obtained from mice 2, 6 and 24 h post-PAMP injection and the levels of AIM were determined by western blotting. The results (Figure 5) show that serum AIM levels are decreased, as compared to basal levels, 2 and 6 h post-injection of sublethal doses of both LPS (1 mg/kg) and Zymosan (500 mg/kg), and that they recover and even surpass those observed in basal conditions after 24 h. This recovery in AIM levels was paralleled by an improvement in mice wellbeing. Moreover, when higher (lethal) doses of the two PAMPs were used (10 and 700 mg/kg, respectively), serum AIM levels behaved in a similar manner to above reported for sublethal doses, except that they remained below basal levels at 24 h post-challenge (data not shown). Since the dose is lethal, almost no mice survive more than 24 h, so it is not possible to know whether AIM levels recover at a later time. These results suggest that AIM can act as a soluble PRR, which can bind to PAMPs, and consequently, its serum levels are affected during microbial challenge.
Figure 5.
AIM serum levels in vivo following PAMP challenge. (a) Mice (n=3 for each time point) were injected i.p. with 1 mg/kg LPS and serum was obtained in basal conditions and 2, 6 and 24 h following LPS injection. AIM serum levels were determined by western blotting using a polyclonal anti-AIM antibody, followed by an HRP-conjugated anti-rabbit IgG. (c) Densitometric measurement of the bands in a expressed in arbitrary units (A.U.). (c) The same as in a, except that mice (n=3 for each time point) were injected i.p. with 500 mg/kg Zymosan. (d) Densitometric measurement of the bands in c. AIM, apoptosis inhibitor of macrophage; HRP, horseradish peroxidase; LPS, lipopolysaccharide; PAMP, pathogen-associated molecular pattern.
Discussion
The present report shows that AIM/Api6/CD5L, a soluble member of the SRCR superfamily found in mouse serum, acts as a pattern-recognition receptor for PAMPs present in bacteria and fungi. This might not seem surprising, considering that Spα, the human homologue of AIM, has been found to act as a PRR for LPS and LTA.10 However, although both proteins share approximately 70% homology, their glycosylation pattern is very different, which might account for functional differences. Indeed, even though binding of LPS and LTA by rSpα was found to be mediated by two fully independent sites, binding of both PAMPs by rAIM has now been found to depend on a single or two closely adjacent sites, judging by how LPS is able to inhibit LTA binding to rAIM and vice versa. This study also describes the expanded spectrum of rSpα PAMP binding, including now not only bacteria but also pathogenic and saprophytic fungi; these binding properties are also shared by rAIM. It should be noted that both rAIM and rSpα also have the ability to aggregate as well as to bind bacteria and fungi, which could be a defensive mechanism used by these proteins in preventing single cell invasion of tissues by microorganisms, as well as facilitating their elimination. The ability to induce aggregation is easily explained by the structure of these proteins, consisting of three tandem repeats of highly homologous SRCR domains.
Both AIM/Api6/CD5L and Spα were shown by our results to bind to whole bacteria and fungi through recognition of well-known bacterial (LPS, LTA, and PGN) and fungal (mannan and β-𝒹-glucans) PAMPs. It is known that PAMPs alone, without accompanying infection by microorganisms, are able to cause tissue damage (and ultimately septic shock) through their pro-inflammatory activity.33 Binding of free PAMPs could thus neutralize the pro-inflammatory effect of these compounds and protect tissues from damage. The results presented herein show that AIM/Api6/CD5L and Spα have the potential to reduce pro-inflammatory cytokine and chemokine secretion by mouse and human cells, respectively, thus suggesting a role for these proteins in tissue protection during exposure to PAMPs.
The fact that AIM/Api6/CD5L and Spα appear to be useful in fighting infection and protecting tissue from inflammation-related damage could lead one to believe that expression of these proteins might be induced following pathogen infection or by the presence of PAMPs. Previous studies, however, have reported lack of AIM mRNA induction in macrophages by PAMPs such as LPS.18 Our results show that AIM and Spα levels are increased in the culture medium of mouse and human monocyte/macrophages, respectively, following treatment with whole bacteria and several bacterial and fungal PAMPs. The reason for the apparent discrepancy could be that AIM and Spα accumulate in cytoplasmic stores ready for rapid release, which is triggered by the presence of PAMPs, independently from mRNA transcription. Indeed, the time frame of AIM/Api6/CD5L release into the culture medium is not consistent with de novo protein synthesis by monocyte/macrophages, peaking as early as 1 h following PAMP exposure. Moreover, release of AIM/Api6/CD5L into the culture medium was not substantially affected by inhibiting transcription, protein synthesis or transport into the Golgi, supporting the notion that AIM/Api6/CD5L is mostly preformed in cells and its release is triggered by PAMPs. Storing these proteins inside cells ready for release would be of great use as a rapid reaction against infection, whereby their release would be triggered by the presence of pathogen compounds which could quickly be neutralized, protecting the tissue. Aggregation of pathogens could also impair the spread of infection and might even facilitate the phagocytic elimination of microorganisms.
The importance of these results lies in the fact that, to date, the only known inducers of AIM/Spα mRNA expression are LXR ligands.34 Triggering of AIM release by PAMPs could therefore be a novel mechanism through which the innate immune system attempts to control infection and reduce inflammatory damage to tissues.
To test this idea, an in vivo challenge with PAMPs was carried out and a time-dependent decrease in AIM serum levels in LPS and Zymosan-treated mice was observed. Interestingly, when animals were treated with low (non-lethal) doses of PAMPs, the decrease of AIM levels in serum was followed by a sharp increase, which even surpassed the basal levels. This suggests that AIM is spent in situations where circulating PAMPs are present, presumably because it binds to PAMPs and contributes to their elimination; however, these levels can recover relatively quickly. Moreover, the recovery of AIM serum levels paralleled the improvement in mice wellbeing. A recent report shows that transfection with AIM enhances macrophage bactericidal ability in a model of infection by Mycobacterium tuberculosis.35 Interestingly, AIM levels increased early in mice serum (24 h) following inoculation of M. tuberculosis, which is in agreement with our results. This increase in AIM levels could, at least in part, be ascribed to PAMP-mediated triggering of AIM release into the circulation; however, the release observed in vitro is much faster than that observed in the in vivo experiments. This suggests that other, still unknown, inflammation-related mechanisms might induce AIM expression and/or release. Indeed, it has been reported that AIM mRNA is induced in peritoneal macrophages following thyoglycollate-induced inflammation.18
Further studies will need to be carried out in order to determine the true importance of AIM expression levels in situations of infection or PAMP-induced sepsis and septic shock syndrome. It should be noted that as well as a pattern recognition receptor, AIM is also a survival factor for macrophages, key contributors to inflammation.18,36,37,38,39 Future experiments need to assess which of the dual functions of AIM predominates in the early immune response against pathogens.
In summary, we have identified AIM as a PRR with similar binding properties as those of its human homologue Spα, capable of binding and aggregating a broad spectrum of microbial agents (bacteria and fungi), thus playing a role in the early response to microbial aggression. These properties are in line with those displayed by other members of the SRCR superfamily such as MARCO,40 SAG/DMBT-1,9,41 sCD611 and sCD163,42 and reinforces the notion that the PRR function could be one unifying trait shared by this superfamily's members. AIM/Api6/CD5L has also shown other immunomodulating properties such as the ability to impair PAMP-induced pro-inflammatory cytokine production, which could be useful in protecting tissues from local inflammation damage, and also from more generalized effects of PAMPs such as sepsis and septic shock syndrome.
Acknowledgments
We would like to thank Dr Rosa Aligué (Cell Biology Unit of the University of Barcelona) and Dr. Jordi Vila (Department of Microbiology of the Hospital Clinic of Barcelona) for their kind help with microbial specimens. This study was supported by grants from the Spanish Ministerio de Economía y Competitividad (SAF2010-19717), Generalitat de Catalunya (2009SGR1101) and Instituto de Salud Carlos III (Spanish Network for Research in Infectious Diseases, REIPI, RD12/0015/0018). VGM is recipient of a fellowship from Ministerio de Economia y Competitividad (JCI-2010-06378). CE-F is recipient of a fellowship from Spanish Ministerio de Economía y Competitividad (BES-2008-005544).
The authors declare no financial interests.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website.
Supplementary Information
References
- Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Resnick D, Pearson A, Krieger M. The SRCR superfamily: a family reminiscent of the Ig superfamily. Trends Biochem Sci. 1994;19:5–8. doi: 10.1016/0968-0004(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Sarrias MR, Gronlund J, Padilla O, Madsen J, Holmskov U, Lozano F. The Scavenger Receptor Cysteine-Rich (SRCR) domain: an ancient and highly conserved protein module of the innate immune system. Crit Rev Immunol. 2004;24:1–37. doi: 10.1615/critrevimmunol.v24.i1.10. [DOI] [PubMed] [Google Scholar]
- Freeman M, Ashkenas J, Rees DJ, Kingsley DM, Copeland NG, Jenkins NA, et al. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc Natl Acad Sci USA. 1990;87:8810–8814. doi: 10.1073/pnas.87.22.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A, Bowen MA, Patel DD, Haynes BF, Starling GC, Gebe JA, et al. CD6–ligand interactions: a paradigm for SRCR domain function. Immunol Today. 1997;18:498–504. doi: 10.1016/s0167-5699(97)01130-4. [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- Brannstrom A, Sankala M, Tryggvason K, Pikkarainen T. Arginine residues in domain V have a central role for bacteria-binding activity of macrophage scavenger receptor MARCO. Biochem Biophys Res Commun. 2002;290:1462–1469. doi: 10.1006/bbrc.2002.6378. [DOI] [PubMed] [Google Scholar]
- Bikker FJ, Ligtenberg AJ, End C, Renner M, Blaich S, Lyer S, et al. Bacteria binding by DMBT1/SAG/gp-340 is confined to the VEVLXXXXW motif in its scavenger receptor cysteine-rich domains. J Biol Chem. 2004;279:47699–47703. doi: 10.1074/jbc.M406095200. [DOI] [PubMed] [Google Scholar]
- Sarrias MR, Rosello S, Sanchez-Barbero F, Sierra JM, Vila J, Yelamos J, et al. A role for human Sp alpha as a pattern recognition receptor. J Biol Chem. 2005;280:35391–35398. doi: 10.1074/jbc.M505042200. [DOI] [PubMed] [Google Scholar]
- Sarrias MR, Farnos M, Mota R, Sanchez-Barbero F, Ibanez A, Gimferrer I, et al. CD6 binds to pathogen-associated molecular patterns and protects from LPS-induced septic shock. Proc Natl Acad Sci USA. 2007;104:11724–11729. doi: 10.1073/pnas.0702815104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J, Fenutria R, Canadas O, Figueras M, Mota R, Sarrias MR, et al. The CD5 ectodomain interacts with conserved fungal cell wall components and protects from zymosan-induced septic shock-like syndrome. Proc Natl Acad Sci USA. 2009;106:1506–1511. doi: 10.1073/pnas.0805846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miro-Julia C, Rosello S, Martinez VG, Fink DR, Escoda-Ferran C, Padilla O, et al. Molecular and functional characterization of mouse S5D-SRCRB: a new group B member of the scavenger receptor cysteine-rich superfamily. J Immunol. 2011;186:2344–2354. doi: 10.4049/jimmunol.1000840. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Oliver P, Davies KE, Platt N. Identification and characterization of murine SCARA5, a novel class A scavenger receptor that is expressed by populations of epithelial cells. J Biol Chem. 2006;281:11834–11845. doi: 10.1074/jbc.M507599200. [DOI] [PubMed] [Google Scholar]
- Fabriek BO, van BR, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, et al. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113:887–892. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- Kim WK, Hwang HR, Kim DH, Lee PY, In YJ, Ryu HY, et al. Glycoproteomic analysis of plasma from patients with atopic dermatitis: CD5L and ApoE as potential biomarkers. Exp Mol Med. 2008;40:677–685. doi: 10.3858/emm.2008.40.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwata K, Watanabe H, Jiang SY, Yamamoto T, Tomiyama-Miyaji C, Abo T, et al. AIM inhibits apoptosis of T cells and NKT cells in Corynebacterium-induced granuloma formation in mice. Am J Pathol. 2003;162:837–847. doi: 10.1016/S0002-9440(10)63880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Hirokami Y, Matsuhashi N, Takatsuka H, Naito M. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J Exp Med. 1999;189:413–422. doi: 10.1084/jem.189.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebe JA, Kiener PA, Ring HZ, Li X, Francke U, Aruffo A. Molecular cloning, mapping to human chromosome 1 q21–q23, and cell binding characteristics of Spalpha, a new member of the scavenger receptor cysteine-rich (SRCR) family of proteins. J Biol Chem. 1997;272:6151–6158. doi: 10.1074/jbc.272.10.6151. [DOI] [PubMed] [Google Scholar]
- Gebe JA, Llewellyn M, Hoggatt H, Aruffo A. Molecular cloning, genomic organization and cell-binding characteristics of mouse Spalpha. Immunology. 2000;99:78–86. doi: 10.1046/j.1365-2567.2000.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrias MR, Padilla O, Monreal Y, Carrascal M, Abian J, Vives J, et al. Biochemical characterization of recombinant and circulating human Spalpha. Tissue Antigens. 2004;63:335–344. doi: 10.1111/j.0001-2815.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- Gangadharan B, Antrobus R, Dwek RA, Zitzmann N. Novel serum biomarker candidates for liver fibrosis in hepatitis C patients. Clin Chem. 2007;53:1792–1799. doi: 10.1373/clinchem.2007.089144. [DOI] [PubMed] [Google Scholar]
- Gray J, Chattopadhyay D, Beale GS, Patman GL, Miele L, King BP, et al. A proteomic strategy to identify novel serum biomarkers for liver cirrhosis and hepatocellular cancer in individuals with fatty liver disease. BMC Cancer. 2009;9:271. doi: 10.1186/1471-2407-9-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Kobayashi M, Sousa EA, Liu W, Cai J, Goldman SJ, et al. Differential proteomic analysis of bronchoalveolar lavage fluid in asthmatics following segmental antigen challenge. Mol Cell Proteomics. 2005;4:1251–1264. doi: 10.1074/mcp.M500041-MCP200. [DOI] [PubMed] [Google Scholar]
- Yu HR, Kuo HC, Sheen JM, Wang L, Lin IC, Wang CL, et al. A unique plasma proteomic profiling with imbalanced fibrinogen cascade in patients with Kawasaki disease. Pediatr Allergy Immunol. 2009;20:699–707. doi: 10.1111/j.1399-3038.2008.00844.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Kurokawa J, Arai S. AIMing at metabolic syndrome. Towards the development of novel therapies for metabolic diseases via apoptosis inhibitor of macrophage (AIM) Circ J. 2011;75:2522–2531. doi: 10.1253/circj.cj-11-0891. [DOI] [PubMed] [Google Scholar]
- Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, et al. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kurokawa J, Arai S, Nakashima K, Nagano H, Nishijima A, Miyata K, et al. Macrophage-derived AIM is endocytosed into adipocytes and decreases lipid droplets via inhibition of fatty acid synthase activity. Cell Metab. 2010;11:479–492. doi: 10.1016/j.cmet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Ravnskov U, McCully KS. Infections may be causal in the pathogenesis of atherosclerosis. Am J Med Sci. 2012;344:391–394. doi: 10.1097/MAJ.0b013e31824ba6e0. [DOI] [PubMed] [Google Scholar]
- Haruta I, Kato Y, Hashimoto E, Minjares C, Kennedy S, Uto H, et al. Association of AIM, a novel apoptosis inhibitory factor, with hepatitis via supporting macrophage survival and enhancing phagocytotic function of macrophages. J Biol Chem. 2001;276:22910–22914. doi: 10.1074/jbc.M100324200. [DOI] [PubMed] [Google Scholar]
- Kohfeldt E, Maurer P, Vannahme C, Timpl R. Properties of the extracellular calcium binding module of the proteoglycan testican. FEBS Lett. 1997;414:557–561. doi: 10.1016/s0014-5793(97)01070-3. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Mandell L, Whitney C, Padgett A, Gosselin K, Newburger PE, et al. Role of toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100:1860–1868. [PubMed] [Google Scholar]
- Ferstl R, Spiller S, Fichte S, Dreher S, Kirschning CJ. Experimental models of acute infection and Toll-like receptor driven septic shock. Methods Mol Biol. 2009;517:313–327. doi: 10.1007/978-1-59745-541-1_19. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Sanjurjo L, Amezaga N, Vilaplana C, Caceres N, Marzo E, Valeri M, et al. The scavenger protein apoptosis inhibitor of macrophages (AIM) potentiates the antimicrobial response against mycobacterium tuberculosis by enhancing autophagy. PLoS ONE. 2013;8:e79670. doi: 10.1371/journal.pone.0079670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SA, Paxian M, Ashburn JH, Clemens MG, Huynh T. Kupffer cell ablation improves hepatic microcirculation after trauma and sepsis. J Trauma. 2005;58:740–749. doi: 10.1097/01.ta.0000158246.74816.18. [DOI] [PubMed] [Google Scholar]
- Traeger T, Kessler W, Hilpert A, Mikulcak M, Entleutner M, Koerner P, et al. Selective depletion of alveolar macrophages in polymicrobial sepsis increases lung injury, bacterial load and mortality but does not affect cytokine release. Respiration. 2009;77:203–213. doi: 10.1159/000160953. [DOI] [PubMed] [Google Scholar]
- Verdrengh M, Tarkowski A. Role of macrophages in Staphylococcus aureus-induced arthritis and sepsis. Arthritis Rheum. 2000;43:2276–2282. doi: 10.1002/1529-0131(200010)43:10<2276::AID-ANR15>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Vollmar B, Ruttinger D, Wanner GA, Leiderer R, Menger MD. Modulation of kupffer cell activity by gadolinium chloride in endotoxemic rats. Shock. 1996;6:434–441. doi: 10.1097/00024382-199612000-00008. [DOI] [PubMed] [Google Scholar]
- Elomaa O, Sankala M, Pikkarainen T, Bergmann U, Tuuttila A, Raatikainen-Ahokas A, et al. Structure of the human macrophage MARCO receptor and characterization of its bacteria-binding region. J Biol Chem. 1998;273:4530–4538. doi: 10.1074/jbc.273.8.4530. [DOI] [PubMed] [Google Scholar]
- Bikker FJ, Ligtenberg AJ, Nazmi K, Veerman EC, van't HW, Bolscher JG, et al. Identification of the bacteria-binding peptide domain on salivary agglutinin (gp-340/DMBT1), a member of the scavenger receptor cysteine-rich superfamily. J Biol Chem. 2002;277:32109–32115. doi: 10.1074/jbc.M203788200. [DOI] [PubMed] [Google Scholar]
- Kneidl J, Loffler B, Erat MC, Kalinka J, Peters G, Roth J, et al. Soluble CD163 promotes recognition, phagocytosis and killing of Staphylococcus aureus via binding of specific fibronectin peptides. Cell Microbiol. 2012;14:914–936. doi: 10.1111/j.1462-5822.2012.01766.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.