Abstract
Interleukin-15 (IL-15) is essential for the survival of memory CD8+ and CD4+ T cell subsets, and natural killer and natural killer T cells. Here, we describe a hitherto unreported role of IL-15 in regulating homoeostasis of naive CD4+ T cells. Adoptive transfer of splenocytes from non-obese diabetic (NOD) mice results in increased homeostatic expansion of T cells in lymphopenic NOD.scid.Il15−/− mice when compared to NOD.scid recipients. The increased accumulation of CD4+ T cells is also observed in NOD.Il15−/− mice, indicating that IL-15-dependent regulation also occurs in the absence of lymphopenia. NOD.scid mice lacking the IL-15Rα chain, but not those lacking the common gamma chain, also show increased accumulation of CD4+ T cells. These findings indicate that the IL-15-mediated regulation occurs directly on CD4+ T cells and requires trans-presentation of IL-15. CD4+ T cells expanding in the absence of IL-15 signaling do not acquire the characteristics of classical regulatory T cells. Rather, CD4+ T cells expanding in the absence of IL-15 show impaired antigen-induced activation and IFN-γ production. Based on these findings, we propose that the IL-15-dependent regulation of the naive CD4+ T-cell compartment may represent an additional layer of control to thwart potentially autoreactive cells that escape central tolerance, while permitting the expansion of memory T cells.
Keywords: BDC2.5, CD4+ T cells, homeostasis, IL-15, NOD mouse
Introduction
IL-15 is a member of the gamma chain (γc, CD132) family of cytokines.1 The IL-15 receptor (IL-15R) complex also shares the IL-2 receptor (IL-2R) beta chain (IL-2/15Rβ, CD122). The unique alpha chain (IL-15Rα, CD215) of the trimeric IL-15R complex binds IL-15 during biosynthesis and trans-presents IL-15 to the IL-15Rβγc complex on the same cell or on adjacent responder cells.2 Trans-presentation of IL-15 is required for the maintenance of several lymphoid cell subsets such as natural killer, natural killer T and memory CD8+ T cells.3,4 While IL-7 is essential for the survival of naive T lymphocyte subsets,5 IL-15 promotes the survival of memory CD8+ T cells.6 However, IL-15 signaling can occur during all stages of CD8+ T cell development.7,8,9 In line with these observations, a recent report suggests that trans-presentation of IL-15 is required to support the expansion of CD8+ single positive thymocytes.9 IL-15 also appears to sustain CD8+ effector T cells generated following weak T-cell receptor (TCR) stimulation by low-affinity antigens.10,11
Whereas the effect of IL-15 on CD8+ T cells has been well documented, its influence on CD4+ T cells is less clear. IL-15 is reported to be required for the maintenance of ‘memory-like' and antigen-specific memory CD4+ T cells, but it is unclear if IL-15 is also required for their activity.12,13 In vitro, IL-15 has been shown increase CD4+ T-cell proliferation by suppressing regulatory CD4+ T cells.14,15 Contrarily, other reports have shown that IL-15 supports the expansion of CD4+CD25+ regulatory T cells.16,17,18 While it is possible that IL-15 may influence both regulatory and target CD4+ T-cell populations in vitro, the scenario may be different in vivo, where additional inflammatory mediators may modulate the effect of IL-15. For instance, even though the frequency of circulating regulatory T cells (Tregs) is increased in patients with rheumatoid arthritis, they are not capable of regulating the pathogenic process, in which IL-15 plays a key role.19
Non-obese diabetic (NOD) mice develop spontaneous autoimmune type 1 diabetes (T1D).20 In this model, CD8+ T cells play a key pathogenic role, but CD4+ T cells are also needed for disease initiation. Recently, we have reported that IL-15 deficiency in NOD mice prevented the development of T1D.21 We have also shown that adoptive transfer of splenocytes from diabetic NOD mice induced T1D in NOD.scid recipients, but not in NOD.scid.Il15−/−recipients.21 In this adoptive transfer setting, CD8+ T-cell numbers gradually diminished in NOD.scid.Il15−/− recipients. Curiously, we consistently recovered higher numbers of CD4+ T cells from these mice. In the present study, we characterized the CD4+ T cells that are undergoing expansion in IL-15-deficient NOD.scid mice. Our results show that IL-15 restrains homeostatic expansion of CD4+ T cells. Furthermore, our results show that CD4+ T cells that had expanded in the absence of IL-15 do not gain regulatory functions, but show impaired antigen-induced activation and reduced ability to produce IFN-γ.
Materials and methods
Animals
Animal experiments were carried out under protocols approved by the Université de Sherbrooke Ethics Committee for Animal Care and Use. Mice were housed in micro-isolated sterile cages under specific pathogen-free conditions. NOD (NOD/ShiLtJ), 8.3 TCR transgenic NOD (NOD.Cg-Tg(TcraTcrbNY8.3)-1Pesa/DvsJ; 8.3-NOD; for brevity, 8.3-NOD), BDC2.5 TCR transgenic NOD (NOD.Cg-Tg(TcraBDC2.5, TcrbBDC2.5)1Doi/DoiJ; for brevity, BDC2.5-NOD), NOD.scid and NOD.scid.gamma (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Generation of NOD.scid.Il15−/− mice has been described previously.21 C57BL/6.Il15ra−/− mice were obtained from the Jackson Laboratory and backcrossed to NOD background for nine generations before crossing them with NOD.scid mice to generate NOD.scid.Il15ra−/− mice. The characteristics of IL-15-related strains used in this study are given in Table 1.
Table 1. IL-15 production and trans-presentation in the mouse strains used in this study.
| Mouse strains | EndogenousIL-15 production | IL-15 trans-presentation |
|---|---|---|
| Wild-type | Yes | Yes |
| NOD | ||
| 8.3-NOD (MHC I-restricted Tg TCR) | ||
| BDC2.5-NOD | ||
| (MHC II-restricted Tg TCR) | ||
| NOD.scid | ||
| IL-15-deficient | No | No |
| NOD.Il15−/− | ||
| NOD.scid.Il15−/− | ||
| IL-15R alpha chain-deficient | Yes | No |
| NOD.Il15ra−/− | ||
| NOD.scid.Il15ra−/− | ||
| Common gamma chain-deficient | Yes | Yes |
| NOD.scid.gamma |
Abbreviations: NOD, non-obese diabetic; TCR, T-cell receptor; Tg, transgenic.
Antibodies and reagents
Antibodies against mouse CD3ε, CD4, CD8α, CD44, CD62L, CD25, CD69 and IFN-γ, conjugated to flurochromesor biotin and streptavidin–flurochromes conjugates were purchased from BD Pharmingen Biosciences (Palo Alto, CA, USA) or eBiosciences (San Diego, CA, USA). RPMI 1640 cell culture medium and fetal bovine serum were from Sigma-Aldrich (Oakville, Canada). 5-(6)carboxyfluoresceindiacetatesuccinimidyl ester (CFSE) was purchased from Molecular Probes, Life Technologies Inc. (Burlington, Ont., Canada). MHC class III-A(g7)-restricted peptide derived from the mouse chromogranin A protein (ChgA29–42; DTKVMKCVLEVISD)22 and MHC class-I H2Kb-restricted cognate peptide of 8.3 TCR derived from the islet-specific autoantigen glucose-6-phosphatase catalytic subunit-related protein (IGRP206–214; VYLKTNKFL)23 were synthesized by GenScript (Scotch Plains, NJ, USA) to >95% purity.
Mononuclear cell preparation, adoptive transfer and in vivo proliferation assay
Mononuclear cell suspensions were prepared from individual lymph nodes or spleen as described previously.11 CD4+ T cells were purified by negative selection using kits from Dynal Beads (Life Technologies Inc., Ont., Canada). In vivo cell proliferation was evaluated using CFSE-dye dilution assayas described before.11 Two million CFSE-labeled or unlabeled splenocytes from the indicated donors were injected intravenously into NOD.scid, NOD.scid.Il15−/−, NOD.scid.Il15ra−/− or NOD.scid.gamma recipients. Mononuclear cell suspensions prepared from individual lymph nodes, or spleen were analyzed for sequential reduction in dye content within CD4+T cells.11
Evaluation of regulatory function of T cells in vitro
CD4+ T cells isolated from CD4+ T cells reconstituted NOD.scid, NOD.scid.Il15−/− or NOD.scid.gamma mice were tested for their regulatory activity. Polyclonal CD4+ T cells or 8.3 TCR Tg CD8+ T cells isolated from pre-diabetic donor NOD or 8.3-NOD mice, respectively, and labeled with CFSE were used as responder cells at a ratio of 1 effector cell to 1 putative regulatory cell (each at a concentration of 1×106 cells/ml) along with 0.2×106 irradiated NOD splenocytes as antigen-presenting cells in 500 µl medium in 24-well plates. To evaluate anti-TCR (2C11)-induced proliferation, the responder and putative regulatory cells were plated together. To evaluate inhibition of antigen-induced proliferation of 8.3 cells, the putative regulatory CD4+ cells were pre-stimulated with anti-CD3 antibody for 24 h and washed to remove excess antibody before adding to responder cells and antigen-presenting cells. After 3 days, proliferation of responder cells was evaluated by monitoring dilution of CFSE fluorescence on gated CD4+ or CD8+ T cells.
Western blotting
Purified 2×106 CD8+ or CD4+ T cells were washed and resuspended in starving medium (medium containing 0.5% fetal bovine serum, 1 mg/ml bovine serum albumin and 50 µM 2-mercaptoethanol) before stimulation with IL-15 (10 ng/ml) in a volume of 0.5 ml. Fifteen minutes after stimulation, cells were lysed by boiling in SDS–PAGE sample buffer (50 mM Tris pH 6.8, 1% (w/v) SDS, 1 mM EDTA, 1 mM dithiothreitol). Equivalent amounts of proteins were separated in SDS–PAGE gels and transferred to polyvinylidene difluoride membranes. The blots were probed with phospho-specific antibodies and developed by enhanced chemiluminescence reagent (GE-Amersham, GE Healthcare, Canada). After incubating in stripping solution (2% SDS, 62.5 mM Tris pH 6.8, 100 mM 2-mercaptoethanol) for 30 min at 55 °C, the blots were blocked and reprobed for total proteins.
Flow cytometry
Expression of cell surface markers was evaluated by flow cytometry using FACS Canto flow cytometer (Becton Dickinson flow cytometry systems, Missisauga, Canada) and the data were analyzed using the FlowJo software (Tree Star Inc., Ashland, OR, USA).
Adoptive transfer and diabetes monitoring
Splenocytes from 6 months old non-diabetic NOD.Il15−/− NOD mice were injected intravenously (3×107 cells) into 1-month-old female NOD recipients. Control mice received PBS. Diabetes was monitored using urine glucose strips (Ketodiastix; Bayer, Canada). Animals with two consecutive readings of >3 (corresponding to 15 mmol/l glucose) were considered diabetic.
Statistical analyses
Cumulative incidence of T1D was analyzed using the Prism software (GraphPad, La Jolla, CA, USA) and statistical significance was calculated using log-rank (Mantel–Cox) test. For all other parameters, significance was calculated by two-way ANOVA.
Results
Trans-presented IL-15 regulates homeostatic expansion of CD4+ T cells
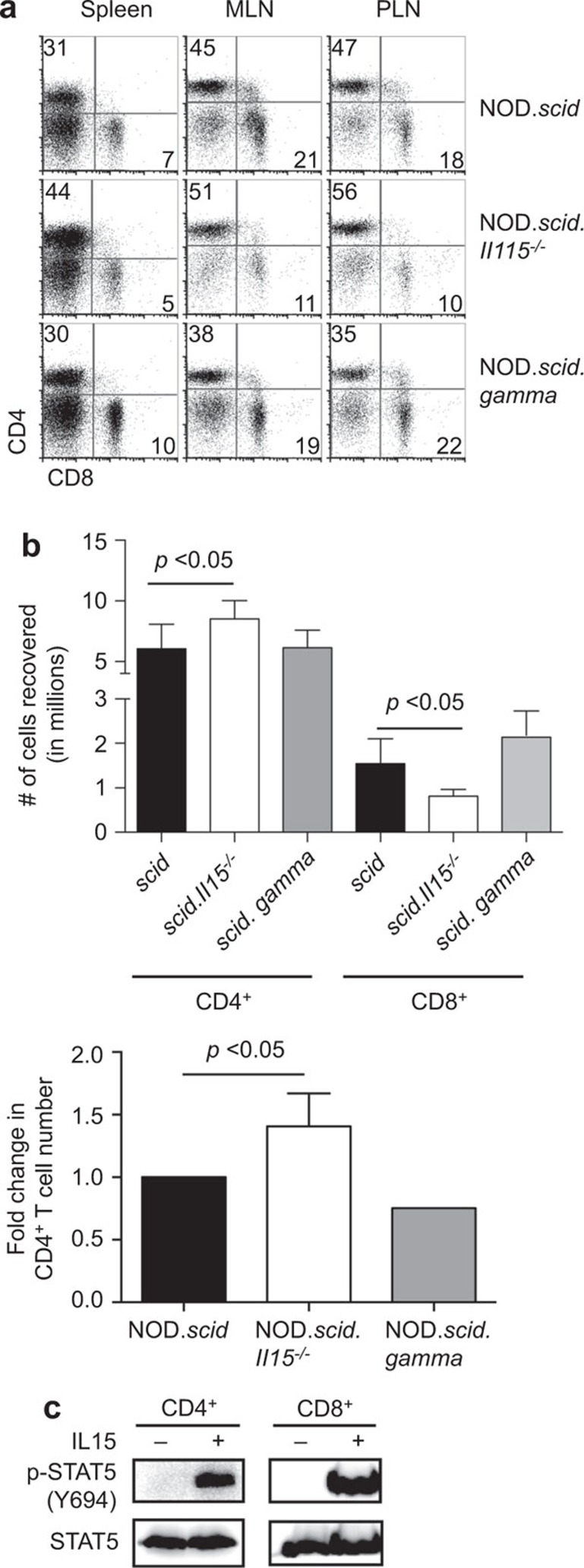
Phenotypic analysis of cells recovered from the NOD.scid and NOD.scid.Il15−/− recipients 2 months after adoptive transfer of splenocytes from NOD mice showed a reduction in the frequency of CD8+ T cells in the lymph nodes ofNOD.scid.Il15−/− hosts 21 (Figure 1a). However, we consistently recovered more CD4+ T cells from NOD.Scid.Il15−/− recipients than from NOD.scid or NOD.scid.gamma recipients (Figure 1b). These observations prompted us to investigate whether donor CD4+ T cells could use IL-15 available in recipient mice, although IL-15 deficiency was not reported to affect the CD4+ T-cell compartment in NOD or C57BL/6 mice.21,24 To this end, we purified CD4+ T cells, exposed them to IL-15 and evaluated downstream STAT signaling. As shown in Figure 1c, IL-15 induced STAT5 phosphorylation in CD4+ T cells as efficiently as in CD8+ T cells. These results indicated that CD4+ T cells possess a functional IL-15 signaling machinery and that IL-15 availability might directly affect the CD4+ T-cell compartment at least during lymphopenic conditions.
Figure 1.
IL-15-deficient NOD.scid mice accumulate more CD4+ T cells following adoptive transfer of NOD splenocytes. (a) Total splenocytes from diabetic NOD mice were adoptively transferred to NOD.scid, NOD.scid.Il15−/− and NOD.scid.gamma mice. Two months later, splenocytes from the recipient mice were counted and the frequency of CD4+ T cells was determined by FACS to determine the absolute number of CD4+ T cells recovered. (b) The numbers of CD4 T cells recovered from NOD.scid.Il15−/− and NOD.scid.gamma recipients two months after adoptive transfer (top panel) are expressed as fold-increase (n=6; mean±standard error of mean) over those recovered from NOD.scid recipients to offset variability in cell numbers between experiments (bottom panel). (c) Purified NOD CD4+ or CD8+ T cells were stimulated with IL-15 (10 ng/ml) for 15 min. Total cell lysates were analyzed by western blot for pSTAT5, stripped and blotted for total STAT5 protein. NOD, non-obese diabetic.
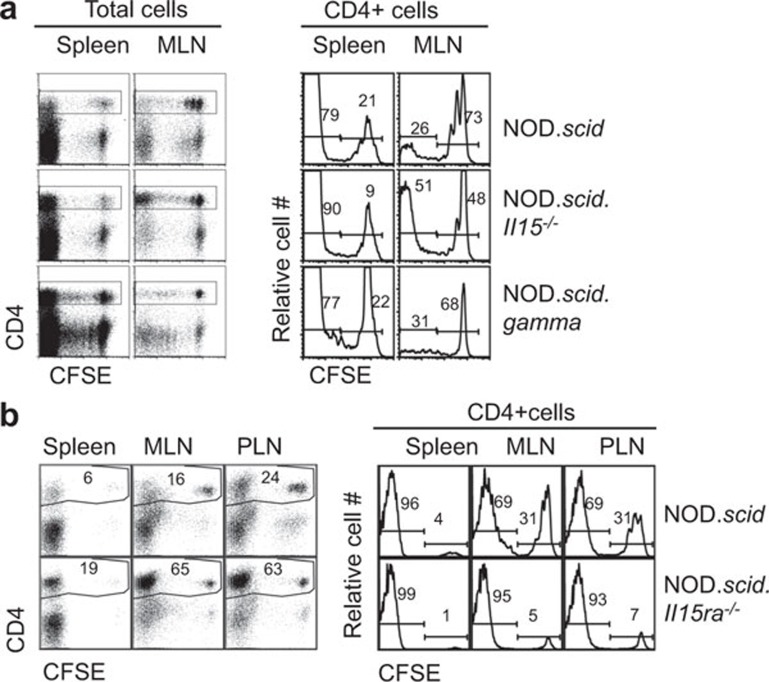
To determine if the elevated CD4+ T-cell numbers in NOD.scid.Il15−/− mice reconstituted with NOD splenocytes was due to increased expansion, we labeled the donor cells with CFSE before adoptive transfer. CD4+ T cells underwent extensive proliferation in lymph nodes and spleen of NOD.scid, NOD.scid.Il15−/− and NOD.scid.gamma recipients 3 days after transfer (Figure 2a). However, this expansion was more pronounced in NOD.scid.Il15−/− mice than in NOD.scid or NOD.scid.gamma recipients. These observations indicated that IL-15 attenuated homeostatic expansion of CD4+ T cells. Next, we assessed if IL-15 needs to be trans-presented to exert this regulation, we used NOD.scid mice lacking IL-15Rα as recipients. As shown in Figure 2b, NOD.scid.Il15ra−/− mice also showed increased expansion of CD4+ T cells, indicating that trans-presentation of IL-15 by non-T cells is needed to control homeostatic expansion of CD4+ T cells.
Figure 2.
Trans-presented IL-15 attenuates homeostatic expansion of CD4+ T cells in NOD.scid mice. (a) Total splenocytes from four weeks-old NOD mice were injected intravenously (1×107 cells) into NOD.scid, NOD.scid.Il15−/− or NOD.scid.gamma recipients. Three days after adoptive transfer, cell suspensions from spleen and MLNs were analyzed by flow cytometry to determine the CFSE-dye dilution in CD4+ or CD8+ T-cell compartments. Numbers in the graph indicate the proportion of cells within the marker boundaries. (b) CFSE-labeled NOD splenocytes were transferred to NOD.scid.Il15ra−/− mice and recipient mice were analyzed 3 days later. Representative data from one animal for each recipient group (5–6 mice/group) from at least two independent experiments are shown. CFSE, 5-(6)carboxyfluorescein diacetate succinimidyl ester; MLN, mesenteric lymph node; NOD, non-obese diabetic.
Regulation of homeostatic expansion of CD4+ T cells by IL-15 occurs in a cell autonomous manner and is influenced by basal TCR signaling
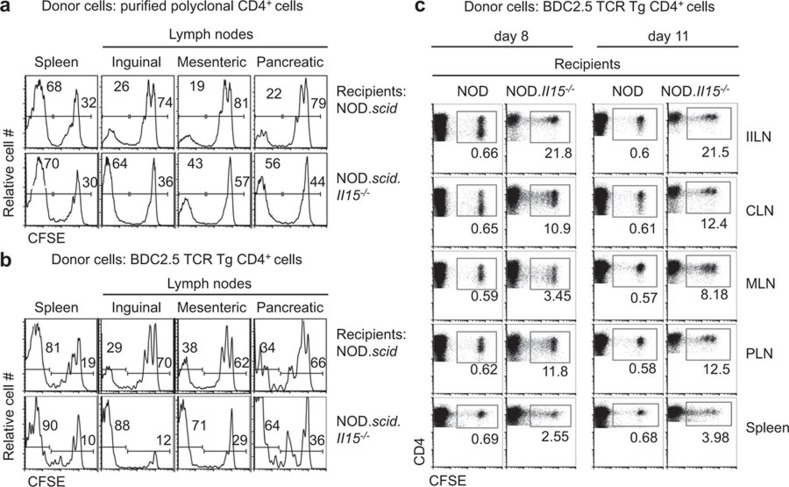
To rule out the possibility that the increased expansion of CD4+ T cells could be a compensatory effect due to the reduced expansion of CD8+ T cells, we evaluated homeostatic expansion of purified CD4+ T cells in NOD.scid and NOD.scid.Il15−/− recipients. We also used splenocytes from MHC II-restricted BDC2.5 TCR Tg mice.25 The BDC2.5 TCR Tg CD4+ T cells recognize a peptide derived from the islet specific antigen chromogranin A.22 Both polyclonal and monoclonal donor CD4+ T-cell populations showed accelerated expansion in NOD.scid.Il15−/−mice compared to NOD.scid recipients (Figure 3a and b), indicating that the increased expansion of CD4+ T cells does not result from the lack of competition from CD8+ T cells. This increased expansion was not restricted to pancreatic draining lymph nodes, but occurred in all lymph nodes with similar efficiency (Figure 3b). This observation suggested that TCR avidity towards self-peptides might influence the IL-15-dependent control of CD4+ T-cell expansion during lymphopenia. We have previously shown that NOD and NOD.Il15−/− mice bearing polyclonal TCR harbor comparable numbers of CD4+ T cells in spleen and lymph nodes.21 Therefore, we tested whether IL-15 deficiency would deregulate homeostasis of BDC2.5 cells under non-lymphopenic conditions. We used BDC2.5 cells because of their higher propensity to undergo homeostatic expansion in NOD.scid.Il15−/− hosts (Figure 3a and b). Surprisingly, BDC2.5 cells underwent robust proliferation in NOD.Il15−/− recipients when compared to wild-type NOD mice (Figure 3c). This finding indicates that IL-15 dependent regulation of CD4+ T-cell homeostasis can occur even under non-lymphopenic conditions.
Figure 3.
Increased homeostatic expansion of CD4+ T cells in NOD.scid.Il15−/− mice occurs in a cell autonomous manner. Purified CD4+ T cells from NOD mice (a) or splenocytes from BDC2.5 mice (b and c) were labeled with CFSE and injected intravenously (0.5×107 cells) into lymphopenic NOD.scid and NOD.scid.Il15−/− recipients (a and b) or lymphoreplete NOD and NOD.Il15−/− recipients (c). Splenocytes and cells from individual lymph nodes of the recipients recovered on day 3 (a, b) or at the indicated days (c) after adoptive transfer were analyzed. Representative data from at least two independent experiments (5–6 mice per group) are shown. CFSE, 5-(6)carboxyfluorescein diacetate succinimidyl ester; NOD, non-obese diabetic.
CD4+ T cells expanding in the IL-15-deficient mice do not develop regulatory functions
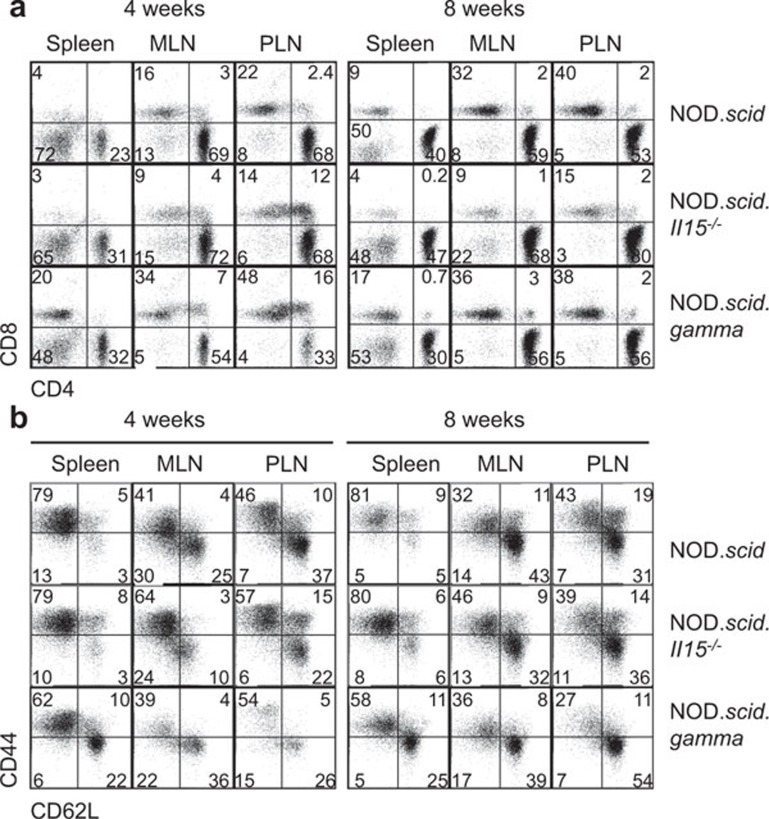
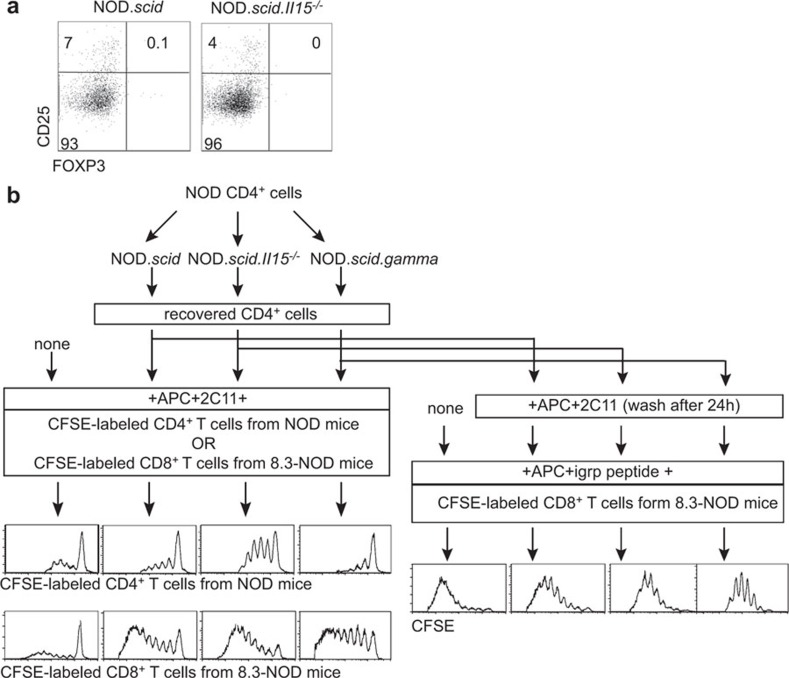
Adoptive transfer of splenocytes from diabetic NOD mice induces T1D in NOD.scid but not in NOD.scid.Il15−/− recipients.21 Even though CD8+ T cells showed impaired reconstitution in NOD.scid.Il15−/− recipients, a significant proportion of these cells displayed CD44hiCD62Llo-activated phenotype as in NOD.scid recipients.21 In contrast to CD8+ T cells, CD4+ T cells undergoing increased expansion in NOD.scid.Il15−/− recipients did not show any dramatic difference in their activation phenotype at 4 or 8 weeks later, compared to cells in NOD.scid recipients21 (Figure 4a and b). Despite the presence of activated CD8+ and CD4+ T cells, splenocytes recovered from NOD.scid.Il15−/− recipients failed to induce diabetes upon subsequent transfer to IL-15-sufficient NOD.scid recipients.21 The loss of pathogenic potential of these cells that had survived in an IL-15-deficient environment for a brief period may result from at least two mutually non-exclusive possibilities: (i) IL-15 is required to maintain the effector T cells that cause diabetes; and (ii) absence of IL-15 might have expanded a subset of regulatory CD4+ T cells (Tregs) that inhibited the pathogenic potential of diabetogenic T cells. To assess whether the increased homeostatic expansion of CD4+ T cells in NOD.scid.Il15−/− mice resulted in the expansion of Tregs, we evaluated the expression of FOXP3 in CD25+CD4+ T cells recovered from NOD.scid.Il15−/− and NOD.scid recipients. In both groups of mice, the proportion of FOXP3+CD25+ CD4+ T cells was negligible (Figure 5a).
Figure 4.
Phenotype of CD4+ cells expanding in IL-15-deficient mice. Total splenocytes from 1-month-old NOD mice expressing a polyclonal TCR repertoire were injected intravenously (1×107 cells) into NOD.scid, NOD.scid.Il15−/− and NOD.scid.gamma recipients. At 4 and 8 weeks after adoptive transfer, cells recovered from the spleen and mesenteric (MLN) and pancreatic (PLN) lymph nodes of recipient mice were analyzed for the expression of CD4 and CD8 (a) and CD44 and CD62L on gated CD4+ cells (b). Representative data from two independent experiments with four mice per group of recipients are shown. NOD, non-obese diabetic; TCR, T-cell receptor.
Figure 5.
CD4+ T cells recovered from NOD.scid.Il15−/− recipients do not inhibit proliferation of T cells or protect NOD mice from diabetes. (a) Splenocytes recovered from the reconstituted NOD.scid and NOD.scid.Il15−/− recipients were stained for CD4, CD25 and intracellular FOXP3. Expression of CD25 and FOXP3 in gated CD4+ cells was evaluated. (b) CD4+ T cells were purified from reconstituted NOD.scid, NOD.scid.Il15−/− and NOD.scid.gamma recipients and tested for their ability to suppress the anti-CD3-induced (CD4+ and 8.3 TCR Tg CD8+ T cells) and antigen-induced (8.3 T cells) proliferation by CFSE-dye dilution assay. CFSE, 5-(6)carboxyfluorescein diacetate succinimidyl ester; NOD, non-obese diabetic; TCR, T-cell receptor.
Next we tested whether CD4+ T cells expanded in NOD.scid.Il15−/− recipients have developed regulatory functions without acquiring the classical Treg phenotype. For this purpose, we purified CD4+ T cells from pooled splenocytes and lymph node cells of NOD.scid, NOD.scid.Il15−/− or NOD.scid.gamma recipients 2 months after reconstitution and tested their ability to suppress TCR-induced proliferation of T cells. As responder cells, we used CFSE-labeled polyclonal CD4+ T cells or 8.3 TCR Tg CD8+ T cells. The putative regulatory population and CFSE-labeled purified naive polyclonal CD4+ T cells or 8.3 TCR Tg CD8+ T cells were cultured together in the presence of beads coated with anti-CD3 mAb 2C11. For 8.3 TCR Tg CD8+ T cell responders, we also evaluated antigen-induced proliferation using the cognate IGRP206–214 peptide. Proliferation of CFSE-labeled CD4+ and CD8+ responder T cells was not reduced by coculture with cells recovered from all the recipients (Figure 5b). Indeed, we observed that the proliferation of responder cells increased in cocultures with cells from NOD.scid.Il15−/− recipients. These results indicate that the CD4+ T cells expanding in reconstituted NOD.scid.Il15−/− recipients do not develop regulatory functions.
CD4+ T cells recovered from IL-15-deficient NOD mice do not inhibit T1D development in NOD mice
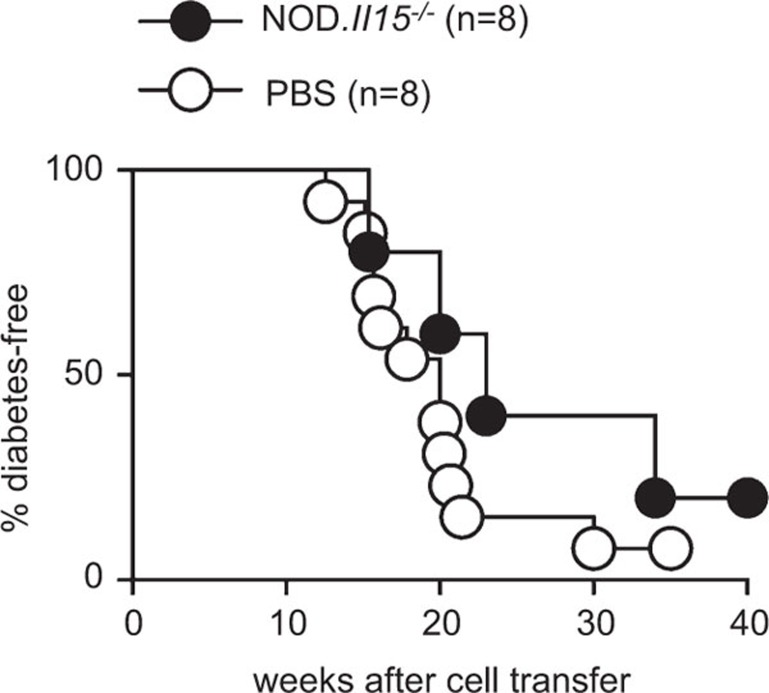
We have previously reported that IL-15 deficiency protected NOD mice from diabetes.21 Even though CD4+ T cells that had expanded in the absence of IL-15 did not show any regulatory property in vitro, it is possible that CD4+ T cells that had developed in IL-15-deficient environment may exert a regulatory role in vivo that could have contributed to the lack of autoimmune diabetes in NOD.Il15−/− mice. To test this possibility, we adoptively transferred pooled splenocytes and lymph node cells from non-diabetic NOD.Il15−/− mice to pre-diabetic female NOD mice. In line with the results obtained in vitro, cells from non-diabetic NOD.Il15−/− mice did not prevent the development of T1D in NOD mice (Figure 6). In fact, the NOD.Il15−/−splenocyte-reconstituted mice and the control mice developed T1D with similar kinetics, indicating that the CD4+ T cells expanding in the absence of IL-15 do not acquire the capacity to regulate T-cell responses in vivo.
Figure 6.
CD4+ T cells recovered from NOD.Il15−/− mice do not protect NOD mice from diabetes. Splenocytes (3×107 cells) pooled from 6- to 7-month-old female diabetes-free NOD.Il15−/− mice were injected into 6-week-old female pre-diabetic NOD mice. Splenocytes from six non-diabetic NOD.Il15−/− mice were pooled and injected into eight female NOD mice. Control group received PBS. The mice were followed for the development of diabetes by monitoring urine glucose levels. NOD, non-obese diabetic; PBS, phosphate-buffered solution.
CD4+ cells undergoing homeostatic expansion in the absence of IL-15 show reduced antigen responsiveness and effector cytokine production
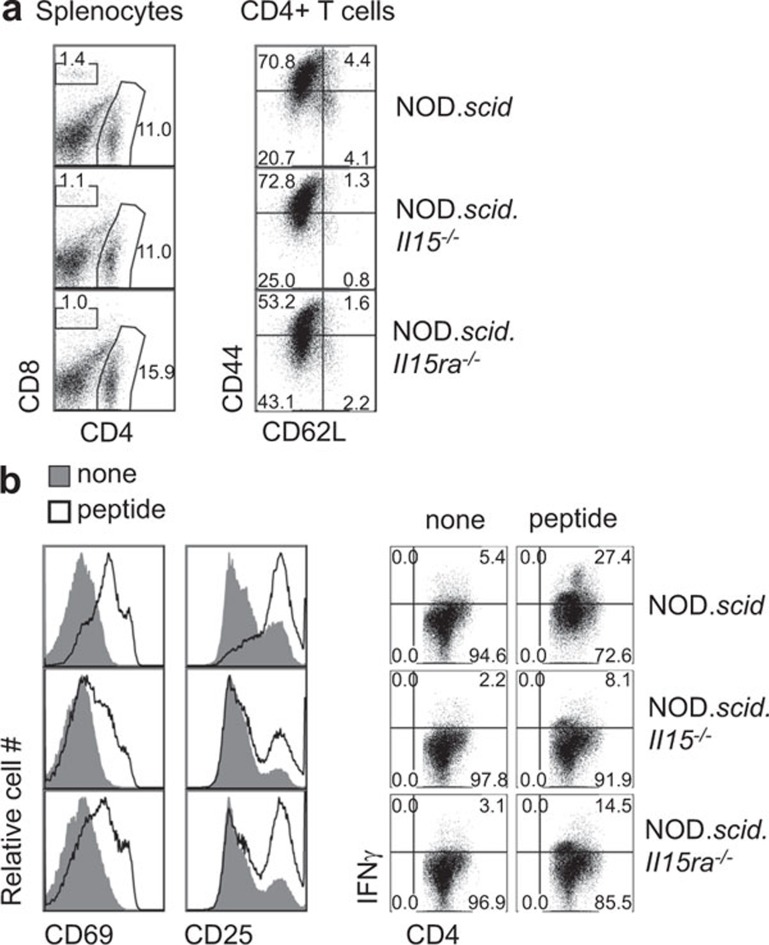
In NOD.scid.Il15−/− recipients, the absolute number of 8.3 TCR Tg CD8+ T cells that had undergone homeostatic expansion was decreased, even though they still retained their ability to proliferate in the presence of cognate antigen.21 To determine if this was also the case in CD4+ T cells, we assessed the antigen reactivity of BDC2.5 cells that had expanded in NOD.scid.Il15−/− mice. As shown in Figure 7a, the phenotype of CD4+ T cells recovered from IL-15- or IL-15Rα-deficient mice 10 days after adoptive transfer was comparable to that recovered from the control NOD.scid mice. However, following stimulation with the cognate peptide, BDC2.5 cells recovered from NOD.scid mice showed efficient upregulation of markers of activation such as CD25 and CD69, whereas cells recovered from NOD.scid.Il15−/− or NOD.scid.Il15ra−/− mice were not activated efficiently (Figure 7b, left panels). In addition, the production of IFN-γ was reduced in cells recovered from IL-15- or IL-15Rα-deficient mice (Figure 7b, right panels). These results suggest that IL-15 and its trans-presentation promote the antigen responsiveness of CD4+ T cells.
Figure 7.
BDC2.5 cells recovered from IL-15-deficient mice show reduced activation and IFN-γ production following stimulation with cognate antigen. (a) Splenocytes from NOD.scid, NOD.scid.Il15−/− or NOD.scid.Il15ra−/− recipients reconstituted with 0.5×107 BDC2.5-NOD splenocytes were recovered 10 days later and labeled for the indicated markers. (b) The recovered splenocytes were activated with the cognate peptide (1 µg/ml) for 24 h. Upregulation of CD25 and CD69 and the expression of IFN-γ were determined on gated CD4+ cells. Representative data from two independent experiments are shown. NOD, non-obese diabetic.
Discussion
Homeostatic expansion of specific subsets of T cells is driven by cytokines such as IL-7 and IL-15, as well as basal TCR signaling through engagement of MHC–self-peptide complexes.26 Naive T cells require IL-7 and TCR–MHC interaction, whereas signals through IL-7R and IL-15R are sufficient for the expansion of memory T cells. In a lymphopenic environment, CD8+ T cells reach normalization by homeostatic expansion, while CD4+ T cells are chronically depleted.27,28 IL-7 has been reported to negatively regulate the expansion of CD4+ T cells by modulating the expression of MHC class II molecules on dendritic cells.29,30 In the present study, we show that IL-15, which promotes homeostasis and survival of CD8+ T cells, may simultaneously control the expansion of CD4+ T cells. In fact, IL-15 may exert differential effect on different subsets of CD4+ T cells as it has been shown to promote the expansion of memory CD4+ T cells in mice and in humans.13,26,31 Our results obtained using T cells from young donor mice suggest that IL-15 inhibits the homeostatic expansion of naïve CD4+ T cells. Moreover, the use of genetically immunodeficient NOD.scid recipients instead of irradiated hosts avoids competition from endogenous thymus-derived T cells. Given that thymus continually exports naive T cells and the size of the T cell pool is limited, we propose that IL-15-mediated regulation of peripheral CD4+ T-cell expansion may facilitate the expansion and survival of memory T-cell subsets. We also speculate that the restriction on the expansion of naive T cells in the periphery may serve as a safety mechanism to contain the expansion of potentially autoreactive CD4+ T cells that are otherwise kept under check by peripheral tolerance mechanisms under steady state. Overall, our findings raise the possibility that cytokines such as IL-7 and IL-15 that promote homeostatic expansion of certain T-cell subsets may also negatively regulate the expansion of certain other T-cell subsets.
The observed increase in the homeostatic expansion of CD4+ T cells is cell intrinsic and is a direct consequence of the absence of trans-presentation of IL-15 by the non-lymphoid cells of the host for the following reasons. The increased expansion is observed in both lymphopenic and non-lymphopenic IL-15-deficient hosts, irrespective of whether the injected cells were purified CD4+ T cells or total splenocytes (Figures 2 and 3). The requirement for trans-presentation of IL-15 by non-T cells is evident from increased expansion in NOD.scid.Il15ra−/− hosts but not in NOD.scid.gamma recipients (Figure 2b). This requirement for trans-presentation also suggests that the physiological amount of soluble IL-15 that is available in circulation may not be able to bind to IL-15Rα that is expressed on donor CD4+ T cells. Thus, CD4+ T cells are also dependent on trans-presented IL-15 in vivo, similar to CD8+ T cells.3 Moreover, the IL-15-mediated suppression of CD4+ T-cell expansion is regulated directly by signals through the IL-15 receptor complex on T cells rather than indirectly through its effect on other cell types because this increased expansion does not occur in NOD.scid.gamma mice lacking the common gamma chain required for IL-15 signaling. It is also possible that the regulation is mediated through cell–cell interaction, of which one component is trans-presented IL-15, as naive CD4 cells do not proliferate in the presence of IL-15.32
Conflicting reports exist on the role of IL-15 in the generation of T cells with regulatory functions.17,18,33 Van Belle et al.15 has shown recently that in the presence of IL-15, the suppressive effects of Tregs was diminished in vitro. On the other hand, IL-15 has been shown to promote the development of CD4+CD25+FOXP3+ Tregs in humans,18 consistent with the fact that signaling via CD122 is required for the development of Tregs in vivo.34 However, the pro-inflammatory effect of IL-15 may play a dominant role on effector T cells in vivo, either directly and/or indirectly via non-lymphoid cells, such that the Tregs are ineffective in suppressing the pathogenicity of effector T cells.19,35 Our results (Figure 5) confirm the observation that the absence of IL-15 does not alter the frequency of Tregs.36 On the other hand, BDC2.5 TCR Tg CD4+ T cells that had expanded in the absence of IL-15 showed reduced antigen-induced differentiation into IFN-γ producing cells (Figure 7). Despite the reduction in IFN-γ production, the ability of BDC2.5 cells to induce T1D in NOD.scid.Il15−/− recipients21 suggests that increase in the frequency of autoantigen specific T cells, as observed with TCR Tg mice, may overcome the critical threshold of islet-antigen specific T cells required to cause pathology.25 Additionally, lack of IL-15 may promote other pathogenic mechanisms. For instance, it has been shown that lack of IL-15 skewed the antigen specific CD4+ T cells to produce IL-10 or IL-17.36,37 Accordingly, autoimmune diseases mediated by Th17 cells such as experimental autoimmune encephalomyelitis are aggravated by the absence of IL-15.36,37
In this study, we have identified a novel role for IL-15 in restraining the homeostatic expansion of CD4+ T cells. Thus, IL-7 and IL-15, the two major cytokines that regulate the survival and homeostasis of different subsets of T cells also restrain the expansion of CD4+ T cells. This additional layer of negative control on CD4+ T cells may be help to thwart the expansion of post-thymic CD4+ T cells that may escape central tolerance, while maintaining the diversity of the T-cell repertoire generated by thymic output. Clearly, further studies in other strains of mice are needed to elucidate molecular mechanisms underlying the IL-15-dependent regulation of the CD4+ T-cell compartment. It is equally important to study this regulation during immunotherapies using IL-15 to boost the effectiveness of CD8+ T cells, for instance against cancer.38
Acknowledgments
This work was supported by the Canadian Institutes of Health Research operating grant (MOP-86530) to SR. XLC and DB are recipients of studentship and post-doctoral fellowship, respectively, from Fonds de Recherché du Québec-Santé. YCD is a recipient of Dr Pierre Cossette studentship from the Faculty of Medicine, Université de Sherbrooke. Centre de Recherche Clinique Etienne-Le Bel is a research centre funded by the Fonds de Recherché du Québec-Santé.
References
- He YW, Malek TR. The structure and function of gamma c-dependent cytokines and receptors: regulation of T lymphocyte development and homeostasis. Crit Rev Immunol. 1998;18:503–524. doi: 10.1615/critrevimmunol.v18.i6.20. [DOI] [PubMed] [Google Scholar]
- Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J Exp Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett. 2010;127:85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S, Ramanathan S, Ning T, La Rose J, Reinhart B, Poussier P, et al. Suppressor of cytokine signaling 1 attenuates IL-15 receptor signaling in CD8+ thymocytes. Blood. 2003;102:4115–4122. doi: 10.1182/blood-2003-01-0175. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Gagnon J, Leblanc C, Rottapel R, Ilangumaran S. Suppressor of cytokine signaling 1 stringently regulates distinct functions of IL-7 and IL-15 in vivo during T lymphocyte development and homeostasis. J Immunol. 2006;176:4029–4041. doi: 10.4049/jimmunol.176.7.4029. [DOI] [PubMed] [Google Scholar]
- Chow KP, Qiu JT, Lee JM, Hsu SL, Yang SC, Wu NN, et al. Selective reduction of post-selection CD8 thymocyte proliferation in IL-15Ralpha deficient mice. PLoS ONE. 2012;7:e33152. doi: 10.1371/journal.pone.0033152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa F, Tagaya Y, Kim BS, Patel HJ, Ishida K, Ohteki T, et al. IL-15 serves as a costimulator in determining the activity of autoreactive CD8 T cells in an experimental mouse model of graft-versus-host-like disease. J Immunol. 2008;181:1109–1119. doi: 10.4049/jimmunol.181.2.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S, Dubois S, Chen XL, Leblanc C, Ohashi PS, Ilangumaran S. Exposure to IL-15 and IL-21 enables autoreactive CD8 T cells to respond to weak antigens and cause disease in a mouse model of autoimmune diabetes. J Immunol. 2011;186:5131–5141. doi: 10.4049/jimmunol.1001221. [DOI] [PubMed] [Google Scholar]
- Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–661. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci USA. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle TL, Dooms H, Boonefaes T, Wei XQ, Leclercq G, Grooten J. IL-15 augments TCR-induced CD4+ T cell expansion in vitro by inhibiting the suppressive function of CD25 high CD4+ T cells. PLoS ONE. 2012;7:e45299. doi: 10.1371/journal.pone.0045299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen HJ, Fasse E, Joosten I. IL-15 and cognate antigen successfully expand de novo-induced human antigen-specific regulatory CD4+ T cells that require antigen-specific activation for suppression. J Immunol. 2003;171:6431–6441. doi: 10.4049/jimmunol.171.12.6431. [DOI] [PubMed] [Google Scholar]
- Xu S, Sun Z, Sun Y, Zhu J, Li X, Zhang X, et al. IL-15 and dendritic cells induce proliferation of CD4+CD25+ regulatory T cells from peripheral blood. Immunol Lett. 2011;140:59–67. doi: 10.1016/j.imlet.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Passerini L, Allan SE, Battaglia M, Di Nunzio S, Alstad AN, Levings MK, et al. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells. Int Immunol. 2008;20:421–431. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- Benito-Miguel M, Garcia-Carmona Y, Balsa A, Perez de Ayala C, Cobo-Ibanez T, Martin-Mola E, et al. A dual action of rheumatoid arthritis synovial fibroblast IL-15 expression on the equilibrium between CD4+CD25+ regulatory T cells and CD4+CD25− responder T cells. J Immunol. 2009;183:8268–8279. doi: 10.4049/jimmunol.0900007. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- Bobbala D, Chen XL, Leblanc C, Mayhue M, Stankova J, Tanaka T, et al. Interleukin-15 plays an essential role in the pathogenesis of autoimmune diabetes in the NOD mouse. Diabetologia. 2012;55:3010–3020. doi: 10.1007/s00125-012-2675-1. [DOI] [PubMed] [Google Scholar]
- Nikoopour E, Sandrock C, Huszarik K, Krougly O, Lee-Chan E, Masteller EL, et al. Cutting edge: vasostatin-1-derived peptide ChgA29–42 is an antigenic epitope of diabetogenic BDC2.5 T cells in nonobese diabetic mice. J Immunol. 2011;186:3831–3835. doi: 10.4049/jimmunol.1003617. [DOI] [PubMed] [Google Scholar]
- Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci USA. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Hakim FT, Cepeda R, Kaimei S, Mackall CL, McAtee N, Zujewski J, et al. Constraints on CD4 recovery postchemotherapy in adults: thymic insufficiency and apoptotic decline of expanded peripheral CD4 cells. Blood. 1997;90:3789–3798. [PubMed] [Google Scholar]
- Capitini CM, Chisti AA, Mackall CL. Modulating T-cell homeostasis with IL-7: preclinical and clinical studies. J Intern Med. 2009;266:141–153. doi: 10.1111/j.1365-2796.2009.02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimond M, Veenstra RG, Grindler DJ, Zhang H, Cui Y, Murphy RD, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009;10:149–157. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CE, Kim DM, Sprent J, Surh CD.Is IL-7 from dendritic cells essential for the homeostasis of CD4+ T cells Nat Immunol 201011547–548.author reply 548. [DOI] [PubMed] [Google Scholar]
- Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, et al. IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc Natl Acad Sci USA. 2004;101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper DM, Kasprowicz DJ, Ziegler SF. IL-2Rbeta links IL-2R signaling with Foxp3 expression. Eur J Immunol. 2007;37:1817–1826. doi: 10.1002/eji.200737101. [DOI] [PubMed] [Google Scholar]
- Zanzi D, Stefanile R, Santagata S, Iaffaldano L, Iaquinto G, Giardullo N, et al. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in celiac disease. Am J Gastroenterol. 2011;106:1308–1317. doi: 10.1038/ajg.2011.80. [DOI] [PubMed] [Google Scholar]
- Chow KP, Lee JM, Qiu JT, Liao SK, Lin SC, Hsu SL, et al. Enhanced IL-10 production by CD4+ T cells primed in IL-15Ralpha-deficient mice. Eur J Immunol. 2011;41:3146–3156. doi: 10.1002/eji.201141746. [DOI] [PubMed] [Google Scholar]
- Pandiyan P, Yang XP, Saravanamuthu SS, Zheng L, Ishihara S, O'Shea JJ, et al. The role of IL-15 in activating STAT5 and fine-tuning IL-17A production in CD4 T lymphocytes. J Immunol. 2012;189:4237–4246. doi: 10.4049/jimmunol.1201476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce M, Orengo AM, Azzarone B, Ferrini S. Immunotherapeutic applications of IL-15. Immunotherapy. 2012;4:957–969. doi: 10.2217/imt.12.92. [DOI] [PubMed] [Google Scholar]