Inflammatory bowel disease (IBD) manifests during Crohn's disease and ulcerative colitis as well as in a group of inflammatory disorders of the gastrointestinal tract.1 The etiology of IBD is thought to be a combination of genetics and environmental factors, including the microbiome and immune system of the individual.1
The initial immune response in the intestine is controlled by macrophages, dendritic cells and intestinal epithelial cells. Intestinal macrophages are phagocytic and bactericidal, but are mostly refractory to inflammatory stimulation. Microbes do not trigger the production of pro-inflammatory cytokines, preventing an unfavorable response to resident commensal flora. These cells do not respond to Toll-like receptor ligands and express anti-inflammatory cytokines. In IBD, Toll-like receptor-responsive macrophages that release pro-inflammatory cytokines arise.2 Activated macrophages are commonly categorized as classically activated (M1-type) or alternatively activated (M2-type). The latter are characterized by the expression of arginase and the mannose receptor CD206.2 However, due to the heterogeneity of tissue macrophages, this categorization is simplified, and resident intestinal macrophages cannot be easily assigned to one of these classes.2
Mesenteric fat hypertrophy is a common feature in IBD, particularly in Crohn's disease.3 Proteins secreted by adipose tissue, such as adiponectin and leptin, regulate immune function, suggesting that increased synthesis of these proteins in adipose tissue and higher systemic levels in IBD may contribute to disease pathogenesis.3 Chemerin, which is mainly expressed in adipocytes and hepatocytes, is also present at higher concentrations in serum from IBD patients.4,5,6 Chemerin is an attractant for immune cells and may play a role in the recruitment of tissue macrophages.4 Chemerin produced by human fetal intestinal epithelial cells constitutes most of the chemotactic macrophage activity observed in cultured fetal intestinal epithelial cells.7 In mature intestine, small amounts of chemerin are expressed, suggesting that the postnatal attraction of immune cells is initiated by other chemokines.7
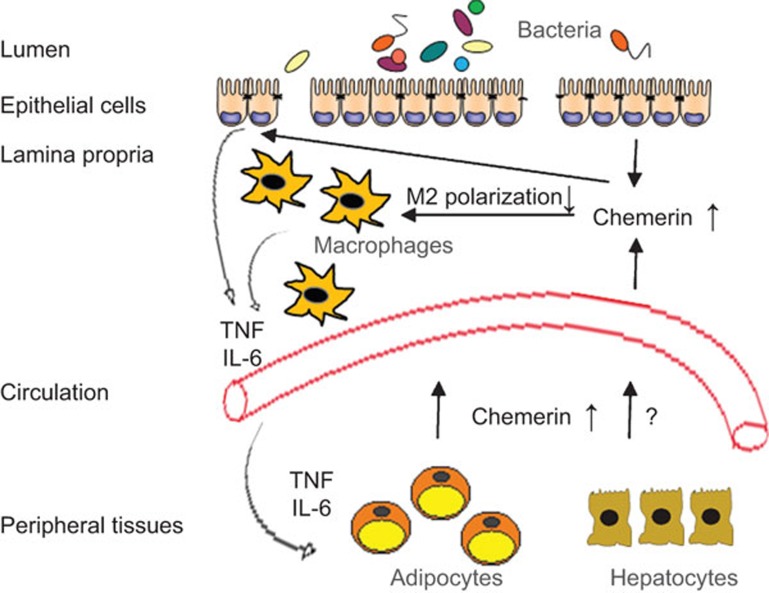
Research on IBD commonly uses dextran sodium sulfate (DSS)-induced colitis in mice. Intestinal hyperpermeability affects the penetration of pathogens, toxic compounds and macromolecules. In this model, mucosal inflammation is maintained by cells of the innate immune system.8 Using this model, Lin and colleagues9 report that chemerin aggravates colitis. Importantly, intraperitoneal injection of chemerin (aa16-157) strongly increases chemerin serum levels, but does not cause inflammation in healthy mice. Circulating chemerin is elevated in experimental colitis (Figure 1) and is further increased by intraperitoneal administration of chemerin. Chemerin-treated mice display significantly greater weight loss, colon shortening, and exaggerated histological damage, as well as a higher disease activity index at day 8 following DSS exposure. TNF and IL-6 serum levels and secretion by colonic cells are markedly induced (Figure 1). Unexpectedly, chemerin administration does not affect the number of dendritic cells, neutrophils, macrophages and natural killer cells in the colon. RT-PCR expression analysis reveals a colitis-associated mRNA upregulation of the M2 genes, including arginase-1 and IL-10, which is completely abrogated by chemerin treatment (Figure 1).
Figure 1.
Role of chemerin in dextran sodium sulfate (DSS) colitis. In DSS colitis, colonic epithelial cells release more chemerin. Chemerin enhances IL-6 and TNF secretion in these cells. It further blocks M2 polarization of macrophages, which is most likely associated with higher release of inflammatory cytokines. Increased pro-inflammatory cytokines in circulation might induce chemerin in mesenteric adipocytes and subsequently contribute to higher systemic levels. Whether chemerin in serum plays a role in the local effects in the bowel requires further study. Hepatocyte chemerin synthesis is not induced by inflammatory cytokines or lipopolysaccharide, suggesting that liver chemerin is not increased in inflammatory bowel disease.
The chemerin receptor CMKLR1 is expressed by macrophages, but not neutrophils or dendritic cells, suggesting that this chemokine may directly affect macrophages function.9 In vitro experiments using peritoneal macrophages demonstrate that (i) chemerin alone has no effect on the expression of the M2 genes analyzed; (ii) chemerin does not enhance lipopolysaccharide-mediated M1 activation, in agreement with recently published findings;10 (iii) chemerin impairs IL-4-induced phosphorylation of STAT6 and M2-induced macrophage polarization; and (iv) IL-4 upregulates expression of CMKLR1 by macrophages.
In mouse peritoneal macrophages, lipopolysaccharide upregulates CMKLR1,11 whereas a second study demonstrates that macrophage CMKLR1 is suppressed by inflammatory cytokines and Toll-like receptor ligands, such as lipopolysaccharide. The immune-suppressive cytokines TGF-beta12 and IL-4 induce CMKLR1 expression.9 Thus, further studies are needed to elucidate whether classically activated macrophages are less responsive to chemerin compared to alternatively activated cells.
To evaluate the contribution of endogenous chemerin to disease severity, an antibody blocking chemerin activity has been tested.9 Administration of this antibody improves histological scores, but not the clinical manifestations of DSS colitis. Lin and colleagues9 speculate that this approach does not efficiently block endogenous chemerin and suggest the use of chemerin knockout mice to perform confirmatory experiments. Despite this limitation, cultured colonic cells from antibody-treated mice produce lower levels of TNF. Furthermore, colonic expression of arginase-1 is induced. Again, the number of inflammatory cells is not affected. Collectively, these data demonstrate that chemerin has a role in IBD pathology.
Elevated systemic chemerin is, however, not related to disease activity in IBD patients.6 In colon biopsies of patients with ulcerative colitis, the expression of chemerin is higher in inflamed tissues and, importantly, is further increased in more severely inflamed tissues.9 Colon cells isolated from DSS-treated mice release chemerin at levels consistent with disease severity. Therefore, only locally produced chemerin is associated with disease severity in rodent and human IBD.6,9
Higher circulating chemerin levels in IBD may result from increased intestinal or colonic secretion. Furthermore, pro-inflammatory cytokines induce adipocytes to express chemerin but have no effect on hepatocytes4,5 (Figure 1). Overall, the study by Lin and colleagues demonstrates a role for chemerin in IBD pathophysiology in a commonly used rodent model.9 CMKLR1 is induced in the colon tissue of DSS-treated mice11 and serves as a receptor for chemerin and resolvin E1. Chemerin exacerbates9 and resolvin E1 prevents DSS colitis,11 and the underlying mechanisms of the opposing effects of these ligands still need to be characterized. Additional studies using different IBD models, other strategies to block endogenous chemerin action and experiments to reveal the role of chemerin in human IBD are needed before chemerin targeting can be used as a novel approach to treat IBD.
Acknowledgments
Professor Dr Charalampos Aslanidis is acknowledged for helpful discussions and Dr Claudia Kunst is acknowledged for providing the template to prepare the figure.
References
- Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- Bain CC, Mowat AM. Intestinal macrophages—specialised adaptation to a unique environment. Eur J Immunol. 2011;41:2494–2498. doi: 10.1002/eji.201141714. [DOI] [PubMed] [Google Scholar]
- Kaser A, Tilg H. “Metabolic aspects” in inflammatory bowel diseases. Curr Drug Deliv. 2012;9:326–332. doi: 10.2174/156720112801323044. [DOI] [PubMed] [Google Scholar]
- Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010;21:660–667. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Krautbauer S, Wanninger J, Eisinger K, Hader Y, Beck M, Kopp A, et al. Chemerin is highly expressed in hepatocytes and is induced in non-alcoholic steatohepatitis liver. Exp Mol Pathol. 2013;95:199–205. doi: 10.1016/j.yexmp.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Weigert J, Obermeier F, Neumeier M, Wanninger J, Filarsky M, Bauer S, et al. Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn's disease. Inflamm Bowel Dis. 2010;16:630–637. doi: 10.1002/ibd.21091. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Kurundkar AR, Shaik SS, Kelly DR, Hartman Y, Zhang W, et al. Epithelial cells in fetal intestine produce chemerin to recruit macrophages. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1–G10. doi: 10.1152/ajpgi.90730.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Lin Y, Yang X, Yue W, Xu X, Li B, Zou L, et al. Chemerin aggravates DSS-induced colitis by suppressing M2 macrophage polarization. Cell Mol Immunol. 2014;4:355–366. doi: 10.1038/cmi.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondue B, de Henau O, Luangsay S, Devosse T, de Nadai P, Springael JY, et al. The Chemerin/ChemR23 system does not affect the pro-inflammatory response of mouse and human macrophages ex vivo. PLoS ONE. 2012;7:e40043. doi: 10.1371/journal.pone.0040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, et al. Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflamm Bowel Dis. 2010;16:87–95. doi: 10.1002/ibd.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel BA, Ohyama T, Zuniga L, Kim JY, Johnston B, Allen SJ, et al. Chemokine-like receptor 1 expression by macrophages in vivo: regulation by TGF-beta and TLR ligands. Exp Hematol. 2006;34:1106–1114. doi: 10.1016/j.exphem.2006.03.011. [DOI] [PubMed] [Google Scholar]