Abstract
Although islet transplantation for individuals with type 1 diabetes has been shown to yield superior blood glucose control, it remains inadequate for long-term control. This is partly due to islet injuries and stresses that can lead to beta cell loss. Inhibition of excess IL-1β activity might minimize islet injuries, thus preserving function. The IL-1 receptor antagonist (IL-1Ra), an endogenous inhibitor of IL-1β, protects islets from cytokine-induced necrosis and apoptosis. Therefore, an imbalance between IL-1β and IL-1Ra might influence the courses of allogeneic and autoimmune responses to islets. Our group previously demonstrated that the circulating serine-protease inhibitor human alpha-1-antitrypsin (hAAT), the levels of which increase in circulation during acute-phase immune responses, exhibits anti-inflammatory and islet-protective properties, as well as immunomodulatory activity. In the present study, we sought to determine whether the pancreatic islet allograft-protective activity of hAAT was mediated by IL-1Ra induction. Our results demonstrated that hAAT led to a 2.04-fold increase in IL-1Ra expression in stimulated macrophages and that hAAT-pre-treated islet grafts exhibited a 4.851-fold increase in IL-1Ra transcript levels, which were associated with a moderate inflammatory profile. Unexpectedly, islets that were isolated from IL-1Ra-knockout mice and pre-treated with hAAT before grafting into wild-type mice yielded an increase in intragraft IL-1Ra expression that was presumably derived from infiltrating host cells, albeit in the absence of hAAT treatment of the host. Indeed, hAAT-pre-treated islets generated hAAT-free conditioned medium that could induce IL-1Ra production in cultured macrophages. Finally, we demonstrated that hAAT promoted a distinct phosphorylation and nuclear translocation pattern for p65, a key transcription factor required for IL-1Ra expression.
Keywords: adaptive immunity, inflammation, innate immunity, macrophages, tolerance
Introduction
Islet beta cells are highly susceptible to local inflammation; indeed, excess amounts of pro-inflammatory cytokines, particularly IL-1β, can compromise islet function and lead to beta cell death.1,2,3 Pancreatic islet allografts are exposed to an inflammatory insult similar to the early stages of autoimmune diabetes,4 and both islet-derived IL-1β and IL-1α contribute to the progression of islet allograft failure.5,6,7,8
Although islet transplantation has been shown to yield superior blood glucose control in individuals with type 1 diabetes, it remains an inadequate long-term therapy.9 During islet transplantation, human islets are embolized into the hepatic portal vein of the recipient patients. The accompanying immunosuppressive protocol is devoid of corticosteroids, as these agents can exert diabetogenic effects, and therefore. the inflammatory IL-1 pathway remains largely intact and can contribute to the myriad responses that immediately follow islet engraftment. These non-antigenic inflammatory events are responsible for up to 70% of the beta cell losses observed within the first 48 h after engraftment.10
The underlying inflammatory flare is most likely initiated by isolation-damaged islets that were severed from the blood supply, exposed to digestive processes and then introduced into the poorly oxygenized portal system. Consequently, islet cells release pro-inflammatory cytokines such as IL-1α, IL-1β and TNF-α as well as important chemokines such as MCP-1 and injurious levels of nitric oxide. Stimulated resident pancreatic islet macrophages and responsive local host macrophages are significant sources of inflammatory mediators at the graft site.10 The inhibition of excess IL-1β activity could therefore minimize islet injury and reduce the consequent immunogenicity.11,12
IL-1 receptor antagonist (IL-1Ra), an endogenous IL-1 inhibitor, binds to IL-1R1 and blocks the recruitment of the IL-1R accessory protein (IL-1RAcP), thus effectively blocking IL-1 receptor activation.13 An imbalance between IL-1β and IL-1Ra was previously established as a possible influence on the courses of allogeneic and autoimmune conditions.14,15 For example, IL-1Ra protects islets from cytokine-induced necrosis and apoptosis.16 Additionally, a clinical study demonstrated that commercially available IL-1Ra (Anakinra, Kineret®,Sobi inc, PA, USA) could reduce both the insulin requirements and levels of glycated hemoglobin (HbA1c) in patients with type 2 diabetes.17 A similar study conducted in type 1 diabetes patients concluded that although IL-1 blockade was safe, the use of both IL-1Ra (Anakinra) and a neutralizing antibody (Canakinumab) was insufficient as a single immunomodulating agent to reduce the insulin requirements and levels of HbA1c.18
IL-1Ra is released from IL-1β-producing cells such as macrophages and hepatocytes19 as well as from human and murine pancreatic islets20,21 in response to inflammation. The IL-1Ra promoter interacts with transcription factors such as NF-κB3 (p65, RelA), AP1, C/EBPβ and C/EBPδ.22 Of these, p65 was found to be essential for IL-1Ra expression.23,24,25,26,27
Alpha-1-antitrypsin (hAAT) is a serine-protease inhibitor that exhibits both tissue-protective and anti-inflammatory properties as well as immunomodulatory activities.28 Both hepatocytes and human pancreatic islets produce hAAT in response to IL-1β.29,30 Indeed, the circulating hAAT concentrations increase by four- to sixfold during acute-phase responses.31 hAAT suppresses the release of IL-1β from several cell types either by directly inhibiting caspase-132,33,34,35,36,37 or through another currently undetermined mechanism. Treatment with plasma-derived affinity-purified hAAT is currently indicated for patients with genetic hAAT deficiency38 and is being evaluated in several clinical trials for the treatment of recent-onset autoimmune diabetes, given its islet-protective properties.34,35,36,37,38,39,40 Although hAAT reduces NF-κB nuclear translocation, including that of p65,41,42,43 it also induces IL-1Ra expression in human peripheral blood mononucleated cells.44 More recently, in vivo hAAT treatment was shown to elevate the IL-1Ra transcript levels in long-lasting islet allografts;34,45 similarly, increased IL-1Ra protein levels were detected in supernatants collected from cultured macrophages that had been exposed to sera from plasmid-derived hAAT-expressing mice.34
In the present study, we wished to determine whether the pancreatic islet allograft-protective activity of hAAT would require IL-1Ra induction.
Materials and methods
Animals
BALB/c mice (Harlan Laboratories Inc. Rehovot, Israel) and IL-1Ra-KO mice (BALB/c background; kind gift from Professor Ron Apte, Ben-Gurion University of the Negev) were used as islet and macrophage donors. C57BL/6 mice (Harlan Laboratories Inc.) and GFP transgenic mice (C57BL/6 background, Jackson Laboratories, Bar Harbor, MA, USA) were used as islet recipients. All mice were housed in the Ben-Gurion University of the Negev animal facility and studied at 6–8 weeks of age. All experiments were approved by the institutional animal care and use committee.
Pancreatic islet isolation
The islets were isolated as described previously.45 Briefly, the mice were anesthetized and the pancreata were inflated with cold collagenase (1 mg/ml, type XI; Sigma-Aldrich, Rehovot, Israel). The inflated pancreata were then excised and incubated for 26 min in a non-shaking 37 °C wet bath with a constant fluid flow. The digested pancreata were vortexed and filtered through 1000-µm and 500-µm sieves, after which the pellet was washed in HBSS containing 0.5% BSA (culture-grade; Sigma-Aldrich). The resulting pellet was resuspended in RPMI 1640 (Sigma-Aldrich) supplemented with 2% FCS (Cellgro, Mediatech, Herndon, VA, USA) and 50 mg/ml each of penicillin, streptomycin and L-glutamine (all from Biological Industries, Kibbutz Beit Haemek, Israel). Islets were collected using a 100-µm cell strainer (BD “Falcon”, Franklin Lakes, New Jersey, USA) and were hand selected and placed directly into culture medium.
Islet culture experiments
IL-1Ra mRNA study
Fifty islets per well were seeded into 24-well plates in RPMI 1640 (Sigma-Aldrich) supplemented with 2% FCS, 50 U/ml of penicillin and 50 mg/ml of streptomycin (Biological Industries). The islets were treated with hAAT (0.5 mg/ml; Glassia; Kamada, Israel) and after a 48-h incubation, were pelleted and treated with an RNA lysis solution (RNA extraction kit, TAMAR Inc., Abu-Gosh, Israel) containing TCEP (1∶100 dilution; 5 Prime, Gaithersburg, MD, USA). The islets were dispersed by pipetting, and the total RNA was extracted.
IL-1Ra protein study
One hundred and fifty islets per well were seeded into 24-well plates. The islets were treated with hAAT (0.5 mg/ml) and, after a 48-h incubation, the supernatants were collected for IL-1Ra ELISA analysis.
Islet transplantation
Islet transplantation was performed as previously described.45 Briefly, 450 isolated islets were gently pelleted at 400 r.p.m. for 1 min and washed twice in HBSS containing 0.5% BSA. The islets were then pre-treated with or without hAAT (0.5 mg/ml) for 48 h and were subsequently washed and grafted into the renal subcapsular spaces of anesthetized recipient mice.
Peritoneal macrophages (pMφ)
A 3% thioglycolate solution (Sigma-Aldrich) was injected into the abdomens of BALB/c mice 3 days prior to macrophage harvesting. Next, 10 ml of cold, sterile PBS (Biological Industries) were injected i.p. and the peritoneal cavity contents were aspirated. For macrophage isolation, the aspirates were filtered through 70-µm sieves (BD Falcon). The washed cells were counted in an automated counter (CountessTM, Invitrogen, Grand Island, NY) and seeded into 24-well plates (1×106 per well) in RPMI 1640 with 2% FCS, 50 U/ml of penicillin and 50 mg/ml of streptomycin.
RT-PCR analysis
Total RNA was extracted from the primary pancreatic islets, pMφ and harvested graft samples using an RNA extraction kit according to the manufacturer's instructions (PerfectPure RNA Tissue Kit; 5 Prime). A micro-volume spectrophotometer (NanoDrop, Wilmington, DL, USA) was used for RNA quantification. Concentration-normalized RNA samples were reverse transcribed using a commercial kit (Verso cDNA Kit; Thermo Scientific, Wilmington, DE, USA). cDNA amplification was performed both semiquantitatively by PCR (XP Cycler, BIOER, Hangzhou, P.R. China) and quantitatively by real-time PCR (Rotor Gene RG-3000, Qiagen, Boston, MA) using primers for murine genes (Table 1).
Table 1. PCR and QPCR primers for murine genes.
| Primer | Forward 5′→3′ | Reverse 5′→3′ |
|---|---|---|
| β-actin | GGTCTCAAACATGATCTGGG | GGGTCAGAAGGATTCCTATG |
| IL-1Ra | GACCCTGCAAGATGCAAGCC | GAGCGGATGAAGGTAAAGCG |
| Insulin | CAGAAACCATCAGCAAGCAGG | TTGACAAAAGCCTGGGTGGG |
| IL-1β | CTCCATGAGCTTTGTACAAGG | TGCTGATGTACCAGTTGGGG |
| TNF-α | CCTGAGTTCTGCAAAGGGAG | AGCAAAAGAGGAGGCAACAA |
Mixed cell cultures
The islets were pre-treated with hAAT (0.5 mg/ml) for 48 h and were subsequently washed; 50 islets/well were placed either inside Transwell Permeable Supports (0.5-µM diameter perforated polycarbonate membranes; Costar; Corning, Tewksbury, MA, USA) or directly over pMφ that had been stimulated with IL-1β (5 ng/ml) and IFN-γ (5 ng/ml; both from R&D systems, Minneapolis, MN, USA). The mixed cultures were incubated for 48 h and the supernatants were subsequently collected for analysis.
Conditioned medium (CM)
Islets (200/well) were cultured in the presence or absence of hAAT (0.5 mg/ml) for 72 h, at which time the medium was replaced and the cells were cultured for an additional 72 h. The cytokine concentrations were determined in the media from the first incubation stage. The 72-h CM was collected and directly added to cultured pMφ (2.5×105/well in 48-well plates) at a 1∶2 volume ratio at 1 h prior to LPS (Sigma-Aldrich) stimulation (10 ng/ml). Supernatants from the stimulated pMφ were analyzed after 48 h.
Evaluation of inflammatory mediators
The IL-1Ra levels were measured with an IL-1Ra ELISA (R&D systems). Nitrite oxide levels were measured with the Griess assay (Promega, Madison, WI, USA). Insulin levels were determined with a mouse insulin ELISA (Mercodia AB, Uppsala, Sweden). LDH activity was measured using LDH-Cytotoxicity Assay (BioVision, San Francisco, CA, USA) . Cytokine levels were measured using a multiplex ELISA (Q-Plex mouse cytokine custom, Quansys Biosciences, Logan, Utah) according to manufacturer's instructions; the ELISA included the following analytes: IL-1β, IL-6, IL-10, MCP-1, IL-17, IFN-γ, TNF-α and KC.
Western blot analysis
RAW 264.7 cells (ATCC) were cultured (8×106/plate in 10-cm tissue culture plates) for 1 h in the presence or absence of hAAT (0.5 mg/ml) and were then washed and stimulated with either LPS or TNF-α (10 ng/ml each; Sigma-Aldrich) for 30 min. The cells were harvested by scraping and were lysed as described elsewhere.46 Briefly, the cells were resuspended in buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, 1 mM DTT (all from Sigma-Aldrich) and protease inhibitor cocktail set III (Calbiochem; Millipore, Billerica, MA, USA)). Triton X-100 (Sigma-Aldrich) was added to a final concentration of 0.1% and the cells were subsequently incubated for 8 min on ice. The nuclei were collected in pellet 1 via low-speed centrifugation (4 min, 1300g, 4 °C). The supernatant was further clarified by high-speed centrifugation (15 min, 20 000g, 4 °C) to remove cell debris and insoluble aggregates and the resulting supernatant was collected (cytosol fraction). The nuclei were washed once in buffer A and then lysed in buffer B (3mM EDTA, 0.2 mM EGTA, 1 mM DTT, (all from Sigma-Aldrich) and protease inhibitor cocktail set III, (Calbiochem®))for 30 min. Insoluble chromatin was separated from the soluble nuclear components (nuclear fraction) by centrifugation (4 min, 1700g, 4 °C); the resulting pellet was washed once in buffer B and centrifuged again under the same conditions (chromatin-enriched fraction). The protein concentrations of all fractions were determined using a Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Next, 50-µg protein aliquots were separated via 10% SDS–PAGE and blotted onto polyvinylidene fluoride membranes (Bio-Rad). The following antibodies were used for protein detection: anti-p65 (poly6226; Biolegend, San Diego, CA, USA), anti-phosphorylated-p65 (49.Ser 311; Santa Cruz Biotechnology, Dallas, TX, USA), anti-α-tubulin (TU-01; Biolegend) and anti-trimethyl-Histone H3 (Lys9; Millipore). To detect primary antibody binding, the blots were incubated with horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies. The immobilized antibodies were detected with an ECL reagent (Advansta, Menlo Park, CA, USA).
Statistical analysis
For all experiments, the Mann–Whitney test was used to assess significance with a confidence interval of 95%. The results are shown as means±s.e.m. P values <0.05 were considered significant. GraphPad Prism software (GraphPad Software, La Jolla, CA, USA) was used for statistical processing.
Results
hAAT increases IL-1Ra expression and secretion in primary islets and macrophages
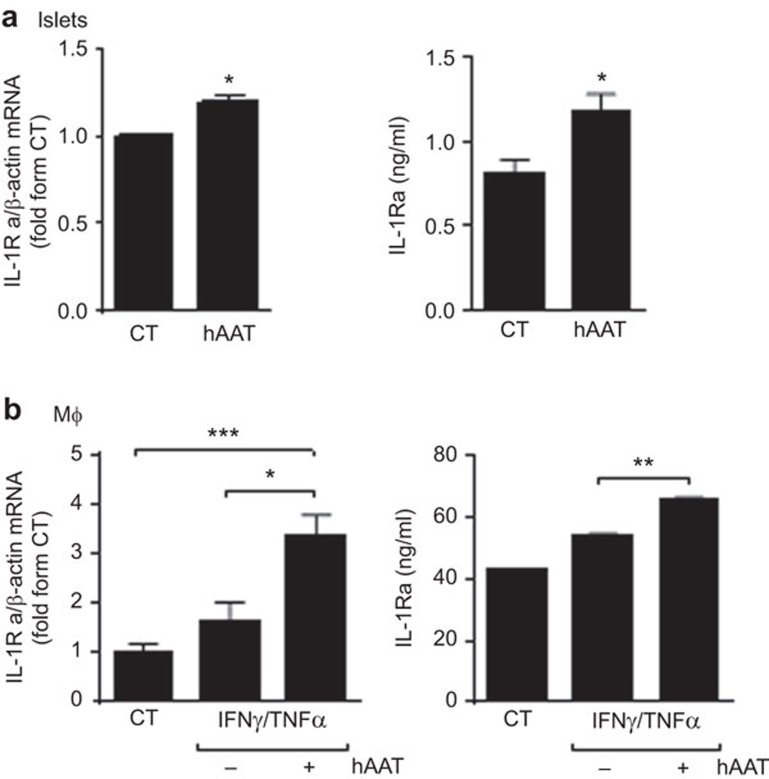
Freshly isolated primary mouse islets (50/well in triplicate) were incubated in the absence or presence of clinical-grade hAAT (0.5 mg/ml). After 48 h, the murine IL-1Ra transcript and Protein levels transcript levels were determined by QPCR. As shown in Figure 1a, incubation with hAAT resulted in a 1.186-fold increase in the IL-1Ra transcript expression levels. IL-1Ra protein secretion was determined using 150 islets per well; hAAT-treated islets exhibited a 1.456-fold increase in IL-1Ra secretion. The 72-h assays revealed a consistent increase in IL-1Ra transcript and Protein levels (not shown).
Figure 1.
IL-1Ra expression and secretion are increased in pancreatic islets and peritoneal macrophages in response to hAAT. (a) IL-1Ra expression and secretion in islets. Primary pancreatic islets were incubated in triplicate in the absence (CT) or presence of 0.5 mg/ml of human hAAT. For QPCR, 50 islets were seeded per well; for supernatant analysis, 150 islets were seeded per well. The murine IL-1Ra transcript levels were determined after 48 h, normalized to β-actin transcript levels and shown as fold changes relative to the CT. The supernatant IL-1Ra levels were determined after 48 h. Data are shown as means±s.e.m.; *P<0.05. (b) IL-1Ra expression and secretion in macrophages. Freshly prepared peritoneal macrophages (1×106/well) were incubated in duplicate in the absence (CT) or presence of 0.5 mg/ml of hAAT for 1 h, followed by the addition of IFN-γ (25 ng/ml) and TNF-α (10 ng/ml). The IL-1Ra transcript levels were determined by QPCR after 6 h, normalized to β-actin transcript levels and shown as fold changes relative to the CT. The supernatant IL-1Ra levels were assessed at 24, 48 and 72 h. Data are shown as means±s.e.m.; *P<0.05, **P<0.01, ***P<0.001. The results are representative of four independent experiments. hAAT, alpha-1-antitrypsin.
Both resting and stimulated macrophages were examined (Figure 1b). Thioglycolate-elicited peritoneal macrophages (1×106 cells/well in triplicate) were stimulated with IFN-γ and TNF-α (25 and 10 ng/ml, respectively). hAAT (0.5 mg/ml) was added to the cultures 1 h prior to cytokine stimulation. Six hours later, the cells were harvested and IL-1Ra gene expression levels were determined by QPCR. As shown in figure 1B, the IL-1Ra expression level increased 2.04-fold in the presence of hAAT relative to stimulated cells and by 3.361-fold relative to resting cells. IL-1Ra protein secretion was analyzed at 72 h after cytokine stimulation. As shown, hAAT treatment resulted in increased IL-1Ra secretion from the stimulated macrophages (1.22-fold compared to stimulated cells without hAAT). In the absence of stimulation, hAAT treatment did not elevate IL-1Ra expression (data not shown).
hAAT-pre-treated islet grafts exhibited elevated IL-1Ra transcript levels and a moderated inflammatory profile
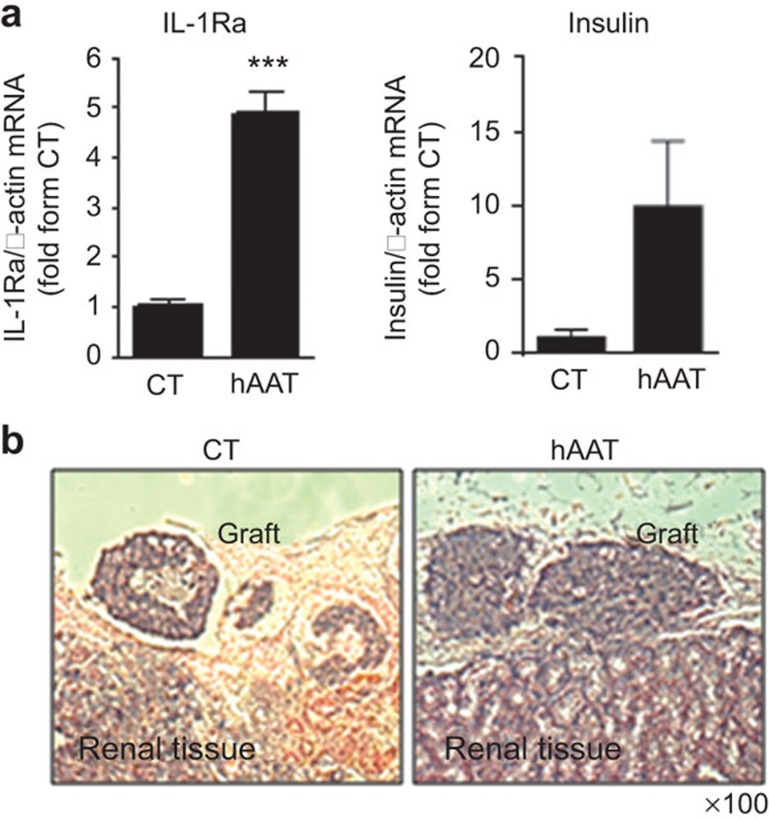
Given the inducible effect of hAAT on IL-1Ra expression in primary islets, we sought to determine whether this response would occur in the islet graft microenvironment upon islet transplantation. Islets were pre-incubated for 48 h in the absence or presence of hAAT (0.5 mg/ml) and were then washed and transplanted under the renal capsules of recipient mice (500 islets per graft; n=5/group). Forty hours later, the islet grafts were excised and subjected to total RNA extraction for QPCR analysis. The IL-1Ra, insulin, IL-1β and TNF-α transcript levels were then determined. As shown in Figure 2, hAAT pre-treatment increased the IL-1Ra expression level by 4.851-fold in explanted 2-day-old grafts, compared with the levels detected in grafts that had not been pre-treated with hAAT. Additionally, the insulin transcript levels were increased by 9.861-fold in the hAAT-pre-treated explanted grafts. In contrast, the IL-1β and TNF-α transcript levels were reduced by 9.5 and 3.291-fold, respectively, in response to hAAT treatment.
Figure 2.
Ex vivo hAAT-pre-treatment increases IL-1Ra expression in grafts in vivo. Islets (500/well) were incubated in the absence or presence of hAAT (0.5 mg/ml) for 48 h. Islet transplantation was performed and after 48 h, the islet grafts were excised and analyzed as follows: (a) gene expression via QPCR. The results were normalized to the β-actin transcript levels and presented as fold changes relative to the CT; (b) cellular components via H&E staining. Representative fields are shown. hAAT, alpha-1-antitrypsin.
hAAT-induced IL-1Ra is derived from host cells rather than from graft cells
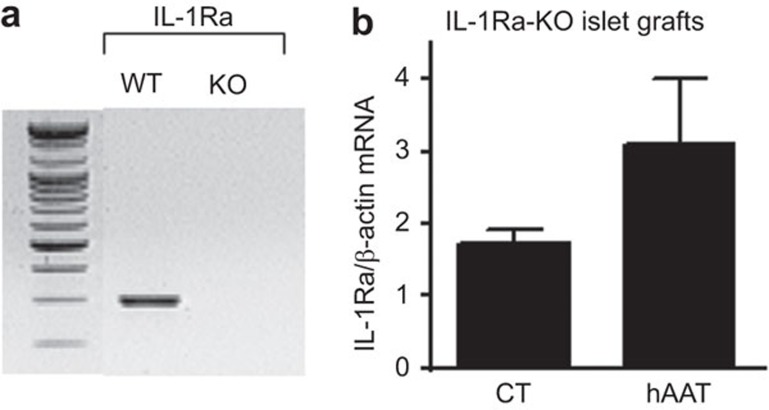
IL-1Ra-KO mice were used as islet donors to investigate whether hAAT pre-treatment led to increased graft IL-1Ra expression from the islets or from host infiltrating cells. IL-1Ra-KO islets were pre-incubated for 48 h in the absence or presence of hAAT (0.5 mg/ml) and were then washed and transplanted under the renal capsules of recipient C57BL/6 mice (500 islets per graft; n=5/group). After 48 h, the islet grafts were excised and subjected to total RNA extraction for QPCR analysis. The IL-1Ra transcript levels were subsequently determined. As shown in Figure 3, although the grafts had been prepared from IL-1Ra-KO mice, the IL-1Ra transcript levels were increased by 2.26-fold in the hAAT-pre-treated grafts relative to the non-treated grafts.
Figure 3.
IL-1Ra expression is detected in 2-day-old hAAT-pre-treated IL-1Ra-KO grafts. (a) RT-PCR for IL-1Ra expression in WT and IL-1Ra-KO mice; (b) IL-1Ra expression in vivo (n=5/group). Data are shown as means±s.e.m.; *P<0.05. hAAT, alpha-1-antitrypsin; WT, wild-type.
Macrophage-islet crosstalk provides a platform for hAAT-induced IL-1Ra expression
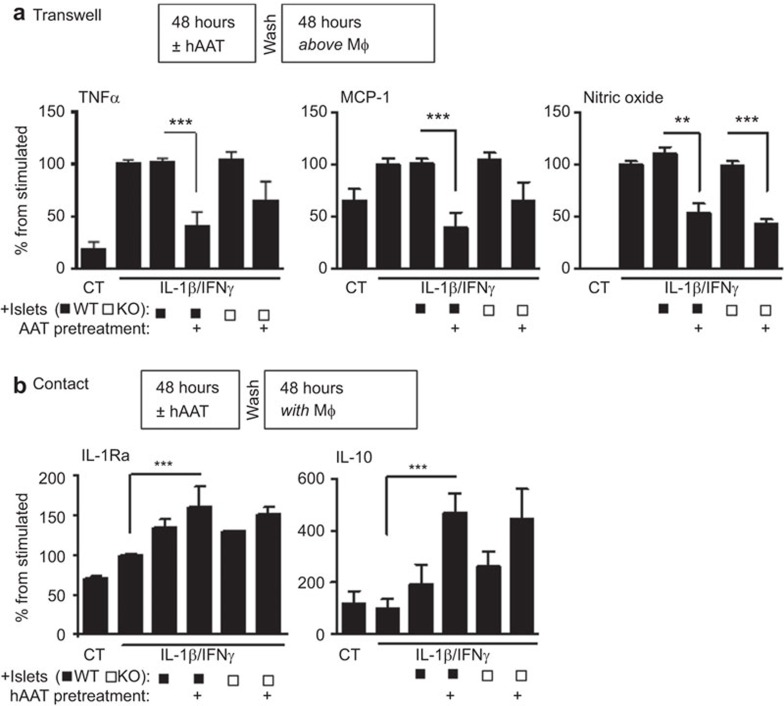
As hAAT-treated islets appear to elicit IL-1Ra expression from the host-derived cells collected with the graft explants during the process of graft excision, we sought to examine whether an islet-derived product could induce IL-1Ra in macrophages. To evaluate mixed cultures comprising macrophages and islets, primary islets were isolated from wild-type (WT) and IL-1Ra-KO mice and cultured in the presence or absence of hAAT (0.5 mg/ml) for 48 h (50 islets/well in triplicate). Peritoneal macrophages were seeded into separate plates (0.5×106/well). After washing hAAT from the islets, 50 islets were further cocultured with the seeded pMφ using one of two methods: direct contact or indirect contact by plating the islets in the top compartment of a Transwell. The culture wells were then stimulated with IFN-γ and IL-β (5 ng/ml each), and the supernatants were collected after 48 h. Figure 4 presents an analysis of the levels of secreted inflammatory cytokines and mediators. In the Transwell cocultures, the TNF-α levels were reduced by 2.535-fold in the hAAT-pre-treated WT cultures and 1.607 in the IL-1Ra-KO cultures. The nitric oxide levels exhibited a consistent behavior and were reduced by 2.061-fold and 2.275-fold in the WT and IL-1Ra-KO cultures, respectively (Figure 4a). In contrast, in the cultures with direct cell-to-cell contact, no significant changes were observed in the levels of these analytes. However, anti-inflammatory markers emerged in these direct contact cultures (Figure 4b). The hAAT-treated WT islets induced a 1.203-fold increase in the IL-1Ra levels upon direct incubation with macrophages; similarly, the hAAT-treated IL-1Ra-KO islets also induced a 1.167-fold increase in the IL-1Ra release levels relative to the control wells. The IL-10 levels displayed a strikingly similar trend with 2.774- and 2.275-fold increases in the wells containing macrophages cultured directly with WT and IL-1Ra-KO islets, respectively.
Figure 4.
hAAT pre-treatment favors an anti-inflammatory cytokine profile in a mixed islet macrophage culture system. Fifty islets each were isolated from WT and IL-1Ra-KO mice and incubated in the presence or absence of hAAT (0.5 mg/ml) for 48 h. pMφ were elicited and seeded (0.5×106/well) onto 24-well plates. The islets were washed and incubated with the pMφ for 48 h at 37 °C; these mixed cell systems were stimulated with IL-1β (5 ng/ml) and IFN-γ (5 ng/ml) and the supernatants were collected and assayed. (a) Transwell assay (no direct contact between the islets and pMφ): TNF-α and MCP-1 were assayed with a multiplex ELISA and NO levels were determined with the Griess assay. Data are shown as means±s.e.m.; **P<0.01, ***P<0.001. (b) Direct contact (islets were plated on the pMφ): IL-1Ra levels were measured with an ELISA and IL-10 levels were assayed with a multiplex ELISA. Data are shown as means±s.e.m.; ***P<0.001. hAAT, alpha-1-antitrypsin; NO, nitric oxide; pMφ, peritoneal macrophages; WT, wild-type.
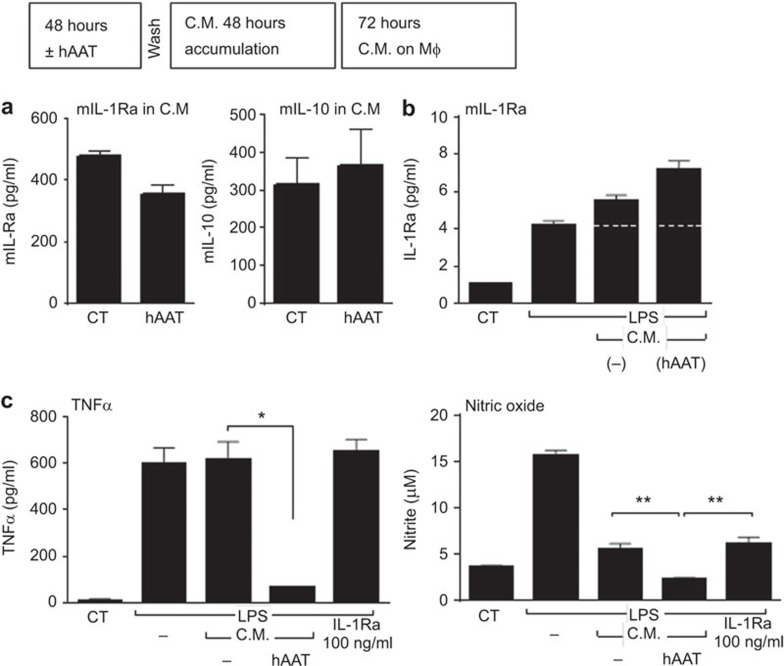
Because hAAT treatment reduced the observed inflammatory cytokine levels in the Transwell islet macrophage coculture system, we established a conditioned medium system to test whether this effect could be isolated to products that were unidirectionally released from islets. To generate CM, islets (200/well) were cultured in the presence or absence of hAAT (0.5 mg/ml) for 72 h and were subsequently washed and allowed to release products into fresh medium without hAAT. To examine the activity contained within this CM, peritoneal macrophages were seeded onto plates (3×105/well in quadruplicate) and treated with CM at a 1∶2 dilution 1 h before the introduction of LPS (10 ng/ml) and/or IL-1Ra (100 ng/ml). The levels of nitric oxide, TNF-α and IL-1Ra released by these CM-treated macrophages were assayed after 48 h (Figure 5a).
Figure 5.
Conditioned medium from hAAT-treated islets alter inflammatory macrophage responses. (a) CM from hAAT-treated islets alters inflammatory macrophage responses: CM was defined as the 48-h supernatants from hAAT-treated islets (0.5 mg/ml for 72 h). Macrophages (3×105/well in quadruplicate) were treated with CM 1 h before LPS (10 ng/ml) and/or IL-1Ra treatment (100 ng/ml). The 48-h macrophage supernatants were collected for nitric oxide and cytokine level analyses. Data are shown as means±s.e.m.; *P<0.05, **P<0.01. (b) Islet survival: islets from WT and IL-1Ra-KO mice were incubated in the presence or absence of hAAT (0.5 mg/ml) for 48 h and were subsequently washed and seeded in triplicate (100 islets/well). The supernatants were assayed after 48 h with an LDH cytotoxicity Assay. Data are shown as means±SEM; *P<0.05, **P<0.01. CM, conditioned medium; hAAT, alpha-1-antitrypsin; WT, wild-type.
Furthermore, we investigated the role of IL-1Ra with respect to the effects of hAAT treatment on islet viability and reactivity. WT and IL-1Ra-KO islets were cultured in the presence or absence of hAAT (0.5 mg/ml) for 48 h and were subsequently washed, seeded (100 islets/well) and stimulated with IL-1β (5 ng/ml) and IFN-γ (5 ng/ml). The LDH levels in the supernatants were assayed 48 h later (Figure 5b).
hAAT promotes a distinct p65 phosphorylation and nuclear translocation pattern
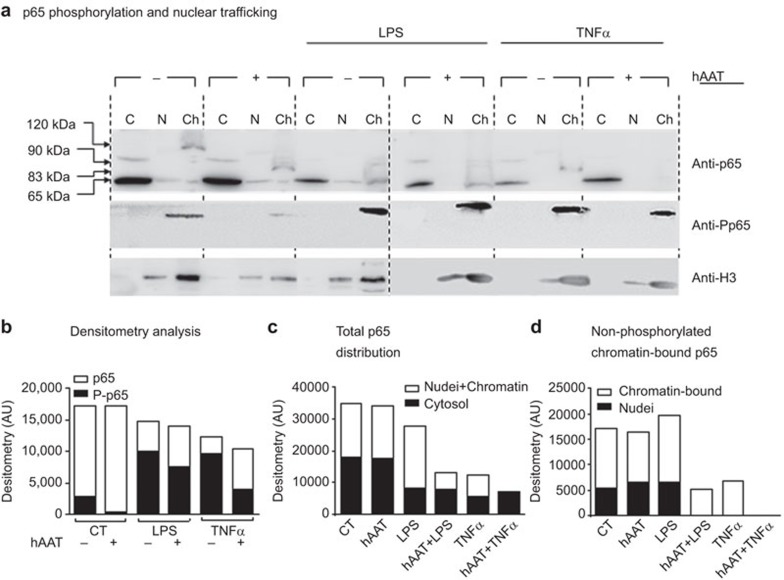
Given the role of p65 in IL-1Ra expression under inflammatory conditions, we sought to determine whether the hAAT-mediated anti-inflammatory environment might modify p65 nuclear translocation and/or covalent modifications (Figure 6). RAW 264.7 cells were pre-treated with hAAT (0.5 mg/ml) for 1 h and were subsequently washed and treated with either TNF-α or LPS (10 ng/ml each). Thirty minutes later, the cells were lysed and fractionated into cytosolic, nuclear (including membrane) and chromatin fractions. The distributions of p65 and phosphorylated p65 were evaluated in a western blot analysis. As shown in Figure 6, cytosolic fractions from the non-stimulated cells and hAAT-treated resting cultures contained total p65. However, the nuclear fraction obtained from the non-stimulated hAAT-treated cells contained uniquely sized p65 variants that were calculated to be approximately 120 kDa in the CT group and 83 kDa in the hAAT group. hAAT pre-treatment prior to cell stimulation resulted in an overall reduction in the total p65 level (2.12-fold less in the hAAT-treated LPS group relative to LPS alone and 1.72-fold less in the hAAT-treated TNF-α group relative to TNF-α alone). The proportion of nuclear phosphorylated p65 (resting cells were set at 100%) varied from 124% to 93% with LPS and 170% to 57% with TNF-α treatment. Additionally, the 120-kDa and 83-kDa modified forms of p65 were absent upon stimulation. Figure 6b illustrates the shift from cytosolic p65 to nuclear p65; Figure 6c illustrates changes in the chromatin-bound p65.
Figure 6.
hAAT promotes a distinct p65 phosphorylation and nuclear translocation pattern. RAW 264.7 cells (8×106 per plate) were pre-treated with hAAT (0.5 mg/ml) for 1 h, washed and treated with LPS (10 ng/ml) or TNF-α (10 ng/ml). After 30 min, the cells were lysed and fractionated into cytosolic (C), nuclear (N) and chromatin-enriched fractions (Ch). The lysates were analyzed via western blotting with anti-p65 and anti-P-p65 antibodies. Tubulin and histone 3 were used as loading controls (tubulin not shown). (a) A representative blot from 10 repeats. (b) Densitometry analysis. (c) p65 distribution between the cytoplasm and nucleus. (d) Non-phosphorylated p65 distribution between all nuclear (the sum of nuclear membrane-associated p65, free nuclear p65 and chromatin-bound p65) and chromatin-enriched fractions. hAAT, alpha-1-antitrypsin; P-p65, phosphorylated p65.
Discussion
Given that multiple studies have demonstrated hAAT-mediated islet protection and prolonged hAAT administration to hAAT-deficient individuals has been proven markedly safe, hAAT is currently under evaluation in several clinical trials of recently diagnosed type 1 diabetes patients (NIH clinical trial registries NCT01183455, NCT01319331, NCT01183468, NCT02005848, NCT01304537 and NCT01661192). In the present study, hAAT treatment led to increased IL-1Ra transcription and expression in pancreatic islets. A 48-h hAAT treatment followed by washing and engraftment yielded superior immediate mouse pancreatic islets graft function and an increase in IL-1Ra expression within the graft site samples. Consistently, hAAT islet-conditioned medium, which was determined via ELISA (sensitivity 4 ng/ml) to be hAAT-free, induced increased IL-1Ra secretion from macrophages. The rationale for testing this conditioned medium was to avoid the apparent damage inflicted upon islets during direct cocultured with stimulated macrophages in a model that would reflect the encounters of islets with host macrophages upon transplantation.
This model and an in vivo transplantation model using IL-1Ra-KO mice clearly demonstrated that while the grafted islets might trigger IL-1Ra release, host macrophages are the IL-1Ra producing cells. Interestingly, the IL-1Ra concentration was lower in the hAAT islet-conditioned medium than in the control islet conditioned medium; we therefore postulated that the hAAT-treated islets were less inflamed and thus secreted less IL-1Ra in comparison to the control islets. Taken together, the finding of elevated IL-1Ra levels in responder cells in the absence of direct hAAT treatment suggests that hAAT-treated cells release a soluble mediator that might then communicate with responder cells to induce IL-1Ra secretion.
As hAAT has been shown to induce increased IL-10 secretion32,45,47,48 and IL-10 is a known inducer of IL-1Ra,49,50,51,52 it is possible that IL-10 could be the soluble mediator responsible for the observed phenomenon; IL-10 has been shown to potentiate p65 binding to the IL-1Ra promoter in STAT3-dependent mechanism.53 Therefore, a resting cell will have a limited drive toward IL-1Ra upregulation, whereas a cell exposed to inflammation or, conversely, to IL-10, might instead secrete more IL-1Ra. However, the IL-10 levels in the conditioned medium were unchanged after hAAT pre-treatment. However, the reduced levels of TNF-α in the conditioned medium might suggest a partial mechanism for the increased IL-1Ra levels. Future studies are required to determine whether might downregulate IL-1Ra production and whether an increase in IL-1Ra expression would be favored by hAAT-mediated TNF-α inhibition.
A significant amount of evidence has recently emerged regarding the hAAT-mediated inhibition of IL-1β secretion in various immunological models. For example, bone marrow-derived dendritic cells that were cultured overnight with 0.5 mg/ml of hAAT and were subsequently washed, loaded with ovalbumin and cultured with CD4+ T cells from OT II mice yielded a threefold reduction in IL-1β secretion.32 Similarly, hAAT-treated allogeneic bone marrow-recipient mice displayed a 5-fold reduction in serum IL-1β levels33 and the IL-1β transcript levels measured within islet allograft samples were 1.8-fold lower in hAAT-treated mice relative to those in non-treated mice.34 In a model of experimental autoimmune encephalomyelitis, day-21 splenocytes were stimulated with MOG-35-55 and then allowed to release IL-1β into their supernatants for 48 h; the resulting supernatant IL-1β levels were found to be 1.8-fold lower in cultures of splenocytes from transgenic hAAT-expressing animals.35 Similarly, in a transient coronary artery ligation model, hAAT treatment led to a threefold reduction in IL-1β.36 Our group and several others have demonstrated the cytoprotective effect of hAAT protein treatment on islet allografts.34,54,55,56,57 However, very little is known about the mechanism that underlies this effect.
The transcription factor p65 is a component of the most common cytoplasmic NF-κB transcription factor family member. NF-κB pathway activation is context-dependent and can sometimes induce contradicting effects. For instance, NF-κB activation is recognized as a contributing factor to the development of several cancers, whereas UV damage and cell stress promote apoptosis by activating the same proteins. A possible explanation might be found among the diverse post-transcriptional covalent p65 modifications. For example, p65 ubiquitination has been found to act as a nuclear transport signal58 and p65 mono-ubiquitination was found to negatively regulate p65 activity in a proteasomal degradation-independent manner.59 Indeed, mono-ubiquitinated p65 reduces the transcription of downstream targets such as IL-6 and MHC class II.60 Very few studies have analyzed the direct impact of hAAT on changes in p65 distribution. In a recent study by Zhou et al.,41 hAAT treatment inhibited p65 translocation to the nucleus by 39% and increased the proportion of cytoplasmic p65 in HIV-infected CD4 lymphocytes. hAAT treatment was also found to reduce the total p65 levels in human monocyte nuclear extracts.61 The nuclear p65 size shift that we observed following hAAT treatment could be explained as a shift from poly- to mono-ubiquitinated p65. An in-depth evaluation of the effect of hAAT on p65 ubiquitination is therefore required.
In summary, we have demonstrated a significant benefit of pre-implantation hAAT treatment of pancreatic islets and that at least two IL-1 family members, IL-1β and IL-1Ra, are involved in this process. As hAAT acts predominantly as an inhibitor, our findings have suggested that hAAT might remove a constitutive downregulatory signal responsible for maintaining steady-state IL-1Ra levels. This treatment might thus encourage the secretion of soluble factors that would affect transplant-site macrophages and enable them to express and secrete higher levels of anti-inflammatory cytokines, thereby suppressing inflammatory pathways in favor of islet function.
References
- Eizirik DL, Mandrup-Poulsen T. A choice of death—the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation. 2007;14:288–297. doi: 10.1111/j.1399-3089.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T, Bendtzen K, Nerup J, Dinarello CA, Svenson M, Nielsen JH. Affinity-purified human interleukin I is cytotoxic to isolated islets of Langerhans. Diabetologia. 1986;29:63–67. doi: 10.1007/BF02427283. [DOI] [PubMed] [Google Scholar]
- van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- Filippi CM, von Herrath MG. Islet beta-cell death—fuel to sustain autoimmunity. Immunity. 2007;27:183–185. doi: 10.1016/j.immuni.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Yamada K, Takane-Gyotoku N, Yuan X, Ichikawa F, Inada C, Nonaka K. Mouse islet cell lysis mediated by interleukin-1-induced Fas. Diabetologia. 1996;39:1306–1312. doi: 10.1007/s001250050574. [DOI] [PubMed] [Google Scholar]
- Suarez-Pinzon WL, Power RF, Rabinovitch A. Fas ligand-mediated mechanisms are involved in autoimmune destruction of islet beta cells in non-obese diabetic mice. Diabetologia. 2000;43:1149–1156. doi: 10.1007/s001250051506. [DOI] [PubMed] [Google Scholar]
- Ylipaasto P, Smura T, Gopalacharyulu P, Paananen A, Seppanen-Laakso T, Kaijalainen S, et al. Enterovirus-induced gene expression profile is critical for human pancreatic islet destruction. Diabetologia. 2012;55:3273–3283. doi: 10.1007/s00125-012-2713-z. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Chhabra P, Brayman KL. Pancreatic islet transplantation in type 1 diabetes mellitus: an update on recent developments. Curr Diabetes Rev. 2013;9:294–311. doi: 10.2174/15733998113099990063. [DOI] [PubMed] [Google Scholar]
- Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- Schwarznau A, Hanson MS, Sperger JM, Schram BR, Danobeitia JS, Greenwood KK, et al. IL-1beta receptor blockade protects islets against pro-inflammatory cytokine induced necrosis and apoptosis. J Cell Physiol. 2009;220:341–347. doi: 10.1002/jcp.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Akash MS, Rehman K, Sun H, Chen S. Sustained delivery of IL-1Ra from PF127-gel reduces hyperglycemia in diabetic GK-rats. PloS ONE. 2013;8:e55925. doi: 10.1371/journal.pone.0055925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarznau A, Hanson MS, Sperger JM, Schram BR, Danobeitia JS, Greenwood KK, et al. IL-1beta receptor blockade protects islets against pro-inflammatory cytokine induced necrosis and apoptosis. J Cell Physiol. 2009;220:341–347. doi: 10.1002/jcp.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32:1663–1668. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- Glas R, Sauter NS, Schulthess FT, Shu L, Oberholzer J, Maedler K. Purinergic P2X7 receptors regulate secretion of interleukin-1 receptor antagonist and beta cell function and survival. Diabetologia. 2009;52:1579–1588. doi: 10.1007/s00125-009-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, et al. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci USA. 2004;101:8138–8143. doi: 10.1073/pnas.0305683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MF., Jr Eidlen D, Brewer MT, Eisenberg SP, Arend WP, Gutierrez-Hartmann A. Human IL-1 receptor antagonist promoter. Cell type-specific activity and identification of regulatory regions. J Immunol. 1992;149:2000–2007. [PubMed] [Google Scholar]
- Andersson J, Bjork L, Dinarello CA, Towbin H, Andersson U. Lipopolysaccharide induces human interleukin-1 receptor antagonist and interleukin-1 production in the same cell. Eur J Immunol. 1992;22:2617–2623. doi: 10.1002/eji.1830221022. [DOI] [PubMed] [Google Scholar]
- Jenkins JK, Arend WP. Interleukin 1 receptor antagonist production in human monocytes is induced by IL-1 alpha, IL-3, IL-4 and GM-CSF. Cytokine. 1993;5:407–415. doi: 10.1016/1043-4666(93)90030-9. [DOI] [PubMed] [Google Scholar]
- Luhm J, Langenkamp U, Hensel J, Frohn C, Brand JM, Hennig H, et al. Beta-(1→3)-D-glucan modulates DNA binding of nuclear factors kappaB, AT and IL-6 leading to an anti-inflammatory shift of the IL-1beta/IL-1 receptor antagonist ratio. BMC Immunol. 2006;7 doi: 10.1186/1471-2172-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Kimura A, Matsushima K, Mukaida N. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kappaB activation and a reciprocal suppression of TGF-beta signal pathway. J Immunol. 2006;176:5598–5606. doi: 10.4049/jimmunol.176.9.5598. [DOI] [PubMed] [Google Scholar]
- Smith MF, Jr, Eidlen D, Arend WP, Gutierrez-Hartmann A. LPS-induced expression of the human IL-1 receptor antagonist gene is controlled by multiple interacting promoter elements. J Immunol. 1994;153:3584–3593. [PubMed] [Google Scholar]
- Lewis EC. Expanding the clinical indications for alpha1-antitrypsin therapy. Mol Med. 2012;18:957–970. doi: 10.2119/molmed.2011.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russi EW. Alpha-1 antitrypsin: now available, but do we need it. Swiss Med Wkly. 2008;138:191–196. doi: 10.4414/smw.2008.11991. [DOI] [PubMed] [Google Scholar]
- Toso C, Serre-Beinier V, Emamaullee J, Merani S, Armanet M, Wojtusciszyn A, et al. The role of macrophage migration inhibitory factor in mouse islet transplantation. Transplantation. 2008;86:1361–1369. doi: 10.1097/TP.0b013e31818bdbef. [DOI] [PubMed] [Google Scholar]
- Pott GB, Chan ED, Dinarello CA, Shapiro L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol. 2009;85:886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeri E, Mizrahi M, Shahaf G, Lewis EC. alpha-1 antitrypsin promotes semimature, IL-10-producing and readily migrating tolerogenic dendritic cells. J Immunol. 2012;189:146–153. doi: 10.4049/jimmunol.1101340. [DOI] [PubMed] [Google Scholar]
- Tawara I, Sun Y, Lewis EC, Toubai T, Evers R, Nieves E, et al. Alpha-1-antitrypsin monotherapy reduces graft-versus-host disease after experimental allogeneic bone marrow transplantation. Proc Natl Acad Sci USA. 2012;109:564–569. doi: 10.1073/pnas.1117665109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahaf G, Moser H, Ozeri E, Mizrahi M, Abecassis A, Lewis EC. alpha-1-antitrypsin gene delivery reduces inflammation, increases T-regulatory cell population size and prevents islet allograft rejection. Mol Med. 2011;17:1000–1011. doi: 10.2119/molmed.2011.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Shahaf G, Ozeri E, Miller LM, Vandenbark AA, Lewis EC, et al. Sustained expression of circulating human alpha-1 antitrypsin reduces inflammation, increases CD4+FoxP3+ Treg cell population and prevents signs of experimental autoimmune encephalomyelitis in mice. Metab Brain Dis. 2011;26:107–113. doi: 10.1007/s11011-011-9239-9. [DOI] [PubMed] [Google Scholar]
- Toldo S, Seropian IM, Mezzaroma E, Van Tassell BW, Salloum FN, Lewis EC, et al. Alpha-1 antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2011;51:244–251. doi: 10.1016/j.yjmcc.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Wang Y, He Y, Abraham B, Rouhani FN, Brantly ML, Scott DE, et al. Cytosolic, autocrine alpha-1 proteinase inhibitor (A1PI) inhibits caspase-1 and blocks IL-1beta dependent cytokine release in monocytes. PloS ONE. 2012;7:e51078. doi: 10.1371/journal.pone.0051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller JK, Aboussouan LS. A review of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185:246–259. doi: 10.1164/rccm.201108-1428CI. [DOI] [PubMed] [Google Scholar]
- Kalis M, Kumar R, Janciauskiene S, Salehi A, Cilio CM. alpha 1-antitrypsin enhances insulin secretion and prevents cytokine-mediated apoptosis in pancreatic beta-cells. Islets. 2010;2:185–189. doi: 10.4161/isl.2.3.11654. [DOI] [PubMed] [Google Scholar]
- Zhang B, Lu Y, Campbell-Thompson M, Spencer T, Wasserfall C, Atkinson M, et al. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes. 2007;56:1316–1323. doi: 10.2337/db06-1273. [DOI] [PubMed] [Google Scholar]
- Zhou X, Shapiro L, Fellingham G, Willardson BM, Burton GF. HIV replication in CD4+ T lymphocytes in the presence and absence of follicular dendritic cells: inhibition of replication mediated by alpha-1-antitrypsin through altered IkappaBalpha ubiquitination. J Immunol. 2011;186:3148–3155. doi: 10.4049/jimmunol.1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ED, Pott GB, Silkoff PE, Ralston AH, Bryan CL, Shapiro L. Alpha-1-antitrypsin inhibits nitric oxide production. J Leukoc Biol. 2012;92:1251–1260. doi: 10.1189/jlb.0212071. [DOI] [PubMed] [Google Scholar]
- Churg A, Dai J, Zay K, Karsan A, Hendricks R, Yee C, et al. Alpha-1-antitrypsin and a broad spectrum metalloprotease inhibitor, RS113456, have similar acute anti-inflammatory effects. Lab Invest. 2001;81:1119–1131. doi: 10.1038/labinvest.3780324. [DOI] [PubMed] [Google Scholar]
- Tilg H, Vannier E, Vachino G, Dinarello CA, Mier JW. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med. 1993;178:1629–1636. doi: 10.1084/jem.178.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EC, Mizrahi M, Toledano M, Defelice N, Wright JL, Churg A, et al. alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc Natl Acad Sci USA. 2008;105:16236–16241. doi: 10.1073/pnas.0807627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez J, Stillman B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol Cell Biol. 2000;20:8602–8612. doi: 10.1128/mcb.20.22.8602-8612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott GB, Chan ED, Dinarello CA, Shapiro L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol. 2009;85:886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawara I, Sun Y, Lewis EC, Toubai T, Evers R, Nieves E, et al. Alpha-1-antitrypsin monotherapy reduces graft-versus-host disease after experimental allogeneic bone marrow transplantation. Proc Natl Acad Sci USA. 2012;109:564–569. doi: 10.1073/pnas.1117665109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline JN, Fisher PA, Monick MM, Hunninghake GW. Regulation of interleukin-1 receptor antagonist by Th1 and Th2 cytokines. Am J Physiol. 1995;269:L92–L98. doi: 10.1152/ajplung.1995.269.1.L92. [DOI] [PubMed] [Google Scholar]
- Marie C, Pitton C, Fitting C, Cavaillon JM. IL-10 and IL-4 synergize with TNF-alpha to induce IL-1ra production by human neutrophils. Cytokine. 1996;8:147–151. doi: 10.1006/cyto.1996.0021. [DOI] [PubMed] [Google Scholar]
- Jenkins JK, Malyak M, Arend WP. The effects of interleukin-10 on interleukin-1 receptor antagonist and interleukin-1 beta production in human monocytes and neutrophils. Lymphokine Cytokine Res. 1994;13:47–54. [PubMed] [Google Scholar]
- Cassatella MA, Meda L, Gasperini S, Calzetti F, Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994;179:1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamassia N, Castellucci M, Rossato M, Gasperini S, Bosisio D, Giacomelli M, et al. Uncovering an IL-10-dependent NF-kappaB recruitment to the IL-1ra promoter that is impaired in STAT3 functionally defective patients. FASEB J. 2010;24:1365–1375. doi: 10.1096/fj.09-145573. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Shahaf G, Ozeri E, Miller LM, Vandenbark AA, Lewis EC, et al. Sustained expression of circulating human alpha-1 antitrypsin reduces inflammation, increases CD4+FoxP3+ Treg cell population and prevents signs of experimental autoimmune encephalomyelitis in mice. Metab Brain Dis. 2011;26:107–113. doi: 10.1007/s11011-011-9239-9. [DOI] [PubMed] [Google Scholar]
- Ozeri E, Mizrahi M, Shahaf G, Lewis EC. Alpha-1 antitrypsin promotes semi-mature, interleukin-10-producing and readily migrating tolerogenic dendritic cells. J Immunol. 2012;189:146–153. doi: 10.4049/jimmunol.1101340. [DOI] [PubMed] [Google Scholar]
- Koulmanda M, Bhasin M, Hoffman L, Fan Z, Qipo A, Shi H, et al. Curative and beta cell regenerative effects of alpha1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc Natl Acad Sci USA. 2008;105:16242–16247. doi: 10.1073/pnas.0808031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Reddy P. Tolerance without toxicity? alpha1-antitrypsin as a novel alternative to immunosuppression. Expert Rev Clin Immunol. 2012;8:397–399. doi: 10.1586/eci.12.33. [DOI] [PubMed] [Google Scholar]
- Thoms HC, Loveridge CJ, Simpson J, Clipson A, Reinhardt K, Dunlop MG, et al. Nucleolar targeting of RelAp65 is regulated by COMMD1-dependent ubiquitination. Cancer Res. 2010;70:139–149. doi: 10.1158/0008-5472.CAN-09-1397. [DOI] [PubMed] [Google Scholar]
- Makowiec F, Koveker G, Weber P, Jenss H, Starlinger M.Crohn's disease: disease activity and recurrence following surgery. Deut Med Wochenschr 19901151659–1664.German. [DOI] [PubMed] [Google Scholar]
- Hochrainer K, Racchumi G, Zhang S, Iadecola C, Anrather J. Monoubiquitination of nuclear RelA negatively regulates NF-kappaB activity independent of proteasomal degradation. Cell Mol Life Sci. 2012;69:2057–2073. doi: 10.1007/s00018-011-0912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nita IM, Serapinas D, Janciauskiene SM. alpha1-Antitrypsin regulates CD14 expression and soluble CD14 levels in human monocytes in vitro. . Int J Biochem Cell Biol. 2007;39:1165–1176. doi: 10.1016/j.biocel.2007.02.017. [DOI] [PubMed] [Google Scholar]