Abstract
Ovariectomy in young, growing rodents results in decreased trabecular bone mineral density (BMD) and increased radial growth of the cortical bone. Both of these effects are reversed by treatment with estrogen. The aim of the present study was to determine the physiological role of estrogen receptor-β (ERβ) on bone structure and bone mineral content (BMC). The BMC was increased in adult (11 weeks old), but not prepubertal (4 weeks old), female ERβ–/– mice compared with wild-type (WT) mice. This increase in BMC in females was not due to increased trabecular BMD, but to an increased cross-sectional cortical bone area associated with a radial bone growth. Male ERβ–/– mice displayed no bone abnormalities compared with WT mice. Ovariectomy decreased the trabecular BMD to the same extent in adult female ERβ–/– mice as in WT mice. The expression levels of osteoblast-associated genes — α1(I) collagen, alkaline phosphatase, and osteocalcin mRNAs — were elevated in bone from adult ERβ–/– females compared with WT mice. These observations provide a possible explanation for the increased radial bone growth seen in female mutants, suggesting a repressive function for ERβ in the regulation of bone growth during female adolescence. In summary, ERβ is essential for the pubertal feminization of the cortical bone in female mice but is not required for the protective effect of estrogens on trabecular BMD.
Introduction
Postmenopausal osteoporosis is a condition primarily caused by the severe decrease of serum estrogen levels after cessation of ovarian function. The absence of estrogen results in an increase in bone turnover (1) and a negative bone remodeling balance, leading to bone loss and an increased fracture risk. The decrease in bone mass can be prevented by treatment with estrogens (2). Although the bone-preserving effect of estrogen replacement is indisputable (1), the cellular mechanism of action for this hormone effect is unclear. Osteoblasts express estrogen receptors (3, 4), indicating a direct effect of estrogens on bone tissue. However, indirect effects of estrogens on bone cannot be excluded.
It has been reported that a man who was homozygous for an inactivating point mutation in the estrogen receptor-α (ERα) gene demonstrated unfused growth plates and severe osteoporosis (5). Based on this clinical finding, it was assumed that ERα is important for growth-plate closure and for adult bone metabolism. In contrast, mice lacking a functional ERα had only minor skeletal abnormalities (6), suggesting that other mechanisms or receptors for estrogen action might be important during skeletal development of the mouse.
The cloning of a novel estrogen receptor, estrogen receptor-β (ERβ), suggested that there may exist alternative mechanisms of action for estrogen (7). In general, ERβ is expressed in the same tissues as ERα, but expression levels vary (8). We and others have demonstrated that ERβ is expressed in growth-plate chondrocytes and osteoblasts, indicating a possible role for ERβ in the regulation of longitudinal bone growth and/or adult bone metabolism (refs. 9–12; and S. H. Windahl, unpublished results). However, the physiological role of ERβ in the regulation of growth and bone metabolism is still unknown. We have recently generated mice devoid of functional ERβ protein, and reported that ERβ is essential for normal ovulation efficiency but not for female or male sexual development, fertility, or lactation (13). The aim of the present study was to determine the physiological role of ERβ for longitudinal bone growth and bone mineral content (BMC) using the ERβ–/– mouse model.
Methods
Animals.
The animals were maintained under standardized environmental conditions, with free access to food and water. Genotyping of tail DNA was performed at 4–5 weeks of age using PCR with partly different primers than described previously (13). The primers used were one primer in intron 2 (βNHD4-25; 5′-AGAATGTTGCACTGCCCCTGCTGC-3′), one in intron 3 (C1wt-27; 5′-GGAGTAGAAACAAGCAATCCAGACATC-3′), and one in the Neo cassette (Neo-25; 5′-GCAGCCTCTGTTCCACATACACTTC-3′). The PCR program used was as follows: 95°C for 2 minutes (95°C for 30 seconds, 64°C for 1 minute, 72°C for 1 minute) for 30 cycles, and 72°C for 7 minutes. A 650-bp product (βNHD4-25 and C1wt-27) was amplified for the homozygous wild-type (WT; +/+) mice, and a 450-bp (βNHD4-25 and Neo-25) product was amplified for the homozygous mutant (–/–) mice; both bands were amplified for the heterozygous (+/–) mice.
Histological examination.
Right femora were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned and stained with Alcian blue/van Gieson. The width of the growth plate was measured using an image-processing system (Easy Image; Bergströms Instrument, Stockholm, Sweden) coupled to a microscope. The average of 30 growth-plate measurements (3 sections, 10 measurements per section) was calculated for each femur.
Dual x-ray absorptiometry.
Areal bone mineral density (BMD; BMC/cm2) and BMC were measured with the pDEXA Sabre and Sabre Research software (both from Norland Medical Systems Inc., Fort Atkinson, Wisconsin, USA).
In vivo measurements of animals were performed to determine total body, spine, and cranium BMC (medium-resolution scan with line spacing set at 0.05 cm). Three mice were analyzed at a time. A mouse that was sacrificed at the beginning of the experiment was included in all the scans as an internal standard in order to avoid interscan variations.
Ex vivo measurements of the left femur and tibiae and vertebrae L5 were performed on excised bones placed on a 1-cm-thick Plexiglas™ table. All bones compared were measured in the same scan (high-resolution scan with line spacing set at 0.01 cm).
Peripheral quantitative computerized tomography.
Computerized tomography was performed with the Stratec peripheral quantitative computerized tomography (pQCT) XCT Research M (software version 5.4B; Norland Medical Systems Inc.) operating at a resolution of 70 μm (14).
Mid-diaphyseal pQCT scans of femora and tibiae were performed to determine the cortical volumetric BMD, the cortical cross-sectional area, the periosteal circumference, the endosteal circumference, the moment of resistance, and the cross-sectional moment of inertia. The mid-diaphyseal region of femora and tibiae in mice contains only cortical bone.
Metaphyseal pQCT scans of left femora and tibiae were performed to measure trabecular volumetric BMD. The scan was positioned in the metaphysis at a distance from the distal growth plate corresponding to 4% of the total length of the femur (an area containing cortical and trabecular bone). The trabecular bone region was defined by setting an inner area to 45% of the total cross-sectional area. The interassay coefficients of variation for the pQCT measurements were less than 2%.
It should be emphasized that dual x-ray absorptiometry (DXA) gives the areal BMD, whereas the pQCT gives the real/volumetric BMD. Thus, DXA gives the mineral content per area, not per volume. Therefore, a factor regulating the outer dimensions of a bone will affect the areal BMD (DXA) but not the volumetric BMD (pQCT).
RNA preparation and RT-PCR.
Total RNA was extracted from individual intact femoral bones of WT and ERβ–/– animals after homogenization in denaturation buffer with a Polytron homogenizer, using the kit ToTALLY RNA (Ambion Inc., Austin, Texas, USA) according to the manufacturer’s instructions. cDNA synthesis was done with 5 μg total RNA and 200 U Superscript Reverse Transcriptase (Life Technologies Inc., Gaithersburg, Maryland, USA) at 42°C for 1 hour, using oligo-(dT)12–18 as primer (GIBCO BRL, Paisley, Scotland, United Kingdom). For the PCR amplification, 5% of the synthesized cDNA was added to a 33-μL PCR mix containing 130 ng of each oligonucleotide primer, 0.5 U Taq DNA-polymerase (Promega Corp., Madison, Wisconsin, USA), and the corresponding buffer containing 15 mM MgCl2. The cDNA was amplified for 25 cycles [α1(I) collagen (Col. I), alkaline phosphatase (ALP), cathepsin K (Cath. K), and tartrate-resistant acid phosphatase (TRAP)] or 20 cycles (osteocalcin [OC] and GAPDH) by incubation at 94°C for 30 seconds, 55°C for 30 seconds, and 74°C for 30 seconds. The oligonucleotide primers used for the amplification are as follows: Col. I (GenBank accession no. X 54876): forward primer 5′-GAAGTCAGCTGCATACAC-3′, corresponding to the sequence from nucleotide 3289 to 3306; reverse primer 5′-AGGAAGTCCAGGCTGTCC–3′, corresponding to the sequence from nucleotide 3932 to 3915; product size 313 bp. ALP (GenBank accession no. J02980): forward primer 5′-AGGCAGGATTGACCACGG-3′, corresponding to the sequence from nucleotide 1181 to 1198; reverse primer 5′-TGTAGTTCTGCTCATGGA-3′, corresponding to the sequence from nucleotide 1620 to 1603; product size 440 bp. OC (GenBank accession no. U11542): forward primer 5′-TCTGCTCACTCTGCTGAC-3′, corresponding to the sequence from nucleotide 33 to 50; reverse primer 5′-GGAGCTGCTGTGACATCC-3′, corresponding to the sequence from nucleotide 420 to 403; product size 388 bp. TRAP (GenBank accession no. S70805): forward primer 5′-TGACAAGAGGTTCCAGGA-3′, corresponding to the sequence from nucleotide 4 to 21; reverse primer 5′-AGCCAGGACAGCTGAGTG-3′, corresponding to the sequence from nucleotide 321 to 304; product size 312 bp. Cath. K (GenBank accession no. X 94444): forward primer 5′-CTTGTGGACTGTGTGACT-3′, corresponding to the sequence from nucleotide 499 to 516; reverse primer 5′-AACACTGCATGGTTCACA-3′, corresponding to the sequence from nucleotide 836 to 819; product size 338 bp.
As a control for cDNA synthesis, PCR amplification was made with GAPDH (GenBank accession no. M32599): forward primer 5′-GTGAACGGGAAGCTCACTGG–3′, corresponding to the sequence from nucleotide 710 to 729; reverse primer 5′-TGAGGTCCACCACCCTGTTG-3′, corresponding to the sequence from nucleotide 1022 to 1003; product size 313 bp.
The PCR products were run on a 2% agarose gel in the presence of ethidium bromide.
Results
Body weight and dimensions of bones.
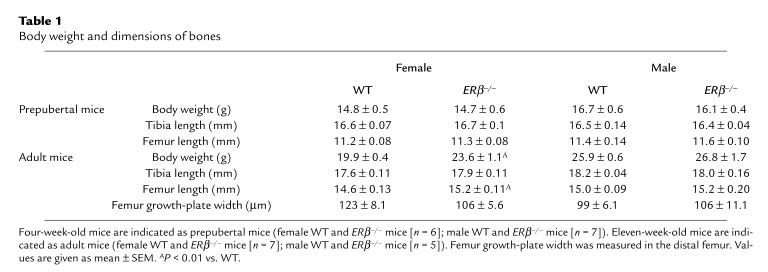
ERβ–/– and WT mice were analyzed at 4 weeks of age (prepubertal) and 11 weeks of age (adult). The body weight and the length of the long bones were unchanged in prepubertal ERβ–/– mice compared with WT mice. No significant difference was seen between prepubertal WT females and WT males (Student’s t test). However, as expected, adult male mice were heavier and had longer tibiae and femora than did adult female mice (Table 1 and Figure 2; Student’s t test). Surprisingly, the adult female ERβ–/– mice were heavier and had longer femora than did adult female WT mice (Table 1).
Table 1.
Body weight and dimensions of bones
Figure 2.
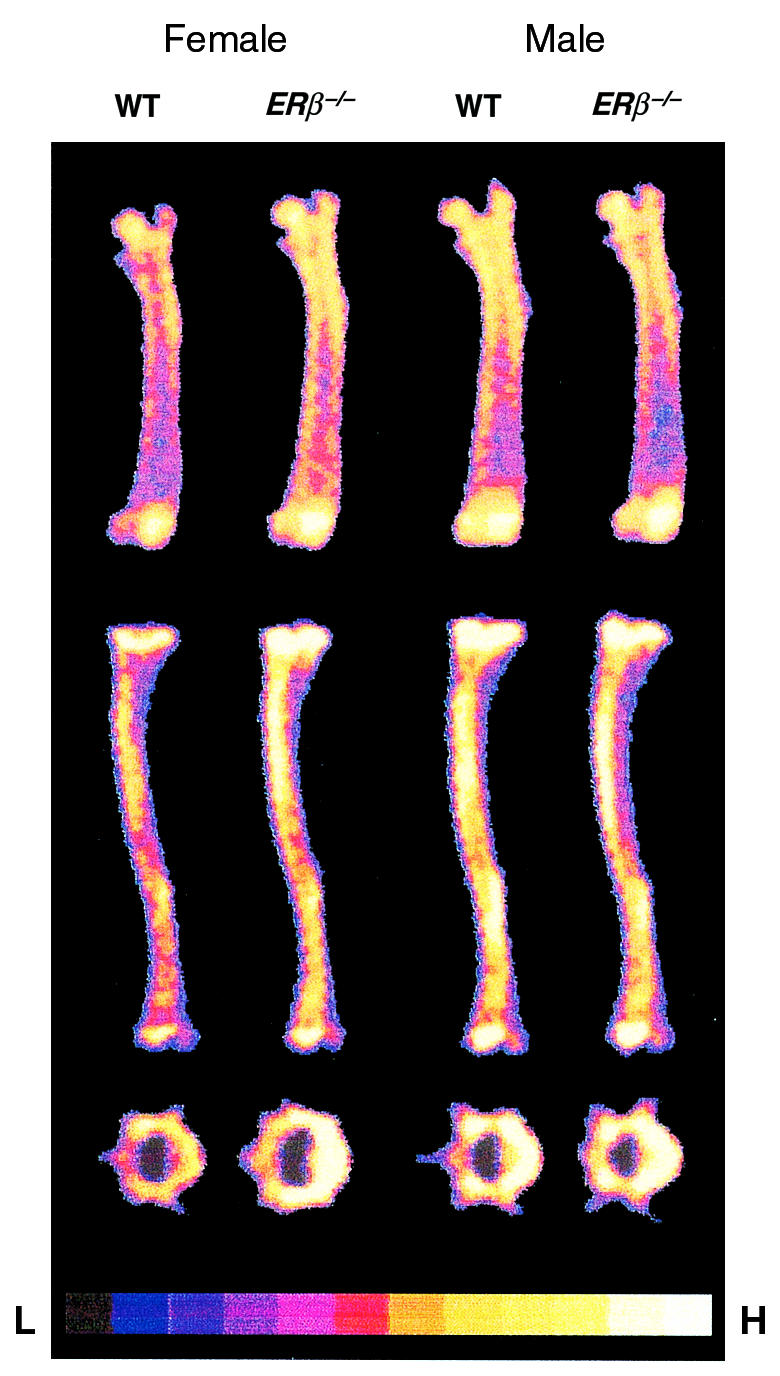
DXA scan of femur (top), tibia (middle), and vertebrae (bottom) from ERβ–/– and WT mice. The relative areal BMD is indicated: H = high density, L = low density.
A disproportional longitudinal bone growth, with a 4% increase in tibial length and a 30% increase in femoral length, was seen from 4 weeks of age to 11 weeks of age in female WT mice (Table 1). Most likely, this difference is due to the fact that most of the tibial growth occurs before puberty, whereas the femur demonstrates a significant adolescent growth spurt. This observation provides a possible explanation for the finding of the present study that femoral length, but not tibial length, is increased in ERβ-deficient female mice. It also suggests that ERβ plays a repressive role during pubertal growth of murine females.
Total BMC and BMC of individual bones.
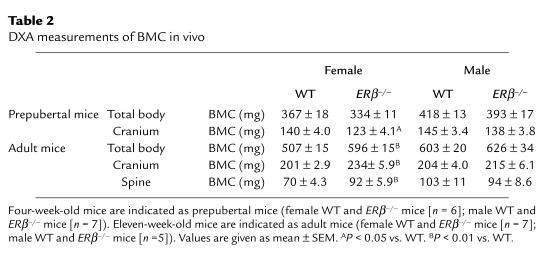
The bones from the ERβ–/– mice were analyzed in detail using DXA and pQCT. The whole-body BMC was increased in adult female ERβ–/– mice, but not in adult male ERβ–/– mice, compared with WT mice — as measured using DXA (Table 2). Actually, the gender difference seen in adult WT mice, with higher total BMC in males compared with females, was not seen in adult ERβ–/– mice (Student’s t test). The effect on individual bones was also studied using DXA. The BMC of cranium, spine, vertebrae L5, tibia, and femur was increased in adult female ERβ–/– mice compared with WT mice (cranium, 16%; spine, 31%; vertebrae L5, 24%; tibia, 20%; and femur, 27%, over that of WT; Table 2 and Figures 1 and 2). WT male mice had higher BMC than did WT females in the spine, the vertebrae L5, and the tibia (Figure 1 and Table 2; Student’s t test). This gender difference was not seen in ERβ–/– mice. No difference in total, spine, tibial, and vertebral BMC was seen between ERβ–/– and WT in prepubertal male and female mice or in adult male mice.
Table 2.
DXA measurements of BMC in vivo
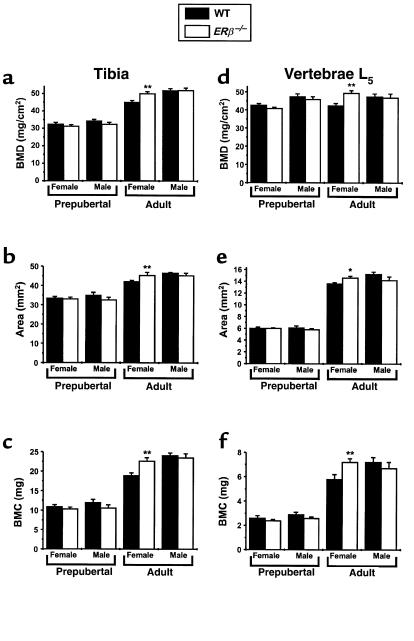
Figure 1.
DXA measurements of bone parameters of excised bones. The area BMD (a and d), bone area (b and e), and BMC (c and f) of excised tibiae (a–c) and vertebrae L5 (d–f) were measured using DXA as described in Methods. Four-week-old mice are indicated as prepubertal mice (female WT and ERβ–/– mice [n = 6]; male WT and ERβ–/– mice [n = 7]). Eleven-week-old mice are indicated as adult mice (female WT and ERβ–/– mice [n =7]; male WT and ERβ–/– mice [n = 5]). Values are given as mean ± SEM. *P < 0.05, **P < 0.01 vs. WT.
The increase in tibial and vertebral BMC in adult female ERβ–/– mice (20% and 24%, respectively, over that of WT) was a result of both an increased bone area (tibia, 8%; vertebrae L5, 11%, over that of WT) and an increased areal BMD (tibia, 11%; vertebrae L5, 16%, over that of WT; Figure 1).
Trabecular BMD.
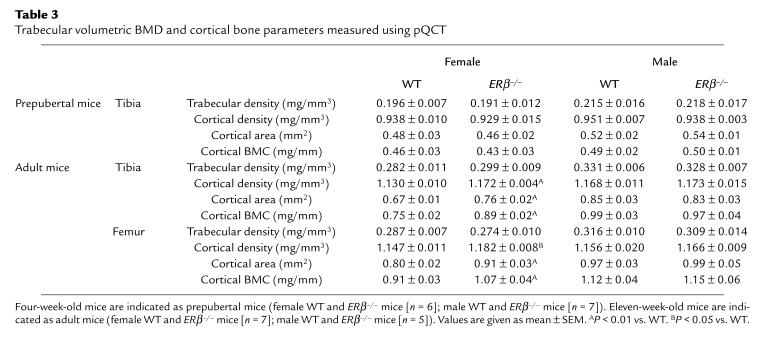
Trabecular volumetric BMD can be measured in the metaphysis of the distal femur and proximal tibia using pQCT. Adult, but not prepubertal, female mice had significantly lower trabecular volumetric BMD than did male mice (Table 3; Student’s t test). In contrast to the total BMC of tibia and femur, no effect was seen on the trabecular volumetric BMD in adult female ERβ–/– mice compared with WT mice. Thus, the increased BMC in female ERβ–/– mice was not caused by an increased trabecular volumetric BMD.
Table 3.
Trabecular volumetric BMD and cortical bone parameters measured using pQCT
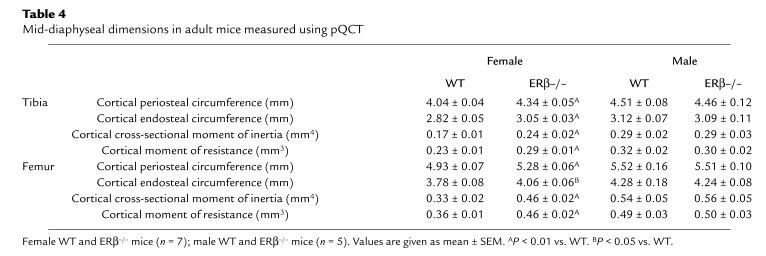
Cortical bone parameters.
As the trabecular volumetric BMD was not changed, the cortical bone parameters were studied in detail. Cortical bone parameters were investigated by mid-diaphyseal pQCT sections. The cortical BMCs of the mid-diaphyseal section of tibia and femur were increased in adult female ERβ–/– mice compared with WT (tibia, 19%; femur, 18%, over that of WT); this increase was mainly due to an increased cross-sectional bone area (tibia, 13%; femur, 14%, over that of WT) and, to a lesser extent, to an increased volumetric BMD (tibia, 4%; femur, 3%, over that of WT; Table 3). The increase in cross-sectional area was associated with an increase of the periosteal circumference and the endosteal circumference (Table 4). The changes in the size and position of the cross-sectional cortical bone area in adult female ERβ–/– mice resulted in a large increase of both the cortical cross-sectional moment of inertia (tibia, 41%; femur, 39%, over that of WT) and the cortical moment of resistance (tibia, 26%; femur, 28%, over that of WT; Table 4). There was a gender difference in all of the cortical bone parameters between adult female WT and male WT mice. However, no gender difference was seen for these parameters between female ERβ–/– mice and male ERβ–/– mice.
Table 4.
Mid-diaphyseal dimensions in adult mice measured using pQCT
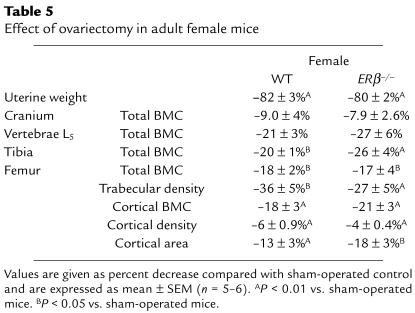
Effect of ovariectomy in adult female mice.
Female ERβ–/– mice were ovariectomized at 13 weeks of age. The bones of the animals were analyzed 8 weeks later. Ovariectomy decreased the uterine weight in both ERβ–/– and WT mice, demonstrating that the ovariectomy was complete (Table 5). Ovariectomy decreased the BMC to the same extent in ERβ–/– mice as in WT mice (Table 5). This decrease in BMC was associated with a large decrease in trabecular volumetric BMD and a small decrease in cortical BMC.
Table 5.
Effect of ovariectomy in adult female mice
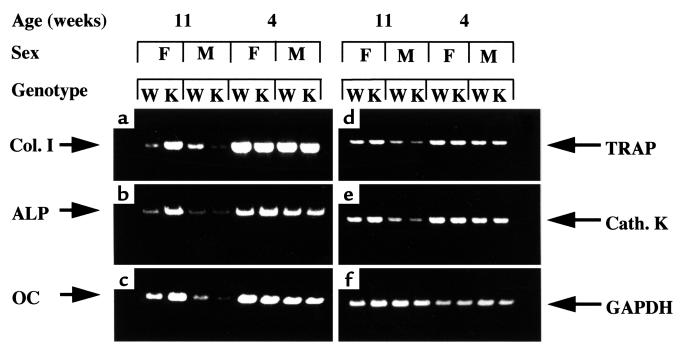
Expression of osteoblast- and osteoclast-associated genes.
To investigate molecular mechanisms behind the bone phenotypic alterations in adult female ERβ–/– mice, the expression of osteoblast-associated mRNAs (Col. I, ALP, OC) and osteoclast-associated mRNAs (TRAP, Cath. K) was assessed by RT-PCR of RNA extracted from long bones of WT and ERβ–/– mice (Figure 3). The expression levels of these genes were generally higher and not sexually differentiated in prepubertal WT mice, consistent with increased bone metabolism and turnover during rapid postnatal growth. In female adult ERβ–/– mice, the expression of the osteoblast-associated genes was, however, increased compared with age-matched WT female mice. As to the osteoclast-associated genes, no difference could be observed between adult ERβ–/– and WT female mice, although expression levels were higher in adult females than in males.
Figure 3.
Expression of osteoblast- and osteoclast-associated genes in long bones of prepubertal (4 weeks old) and adult (11 weeks old) male (M) and female (F) wild-type (W) mice and ERβ–/– knockout (K) mice by RT-PCR. Osteoblast markers: (a) Col. I, (b) ALP, and (c) OC. Osteoclast markers: (d) TRAP, (e) Cath. K, and (f) GAPDH (the control).
Discussion
The present study demonstrated that the adult female ERβ–/– mice had an increased total BMC caused by increased cortical BMC, whereas the trabecular volumetric BMD was unchanged. This increase in cortical BMC was mainly caused by an increase in the cortical cross-sectional area, associated with radial cortical growth (increased periosteal circumference) of the bones. No effect was seen in male ERβ–/– mice. Actually, the gender differences seen in adult WT mice (higher total BMC, increased cross-sectional cortical area, and increased periosteal circumference in males compared with females) were not seen in adult ERβ–/– mice. Thus, it appears that the cortical bone in female ERβ–/– mice displays a loss of feminization. It has previously been demonstrated that gonadectomy of young rats results in decreased radial cortical bone growth in males and increased radial growth in females, such that the gender difference is largely eliminated. The gender difference is reestablished in ovariectomized rats by administration of estrogen to females (1, 15). This finding, together with our present result of a loss of feminization of the cortical bone in adult female ERβ–/– mice, indicates that ERβ is involved in feminization of the cortical bone in rodents. The effect of ERβ on cortical bone mass may include direct effects on the bone tissue or indirect effects such as regulation of systemic hormones. A direct effect on the bone tissue is made possible by recent findings that ERβ is expressed in osteoblasts (refs. 9–11; and S. H. Windahl, unpublished results).
In the tibial long bone, which has almost completed its longitudinal growth before the onset of puberty, an increase in BMC, but not bone length, was seen in the adult female ERβ–/– mice compared with WT mice. This finding demonstrates that the increased BMC in adult female ERβ–/– mice was not caused solely by a stimulation of longitudinal bone growth.
It is well known from clinical and rat studies that ovariectomy results in a decrease in BMC, and that this decrease is predominantly caused by a reduction of the trabecular volumetric BMD (1). The ovariectomy mouse model is less well characterized. However, we recently demonstrated, by using the same techniques as were used in the present study, that ovariectomy in mice also results in a large decrease in trabecular volumetric BMD (16). Interestingly, in the present study, no reduction in trabecular volumetric BMD was seen in ERβ–/– females compared with WT mice. Furthermore, ovariectomy decreased the trabecular volumetric BMD to the same extent in ERβ–/– and WT mice, demonstrating that ovariectomy-induced reduction in trabecular volumetric BMD does not depend solely on ERβ. Therefore, one may speculate that either ERα by itself or ERα and ERβ in a redundant manner protect against ovariectomy-induced trabecular bone loss. Future experiments with ovariectomy of ERα and ERαβ double-knockout mice will provide more insight into the receptor specificity of estrogens on bone mass. A functional receptor specificity for ERα versus ERβ in cardiovascular homeostasis has recently been described (17). It was demonstrated that estrogen inhibits vascular injury response by a mechanism that is independent of the ERα, suggesting that ERβ was involved in this response. In contrast, the same degree of decrease in uterine weight in ERβ–/– and WT mice after ovariectomy, as demonstrated in the present study, indicates that ERβ is not required as a mediator for the effect of estrogens on the uterus. Thus, ERα and ERβ may have both independent and common functions in normal physiology as well as pathophysiology.
The molecular mechanisms of action for ERα versus ERβ have recently been investigated. ERα and ERβ have almost identical DNA-binding domains, and studies in vitro have demonstrated that the 2 receptors have some similarites in the affinity for estrogenic compounds (7, 18, 19). The amino acid sequence of ERβ differs from ERα in the NH2- and COOH-terminal transactivating regions. Therefore the transcriptional activation mediated by ERβ may be distinct from that of ERα (20). Considering the great similarities in DNA-binding specificity, it has been speculated that a differential tissue distribution of estrogen receptors may be important for mediating tissue-specific responses to estrogens (21). Thus, the unique transactivating domains of the 2 receptor subtypes, in combination with differential tissue distribution, could be important factors in determining the estrogen response in target tissues.
In conclusion, ERβ–/– mice have unchanged trabecular BMD. In contrast, adult female mice devoid of ERβ have an increased cortical BMC caused by an increased cross-sectional bone area, resulting in a loss of feminization of the cortical bone. This finding indicates that ERβ is essential for the endogenous estrogen effect on cortical bone in female mice. As suggested by the pattern of osteoblast and osteoclast gene expression, the absence of active ERβ prevents the estrogen-induced downregulation of osteoblast-associated genes occurring in WT mice during adolescence. These observations confirm, and provide a potential explanation at the molecular level for, the sexually differentiated bone phenotype in female mice and, moreover, indicate a repressive role for ERβ in regulating osteoblast function.
Acknowledgments
This study was supported by the Swedish Medical Research Council (to G. Andersson and C. Ohlsson), the Bergvall Foundation, the Emil and Vera Cornell Foundation, the Lundberg Foundation, and the Swedish Association Against Rheumatic Disease.
References
- 1.Turner RT, Riggs BL, Spelsberg TC. Skeletal effects of estrogen. Endocr Rev. 1994;15:275–300. doi: 10.1210/edrv-15-3-275. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay R, et al. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet. 1976;1:1038–1041. doi: 10.1016/s0140-6736(76)92217-0. [DOI] [PubMed] [Google Scholar]
- 3.Eriksen EF, et al. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988;241:84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- 4.Komm BS, et al. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science. 1988;241:81–84. doi: 10.1126/science.3164526. [DOI] [PubMed] [Google Scholar]
- 5.Smith EP, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 6.Taki M, et al. Effects of estrogen receptor destruction on femurs from male mice. J Bone Miner Res. 1997;12:S460. (Abstr.) [Google Scholar]
- 7.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couse JF, et al. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 9.Onoe Y, Miyaura C, Ohta H, Nozawa S, Suda T. Expression of estrogen receptor beta in rat bone. Endocrinology. 1997;138:4509–4512. doi: 10.1210/endo.138.10.5575. [DOI] [PubMed] [Google Scholar]
- 10.Arts J, et al. Differential expression of estrogen receptors alpha and beta mRNA during differentiation of human osteoblast SV-HFO cells. Endocrinology. 1997;138:5067–5070. doi: 10.1210/endo.138.11.5652. [DOI] [PubMed] [Google Scholar]
- 11.Vidal O, Kindblom LG, Ohlsson C. Expression and localization of estrogen receptor-beta in murine and human bone. J Bone Miner Res. 1999;14:923–929. doi: 10.1359/jbmr.1999.14.6.923. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson LO, et al. Demonstration of estrogen receptor-beta immunoreactivity in human growth plate cartilage. J Clin Endocrinol Metab. 1999;84:370–373. doi: 10.1210/jcem.84.1.5531. [DOI] [PubMed] [Google Scholar]
- 13.Krege JH, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen HN, et al. Differentiating between orchiectomized rats and controls using measurements of trabecular bone density: a comparison among DXA, histomorphometry, and peripheral quantitative computerized tomography. Calcif Tissue Int. 1995;57:35–39. doi: 10.1007/BF00298994. [DOI] [PubMed] [Google Scholar]
- 15.Turner RT, Vandersteenhoven JJ, Bell NH. The effects of ovariectomy and 17 beta-estradiol on cortical bone histomorphometry in growing rats. J Bone Miner Res. 1987;2:115–122. doi: 10.1002/jbmr.5650020206. [DOI] [PubMed] [Google Scholar]
- 16.Andersson N, et al. Human parathyroid hormone hPTH(1-84). Effects in distal femur following intermittent or continuous administration in rats. Bone. 1998;23:S631. (Abstr.) [Google Scholar]
- 17.Iafrati MD, et al. Estrogen inhibits the vascular injury response in estrogen receptor alpha-deficient mice. Nat Med. 1997;3:545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 18.Kuiper GG, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 19.Tremblay GB, et al. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 20.Paech K, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 21.Kuiper GG, Gustafsson JA. The novel estrogen receptor-beta subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997;410:87–90. doi: 10.1016/s0014-5793(97)00413-4. [DOI] [PubMed] [Google Scholar]