Abstract
Despite extensive studies on CD4+CD25+ regulatory T cells (Tregs) during the past decade, the progress on their clinical translation remains stagnant. Mounting evidence suggests that naturally occurring CD8+CD122+ T cells are also Tregs with the capacity to inhibit T-cell responses and suppress autoimmunity as well as alloimmunity. In fact, they are memory-like Tregs that resemble a central memory T cell (TCM) phenotype. The mechanisms underlying their suppression are still not well understood, although they may include IL-10 production. We have recently demonstrated that programmed death-1 (PD-1) expression distinguishes between regulatory and memory CD8+CD122+ T cells and that CD8+CD122+ Tregs undergo faster homeostatic proliferation and are more potent in the suppression of allograft rejection than conventional CD4+CD25+ Tregs. These findings may open a new line of investigation for accelerating effective Treg therapies in the clinic. In this review, we summarize the significant progress in this promising field of CD8+CD122+ Treg research and discuss their phenotypes, suppressive roles in autoimmunity and alloimmunity, functional requirements, mechanisms of action and potential applications in the clinic.
Keywords: autoimmunity and transplant immunology, CD8+CD122+ T cells, immune regulation, regulatory T cells
Introduction
Regulatory T cells (Tregs) are essential for maintaining immune tolerance by regulating both autoimmunity and alloimmunity. In addition to CD4+CD25+ Tregs, CD8+ Tregs are emerging as an important subset of T suppressors. Since the original reports showing that CD8+ T cells also possess Treg properties,1,2,3 tremendous progress in understanding their suppression of immune responses has been made. Liu et al.4 have demonstrated the specific suppression of T helper activity by CD8+CD28− T cells. The same cells also reportedly induced CD4+ T-cell anergy5 and regulated reactivation of T and NKT cells.6 Furthermore, antigen-induced CD8+CD103+ Tregs have been shown to suppress effector T-cell function7 and to be significantly increased in spontaneously tolerant recipients of liver allografts.8 It has also been reported that Qa-1-restricted CD8+ T cells regulate the activation of T cells as well as NKT cells.6 Therefore, CD8+CD28−, CD8+Qa-1+ or CD8+CD103+ Treg subsets may share the responsibility of maintaining immune homeostasis with other regulatory cell families, including CD4+CD25+FoxP3+ and CD8+CD122+ Tregs, although it remains to be defined whether and how the latter cells are associated with CD8+CD28−, CD8+CD103+ or other CD8+ Treg subsets. Understanding the relationship between CD8+CD122+ and other subsets of CD8+ Tregs through future studies may help in the design of new strategies to optimize their suppressive efficacy.
Since the pioneering work by Suzuki's group provided the first evidence that CD8+CD122+ T cells maintain T-cell homeostasis,9 many recent studies have demonstrated that naturally occurring CD8+CD122+ T cells indeed are Tregs that suppress conventional T-cell responses9,10,11,12,13,14,15 and regulate autoimmune diseases.16,17 We have also found that CD8+CD122+ T cells are memory-like Tregs that suppress allograft rejection in a murine model.18 Hence, CD8+CD122+ Tregs correspond to CD4+CD25+ Tregs, given that CD122 is the β subunit of the IL-2 receptor on T cells, while CD25 is the α subunit of the same receptor,19 which indicates that both subunits of the receptor are involved in Treg regulation. However, CD8+CD122+ Tregs are recently emerging and relatively understudied in comparison with CD4+CD25+ Tregs. Importantly, we have recently shown that CD8+CD122+ Tregs are more potent in the suppression of allograft rejection than conventional CD4+CD25+ Tregs.20 Therefore, it is imperative to conduct more studies to lay the groundwork for clinical trials using CD8+CD122+ Tregs in the future.
CD8+CD122+ T cells have been traditionally described as antigen-specific memory T cells.21,22,23 However, are they true memory T cells or Tregs? It is very important to answer this question, given their critical roles in long-term immunity versus immune tolerance. We have recently demonstrated that naturally arising CD8+CD122+ T cells contain a substantial subset of programmed death-1 (PD-1)+ cells that are Treg cells, while antigen-specific CD8+CD122+PD-1− cells are true memory T cells.18 This finding may have important implications for the induction of immune tolerance. In this review, we will discuss the CD8+CD122+ Treg phenotypes and functions, functional requirements, mechanisms of action and efficacy in suppression in comparison with that of conventional CD4+CD25+ Tregs, and we will summarize the significant progress that have been made during the last six to eight years.
CD8+CD122+ Treg phenotypes and their associated functions
Previous studies have largely focused on naturally occurring CD4+CD25+ Tregs.24,25 They carry a surface marker, CD25 (IL-2 receptor α), with specific intracellular expression of FoxP3.24,25,26 They are also CD44high with some nonspecific cell surface markers such as glucocorticoid-induced TNF receptor.27 In contrast, CD8+CD122+ Tregs have been relatively understudied. In particular, many immunologists have regarded CD8+CD122+ T cells as antigen-specific memory T cells,21,22,23 but mounting evidence has shown that they are indeed Tregs. These cells now appear to be memory-like Tregs, which indicates that they exhibit memory T cell phenotypes with a regulatory function. Murine CD8+CD122+ Tregs carry CD122 or IL-2Rβ, but not CD25, while CD4+CD25+ Tregs do not express CD122, although both subsets of Tregs are CD44high, CD62Lhigh and mostly CD127-negative (Figure 1).18,28 It is unknown whether CD8+CD122+ Tregs, such as CD4+CD25+ Tregs, also express glucocorticoid-induced TNF receptor. Unlike CD4+CD25+FoxP3+ Tregs, they are FoxP3-negative,18 suggesting that they represent a different population from induced CD8+FoxP3+ Tregs.29 It remains to be defined if the latter can be developed from naturally arising CD8+CD122+ Tregs.
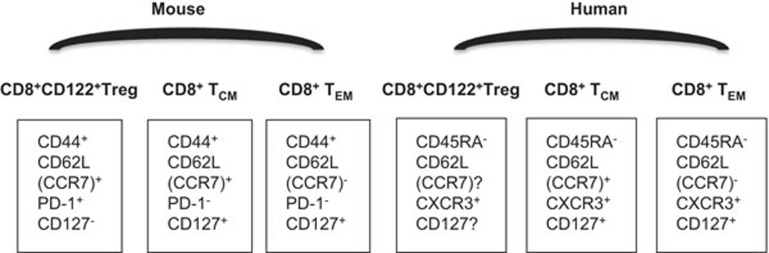
Figure 1.
CD8+CD122+ regulatory versus memory T-cell phenotypes. Overall, murine CD8+CD122+ Tregs are CD44highCD62LhighCCR7+PD-1+CD127−, while CD8+CD122+ memory T cells are CD44highCD62LhighCCR7+PD-1−CD127+ (TCM) or CD44highCD62LlowCCR7-PD-1−CD127+ (TEM). Human CD8+CD122+ Tregs are not well characterized, with simple phenotypes of CD45RA−CXCR3+, while human CD8+ memory T cells are CD45RA−CCR7+CXCR3+CD127+ (TCM) or CD45RA−CCR7−CXCR3+CD127+ (TEM). TCM, central memory T cell; TEM, effector memory T cell; Treg, regulatory T cell; PD-1, programmed death-1.
Human CD8+CD122+ Tregs are not well characterized. Murine CD8+CD122+ Tregs are CXCR3+, while human CD8+CXCR3+ Tregs are largely CD122−.30 It is known if these cells are CD8+CXCR3+CD45RA− and exhibit a feature of in vitro suppression.30 However, it remains controversial whether CXCR3 can distinguish memory from regulatory subsets of CD8+ T cells. Although Shi et al. have shown that murine CD8+CD122+ Tregs are CXCR3+, studies by others have demonstrated that murine CXCR3+CD8+ T cells are memory T cells that are critical for protection against the viral infection.31 Therefore, further studies are needed to draw a conclusion regarding the role of the CXCR3 marker in defining the CD8+CD122+ Treg subset.
CD8+CD122+ T cells have been traditionally described as antigen-specific memory T cells.21,22,23 In particular, the phenotype of CD8+CD122+ Tregs generally resemble a central memory T cell phenotype. Are they true memory, regulatory or memory-like Tregs? Given their essential and opposing roles in long-term immunity versus immune tolerance, it is important to reconcile this dichotomy. These questions of fundamental immunology are too important to be ignored.
It has been reported that PD-1 expression on CD8+ T cells is responsible for their exhaustion during chronic viral infection and that PD-1 blockade restores their function.32,33,34,35 Moreover, previous studies have shown that homeostatically proliferated T cells contain PD-1+ and PD-1− fractions and that the PD-1+ subset of the cells are dysfunctional.36 We have sought to determine if naturally occurring CD8+CD122+ T cells also contain PD-1+ and PD-1− populations. We have found that CD8+CD122+ T cells indeed contain a substantial subset of PD-1+ cells that are Treg cells, while the antigen-specific PD-1− component of CD8+CD122+ T cells are true memory T cells.18 Therefore, PD-1 expression appears to define murine CD8+CD122+ T cells as memory versus regulatory T cells. It is possible that PD-1 is not the only marker that distinguishes between CD8+CD122+ central memory cells and CD8+CD122+ Tregs. We have previously demonstrated that ‘bystander' or ‘third-party' central memory CD8+ T cells are Tregs, while their ‘antigen-specific' counterparts are memory CD8+ T cells.37 Further studies are warranted to identify biomarkers that accurately distinguish CD8+CD122+ Tregs from central memory CD8+ T cells. We also found that CD8+CD122+ Treg suppression is partially dependent on their production of IL-10, while CD8+CD122+PD-1+ cells produce much more IL-10 than their PD-1-negative counterparts.18 In contrast, we have shown that antigen-specific CD8+CD122+PD-1− T cells are bona fide memory T cells that are specific for a previously encountered antigen, but not for a third-party donor antigen. Therefore, CD8+CD122+ T cells can either be Tregs or memory T cells, depending on their PD-1 expression and antigen specificity. Our results may have dual implications for the induction of long-term immunity versus immune tolerance.
Origin of pre-existing CD8+CD122+ Tregs
The exact origin of peripheral CD8+CD122+ Tregs is unclear. Previous studies have shown that CD8+CD122+ Tregs account for nearly 50% of the CD8+ T cells in mice within the first two weeks of life, and these numbers decline in number to approximately 10% at 8–10 weeks after birth.9 Therefore, like naturally occurring CD4+CD25+ Tregs, they most likely originate mostly from thymic emigrates during young age. After 10 weeks, these cells gradually and slowly increase again over time, perhaps because aging mice have likely encountered environmental antigens and developed antigen-specific memory CD8+CD122+ T cells.21,22,23 Studies by Suzuki's teams have also demonstrated that CD8+CD122+ Tregs contain clonally expanded cells with identical CDR3 sequences of the T-cell receptor (TCR) β-chain,38 suggesting that they can be partially generated in the periphery. This finding warrants further investigation into the origin of CD8+CD122+ Tregs.
CD8+CD122+ Tregs in suppression of autoimmunity and alloimmunity
Since Rifa'i et al.9 provided the first evidence that naturally arising CD8+CD122+ Tregs keep potentially dangerous T cells in check, many studies have demonstrated that they indeed play an important role in the suppression of immune responses, especially in the context of autoimmune diseases. Lee et al.39 demonstrated that the transfer of CD8+CD122+ Tregs at the peak score of experimental autoimmune encephalomyelitis (EAE) significantly improved clinical symptoms, indicating a role for these Tregs in the recovery phase of EAE. Others found that brain-localized DCs expressing the B7-H1 molecule recruited CD8+CD122+ Tregs to the inflammatory site, resulting in a decrease in clinical EAE peak values.40 Interestingly, CD8+CD38+CD122+ Tregs suppressed effector CD4+ T-cell proliferation in an nonspecific-antigen manner and mitigated murine EAE by reducing the clinical score and delaying the disease occurrence.41 A recent study based on EAE models of HLA-DR3 transgenic mice also showed that CD8+CD122+ T cells played a regulatory role, while CD8+CD122− T cells acted as a pathogenic subset.17 Moreover, CD8+CD122+ Tregs could suppress other autoimmune diseases in animal models. Saitoh et al.42 found that depletion of CD8+CD122+ T cells increased the incidence of autoimmune Graves' hyperthyroidism in a murine model, suggesting that they play an essential role in suppressing autoimmune hyperthyroidism. CD8+CD122+ and CD4+CD25+ Tregs cooperatively prevented CD4+ T cell-mediated colitis.14 The development of systemic lupus erythematosus-like disease in B6-Yaa mutant mice was associated with a defect in CD8+CD122+ Treg cell activity, indicating that this Treg subset represents an effective therapeutic approach to systemic lupus erythematosus-like autoimmune disease.16 Taken together, CD8+CD122+ Tregs play an important role in the suppression or prevention of various autoimmune diseases in experimental animal models.
Recent studies have also shown that CD8+CD122+ Tregs play a regulatory role in alloimmunity as well as tumor immunity. We have provided the first evidence that CD8+CD122+ Tregs can suppress allograft rejection, although the PD-1+component of these Tregs are more effective than unfractionated CD8+CD122+ Tregs.18 We have found that antigen-specific CD8+CD122+PD-1− cells are true memory T cells that respond to a previously encountered antigen other than a specific donor antigen. However, they could also play an unwanted regulatory role in tumor immunity. Wang et al.15 found that removal of CD8+CD122+ Tregs resulted in a greater expansion of tumor-specific T cells and tumor infiltration of functional effector/memory T cells. They demonstrated that the proliferation of CD8+CD122+ Tregs in reconstituted, lymphodepleted mice limited the antitumor efficacy of DC vaccination in conjunction with adoptive transfer of tumor-specific T cells. Simultaneous inhibition of CD4+CD25+ and CD8+CD122+ Tregs by blocking CTLA-4 and PD-L1 protected T-cell activation from their regulation and enhanced antitumor immunity,43 implying that CD8+CD122+ Tregs hinder antitumor immunity. Taken together, CD8+CD122+ Tregs regulate not only autoimmunity but also alloimmunity and tumor immunity, suggesting a broad involvement of CD8+CD122+ Tregs in immune regulation.
Antigen-specificity of CD8+CD122+ Tregs
It remains unclear whether the suppression by naturally arising CD8+CD122+ Tregs is antigen-specific. A recent study by Okuno et al.38 has demonstrated that CD8+CD122+ Tregs contain clonally expanded cell populations with identical CDR3 sequences of the T-cell receptor β-chain, suggesting that they may specifically recognize certain antigens via their TCR-β-chain. We have previously shown that ‘bystander' memory CD8+ T cells with a central memory T cell (TCM) phenotype suppress allograft rejection.37 We also have found that the suppressive capacity of CD8+CD122+ Tregs is partially dependent on IL-10 and that their suppression in vitro is donor-nonspecific.18 Our findings suggest that the suppression mediated by naturally occurring CD8+CD122+ Tregs is not antigen-specific. It is possible that their suppression involves both antigen-specific and nonspecific-antigen mechanisms. Studies using antigen-specific TCR-transgenic CD8+CD122+ T cells would shed light on their antigen specificity.
Mechanisms underlying CD8+CD122+ Treg-mediated suppression
Although emerging evidence has shown that naturally arising CD8+CD122+ T cells are Tregs in various animal models of diseases, their mechanisms of action are not well understood. As shown in Figure 2, IL-10 produced by CD8+CD122+ Tregs appears to be a main mechanism responsible for their suppression.10,18,44 Endharti et al.10 presented the first data showing that CD8+CD122+ Tregs suppressed IFN-γ production and proliferation of CD8+ T cells by producing IL-10 in vitro.10 The CD8+ Tregs also recognized activated T cells via the interaction of MHC class I-αβ TCR and regulated target T cells by producing IL-10.44 We also found that suppression of allograft rejection by IL-10-deficient CD8+CD122+ Tregs was significantly reduced.18 However, IL-10 did not account for all mechanisms underlying CD8+CD122+ Treg suppression.18 Other mechanisms, in addition to IL-10 production, could be involved in their suppression. In particular, CD8+CD122+ Tregs also released IFN-γ and TGF-β, which suppress CD4+ T-cell activation.17 It remains to be defined if CTL activities generated by CD8+CD122+ Tregs are also involved in regulating effector T cells. Despite previous findings, more studies will be needed to fully understand the mechanisms underlying their suppression of immune responses.
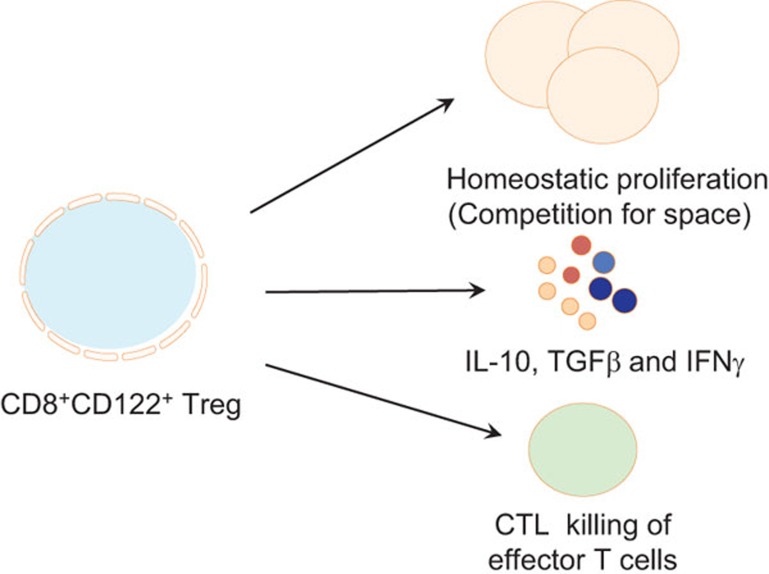
Figure 2.
Mechanisms underlying CD8+CD122+ Treg suppression. CD8+CD122+ Treg suppression of autoimmunity and alloimmunity is mainly mediated by their production of IL-10, while other immunosuppressive cytokines, including TGF-β and IFN-γ, could be involved in the suppression. We have demonstrated that CD8+CD122+ Tregs undergo homeostatic proliferation more rapidly than their CD4+CD25+ counterparts, suggesting that the quickly expanded CD8+CD122+ Tregs may compete for spaces/niches with effector T cells. Finally, the CD8+ Treg-mediated CTL killing of effector T cells may also play a role in CD8+CD122+ Treg suppression. Treg, regulatory T cell.
Functional requirement for CD8+CD122+ Treg-mediated suppression
Many previous studies have shown that the B7/CD28 costimulatory pathway is essential for the homeostasis of CD4+CD25+FoxP3+ Tregs,45 while IL-2 is also critical for their development and function.46 However, the functional requirement of costimulatory pathways and cytokines for CD8+CD122+ Tregs is not clear. Shi et al.12 reported that B7/CD28 costimulatory blockade by antibodies or using CD28-deficient CD8+CD122+ Tregs abrogated their suppressive activity, indicating that they exert their suppression via the B7/CD28 interaction that is essential for them to produce suppressive cytokines, such as IL-10. We have recently shown that the presence of both the B7/CD28 costimulatory and PD-L1/PD-1 co-inhibitory pathways is required for their optimal production of IL-10,18 suggesting that they do need some costimulatory signaling to acquire their suppressive function by producing IL-10. However, IL-2 could be involved in the generation and function of CD8+CD122+ Tregs. IL-2 has been shown to enhance the function of memory-like autoregulatory CD8+CD122+ T cells in autoimmune diabetic NOD mice and suppress their development via the induction of CD4+CD25+FoxP3+ Tregs,47 indicating a dual role for IL-2 in the generation and function of CD8+CD122+ Tregs. Perhaps that is why we found that administration of IL-2 did not significantly promote CD8+CD122+ Treg suppression of allograft rejection.20 Instead, we demonstrated that administration of low doses of IL-15 promoted their homeostatic proliferation and enhanced their suppression of allograft rejection.20 Taken together, both the B7/CD28 costimulatory and PD-L1/PD-1 coinhibitory pathways are required for optimal CD8+CD122+ Treg-mediated suppression, while IL-15 boosts their suppressive capacity via promoting their proliferation or expansion. However, it remains unknown whether CD8+CD122+ Tregs can modulate antigen-presenting cells, which in turn, regulate aggressive effector CD4+ or CD8+ T cells.
CD8+CD122+ Tregs are more potent suppressors than conventional CD4+CD25+ Tregs
It is now known that both CD4+CD25+ and CD8+CD122+ T cells are Tregs, although the former has been studied more extensively than the latter. Interestingly, CD8+CD122+ Tregs correspond to CD4+CD25+ Tregs because CD122 is the β subunit of the IL-2 receptor on T cells, while CD25 is the α subunit of the same receptor.19 To identify more potent Tregs for potential clinical applications, we evaluated the suppression efficacy of naturally occurring CD8+CD122+ versus CD4+CD25+ Tregs. We found that CD8+CD122+ Tregs were much more effective in the suppression of allograft rejection and underwent faster homeostatic proliferation than CD4+CD25+ Tregs.20 Moreover, they produced more IL-10 and were more potent in the suppression of in vitro T-cell proliferation than CD4+CD25+ Tregs. Most importantly, transfer of CD8+CD122+, but not CD4+CD25+, Tregs, together with administration of recombinant IL-15, significantly prolonged allograft survival in immunocompetent, wild-type mice. In contrast, transfer of CD4+CD25+ Tregs, along with administration of recombinant IL-2 or IL-15, did not significantly prolong allograft survival. We speculated that administration of IL-2 likely promoted the expansion or activation of both CD4+CD25+ Tregs and conventional effector T cells, resulting in an unaltered immune balance, whereas administering IL-15 enhanced the expansion and function of CD8+CD122+ Tregs, but not effector T cells. Therefore, cell therapies of allograft rejection using CD8+CD122+ Tregs appear to be promising. Our findings may help find remedies for the cure of autoimmune diseases or allograft rejection.
Conclusions
Naturally occurring CD8+CD122+ T cells clearly display immunoregulatory properties that inhibit T-cell responses and suppress autoimmunity as well as alloimmunity. They are memory-like Tregs, resembling a TCM phenotype, while PD-1 serves as a critical biomarker that can distinguish between memory and regulatory CD8+CD122+ T cells. The mechanisms underlying their suppression mainly include IL-10 production and possible CTL-mediated killing of activated T cells. More studies are warranted to fully understand their mechanisms of action. We have recently demonstrated that CD8+CD122+ Tregs, in addition to conventional CD4+CD25+ Tregs, are another choice for suppressing allograft rejection. Could they represent a better therapeutic approach than CD4+CD25+ Tregs? Further studies from independent groups are needed to draw a definite conclusion on whether CD8+CD122+ Tregs are more effective in suppression than conventional CD4+CD25+ Tregs. Our findings could lay the groundwork for clinical trials using CD8+CD122+ Treg therapies and may lead to new strategies for the cure of human autoimmune diseases or allograft rejection.
The authors declare that there is no conflict of interest in this review.
References
- Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–737. [PMC free article] [PubMed] [Google Scholar]
- Cantor H, Shen FW, Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976;143:1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Cantor H. Generation and regulation of CD8+ regulatory T cells. Cell Mol Immunol. 2008;5:401–406. doi: 10.1038/cmi.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Tugulea S, Cortesini R, Suciu-Foca N. Specific suppression of T helper alloreactivity by allo-MHC class I-restricted CD8+CD28− T cells. Int Immunol. 1998;10:775–783. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- Colovai AI, Liu Z, Ciubotariu R, Lederman S, Cortesini R, Suciu-Foca N. Induction of xenoreactive CD4+ T-cell anergy by suppressor CD8+CD28− T cells. Transplantation. 2000;69:1304–1310. doi: 10.1097/00007890-200004150-00016. [DOI] [PubMed] [Google Scholar]
- Varthaman A, Khallou-Laschet J, Clement M, Fornasa G, Kim HJ, Gaston AT, et al. Control of T cell reactivation by regulatory Qa-1-restricted CD8+ T cells. J Immunol. 2010;184:6585–6591. doi: 10.4049/jimmunol.0903109. [DOI] [PubMed] [Google Scholar]
- Koch SD, Uss E, van Lier RA, ten Berge IJ. Alloantigen-induced regulatory CD8+CD103+ T cells. Hum Immunol. 2008;69:737–744. doi: 10.1016/j.humimm.2008.08.281. [DOI] [PubMed] [Google Scholar]
- Lu L, Yu Y, Li G, Pu L, Zhang F, Zheng S, et al. CD8+CD103+ regulatory T cells in spontaneous tolerance of liver allografts. Int Immunopharmacol. 2009;9:546–548. doi: 10.1016/j.intimp.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Rifa'i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200:1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endharti AT, Rifa IM, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- Chen X, Priatel JJ, Chow MT, Teh HS. Preferential development of CD4 and CD8 T regulatory cells in RasGRP1-deficient mice. J Immunol. 2008;180:5973–5982. doi: 10.4049/jimmunol.180.9.5973. [DOI] [PubMed] [Google Scholar]
- Shi Z, Rifa'i M, Lee YH, Shiku H, Isobe K, Suzuki H. Importance of CD80/CD86−CD28 interactions in the recognition of target cells by CD8+CD122+ regulatory T cells. Immunology. 2008;124:121–128. doi: 10.1111/j.1365-2567.2007.02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy MJ, Zhang W, Usherwood EJ. Suppressive CD8+ T cells arise in the absence of CD4 help and compromise control of persistent virus. J Immunol. 2011;186:6218–6226. doi: 10.4049/jimmunol.1003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endharti AT, Okuno Y, Shi Z, Misawa N, Toyokuni S, Ito M, et al. CD8+CD122+ regulatory T cells (Tregs) and CD4+ Tregs cooperatively prevent and cure CD4+ cell-induced colitis. J Immunol. 2011;186:41–52. doi: 10.4049/jimmunol.1000800. [DOI] [PubMed] [Google Scholar]
- Wang LX, Li Y, Yang G, Pang PY, Haley D, Walker EB, et al. CD122+CD8+ Treg suppress vaccine-induced antitumor immune responses in lymphodepleted mice. Eur J Immunol. 2010;40:1375–1385. doi: 10.1002/eji.200839210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Wang X, Radfar S, Sproule TJ, Roopenian DC, Cantor H. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc Natl Acad Sci USA. 2011;108:2010–2015. doi: 10.1073/pnas.1018974108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalam AK, Luckey D, Giri S, Smart M, Pease LR, Rodriguez M, et al. Two discreet subsets of CD8 T cells modulate PLP(91–110) induced experimental autoimmune encephalomyelitis in HLA-DR3 transgenic mice. J Autoimmun. 2012;38:344–353. doi: 10.1016/j.jaut.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Wan N, Zhang S, Moore Y, Wan F, Dai Z. Cutting edge: programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J Immunol. 2010;185:803–807. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunological self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25)-breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Dai Z, Zhang S, Xie Q, Wu S, Su J, Li S, et al. Natural CD8+CD122+ T cells are more potent in suppression of allograft rejection than CD4+CD25+ regulatory T cells. Am J Transplant. 2014;14:39–48. doi: 10.1111/ajt.12515. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Shi Z, Okuno Y, Isobe K. Are CD8+CD122+ cells regulatory T cells or memory T cells. Hum Immunol. 2008;69:751–754. doi: 10.1016/j.humimm.2008.08.285. [DOI] [PubMed] [Google Scholar]
- Lerret NM, Houlihan JL, Kheradmand T, Pothoven KL, Zhang ZJ, Luo X. Donor-specific CD8+ Foxp3+ T cells protect skin allografts and facilitate induction of conventional CD4+ Foxp3+ regulatory T cells. Am J Transplant. 2012;12:2335–2347. doi: 10.1111/j.1600-6143.2012.04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Okuno Y, Rifa'i M, Endharti AT, Akane K, Isobe K, et al. Human CD8+CXCR3+ T cells have the same function as murine CD8+CD122+ Treg. Eur J Immunol. 2009;39:2106–2119. doi: 10.1002/eji.200939314. [DOI] [PubMed] [Google Scholar]
- Slutter B, Pewe LL, Kaech SM, Harty JT. Lung airway-surveilling CXCR3hi memory CD8+ T cells are critical for protection against influenza A virus. Immunity. 2013;39:939–948. doi: 10.1016/j.immuni.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Peacock CD, Bahl K, Welsh RM. Programmed death-1 (PD-1) defines a transient and dysfunctional oligoclonal T cell population in acute homeostatic proliferation. J Exp Med. 2007;204:2321–2333. doi: 10.1084/jem.20062150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan N, Dai H, Wang T, Moore Y, Zheng XX, Dai Z. Bystander central memory but not effector memory CD8+ T cells suppress allograft rejection. J Immunol. 2008;180:113–121. doi: 10.4049/jimmunol.180.1.113. [DOI] [PubMed] [Google Scholar]
- Okuno Y, Murakoshi A, Negita M, Akane K, Kojima S, Suzuki H. CD8+ CD122+ regulatory T cells contain clonally expanded cells with identical CDR3 sequences of the T-cell receptor beta-chain. Immunology. 2013;139:309–317. doi: 10.1111/imm.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Ishida Y, Rifa'i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825–832. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- Zozulya AL, Ortler S, Fabry Z, Sandor M, Wiendl H. The level of B7 homologue 1 expression on brain DC is decisive for CD8 Treg cell recruitment into the CNS during EAE. Eur J Immunol. 2009;39:1536–1543. doi: 10.1002/eji.200839165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahri R, Bollinger A, Bollinger T, Orinska Z, Bulfone-Paus S. Ectonucleotidase CD38 demarcates regulatory, memory-like CD8+ T cells with IFN-gamma-mediated suppressor activities. PLoS ONE. 2012;7:e45234. doi: 10.1371/journal.pone.0045234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh O, Abiru N, Nakahara M, Nagayama Y. CD8+CD122+ T cells, a newly identified regulatory T subset, negatively regulate Graves' hyperthyroidism in a murine model. Endocrinology. 2007;148:6040–6046. doi: 10.1210/en.2007-0300. [DOI] [PubMed] [Google Scholar]
- Yu P, Steel JC, Zhang M, Morris JC, Waitz R, Fasso M, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci USA. 2012;109:6187–6192. doi: 10.1073/pnas.1203479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifa'i M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe K, et al. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008;20:937–947. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Shameli A, Yamanouchi J, Tsai S, Yang Y, Clemente-Casares X, Moore A, et al. IL-2 promotes the function of memory-like autoregulatory CD8+ T cells but suppresses their development via FoxP3+ Treg cells. Eur J Immunol. 2013;43:394–403. doi: 10.1002/eji.201242845. [DOI] [PubMed] [Google Scholar]