Abstract
VEGF165, the most abundant isoform in man, is an angiogenic cytokine that also regulates vascular permeability. Its function in the renal glomerulus, where it is expressed in visceral epithelial and mesangial cells, is unknown. To assess the role of VEGF165 in glomerular disease, we administered a novel antagonist — a high-affinity, nuclease-resistant RNA aptamer coupled to 40-kDa polyethylene glycol (PEG) — to normal rats and to rats with mesangioproliferative nephritis, passive Heymann nephritis (PHN), or puromycin aminonucleoside nephrosis (PAN). In normal rats, antagonism of VEGF165 for 21 days failed to induce glomerular pathology or proteinuria. In rats with mesangioproliferative nephritis, the VEGF165 aptamer (but not a sequence-scrambled control RNA or PEG alone) led to a reduction of glomerular endothelial regeneration and an increase in endothelial cell death, provoking an 8-fold increase in the frequency of glomerular microaneurysms by day 6. In contrast, early leukocyte influx and the proliferation, activation, and matrix accumulation of mesangial cells were not affected in these rats. In rats with PHN or PAN, administration of the VEGF165 aptamer did not influence the course of proteinuria using various dosages and administration routes. These data identify VEGF165 as a factor of central importance for endothelial cell survival and repair in glomerular disease, and point to a potentially novel way to influence the course of glomerular diseases characterized by endothelial cell damage, such as various glomerulonephritides, thrombotic microangiopathies, or renal transplant rejection.
Introduction
The renal glomerulus is a unique capillary structure within the body. Intracapillary pressures range from 35 to 90 mmHg. It contains a highly fenestrated endothelium without pore diaphragms. The outer support structure of the capillary consists of a single cell layer — the podocytes. Early glomerular endothelial injury is a feature of many human diseases, including preeclampsia, hemolytic uremic syndrome, lupus nephritis, most types of vasculitides, many glomerulonephritides, as well as renal transplant rejection (1). Glomerular endothelial damage also characterizes a variety of conditions associated with glomerular hypertension and hyperperfusion (2). Importantly, it has been shown that after subtotal (five-sixths) nephrectomy in the rat (a model commonly used to examine processes that govern progression of renal disease), one of the first discernible pathological events is capillary endothelial damage (3). Consequently, knowledge about factors that maintain the integrity of the glomerular capillary wall may be of central importance in understanding the pathophysiology of progressive renal disease.
VEGF (also known as vascular permeability factor) is a growth factor with significant roles in angiogenesis, tumor growth, development, and potentially in atherosclerosis (4–7). It is a dimeric protein composed of 121–, 165–, 189–, or 206–amino acid subunits (8). In rodents, the subunits are 1 amino acid shorter (i.e., VEGF120, VEGF164, and VEGF188; ref. 9), but we will refer to these as the more widely known human equivalents throughout this paper. Whereas VEGF121 and VEGF165 are soluble secreted forms, VEGF189 and VEGF206 are mostly bound to the cell surface or to the extracellular matrix (8). Two VEGF receptors have been identified: flt-1 and KDR/flk-1 (10).
In normal human and rat kidney, VEGF expression is confined to podocytes, distal duct epithelia, and collecting-duct epithelia (11–18). The main VEGF isoform expressed by podocytes is VEGF165 (19, 20), which is similar to observations in many other cell types (21). VEGF synthesis (isoforms 121, 165, and 189) has also been demonstrated in activated mesangial cells in vitro and in vivo during human and experimental mesangioproliferative nephritis (12, 16, 22–24). In normal human kidney, expression of KDR and flt-1 mRNA, and binding of 125I-VEGF165, have been localized to glomerular and peritubular capillaries and to pre- and postglomerular vessels (11, 15, 18, 25).
Despite the extensive descriptive information on the expression of VEGF and VEGF receptors in glomerular cells, there is at present no data on the physiological or pathophysiological roles of glomerular VEGF in vivo. Extrapolations based on findings in other organs or other parts of the vasculature are difficult, given the unique features of glomerular capillaries (see above). In this study, we have attempted to gain insight into the physiological and pathophysiological roles of glomerular VEGF using a recently developed oligonucleotide-based antagonist with specificity for the VEGF165 isoform (26). To examine the role of VEGF165 in different glomerular conditions, we have treated several groups of rats with the VEGF165 antagonist. Besides normal rats, treatment groups included rats with immune-mediated mesangial and secondary glomerular endothelial injury (mesangioproliferative anti–Thy 1.1 nephritis), rats with immune-mediated podocyte injury (passive Heymann nephritis [PHN]), and rats with toxic podocyte injury (puromycin aminonucleoside nephrosis [PAN]).
Methods
Aptamer-based antagonist against VEGF165.
A nuclease-resistant, 2′-fluoropyrimidine RNA oligonucleotide aptamer to human VEGF165 was identified using systematic evolution of ligands by exponential enrichment (SELEX) as described previously (26). To further improve nuclease resistance, all but 2 purine nucleotides were substituted with 2′-O-methyl nucleotides to yield aptamer t44-OMe (26). The aptamer was further modified with 40-kDa polyethylene glycol (PEG) to increase its residence time in plasma. The 2′-O-methyl–substituted, 2′-fluoropyrimidine aptamer, conjugated to 40-kDa PEG (also known as NX1838), exhibited a half-life in rat plasma of 6–7 hours. Intravenous and intraperitoneal routes of administration yielded virtually superimposable plasma levels of the aptamer as early as 1 hour after bolus injections (Stanley C. Gill, unpublished results). The VEGF165 aptamer exhibited similar affinity to murine VEGF164 and human VEGF165, but did not recognize VEGF121 or the smaller isoform of placenta growth factor (PIGF129), which exhibits considerable sequence homology to VEGF (26). Although recombinant rat VEGF164 was not available for testing, it is highly likely that the aptamer also binds rat VEGF164 with high affinity, because it is 91% homologous to murine VEGF164 and 96% homologous to human VEGF165 (27). Furthermore, the exon 7–encoded domain, which is critical for aptamer binding, is completely conserved between rat and human proteins. As a control, we used the sequence-scrambled analogue of the VEGF165 aptamer conjugated to 40-kDa PEG (26).
In pilot studies, we determined the pharmacokinetic properties of the VEGF165 aptamer after intravenous or intraperitoneal bolus injection of 1 mg/kg body weight into 9 normal rats each. The data showed that similar plasma levels were achieved between 1 and 24 hours after injection for the 2 routes of administration (data not shown).
Rat corneal pocket angiogenesis assay.
Aptamer and scrambled control aptamer conjugated to 40-kDa PEG were tested for their ability to reduce VEGF165-induced corneal angiogenesis in the normally avascular rat cornea. Briefly, biopolymer (Hydron) pellets with or without recombinant human VEGF165 protein (3 pmol; R&D Systems Inc., Minneapolis, Minnesota, USA) were prepared approximately 18 hours before use by adding the protein or carrier solution to 12% biopolymer in 95% ethanol. Adult Sprague-Dawley rats (200–240 g) were anesthetized by intraperitoneal injection of ketamine hydrochloride (50 mg/kg) and xylazine (10 mg/kg). The left eye was prepared by topical administration of tetracaine hydrochloride (1%) for local anesthesia, followed by application of dilute povidone-iodine solution and subsequent rinsing with isotonic saline solution. A vertical partial-thickness incision was made in the midcornea. A midstromal pocket was dissected caudally toward the lateral canthus, extending to within 1.5 mm of the limbus. A pellet was then inserted into and pushed to the caudal limit of the pocket. Residual air was gently massaged out of the pocket, and a drop of chloramphenicol ophthalmic solution was applied to the eye. The animal was rolled over and the procedure was repeated on the right eye. Upon completion of pellet insertion in each eye, each animal was intravenously administered either PBS (volume matched to aptamer-formulation group) or aptamer (1–10 mg/kg), twice daily as indicated (2–20 mg/kg per day). After 5 days, each animal was anesthetized, and images were obtained with a color video camera (Hitachi, Ltd. Tokyo, Japan) mounted on a dissecting microscope (Leica Microsystems,Wetzler, Germany). Each eye was evaluated for the angiogenic response by measuring the maximum length of vessel growth (0–5; 0 = no vessel growth, 5 = vessels covering the pellet), the density of vessel growth (0–4; 0 = no vessels, 4 = maximal density) adjacent to the implanted pellet, and the circumference of the eye (where angiogenesis was occurring) (0–1; 0 = 0° involvement, 0.5 = 180° involvement, 1 = 360° involvement). An angiogenic index was then determined as the product of length × density × circumference.
Animal treatment groups.
All animal studies were approved by the Institutional Review Boards of both NeXstar Pharmaceuticals and Hannover Medical School.
Normal rats.
Six male Wistar rats (Charles River Wiga GmbH, Sulzfeld, Germany), weighing 140–150 g at the start of the experiments, received twice-daily intravenous bolus injections of 5 mg/kg per day of the VEGF165 aptamer (coupled to 22 mg PEG; n = 3) or an equivalent amount of PEG alone (n = 3) for 21 days. The rats were sacrificed 4 hours after an intraperitoneal injection of the thymidine analogue 5-bromo-2′-deoxyuridine (BrdU; 100 mg/kg; Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany). After sacrifice, both kidneys were quickly removed and a longitudinal section (3–4 mm thick) of the left kidney was obtained for immunohistology. Renal tissue was studied to assess overall morphology, cell proliferation, and ultrastructure of glomeruli.
Mesangioproliferative anti–Thy 1.1 nephritis.
Anti–Thy 1.1 nephritis was induced in 22 male Wistar rats (Charles River Wiga GmbH; 160–180 g at the start of the experiment) as described (28). Every 12 hours, rats received intravenous bolus injections of 2.5 mg/kg (5 mg/kg per day) VEGF165 aptamer (coupled to 22 mg PEG; n = 6), scrambled control aptamer coupled to PEG (n = 5), an equivalent amount of PEG alone (n = 6), or an equivalent amount of PBS (n = 5) on days 1–5 after disease induction. Renal biopsies for histological evaluation were obtained by intravital biopsy on day 2 and post mortem on day 6 after disease induction. Systolic arterial pressure was measured on day 4 after disease induction. Twenty-four-hour urine collections were performed from day 1 to 2 and from day 5 to 6 after disease induction. BrdU was injected intraperitoneally at 36 hours and again at 40 hours after disease induction.
Six additional rats with nephritis were treated with VEGF165 aptamer (n = 3) or PEG alone (n = 3) on days 1–5, and were then followed until day 33.
PHN.
PHN was induced in 35 male Sprague-Dawley rats (Charles River Wiga GmbH; weighing 230–240 g at the start of the experiment), by intravenous injection of 0.8 mL sheep anti-Fx1a antibody per rat (29). Rats were treated on days 1–7 after disease induction with twice-daily intraperitoneal injections of 2 mg/kg per day (n = 5) or 5 mg/kg per day (n = 5) VEGF165 aptamer (coupled to 9 and 22 mg PEG, respectively), 2 mg/kg per day (n = 6) or 5 mg/kg per day (n = 6) of scrambled control aptamer (coupled to PEG), 22 mg PEG alone (n = 5), or PBS (n = 8). Twenty-four-hour urine collections were obtained on days 3, 5, and 7 after disease induction. Renal biopsies for histological evaluation were obtained by intravital biopsy on day 4 and post mortem on day 8.
PAN.
PAN was induced in 6 male Sprague-Dawley rats (Charles River Wiga GmbH; weighing 210–230 g) by intravenous injection of 150 mg/kg puromycin (Sigma-Aldrich Chemie GmbH) dissolved in normal saline. The animals were treated on days 2–6 after disease induction with twice-daily intraperitoneal injections of 2 mg/kg per day (n = 2) or 10 mg/kg per day (n = 2) VEGF165 aptamer (coupled to 9 mg or 44 mg PEG, respectively) or 44 mg PEG alone (n = 2). Twenty-four-hour urine collections were obtained on days 3 and 6. Renal biopsies were obtained after sacrifice on day 7.
Renal morphology.
Tissue for light microscopy and immunoperoxidase staining was fixed in methyl Carnoy’s solution and embedded in paraffin. In periodic acid-Schiff–stained (PAS-stained) sections, the number of mitoses and polymorphonuclear leukocytes within 50–100 glomerular tufts was determined. Using 1,000-fold magnification, mitoses were differentiated into those that clearly localized to the edge of a glomerular capillary lumen and were inside of the glomerular basement membrane (hereafter referred to as proliferating endothelial cells), and mitoses in any other location within the glomerular tuft. Mesangiolysis was graded on a semiquantitative scale: 0 = no mesangiolysis, 1 = segmental mesangiolysis, 2 = global mesangiolysis, 3 = microaneurysm. Focal segmental glomerulosclerosis and tubulointerstitial injury were evaluated as described previously (30).
Immunoperoxidase staining.
Four-micrometer sections of methyl Carnoy’s–fixed biopsy tissue were processed as described previously (28). Primary antibodies were identical to those described (28, 30, 31). In addition, a murine IgM mAb (clone F7-26) to single-stranded DNA (Alexis Corp., San Diego, California, USA) was used to detect cells in the early stages of apoptosis (32). All slides were evaluated by a blinded observer.
To obtain mean numbers of proliferating cells, apoptotic cells, and infiltrating monocytes/macrophages in glomeruli, 30–100 consecutive cross-sections of glomeruli were evaluated and mean values per kidney were calculated. Proliferating and apoptotic cells were differentiated into endothelial and nonendothelial cells as described above. For the evaluation of the immunoperoxidase stains for α-smooth muscle actin, PDGF B-chain, collagen IV, and fibronectin, each glomerular area was graded semiquantitatively, and the mean score per biopsy was calculated as described (28).
Immunofluorescence staining.
Immunofluorescence detection of glomerular mouse IgG and complement C3c was carried out on 4-μm sections of frozen kidney tissue using a direct immunofluorescence procedure. Antibodies included a biotinylated IgG fraction of polyclonal horse anti-mouse IgG (Vector Laboratories Ltd., Peterborough, United Kingdom) and a biotinylated IgG fraction of polyclonal rabbit anti-human C3c (DAKO A/S, Glostrup, Denmark). Biotin was detected with streptavidin-FITC (Amersham Buchler GmbH, Braunschweig, Germany). In control sections, the primary antibody was substituted with equivalent concentrations of a biotinylated or nonbiotinylated irrelevant murine mAb or normal rabbit or goat IgG. Glomerular cross-sections were graded semiquantitatively as either 0 (indistinguishable from control) or positive according to the degree of glomerular fluorescence (0.5 = trace, 1 = weak, 2 = moderate, 3 = intense), and a mean score per biopsy was calculated.
Immunohistochemical double staining.
Double immunostaining for identification of the type of proliferating cells was performed as reported previously (28). First, sections were stained for proliferating cells with the BrdU antibody (28) using an immunoperoxidase procedure. Sections were then incubated with the IgG1 mAb ED-1 against monocytes/macrophages or the mAb clone 3 against endothelial nitric oxide synthase (ecNOS; Dianova, Hamburg, Germany) using an immunoalkaline phosphatase procedure. Cells were identified as proliferating monocytes/macrophages if they showed positive nuclear staining for BrdU and if the nucleus was completely surrounded by cytoplasm positive for the ED-1 antigen. Endothelial cells were identified by positive nuclear staining for BrdU and a nucleus completely surrounded by cytoplasm positive for ecNOS. Negative controls included omission of either of the primary antibodies, in which case no double staining was noted.
TUNEL staining for the detection of renal cell death.
In situ detection of cell death was performed using TUNEL (terminal deoxynucleotidyl transferase–mediated dUTP nick end-labeling) as described (33).
In situ detection of glomerular oxygen-radical production.
Glomerular in situ production of hydrogen peroxide was determined as described elsewhere (34).
Electron microscopy.
Blocks of renal tissue (1 mm3) were fixed in a solution of 2% formaldehyde and 2.5% glutaraldehyde according to Karnovsky’s method, with cacodylate buffer (0.2 M, pH 7.4). After 48 hours of fixation, the samples were embedded in araldite and processed for transmission electron microscopy by standard procedures.
To quantify glomerular endothelial fenestrations, the number of fenestrae was counted in 5–15 different capillary loops of 1–3 glomeruli per biopsy. This corresponded to an evaluated total capillary length of 12–21 μm per animal.
Miscellaneous measurements.
Urinary protein was measured using the Bio-Rad Protein Assay (Bio-Rad Laboratories GmbH, Munich, Germany) and BSA (Sigma-Aldrich Chemie GmbH) as a standard.
Statistical analysis.
All values are expressed as mean ± SD unless otherwise noted. Statistical significance (defined as P < 0.05) was evaluated using Student t tests or Mann-Whitney U tests where appropriate.
Results
Effect of the VEGF165 aptamer on VEGF-induced corneal angiogenesis.
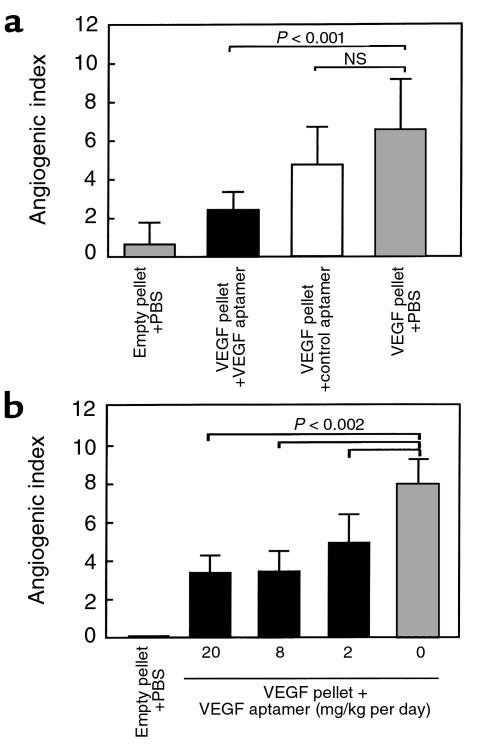
The ability of the VEGF165 aptamer or the scrambled control to block VEGF165-induced angiogenesis in the rat was tested in the corneal micropocket angiogenesis assay (Figure 1). In the first set of experiments, the aptamer and the scrambled control were delivered by intravenous injections twice daily at the dose of 20 mg/kg per day. Aptamer reduced the angiogenic response by about 60%, whereas the scrambled control aptamer did not have a statistically significant effect (Figure 1a). In the second set of experiments, the effect of the aptamer at doses of 20, 6, and 2 mg/kg per day was tested (Figure 1b). Comparable inhibitory effects were observed with the 20 and 6 mg/kg per day doses; a somewhat lower effect (about 40% inhibition) resulted from the 2 mg/kg per day dose.
Figure 1.
Evaluation of VEGF165 aptamer attenuation of VEGF165-induced corneal angiogenesis. Hydron pellets with or without VEGF165 (3 pmol) were implanted in the corneal stroma of rats. Animals were treated intravenously twice daily with either PBS, aptamer, or the control aptamer (20 mg/kg per day) (a) or with the indicated doses of aptamer (b). Corneal neovascularization index was derived from estimates of the length and density of new vessels and the circumference of the neovascular response. Each group contained 8 eyes.
Effects of the VEGF165 aptamer in normal rats.
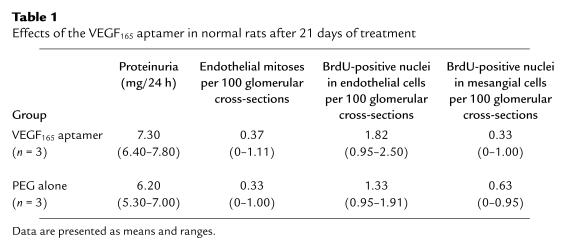
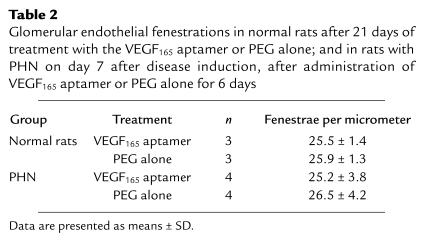
Kidneys from rats receiving the VEGF165 aptamer and those receiving PEG alone for 21 days exhibited a normal renal — and in particular glomerular — morphology on day 21 (data not shown). No pathological proteinuria developed in any of the 6 rats (Table 1). To specifically assess the effect of the aptamer on glomerular cell proliferation, mitotic figures were counted in PAS-stained sections of kidneys. Alternatively, nuclei incorporating the thymidine analogue BrdU were evaluated. In both instances, no differences were detected between the 2 groups (Table 1). By electron microscopy, no pathological findings were noted in either group, and the number of glomerular endothelial fenestrations was not affected by treatment with the VEGF165 aptamer (Table 2).
Table 1.
Effects of the VEGF165 aptamer in normal rats after 21 days of treatment
Table 2.
Glomerular endothelial fenestrations in normal rats after 21 days of treatment with the VEGF165 aptamer or PEG alone; and in rats with PHN on day 7 after disease induction, after administration of VEGF165 aptamer or PEG alone for 6 days
Effects of the VEGF165 aptamer in rats with mesangioproliferative anti–Thy 1.1 nephritis
Effects on glomerular endothelial cell turnover.
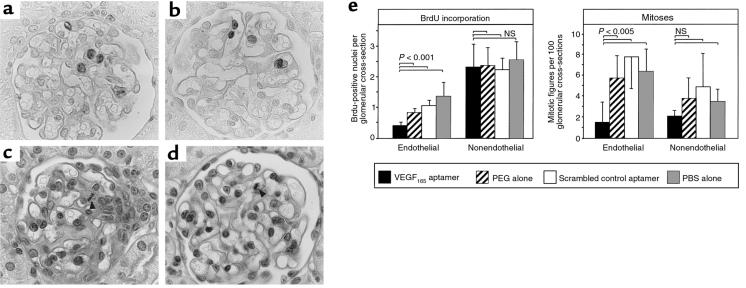
Administration of the VEGF165 aptamer led to a 50% reduction of glomerular endothelial cell proliferation (as determined by nuclear BrdU incorporation) at 48 hours, compared with nephritic rats receiving PEG alone (Figure 2, a, b, and e). Similarly, mitotic figures in glomerular endothelial cells were reduced by 74% at 48 hours after disease induction compared with rats receiving PEG alone (Figure 2, c–e). In contrast, proliferation of nonendothelial cells within the glomerular tuft at 48 hours did not differ significantly between rats receiving the VEGF165 aptamer or PEG alone (Figure 2, a–e). Differences between the relative contribution of nonendothelial cells in the evaluation of glomerular mitotic figures and BrdU-positive nuclei (Figure 2e) probably relate to the fact that BrdU labeled the time period from 36 to 48 hours after disease induction (see Methods), whereas mitotic figures reflected the status at 48 hours only. In additional experiments, proliferating glomerular endothelial cells were also identified by double immunostaining for BrdU and ecNOS. This again confirmed a significant reduction of endothelial cell proliferation in rats receiving the VEGF165 aptamer (0.21 ± 0.12 BrdU+/ecNOS+ cells per glomerulus vs. 0.54 ± 0.18 such cells in rats receiving scrambled aptamer; P < 0.05).
Figure 2.
Glomerular cell proliferation at 48 hours after induction of anti–Thy 1.1 nephritis in rats receiving the VEGF165 aptamer (n = 6), PEG alone (n = 6), scrambled VEGF165 aptamer (n = 5), or PBS alone (n = 5). (a) BrdU incorporation in a rat receiving VEGF165 aptamer. Labeled cells are mostly localized to the mesangium (cluster of 3 cells in the center of the glomerulus); only 1 glomerular endothelial cell (top edge of the glomerulus) is labeled. ×1,000. (b) BrdU incorporation in a rat receiving PEG alone. Labeled cells are mostly endothelial cells. ×1,000. (c) PAS-stained renal section from a rat receiving VEGF165 aptamer. A mesangial cell mitosis is present (arrow). ×1,000. (d) PAS-stained renal section of a rat receiving PEG alone. An endothelial cell mitosis is present (arrow). ×1,000. (e) Quantitative evaluation of endothelial vs. nonendothelial glomerular cell proliferation (as defined by counts of mitotic figures or nuclei incorporating BrdU). NS, not significant.
To rule out the possibility that the above observations represented nonspecific effects of oligonucleotides or that PEG influenced the course of the disease, rats with anti–Thy 1.1 nephritis were also treated with scrambled control aptamer or PBS alone. As shown in Figure 2e, under these circumstances we again failed to note any reduction of glomerular endothelial proliferation similar to that found in rats treated with the VEGF165 aptamer.
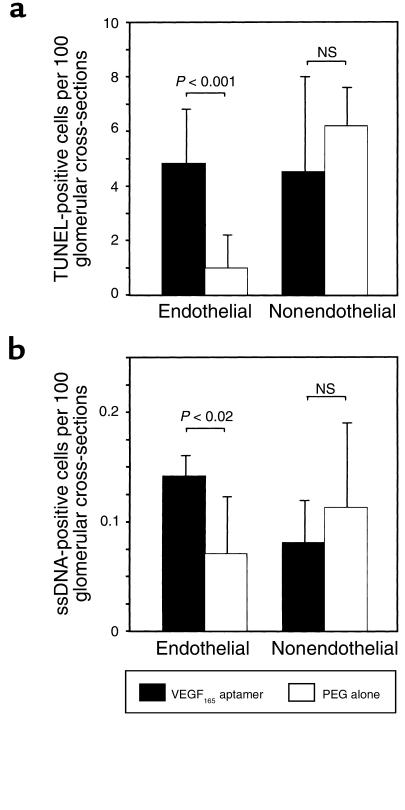
Glomerular cell death rates on day 2, as detected by TUNEL, increased 4.8-fold in endothelial cells of rats receiving the VEGF165 aptamer compared with those receiving PEG alone (Figure 3). In contrast, cell death frequencies in glomerular nonendothelial cells were not affected by the aptamer (Figure 3). Immunostaining with an antibody to single-stranded DNA, a more specific marker of apoptosis (32), confirmed a significant increase in apoptotic endothelial cells in rats receiving the VEGF165 aptamer (Figure 3).
Figure 3.
Glomerular cell death and apoptosis detected by (a) TUNEL staining and (b) staining for nuclei containing single-stranded DNA (ssDNA) at 48 hours after induction of anti–Thy 1.1 nephritis in rats receiving the VEGF165 aptamer (n = 6) or PEG alone (n = 6). TUNEL-stained and ssDNA-positive cells were separated into those that localized to endothelial cell layers and others.
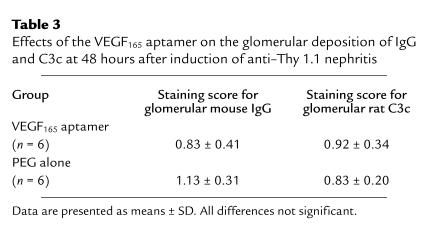
Treatment with the VEGF165 aptamer was started 24 hours after injection of the anti–Thy 1.1 antibody, i.e., after maximal binding of the antibody to mesangial cells had occurred (35, 36). It therefore appears unlikely that the observed effects resulted from direct effects of the aptamer on the induction of disease. Furthermore, at 48 hours, glomerular deposition of IgG and C3c was indistinguishable among rats receiving the aptamer or PEG alone (Table 3). Early glomerular monocyte/macrophage influx was also unaffected by the aptamer (see below).
Table 3.
Effects of the VEGF165 aptamer on the glomerular deposition of IgG and C3c at 48 hours after induction of anti–Thy 1.1 nephritis
Effects on glomerular capillary repair.
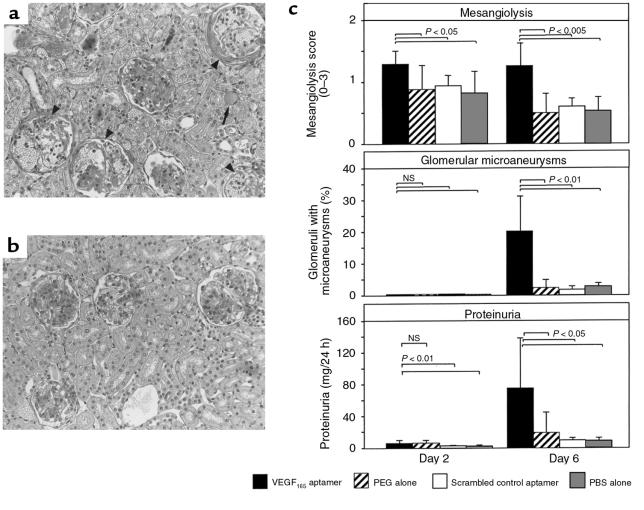
In parallel to the reduction of endothelial repair, mesangiolytic changes were more pronounced on day 2 (i.e., at 48 hours) and on day 6 after disease induction in VEGF165 aptamer–treated rats compared with rats receiving PEG alone (Figure 4, a–c). More importantly, treatment with the VEGF165 aptamer led to a 7.9-fold increase in the frequency of glomerular microaneurysms on day 6 compared with rats receiving PEG alone (Figure 4, a–c). This augmentation of glomerular capillary damage was associated with a 3.9-fold increase in proteinuria on day 6 (Figure 4c). Two additional groups of control rats, which were treated with either the scrambled VEGF165 aptamer or PBS alone, were indistinguishable from the group receiving PEG alone (Figure 4c).
Figure 4.
Mesangiolysis, glomerular microaneurysms, and proteinuria on days 2 and 6 after induction of anti–Thy 1.1 nephritis in rats receiving the VEGF165 aptamer (n = 6), PEG alone (n = 6), scrambled VEGF165 aptamer (n = 5), or PBS alone (n = 5). (a) Overview of histological changes in a rat receiving the VEGF165 aptamer on day 6 after disease induction. Four of 6 glomeruli show marked mesangiolysis, capillary dilation, and microaneurysm formation (arrowheads). A protein cast is present in 1 tubule (arrow). ×200. (b) Overview of histological changes in a rat receiving PEG alone on day 6 after disease induction. Marked mesangioproliferative changes are present in 2 glomeruli; the other 2 glomeruli exhibit milder degrees of mesangial expansion. No evidence of persistent mesangiolysis is present. ×200. (c) Semiquantitative evaluation of mesangiolysis, frequency of microaneurysms, and proteinuria.
We also determined that the effects of the VEGF165 aptamer were not caused by changes in systemic hemodynamics. No effect of the VEGF165 aptamer on systolic blood pressure was noted on day 4 after disease induction (nephritic rats receiving PEG alone: 115 ± 8 mmHg; nephritic VEGF165 aptamer–treated rats: 117 ± 11 mmHg).
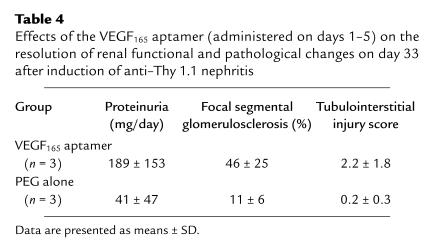
When nephritic rats were followed for 4 weeks after treatment with either the VEGF165 antagonist or PEG, a marked aggravation of chronic renal damage was noted in the VEGF165 group (Table 4).
Table 4.
Effects of the VEGF165 aptamer (administered on days 1–5) on the resolution of renal functional and pathological changes on day 33 after induction of anti–Thy 1.1 nephritis
Effects on glomerular mesangial cell activation, proliferation, and matrix accumulation.
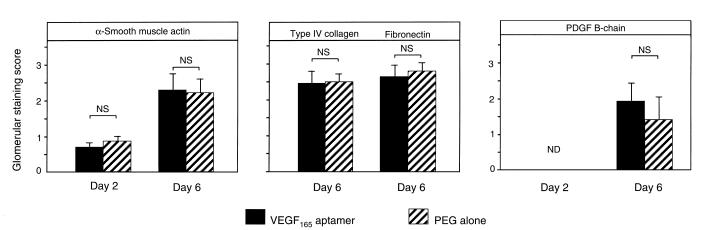
We next investigated whether antagonism of VEGF165 in anti–Thy 1.1 nephritis affected mesangial cell behavior. In addition to demonstrating that the aptamer did not influence the proliferation of nonendothelial cells at 48 hours, we analyzed the glomerular expression of α-smooth muscle actin and PDGF B-chain. Both are exclusively expressed in activated and proliferating mesangial cells during anti–Thy 1.1 nephritis (37–39). The data showed that the glomerular expression of α-smooth muscle actin and PDGF B-chain was not different between rats receiving the aptamer and those receiving PEG on days 2 and 6 after disease induction (Figure 5).
Figure 5.
Mesangial changes on days 2 and 6 after induction of anti–Thy 1.1 nephritis in rats receiving the VEGF165 aptamer (n = 6) or PEG alone (n = 6). Shown is a semiquantitative evaluation of the glomerular de novo expression of α-smooth muscle actin, PDGF B-chain, type IV collagen, and fibronectin.
Another consequence of mesangial cell activation in anti–Thy 1.1 nephritis is overproduction of extracellular-matrix proteins (40). Analysis of the glomerular immunostaining scores of type IV collagen and fibronectin again failed to demonstrate differences between rats receiving the VEGF165 aptamer and those receiving PEG alone (Figure 5).
Effects on glomerular leukocyte influx and activation.
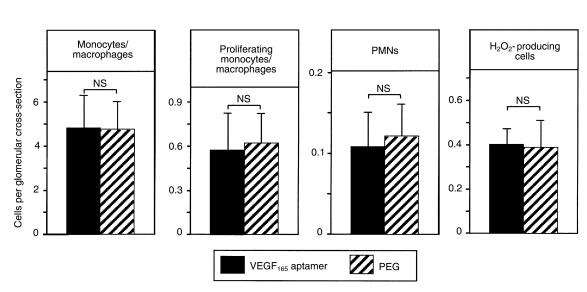
A third early event in anti–Thy 1.1 nephritis is an influx of granulocytes into glomeruli, followed by monocytes/macrophages (41). At 48 hours after disease induction (i.e., after 24 hours of treatment), we failed to detect a significant effect of the VEGF165 aptamer on numbers of polymorphonuclear cells, leukocytes producing hydrogen peroxide, or monocytes/macrophages in glomeruli (Figure 6). Furthermore, the intraglomerular proliferation of monocytes/macrophages (as determined by double immunostaining for the ED-1 antigen and BrdU) was not affected by treatment with the VEGF165 aptamer at 48 hours (Figure 6).
Figure 6.
Glomerular leukocyte influx on day 2 (48 hours) after induction of anti–Thy 1.1 nephritis in rats receiving the VEGF165 aptamer (n = 6) or PEG alone (n = 6). Shown is a quantitative evaluation of glomerular numbers of ED-1–positive monocytes/macrophages, proliferating ED-1–positive cells, polymorphonuclear neutrophils (PMNs), and hydrogen peroxide–producing leukocytes.
Effects of the VEGF165 aptamer in rats with PHN.
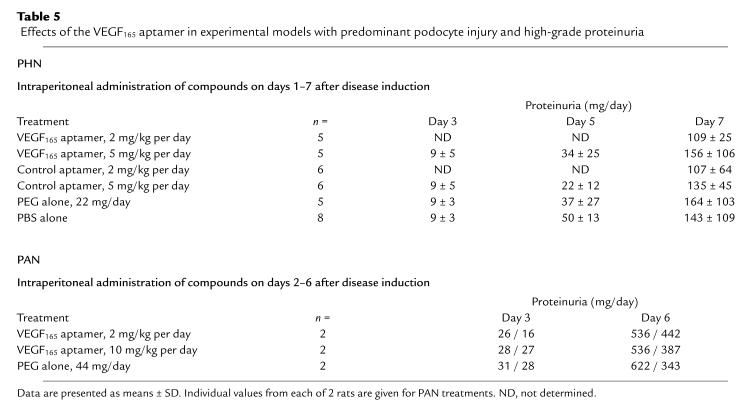
As shown in Table 5, intraperitoneal treatment of PHN rats with 2–5 mg/kg per day of the VEGF165 aptamer affected neither the onset nor the extent of proteinuria. Similar data were obtained in separate pilot experiments in which up to 10 mg/kg per day of the aptamer was given intraperitoneally, or up to 20 mg/kg per day was given intravenously (data not shown).
Table 5.
Effects of the VEGF165 aptamer in experimental models with predominant podocyte injury and high-grade proteinuria
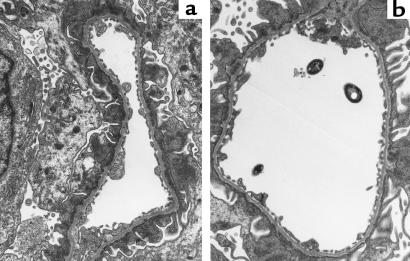
Glomerular light microscopic histology in all rats with PHN, regardless of the mode of treatment, was only remarkable for some mild podocyte swelling on days 7 and 8 of the disease (data not shown). By electron microscopy, the typical characteristics of PHN — foot-process fusion and extensive subepithelial immune deposits — were noted in all proteinuric animals examined (Figure 7). These changes were not affected by the mode of treatment. More importantly, neither control rats with PHN nor those receiving the VEGF165 aptamer exhibited alterations of the endothelial fenestrations (Table 2).
Figure 7.
Glomerular ultrastructure in rats with PHN on day 7 after disease induction in a rat receiving the VEGF165 aptamer (a) and a rat receiving PEG alone (b). Both rats exhibit subepithelial immune deposits and foot-process fusion to an equal degree. There is no difference in endothelial cell structure or the number of endothelial fenestrations. ×12,000.
Effects of the VEGF165 aptamer in rats with PAN.
Similar to the findings in rats with PHN, treatment with the VEGF165 aptamer failed to affect the onset or extent of proteinuria in rats with PAN, despite the fact that proteinuria in these rats was about 4-fold higher than in PHN rats (Table 5). By light microscopy, glomeruli of rats with PAN on day 7 after disease induction exhibited minor alterations, such as mild podocyte swelling, which were not affected by the aptamer. By electron microscopy, all animals showed foot-process fusion but normal endothelial fenestrations (data not shown).
Discussion
In this study, we first established that the VEGF165 aptamer effectively and specifically antagonized VEGF165 in vivo. In this respect, we demonstrated that the aptamer, when administered systemically to rats, dose dependently inhibited VEGF165-induced corneal angiogenesis. Specificity of the aptamer action can be inferred from our observation that a scrambled version of the aptamer did not significantly affect corneal angiogenesis. Furthermore, in vitro, the VEGF165 aptamer did not affect endothelial cell proliferation induced by fibroblast growth factor-2 (FGF-2) (N. Janjic, unpublished data). More importantly, it did not affect the proliferation induced by VEGF121 (42). The latter observation provides a strong argument against nonspecific actions of the VEGF165 aptamer on endothelial cells, because VEGF165 and VEGF121 share intracellular signaling pathways.
In normal glomeruli, endothelial cells account for about 80% of cell turnover (43). Because VEGF165 — the major isoform present in normal podocytes and activated mesangial cells (20, 24) — exerts mitogenic effects on glomerular endothelial cells in vitro (44), it was a prime candidate for the mediation of physiological endothelial cell proliferation. However, under our study conditions, we failed to demonstrate any adverse effects of the aptamer on glomerular morphology, and in particular on the normal rate of endothelial cell turnover.
Another important issue, in normal rats, was the question of whether antagonism of VEGF165 would affect the constitutive glomerular endothelial fenestrations. VEGF was originally discovered as a tumor-secreted protein that rendered vessels hyperpermeable to macromolecules (4). Intradermal injection of VEGF165 rapidly induced endothelial fenestrations and hyperpermeability in normal nonfenestrated capillaries and venules (45). In contrast, our data suggest that the constitutive fenestration of glomerular endothelium is not maintained by VEGF165.
In a second series of experiments, we investigated the effects of the VEGF165 antagonist in rats with anti–Thy 1.1 nephritis. In this model, immune-mediated mesangial cell damage is accompanied by secondary glomerular capillary ballooning, microaneurysm formation, and loss of endothelial cells (16). Subsequent glomerular repair includes enhanced endothelial cell proliferation as well as features of angiogenesis (16). The major finding of this study is that this proliferative endothelial response appears to be driven in large part by VEGF165. Treatment with the VEGF165 antagonist not only reduced glomerular endothelial cell proliferation by 50–74%, but also led to a dramatic amplification of capillary damage. The mechanisms by which the VEGF165 antagonist effected these changes included not only the lack of an endothelial cell mitogen but also an increase in glomerular endothelial cell death/apoptosis. Our data thereby provide the renal correlation of in vitro findings and observations in retinal and tumor vasculature that demonstrated that VEGF165 is a survival factor for endothelial cells (46–48).
A remarkable observation in the anti–Thy 1.1 nephritis experiments was the high selectivity of the effects induced by VEGF165 antagonism. In all previous intervention studies in the anti–Thy 1.1 model (e.g., with PDGF or FGF-2 antagonists, heparins, or C-natriuretic peptide), we had noted effects on almost all levels of glomerular pathology (30, 31, 41, 49, 50). In contrast, treatment with the VEGF165 antagonist in this study had no discernible effects on mesangial cell proliferation, activation (as assessed by the de novo expression of α-smooth muscle actin and PDGF B-chain), or matrix accumulation. In agreement with these observations, VEGF165 exerted no mitogenic effect in cultured rat mesangial cells (51). Antagonism of VEGF165 in our study also did not affect glomerular leukocyte counts (in particular monocyte/macrophage counts) or local proliferation of monocytes/macrophages. Therefore, chemotactic and activating effects of VEGF165 on monocytes that have been observed in vitro (52) do not appear to be relevant under our in vivo study conditions.
Could hemodynamic actions of VEGF165 have contributed to the observations in anti–Thy 1.1 nephritis? Klanke et al. showed that infusion of VEGF165 into a preconstricted, isolated, perfused kidney led to a transient, nitric oxide–mediated increase in renal blood flow of about 10% (53). Systemic bolus injections of 0.5–10 μg VEGF165 into rats induced transient hypotension (54). Therefore, inhibition of VEGF165 might result in systemic or intrarenal hypertension and thereby damage the kidney. However, in rats with anti–Thy 1.1 nephritis, we failed to demonstrate any effect of chronic VEGF165 antagonism on systemic blood pressure. Two observations also argue against effects of the aptamer on intraglomerular hemodynamics. First, exposure of rat mesangial cells in vitro to VEGF165 did not affect their nitric oxide production (51). Second, proteinuria in rats with PHN or PAN was not affected by the VEGF165 antagonist; this would be expected to increase in the presence of intraglomerular hypertension or hyperperfusion.
Iruela-Arispe et al. recently reported that FGF-2 mediates glomerular endothelial cell proliferation in the anti–Thy 1.1 nephritis model (16). In that study, injection of a neutralizing antibody to FGF-2 at 1 hour before disease induction led to a 38% reduction of glomerular endothelial cell proliferation on day 2. However, in light of a more recent study, in which we identified FGF-2 as an early mediator of cytotoxicity in anti–Thy 1.1 nephritis (41), it appears more likely that the effect of anti–FGF-2 treatment observed by Iruela-Arispe et al. was caused by diminution of glomerular damage than by any direct effects of FGF-2 on endothelial cell growth. In agreement with this interpretation, anti–FGF-2 treatment led to a marked reduction of microaneurysm formation in anti–Thy 1.1 nephritis (41). That result contrasts with our own study, in which VEGF165 antagonism substantially augmented capillary damage.
A last series of experiments was devoted to the question of whether VEGF165 modulates proteinuria in glomerular disease, given its effect of increasing permeability in other parts of the vasculature (4, 45). Although proteinuria was indeed increased in rats with anti–Thy 1.1 nephritis after treatment with the VEGF165 antagonist (probably as a consequence of increased capillary damage), we failed to observe any effect of the aptamer on proteinuria in 2 models with primary podocyte injury and high-grade proteinuria. The latter observation is consistent with data obtained in the isolated perfused kidney, in which Klanke et al. failed to demonstrate any direct effect of VEGF165 on glomerular protein permeability (53). However, it is important to remember that the aptamer used here is a specific inhibitor of VEGF165, leaving open the possibility that VEGF121 (or other isoforms not inhibited by the aptamer) may in part mediate the above effects.
In summary, our study identifies VEGF165 as a central and highly selective mediator of glomerular endothelial cell proliferation and survival in damaged but apparently not normal glomerular capillaries. These findings bear remarkable similarity to observations in tumors, where nascent endothelial tubes require VEGF for survival, but where this growth factor dependence is lost once forming vessels associate with mural cells (48, 55). Our observations are also consistent with those of Gerber et al., who have shown that VEGF is required (among other organ systems) for the development of glomerular capillaries in the early postnatal period in mice, but that VEGF ceases to be required in adulthood (56). It is relevant to note in this context that during development, VEGF120 is not sufficient for proper capillary ingrowth in renal glomeruli or the assembly of smooth muscle cells with endothelial cells (9). Our data do not confirm hypotheses that VEGF165 is involved in the maintenance of the fenestrated glomerular endothelial phenotype or in the pathogenesis of proteinuria. Our findings indicate that systemic inhibition of angiogenesis, in particular of VEGF165, should not affect the function of normal kidneys, but may be harmful in situations of ongoing glomerular endothelial repair. Therefore, systemic administration of VEGF165 may provide a novel therapeutic approach to glomerular diseases characterized by extensive endothelial damage, such as various glomerulonephritides, thrombotic microangiopathies, renal transplant rejection, or preeclampsia.
Acknowledgments
The technical help of Monika Kregeler, Yvonne Schönborn, and Ferdinand Bahlmann is gratefully acknowledged. This study was supported by the German Research Foundation (SFB 244/C12, SFB 265/C8, and a Heisenberg stipend to J. Floege).
Footnotes
Dwight D. Henninger’s present address is: Proctor & Gamble Pharmaceuticals, Mason, Ohio 45040, USA.
References
- 1.Ballerman, B.J. 1997. Endothelial responses to immune injury. In Immunologic renal diseases. E.G. Neilson and W.G. Couser, editors. Lippincott-Raven Publishers. Philadelphia, PA. 627–654.
- 2.Rennke HG. Glomerular adaptations to renal injury or ablation. Role of capillary hypertension in the pathogenesis of progressive glomerulosclerosis. Blood Purif. 1988;6:230–239. doi: 10.1159/000169549. [DOI] [PubMed] [Google Scholar]
- 3.Lee LK, Meyer TW, Pollock AS, Lovett DH. Endothelial cell injury initiates glomerular sclerosis in the rat remnant kidney. J Clin Invest. 1995;96:953–964. doi: 10.1172/JCI118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 5.Connolly DT. Vascular permeability factor: a unique regulator of blood vessel function. J Cell Biochem. 1991;47:219–223. doi: 10.1002/jcb.240470306. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Huynh-Do U, Daniel TO. Renal microvascular assembly and repair: power and promise of molecular definition. Kidney Int. 1998;53:826–835. doi: 10.1111/j.1523-1755.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 7.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991;47:211–218. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 10.Neufeld G, Tessler S, Gitay-Goren H, Cohen T, Levi BZ. Vascular endothelial growth factor and its receptors. Prog Growth Factor Res. 1994;5:89–97. doi: 10.1016/0955-2235(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 11.Simon M, et al. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268:F240–F250. doi: 10.1152/ajprenal.1995.268.2.F240. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi K, et al. Activated mesangial cells produce vascular permeability factor in early-stage mesangial proliferative glomerulonephritis. J Am Soc Nephrol. 1998;9:1815–1825. doi: 10.1681/ASN.V9101815. [DOI] [PubMed] [Google Scholar]
- 13.Monacci WT, Merrill MJ, Oldfield EH. Expression of vascular permeability factor/vascular endothelial growth factor in normal rat tissues. Am J Physiol. 1993;264:C995–C1002. doi: 10.1152/ajpcell.1993.264.4.C995. [DOI] [PubMed] [Google Scholar]
- 14.Brown LF, et al. Vascular permeability factor mRNA and protein expression in human kidney. Kidney Int. 1992;42:1457–1461. doi: 10.1038/ki.1992.441. [DOI] [PubMed] [Google Scholar]
- 15.Grone HJ, Simon M, Grone EF. Expression of vascular endothelial growth factor in renal vascular disease and renal allografts. J Pathol. 1995;177:259–267. doi: 10.1002/path.1711770308. [DOI] [PubMed] [Google Scholar]
- 16.Iruela-Arispe L, et al. Participation of glomerular endothelial cells in the capillary repair of glomerulonephritis. Am J Pathol. 1995;147:1715–1727. [PMC free article] [PubMed] [Google Scholar]
- 17.Shulman K, Rosen S, Tognazzi K, Manseau EJ, Brown LF. Expression of vascular permeability factor (VPF/VEGF) is altered in many glomerular diseases. J Am Soc Nephrol. 1996;7:661–666. doi: 10.1681/ASN.V75661. [DOI] [PubMed] [Google Scholar]
- 18.Brown LF, et al. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993;143:1255–1262. [PMC free article] [PubMed] [Google Scholar]
- 19.Schroppel B, Huber S, Horster M, Schlondorff D, Kretzler M. Analysis of mouse glomerular podocyte mRNA by single-cell reverse transcription-polymerase chain reaction. Kidney Int. 1998;53:119–124. doi: 10.1046/j.1523-1755.1998.00742.x. [DOI] [PubMed] [Google Scholar]
- 20.Kretzler M, et al. Detection of multiple vascular endothelial growth factor splice isoforms in single glomerular podocytes. Kidney Int Suppl. 1998;67:S159–S161. doi: 10.1046/j.1523-1755.1998.06733.x. [DOI] [PubMed] [Google Scholar]
- 21.Houck KA, et al. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, et al. Protein tyrosine kinases expressed in glomeruli and cultured glomerular cells: Flt-1 and VEGF expression in renal mesangial cells. Biochem Biophys Res Commun. 1995;209:218–226. doi: 10.1006/bbrc.1995.1492. [DOI] [PubMed] [Google Scholar]
- 23.Gruden G, et al. Mechanical stretch induces vascular permeability factor in human mesangial cells: mechanisms of signal transduction. Proc Natl Acad Sci USA. 1997;94:12112–12116. doi: 10.1073/pnas.94.22.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iijima K, Yoshikawa N, Connolly DT, Nakamura H. Human mesangial cells and peripheral blood mononuclear cells produce vascular permeability factor. Kidney Int. 1993;44:959–966. doi: 10.1038/ki.1993.337. [DOI] [PubMed] [Google Scholar]
- 25.Jakeman LB, Winer J, Bennett GL, Altar CA, Ferrara N. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J Clin Invest. 1992;89:244–253. doi: 10.1172/JCI115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruckman J, et al. 2’-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem. 1998;273:20556–20567. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- 27.Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 28.Floege J, et al. A novel approach to specific growth factor inhibition in vivo: antagonism of PDGF in glomerulonephritis by aptamers. Am J Pathol. 1999;154:169–179. doi: 10.1016/S0002-9440(10)65263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salant DJ, Darby C, Couser WG. Experimental membranous glomerulonephritis in rats. Quantitative studies of glomerular immune deposit formation in isolated glomeruli and whole animals. J Clin Invest. 1980;66:71–81. doi: 10.1172/JCI109837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burg M, Ostendorf T, Mooney A, Koch KM, Floege J. Treatment of experimental mesangioproliferative glomerulonephritis with non-anticoagulant heparin: therapeutic efficacy and safety. Lab Invest. 1997;76:505–516. [PubMed] [Google Scholar]
- 31.Canaan-Kuhl S, Ostendorf T, Zander K, Koch KM, Floege J. C-type natriuretic peptide inhibits mesangial cell proliferation and matrix accumulation in vivo. Kidney Int. 1998;53:1143–1151. doi: 10.1046/j.1523-1755.1998.00895.x. [DOI] [PubMed] [Google Scholar]
- 32.Frankfurt OS, Robb JA, Sugarbaker EV, Villa L. Monoclonal antibody to single-stranded DNA is a specific and sensitive cellular marker of apoptosis. Exp Cell Res. 1996;226:387–397. doi: 10.1006/excr.1996.0240. [DOI] [PubMed] [Google Scholar]
- 33.Baker AJ, et al. Mesangial cell apoptosis: the major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis. J Clin Invest. 1994;94:2105–2116. doi: 10.1172/JCI117565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poelstra K, Hardonk MJ, Koudstaal J, Bakker WW. Intraglomerular platelet aggregation and experimental glomerulonephritis. Kidney Int. 1990;37:1500–1508. doi: 10.1038/ki.1990.141. [DOI] [PubMed] [Google Scholar]
- 35.Johnson RJ, Garcia RL, Pritzl P, Alpers CE. Platelets mediate glomerular cell proliferation in immune complex nephritis induced by anti-mesangial cell antibodies in the rat. Am J Pathol. 1990;136:369–374. [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto T, Wilson CB. Quantitative and qualitative studies of antibody-induced mesangial cell damage in the rat. Kidney Int. 1987;32:514–525. doi: 10.1038/ki.1987.240. [DOI] [PubMed] [Google Scholar]
- 37.Johnson RJ, et al. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991;87:847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshimura A, et al. Demonstration of PDGF B-chain mRNA in glomeruli in mesangial proliferative nephritis by in situ hybridization. Kidney Int. 1991;40:470–476. doi: 10.1038/ki.1991.234. [DOI] [PubMed] [Google Scholar]
- 39.Iida H, et al. Platelet-derived growth factor (PDGF) and PDGF receptor are induced in mesangial proliferative nephritis in the rat. Proc Natl Acad Sci USA. 1991;88:6560–6564. doi: 10.1073/pnas.88.15.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Floege J, et al. Increased synthesis of extracellular matrix in mesangial proliferative nephritis. Kidney Int. 1991;40:477–488. doi: 10.1038/ki.1991.235. [DOI] [PubMed] [Google Scholar]
- 41.Floege J, et al. Endogenous fibroblast growth factor-2 mediates cytotoxicity in experimental mesangioproliferative glomerulonephritis. J Am Soc Nephrol. 1998;9:792–801. doi: 10.1681/ASN.V95792. [DOI] [PubMed] [Google Scholar]
- 42.Bell, C., Lynam, E., Landfair, D.J., Janjic, N., and Wiles, M.E. 1999. Oligonucleotide NX1838 inhibits VEGF165-mediated cellular responses in vitro. In Vitro Cell. Dev. Biol. In press. [DOI] [PubMed]
- 43.Pabst R, Sterzel RB. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 1983;24:626–631. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- 44.Uchida K, et al. Glomerular endothelial cells in culture express and secrete vascular endothelial growth factor. Am J Physiol. 1994;266:F81–F88. doi: 10.1152/ajprenal.1994.266.1.F81. [DOI] [PubMed] [Google Scholar]
- 45.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 46.Gerber HP, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 47.Alon T, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 48.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Floege J, Eng E, Young BA, Couser WG, Johnson RJ. Heparin suppresses mesangial cell proliferation and matrix expansion in experimental mesangioproliferative glomerulonephritis. Kidney Int. 1993;43:369–380. doi: 10.1038/ki.1993.55. [DOI] [PubMed] [Google Scholar]
- 50.Johnson RJ, et al. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992;175:1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trachtman H, Futterweit S, Franki N, Singhal PC. Effect of vascular endothelial growth factor on nitric oxide production by cultured rat mesangial cells. Biochem Biophys Res Commun. 1998;245:443–446. doi: 10.1006/bbrc.1998.8454. [DOI] [PubMed] [Google Scholar]
- 52.Clauss M, et al. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klanke B, et al. Effects of vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF) on haemodynamics and permselectivity of the isolated perfused rat kidney. Nephrol Dial Transplant. 1998;13:875–885. doi: 10.1093/ndt/13.4.875. [DOI] [PubMed] [Google Scholar]
- 54.Malavaud B, et al. Activation of Flk-1/KDR mediates angiogenesis but not hypotension. Cardiovasc Res. 1997;36:276–281. doi: 10.1016/s0008-6363(97)00177-6. [DOI] [PubMed] [Google Scholar]
- 55.Darland DC, D’Amore PA. Blood vessel maturation: vascular development comes of age. J Clin Invest. 1999;103:157–158. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerber HP, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]