Abstract
The autosomal recessive form of type I pseudohypoaldosteronism (PHA-I) is an inherited salt-losing syndrome resulting from diminution-of-function mutations in the 3 subunits of the epithelial Na+ channel (ENaC). A PHA-I stop mutation (αR508stop) of the ENaC α subunit is predicted to lack the second transmembrane domain and the intracellular COOH-terminus, regions of the protein involved in pore function. Nonetheless, we observed a measurable Na+ current in Xenopus laevis oocytes that coexpress the β and γ subunits with the truncated α subunit. The mutant α was coassembled with β and γ subunits and was present at the cell surface at a lower density, consistent with the lower Na+ current seen in oocytes with the truncated α subunit. The single-channel Na+ conductance for the mutant channel was only slightly decreased, and the appearance of the macroscopic currents was delayed by 48 hours with respect to wild-type. Our data suggest novel roles for the α subunit in the assembly and targeting of an active channel to the cell surface, and suggest that channel pores consisting of only the β and γ subunits can provide significant residual activity. This activity may be sufficient to explain the absence of a severe pulmonary phenotype in patients with PHA-I.
Introduction
Type I pseudohypoaldosteronism (PHA-I) is an inherited disorder that manifests soon after birth and is characterized by failure to thrive, salt wasting, metabolic acidosis, life-threatening hyperkalemia, and dehydration. Despite clinical evidence for hypoaldosteronism, plasma aldosterone and plasma renin activity are elevated, suggesting a defect in aldosterone target tissues. Two kinds of inheritance have been described for PHA-I, with distinct clinical and pathophysiological features (1): the autosomal dominant renal form (OMIM 177735), which was recently linked to mutations in the mineralocorticoid receptor gene (2); and the recessive systemic form (OMIM 264350), which presents with severe Na+ transport defects in all aldosterone target tissues. It is caused by diminution-of-function mutations in genes encoding the subunits of the amiloride-sensitive epithelial Na+ channel (ENaC) (3).
ENaC constitutes the rate-limiting step of Na+ reabsorption through aldosterone-responsive epithelia found in kidneys, colon, salivary and sweat glands, and glucocorticoid-responsive lung epithelia. ENaC is a heteromultimeric protein made of 3 subunits (α, β, and γ), sharing 35% homology at the protein level and the same membrane topology (4). A heterotetrameric structure (αβαγ) has recently been proposed for ENaC (5, 6). It is therefore likely that all cation channels belonging to this gene family are tetrameric, even though a nonameric architecture has been proposed by others (7). Mutagenic studies identified amino acid residues involved in ion permeation and critical for channel block by amiloride in a short segment preceding the second transmembrane domain (the pre-M2 segment) of each of the 3 subunits (8). This demonstrated that all 3 subunits contribute to the formation of the conductive pore. The α subunit plays a specific role in channel assembly and/or targeting to the plasma membrane. This conclusion is based on earlier observations in the Xenopus oocyte system showing that only complexes containing the α subunit (α alone or αβ or αγ complexes) are able to form functional channels. When only βγ subunits were present, no detectable amiloride-sensitive current could be measured (4). Taken together, these data suggest that the α subunit contains some specific information needed to get functional channels to the cell surface — information that is lacking in either β or γ subunits.
Homozygous mutations have been identified in all 3 ENaC subunits, causing the recessive form of PHA-I (3). Three mutations in the α subunit have been described: a frameshift (αI68fr), a missense mutation of a conserved cysteine (αC133Y), and a premature stop codon (αR508stop). Truncation of the α subunit is predicted to abolish ENaC activity in all tissues because it deletes the pore-forming region of the subunit present in at least 2 copies in the channel complex. This prediction is supported by gene disruption of the mouse α gene locus. All αENaC knockout pups present a postnatal lethal pulmonary edema, demonstrating the importance of this subunit in lung liquid clearance at birth (9). By contrast, patients with PHA-I have no neonatal lung phenotype, despite impressive truncations of the α subunit in some kindreds (αI68fr or αR508stop). To explain this discrepancy, we considered the following, not mutually exclusive, hypotheses:
(a) Species differences, such as the presence of species-specific channels and subunit or regulatory proteins expressed in the lung, could account for this contradiction. (b) The truncated α subunit may retain some information, leading to some residual activity. This concept is supported by recent in vivo studies demonstrating that less than 20% of ENaC activity in mouse lung is enough to prevent a neonatal lung edema, but not the development of a mild (10) or severe (11) renal phenotype.
To test the latter hypothesis, we introduced the αR508stop mutation in human ENaC and the equivalent αL535stop in rat ENaC, and expressed them in the Xenopus oocyte system. We found that when coexpressed with β and γ, the truncated α subunit leads to significant channel activity, although with a delayed time course. If similar activity were present in the lungs of patients with PHA-I carrying such a mutated allele, it might be sufficient to clear their lungs of fluid at birth. This may partially explain the difference in pulmonary phenotype observed between the αENaC-defective mouse and patients with PHA-I, and gives new insights into the comprehension of this disease.
Methods
Site-directed mutagenesis and expression in Xenopus laevis oocytes.
Point mutations were introduced into α human (αR508stop) and rat (αL535stop) ENaC cDNAs by PCR-based mutagenesis. Constructs were checked by sequencing. Capped cRNAs were obtained from linearized cDNAs by in vitro transcription and were injected (3 ng for each subunit) into stage V–VI X. laevis oocytes. Oocytes were kept at 19°C in low-Na+ modified Barth’s saline (MBS) (10 mM NaCl, 90 mM NMDG-Cl, 5 mM KCl, 0.41 mM CaCl2, 0.33 mM CaNO3, 0.82 mM MgSO4) supplemented with penicillin (100 U/mL) and streptomycin (100 mg/mL).
Whole-cell current was determined with the 2-electrode voltage-clamp method described previously (4). The amiloride-blockable fraction of the inward current at –100 mV, representative of the ENaC current, was measured as the difference between currents obtained before and after the addition of 100 μM amiloride in solutions containing Na+ (INa+) or Li+ (ILi+). Ionic selectivity was assessed as the ratio of amiloride-sensitive macroscopic currents in a bathing solution containing 120 mM of NaCl, LiCl, or KCl. Amiloride sensitivity was determined by blocking channel activity with increasing concentrations of amiloride in 120 mM NaCl at –100 mV.
Binding assay.
Measurement of ENaC surface expression was achieved according to Firsov et al. (12), by counting specific binding of [125I]M2IgG1 (M2Ab) at the surface of oocytes injected with indicated ENaC FLAG-tagged subunits and incubated in low-Na+ MBS. Anti-FLAG mAb’s were purchased from Eastman Kodak Co. (Rochester, New York, USA) and used at a final concentration of 12 nM. Background activity was determined on water- or untagged ENaC–injected oocytes, and was subtracted from values of each condition. The same oocytes were subsequently used for determination of whole-cell Na+ channel current.
Immunoprecipitation.
Oocytes were injected with FLAG-tagged and untagged subunits as specified, and labeled for 12 hours with 1 mCi/mL of [35S]methionine in MBS. Microsomal membranes were obtained as described (13) and solubilized in a digitonin (Calbiochem-Novabiochem Corp., San Diego, California, USA) washing buffer (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 0.5% digitonin, 1 mM phenylmethylsulfonyl fluoride, leupeptin, antipain, pepstatin A). For nondenaturant immunoprecipitations, we used the anti-FLAG M2IgG1 mAb (Kodak). A 1:1 mixture of protein G/protein A Sepharose recovered the immunoprecipitates, and these were loaded, after washing, on a 5–8% gradient SDS-PAGE. Denaturant immunoprecipitations were performed with an anti–ratENaC (anti-rENaC) antibody in a 1% TENT solution (50 mM Tris, 5 mM EDTA, 150 mM NaCl, 1% Triton X-100; pH 7.4). Immunoprecipitates were recovered by protein A Sepharose and subjected to electrophoresis. The gels were exposed to XB-1 Kodak film for 2–5 days.
Single-channel recordings.
Single-channel recordings were obtained in the cell-attached configuration with a List LM EPC 7 patch-clamp amplifier (List Electronics, Darmstadt, Germany), displayed on an oscilloscope (Tektronix Inc., Heerenveen, the Netherlands) and stored on a digital tape recorder (Biologic Co., Claix, France). Borosilicate glass (Corning Inc., New York, New York, USA) pipettes with a 10- to 20-MΩ resistance, filled with 120 mM LiCl or 120 mM NaCl solutions, were used. The bathing solution was 120 mM KCl buffered to pH 7.4 with 10 mM Na-HEPES. Single-channel conductances for Na+ and Li+ were calculated from current/voltage relationships between –150 and +10 mV. Current signals were filtered at 200 Hz with an 8-pole Bessel filter (Frequency Devices Inc., Haverhill, Massachusetts, USA) and digitized at 1 kHz using a Labmaster analog-digital interface and Fetchex software (Axon Instruments Inc., Foster City, California, USA).
Immunofluorescence assay.
Four days after injection, oocytes were fixed with 3% paraformaldehyde (Merck KGaA, Darmstadt, Germany) in PBS for 4 hours at 4°C. For immunochemistry, the tyramide signal amplification kit (TSA-Direct; NEN Life Science Products Inc., Boston, Massachusetts, USA) was used. Cryosections (6-μm thick) were incubated for 1 hour with a 1:100 diluted anti-FLAG IgG antibody (Kodak). After washings, they were incubated for 1 hour with a 1:100 dilution of a horseradish peroxidase–conjugated sheep anti-mouse immunoglobulin (Amersham Pharmacia Biotech AB, Uppsala, Sweden), subsequently revealed by incubation for 10 minutes with the provided fluorescein-tyramide conjugates diluted 1:50. To label the F-actin cytoskeleton of the oocyte plasma membrane, a 1:50 dilution of rhodamine-conjugated phalloidin (Molecular Probes Inc., Eugene, Oregon, USA) was added to the amplification buffer. Sections were mounted with DAKO-Glycergel (DAKO Corp., Glostrup, Denmark) containing 2.5% of 1,4-diazabicyclo[2.2.2]octane as a fading retardant (Sigma Chemical Co., Buchs, Switzerland) and were studied by epifluorescence with a Polyvar microscope (Reichert-Jung, Vienna, Austria). Digitized images were acquired with a VISICAM CCD camera (Visitron, Puchheim, Germany) and processed by Image-Pro Plus software (version 3.0; Media Cybernetics, Silver Spring, Maryland, USA). Identical settings were used for each oocyte.
Results
The rat and human αENaC stop mutations allow significant expression of active channels.
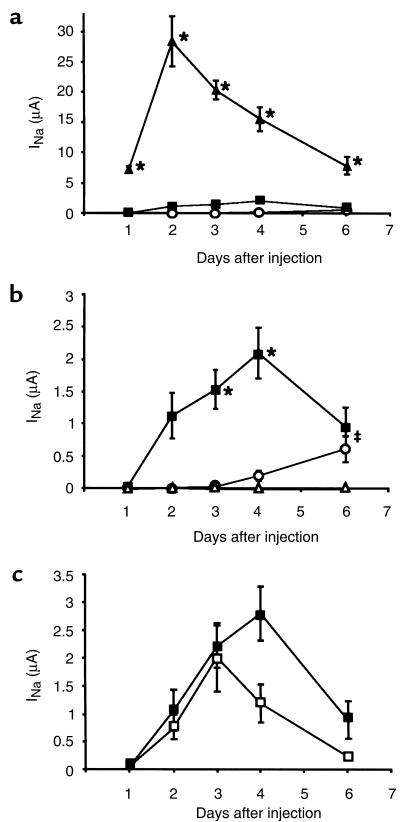
The stop mutation (αR508stop) found in patients with PHA-I interrupts the αENaC protein 46 amino acids before the predicted α-helix of the second transmembrane domain (M2), deleting this domain, the domain preceding M2 (pre-M2), and the intracellular COOH-terminus. It does, however, conserve a large portion of the extracellular loop and the 2 cysteine-rich boxes, characteristic of the ENaC gene superfamily (14) (Figure 1). We introduced the stop mutation in the rat αENaC subunit at the equivalent residue (αL535stop) and coexpressed it with β and γ rat ENaC. When measured with the 2-electrode voltage-clamp method, reproducible amiloride-sensitive currents were recorded from αL535stopβγ-injected oocytes, reaching 13% of currents measured in αβγ-injected oocytes 4 days after injection (Figure 2, a–c). These currents were significantly higher than those recorded in oocytes injected with βγ subunits alone (P < 0.001 at days 3 and 4 after injection) or with water (P < 0.001 at days 3–6) (Figure 2b). Three different kinetics for the appearance of macroscopic amiloride-sensitive Na+ current could be observed during the postinjection period (Figure 2, a and b). (a) In αβγ rENaC-injected oocytes, amiloride-sensitive currents were already detectable after a few hours, and reached their peak on the second day after injection. (b) Oocytes injected with αL535stopβγ display slower kinetics, reaching their peak after 4 days. (c) Surprisingly, amiloride-sensitive currents were recorded from oocytes injected with βγ rENaC (between 0.5 and 2 μA) 6 days after injection (significantly higher than water-injected oocytes; P < 0.001). Their maximal expression was recorded at the seventh day.
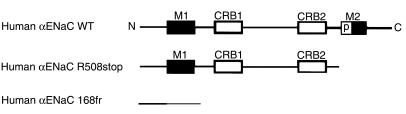
Figure 1.
α ENaC subunit truncations reported in recessive PHA-I. Primary structure of wild-type (WT) and predicted deletions of α subunits are illustrated. M1 and M2 denote first and second transmembrane domains; P denotes the pre-M2 domain of the second transmembrane domain. CRB1 and CRB2 stand for cysteine-rich boxes 1 and 2. Both deletion mutations reported for patients with PHA-I (human αR508stop and human αI68fr) predict an interruption of the α subunit before the M2 and the M1 membrane-spanning domains, respectively.
Figure 2.
(a) Time courses of macroscopic amiloride-sensitive current of wild-type, αL535stopβγ, and βγ rat ENaC–injected oocytes. Oocytes were injected with 3 ng cRNA of each rat ENaC subunit as follows: αβγ (filled triangles), αL535stopβγ (filled squares), or βγ (open circles). Amiloride-sensitive (100 μM) Na+ current was determined every day in a bathing solution containing 120 mM NaCl. Oocytes were clamped at –100 mV and measured with the 2-electrode voltage-clamp method. Currents are reported as the mean ± SEM obtained from 17–34 oocytes (6 independent experiments). *P < 0.001, αβγ channels vs. mutant αL535stopβγ or βγ channels. (b) Focus on mutant αL535stopβγ (filled squares) macroscopic current time courses, compared with βγ-injected (open circles) and water-injected (open triangles) oocytes. *P < 0.001, mutant αL535stopβγ vs. βγ- or water-injected oocytes. ‡P < 0.001, mutant αL535stopβγ- and βγ-injected oocytes vs. water-injected oocytes. (c) Comparison of human and rat mutant macroscopic current time courses. Human (αR508stopβγ; open squares) and rat (αL535stopβγ; filled squares) ENaC mutants have the same expression pattern over time. No significant difference was detected. Each point represents the mean ± SEM of macroscopic Na+ channel currents obtained from 15–26 oocytes (4 different batches).
To assess putative species difference in the expression of this mutant, as related in vivo between humans and mice, we studied the human αR508stop mutation. When coinjected with human β and γ ENaC, the αR508stop led to similar amiloride-sensitive current expression as the rat mutant αL535stopβγ (Figure 2c). Both mutants had the same time course, with a delayed peak of activity compared with wild-type channels. Human βγ currents were significantly smaller than the αR508stopβγ currents, but significantly higher than water-injected oocytes, at day 3 after injection (data not shown).
Differences in macroscopic current properties between human and rat channels.
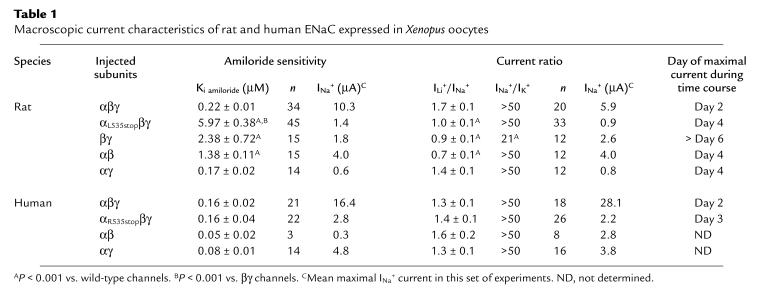
Because the second transmembrane domain forms the channel pore and participates in the selectivity filter and amiloride sensitivity (8), we characterized the macroscopic currents resulting from coinjection of human αR508stop and rat αL535stop with their respective β and γ subunits. They demonstrated subtle differences (Table 1). Human αR508stopβγ channels had similar amiloride sensitivity (Ki amiloride = 0.16 ± 0.04 μM) and ionic selectivity, determined as Li+/Na+ or Na+/K+ amiloride–sensitive current ratio (ILi+/INa+ = 1.4 ± 0.1; INa+/IK+ > 50), as human wild-type channels. By contrast, rat αL535stopβγ channels had a lower amiloride sensitivity than wild-type channels (Ki amiloride = 5.97 ± 0.38 μM; P < 0.001), and lost Li+/Na+ selectivity (ILi+/INa+ = 1.0 ± 0.1; P < 0.001). But they conserved selectivity for Na+ over K+ (INa+/IK+ > 50). Currents from oocytes injected with rat βγ ENaC displayed characteristics close to those of rat αL535stopβγ. Amiloride sensitivity was, however, slightly higher (Ki amiloride = 2.38 ± 0.72 μM; P < 0.001) compared with mutant αL535stopβγ. Selectivity for Na+ over K+ was statistically different from wild-type, but remained high (INa+/IK+ = 21; P < 0.001). Unfortunately, we have not been able to perform similar studies on human βγ channels because currents were too low.
Table 1.
Macroscopic current characteristics of rat and human ENaC expressed in Xenopus oocytes
As reported previously (15), rat αβ and αγ channels have different macroscopic characteristics (Table 1): αγ channels resemble wild-type channels, whereas αβ channels demonstrate amiloride resistance and a loss of Li+/Na+ selectivity. By contrast, human αβ and αγ channels displayed the same amiloride sensitivity (αβ: Ki amiloride = 0.05 ± 0.02 μM; and αγ: Ki amiloride = 0.08 ± 0.01 μM) and selectivity (αβ: ILi+/INa+ = 1.6 ± 0.2, INa+/IK+ > 50; and αγ: ILi+/INa+ = 1.3 ± 0.1, INa+/IK+ > 50) as human wild-type channels, thus confirming species differences depending on subunit arrangements.
In summary, although some features were distinct to either the human or the rat Na+ mutant channel, the important and common feature for both species is that a surprisingly large, macroscopic amiloride-sensitive current was observed, presenting the same delayed kinetics in both species. Subsequent studies were performed on the rat channel, for which more information concerning cell-surface expression is available (12).
The diminution of expressed activity of the mutated channel is due to a decreased number of channels at the cell surface.
Whole-cell Na+ current can be summarized by the following equation:
(Equation 1)
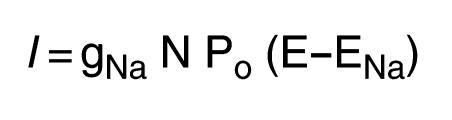 |
1 |
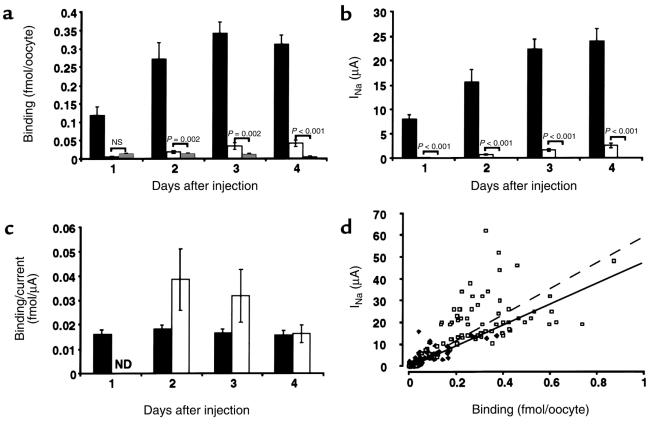
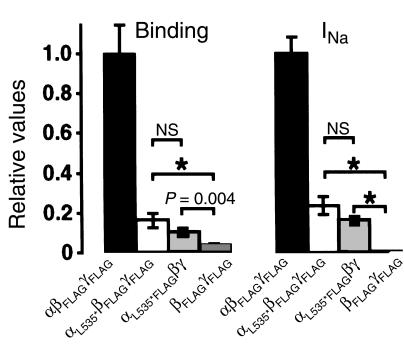
where gNa is the single-channel conductance for Na+, N is the total number of channels for a given cell, Po is the mean open probability for a single channel, E is the membrane potential, and ENa is the reverse potential for Na+ (16). The driving force for Na+ ions (E – ENa) was kept approximately constant by clamping the oocyte membrane potential at –100 mV, and cannot account for the observed decreased mutant channel current. To determine conductance and Po, single-channel recordings were obtained 4 days after injection in the cell-attached patch configuration from αL535stopβγ-injected oocytes. Single-channel conductances determined between –150 and +10 mV were 4.03 ± 0.26 pS (n = 12) with Na+ in the pipette, and 5.08 ± 0.07 pS (n = 8) in the presence of Li+. They were both slightly lower than values given for the wild-type ENaC, but this difference could not explain the diminution of macroscopic current. The low Li+ conductance confirmed the relative loss of selectivity for Li+ compared with Na+ observed in macroscopic current experiments. As described by others (17), Po was highly variable and no obvious difference was observed. We addressed the question of the number of channels at the cell surface by a binding assay, described previously (12). Oocytes were injected with αβFLAGγFLAG, αL535stopβFLAGγFLAG, or βFLAGγFLAG. Binding assays with iodinated anti-FLAG antibodies were performed daily during 4 days. The number of mutant channels detected at the cell surface was rising as late as 4 days after injection (Figure 3a). At this day, they reached a highly significant binding level over βγ-injected oocytes. The number of detected channels at the cell surface paralleled the appearance of the Na+ channel current (Figure 3b), leading to a constant binding/current ratio (Figure 3c). This indicates that the number of mutant channels at the cell surface was decreased in parallel to the decrease of INa+. This can also be illustrated by plotting binding versus INa+ (Figure 3d). A linear relationship existed between the number of channels at the cell surface (reported as binding per oocyte) and the macroscopic amiloride-sensitive Na+ current for both mutant and wild-type channels, but the slopes of the regression lines were not significantly different. These results indicate that the observed αL535stopβγ current reduction is mainly due to a proportional decrease of the channel number at the cell surface.
Figure 3.
(a) Quantitative determination of the number of channel molecules present at the cell surface. Epitope-tagged βFLAG and γFLAG rENaC subunits were coinjected with α wild-type (filled bars), αL535stop (open bars), or water (gray bars) in Xenopus oocytes. Values are mean ± SEM of 2 different experiments with 12 oocytes per experiment and per condition. P values of unpaired Student’s t test are indicated. (b) Whole-cell amiloride-sensitive Na+ currents. βγ channel currents are undetectable. (c) Ratio of the bound anti-FLAG IgG (binding; a) to the whole-cell Na+ current (b) for αβγ and mutant αL535stopβγ channels. ND, not determined. (d) Representation of binding values plotted against INa+. The slopes of the linear regression curves are not statistically different (y = 59x, r2 = 0.44 for αβγ channels [open squares and dashed line]; and y = 48x, r2 = 0.54 for the mutant channel αL535stopβγ [filled diamonds and solid line].
The decreased number of channels at the cell surface is due to a retention of channel proteins in intracellular compartments.
To observe the subcellular distribution of wild-type and mutant channels, we used immunofluorescence to detect the localization of epitope-tagged subunits in cryosections of oocytes that were injected with either water, αβFLAGγFLAG, αL535stopβFLAGγFLAG, or βFLAGγFLAG. Oocytes injected with αβFLAGγFLAG exhibited a bright immunofluorescent signal at the circumference of the oocyte and a weaker staining in the intracellular compartment (Figure 4b). Higher magnification and costaining of the actin cytoskeleton, which is highly abundant in the microvilli of the cell surface, confirmed that the immunofluorescence at the periphery of the oocyte represents FLAG-tagged ENaC subunits present at the cell surface (Figure 4, f and j). Oocytes expressing αL535stopβFLAGγFLAG exhibited only a weak signal in the plasma membrane, but the intracellular staining was clearly increased, indicating a retention of channels in an intracellular compartment (Figure 4, c, g, and k). αL535stopβFLAGγFLAG was, like αβFLAGγFLAG, predominantly found at the vegetal pole of the oocyte (Figure 4, b and c), suggesting that the trafficking of αL535stopβFLAGγFLAG to the cell surface, although less efficient, obeys the same rules as that of αβFLAGγFLAG. In oocytes expressing βFLAGγFLAG (Figure 4, d, h, and l), no ENaC-related plasma membrane staining was observed 4 days after injection; FLAG-tagged ENaC subunits were exclusively found in intracellular compartments. In water-injected oocytes (Figure 4, a, e, and i), no immunofluorescent signal for ENaC was observed. Actin cytoskeleton was stained as in the other groups of oocytes.
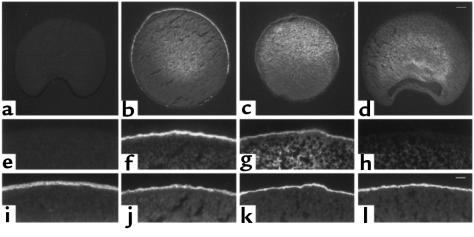
Figure 4.
Immunofluorescence of oocytes injected either with αβFLAGγFLAG (b, f, and j), αL535stopβFLAGγFLAG (c, g, and k), βFLAGγFLAG (d, h, and l), or water (a, e, and i). Detection of FLAG-tagged ENaC subunits with an anti-FLAG mAb (a–h), and of the microvilli actin cytoskeleton with rhodamine-conjugated phalloidin (i–l). (a–d) Low-power magnifications; scale bar in d: 100 μm. (e–l) High-power magnifications; scale bar in l: 20 μm.
In addition to the slower appearance and/or the faster retrieval of the mutant channel at the cell surface, the decreased channel number could be due to an early degradation of the channel complex (shorter half-life), a misassembly of the 3 subunits, a mistrafficking, or a combination of any of these possibilities.
The faithful translation of the protein (wild-type or truncated) can be assessed by a denaturant immunoprecipitation assay (Figure 5a). Using an anti-rat αENaC antibody, we were able to efficiently immunoprecipitate both rat and human αENaC subunits. This experiment showed efficient translation of both mutant proteins. Importantly, no evidence for a significant read-through of the translational stop codon was detected (Figure 5a). No early degradation of the mutant complex or difference in protein turnover could be demonstrated by pulse-chase experiments (not shown).
Figure 5.
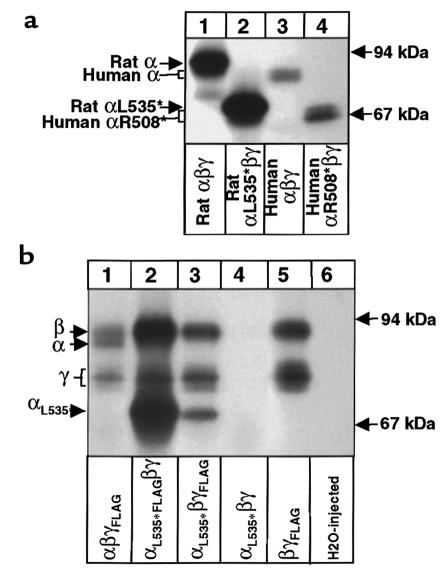
(a) Denaturant immunoprecipitation with an anti-rat αENaC antibody. Oocytes were injected with rat wild-type αβγ (lane 1), rat αL535stopβγ (lane 2), human wild-type αβγ (lane 3), or human αR508stopβγ (lane 4) and were metabolically labeled. Equal counts of microsomal preparation were subjected to immunoprecipitation by a rat anti-α antibody. Recovered immunoprecipitates were run on a 5–8% SDS-polyacrylamide gel. Molecular weight is indicated. (b) Nondenaturant coimmunoprecipitation of wild-type and mutant rENaC. Oocytes were injected with γFLAG subunits (lanes 1, 3, and 5), with αL535stopFLAG subunit (lane 2), or without FLAG subunit (lanes 4 and 6), coinjected with wild-type rENaC or mutant αL535stop subunits, as indicated. Nondenaturant coimmunoprecipitation was performed with an anti-FLAG mAb. Specificity of the antibody is demonstrated on lanes 4 and 6 (no FLAG-tagged subunits injected). Specificity of the coimmunoprecipitation is demonstrated on lanes 2, 3, and 5 (no α subunit injected).
Assembly of αL535stop with β and γ subunits was assessed by nondenaturant immunoprecipitation of microsomal membranes prepared from [35S]methionine-labeled ENaC-injected oocytes. Figure 5b shows an autoradiogram of an SDS-polyacrylamide gel, demonstrating assembly of the FLAG-tagged αL535stop together with β and γ ENaC subunits (lane 2). Specificity of the assembly was assessed by changing the FLAG-tagged subunit of this complex (lane 3). The γ FLAG-tagged subunit was also able to coimmunoprecipitate the αL535stop with the β subunit. Specificity of the antibody is demonstrated on lanes 4 and 6, where nontagged mutant subunits and water-injected oocytes were used, respectively. Finally, the specificity of the coimmunoprecipitation is demonstrated by oocytes injected with βγ (lane 5), in which the 2 proteins are coassembled. These results indicate that the mutated αL535stop subunit coassembles with β and γ, despite the deletion of its second transmembrane domain and the COOH-terminal intracellular tail.
To assess the presence of all 3 subunits at the cell surface, we also used the binding assay with epitope-tagged subunits. Three days after injection, binding signal for oocytes injected with αL535stopFLAGβγ was in the same range as that for oocytes injected with αL535stopβFLAGγFLAG, and the amiloride-sensitive currents were identical (Figure 6). Together with the results from the binding assay (Figure 3a) and the coimmunoprecipitation assay (Figure 5b), our data indicate that the 3 ENaC subunits are present at the cell surface and coassembled to form an active channel.
Figure 6.
Cell-surface detection of FLAG-tagged subunits. Oocytes were injected with αβFLAGγFLAG (filled bars), αL535stopβFLAGγFLAG (open bars), αL535stopFLAGβγ (light gray bars), or βFLAGγFLAG (dark gray bars). After 3 days of incubation, binding assay was performed, and the number of FLAG-tagged molecules present at the cell surface was determined. Values represent the means of 24 oocytes ± SEM (2 batches). Unpaired t test was used when indicated (*P < 0.001). NS, not significant.
Discussion
Assembly domain and targeting signal of the α subunit.
The 3 subunits of the mutated channel (αL535stop, β, and γ) are coassembled, suggesting that the second transmembrane domain (M2) and the COOH-terminal part of αENaC subunit are not necessarily required for assembly. This is in agreement with previous experiments performed on the γ subunit (18). We propose here that an assembly domain is present in the first 535 residues of the α subunit.
Measurable currents were observed after injection of αL535stopβγ or βγ in X. laevis oocytes. These currents could be due to the preferential association of the injected β and γ subunits with an endogenous subunit. Despite some evidence for endogenous amiloride-sensitive currents (19), to date, no significant endogenous expression of ENaC subunits have been identified in Xenopus oocytes.
The 3 coassembled subunits (αL535stop, β, and γ) were detected at the cell surface of the oocyte by the antibody-binding assay, although with a decreased density. This indicates that a significant part of mutated channels reaches the plasma membrane. This impaired trafficking or faster retrieval results in a delayed appearance and a decreased number of channels at the cell surface (proportional to the decrease in amiloride-sensitive macroscopic current). The mechanisms by which ENaC is targeted to the plasma membrane are not known. In the oocyte, the α subunit can reach the cell membrane on its own, as well as in complexes containing α (αβ and αγ), but to a lesser extent compared with complexes containing all 3 subunits (αβγ) (4). The importance of α for the targeting of ENaC complexes to the cell surface is highlighted by the kinetics of macroscopic currents studied in the postinjection period. Comparison of kinetics of βγ subunits coinjected alone or coinjected with the truncated αL535stop or with the full-length αENaC showed a rapid and efficient trafficking with the wild-type αENaC, a short delay and a decrease in the number of channels reaching the surface with the truncated αL535stop, and a long delay and a decreased number of channels when no α subunit was present. This suggests that trafficking signals are contained in the α subunit in at least 2 positions, 1 before and 1 after amino acid 535. In a previous study (20), a proline-rich domain in the intracellular COOH-terminal part of the α subunit was identified as interacting with the SH3 domain of α-spectrin, a component of the cytoskeleton. This proline-rich domain could target the wild-type ENaC complex to the apical cell surface. Its deletion in the αL535stop mutation could account for the disturbed trafficking. We therefore propose that the first 535 residues of αENaC contain enough sorting information to permit a trafficking of βγ subunits to the cell surface, yet with a slower time course. The reduction in the number of channels at the cell surface is enough to account for the observed diminution of the INa+. Thus, the mutant αL535stop coexpressed with β and γ in the Xenopus oocyte gives rise to functional channels with a Na+ conductance close to wild-type ENaC. This raises an intriguing question about the structure of the pore of αL535stopβγ channels.
The rat αL535stopβγ channel presents a 27-fold decreased sensitivity to amiloride and a loss of selectivity of Li+ over Na+. It is remarkable that a complete deletion of the pre-M2 and M2 domains of the α subunit (αL535stop) causes changes in channel properties nearly identical to those observed previously with point mutations (αS583C) (8) or chimeric constructs (21). Rat αL535stopβγ channels have the same macroscopic characteristics (amiloride resistance and Li+ loss of selectivity) as βγ channels, except for 2 relatively small quantitative differences (amiloride sensitivity and K+ selectivity). Overall, the resemblance between currents obtained from αL535stopβγ and βγ channels is striking. We would like to propose a model wherein the pore is made by pre-M2 and M2 domains from βγ subunits only.
Functional role of αL535stopβγ channels in PHA-I phenotype.
Disruption of the α subunit results in 2 different phenotypes, depending on whether the disruption is in humans (patients with PHA-I) or in mice (knockout experiment). In mice, α knockout pups die within 40 hours after birth, mainly from an inability to clear their lungs of fluid (9). No residual ENaC activity could be recorded in these animals. This pulmonary phenotype can be rescued by low levels of ENaC activity in the mouse lung (<20%), as recently reported for disruption of the γ (11) and β (10) subunits and for an α knockout mouse rescued by a transgenic rat α subunit (22). Thus, a low level of ENaC activity seems to be sufficient in vivo to rescue the pulmonary phenotype. In humans, no neonatal pulmonary phenotype was described for patients with PHA-I, but clinical evidence for light pulmonary symptoms arises from careful examination of young patients (23–25). These patients present early respiratory events such as wheezing or recurrent viral infections and, in 1 case, recurrent Pseudomonas infections (26). Our data suggest that the channel activity measured in vitro could be sufficient in vivo to explain the absence of neonatal pulmonary symptoms in patients with PHA-I suffering from the αR508stop mutation. For the more severe truncations, this explanation may not be sufficient. The shorter truncation described in PHA-I kindred, αI68fr (Figure 1), leads to a protein of 143 residues with a deletion of most of the α subunit, leaving only the NH2-terminus. When expressed in the oocyte in association with human β and γ subunits, amiloride-sensitive currents are very low (0.1% of wild-type αβγ) (27). Thus, 2 truncations of different lengths of the α subunit lead in vivo to the same renal phenotype in humans, despite a 100-fold difference in their expression in the oocyte system. Whether such a low residual activity observed in vitro would be enough to prevent a lung phenotype in vivo remains to be seen. Species-specific factors may have an additional role to explain the phenotype difference between mice and humans. In addition, the oocyte expression system may lack specific renal factors that could modulate the expression of the channel. Expression of wild-type and mutated channels in the highly differentiated cortical collecting duct principal cell line (28), or expression of αI68fr or αL535stop mutations in mice by homologous recombination, should help in resolving these issues.
Acknowledgments
We thank M. Lazdunsky for the generous gift of the human αβγ ENaC cDNA, and M. Robert-Nicoud, S. Kohler, and P. Greasley for help in the elaboration of the manuscript. Many thanks to L. Schild, D. Firsov, and D. Bertrand for helpful discussions, and to N. Skarda-Coderey for secretarial help. O. Bonny is supported by a grant from the Swiss National Science Foundation (31-48141.96, M.D.-Ph.D. program of the Swiss Academy of Medical Sciences). B.C. Rossier is also supported by a grant from the Swiss National Science Foundation (31-43384.95).
References
- 1.Hanukoglu A. Type I pseudohypoaldosteronism includes two clinically and genetically distinct entities with either renal or multiple target organ defects. J Clin Endocrinol Metab. 1991;73:936–944. doi: 10.1210/jcem-73-5-936. [DOI] [PubMed] [Google Scholar]
- 2.Geller DS, et al. Mutations in the mineralocorticoid receptor gene cause autosomal dominant pseudohypoaldosteronism type I. Nat Genet. 1998;19:279–281. doi: 10.1038/966. [DOI] [PubMed] [Google Scholar]
- 3.Rossier BC. 1996 Homer Smith Award Lecture. Cum grano salis: the epithelial sodium channel and the control of blood pressure. J Am Soc Nephrol. 1997;8:980–992. doi: 10.1681/ASN.V86980. [DOI] [PubMed] [Google Scholar]
- 4.Canessa CM, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 5.Firsov D, Gautschi I, Merillat AM, Rossier BC, Schild L. The heterotetrameric architecture of the epithelial sodium channel. EMBO J. 1998;17:344–352. doi: 10.1093/emboj/17.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kosari F, et al. Subunit stoichiometry of the epithelial sodium channel. J Biol Chem. 1998;273:13469–13474. doi: 10.1074/jbc.273.22.13469. [DOI] [PubMed] [Google Scholar]
- 7.Snyder PM, Cheng C, Prince LS, Rogers JC, Welsh MJ. Electrophysiological and biochemical evidence that DEG/ENaC cation channels are composed of nine subunits. J Biol Chem. 1998;273:681–684. doi: 10.1074/jbc.273.2.681. [DOI] [PubMed] [Google Scholar]
- 8.Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the alpha, beta, and gamma subunits of the epithelial sodium channel involved in amiloride block and ion permeation. J Gen Physiol. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hummler E, et al. Early death due to defective neonatal lung liquid clearance in alpha-ENaC–deficient mice. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- 10.Pradervand S, et al. Salt restriction induces pseudohypoaldosteronism type 1 in mice expressing low levels of the beta-subunit of the amiloride-sensitive epithelial sodium channel. Proc Natl Acad Sci USA. 1999;96:1732–1737. doi: 10.1073/pnas.96.4.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker PM, et al. Role of gamma ENaC subunit in lung liquid clearance and electrolyte balance in newborn mice. Insights into perinatal adaptation and pseudohypoaldosteronism. J Clin Invest. 1998;102:1634–1640. doi: 10.1172/JCI3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firsov D, et al. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci USA. 1996;93:15370–15375. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geering K, Theulaz I, Verrey F, Hauptle MT, Rossier BC. A role for the beta-subunit in the expression of functional Na+-K+-ATPase in Xenopus oocytes. Am J Physiol. 1989;257:C851–C858. doi: 10.1152/ajpcell.1989.257.5.C851. [DOI] [PubMed] [Google Scholar]
- 14.Firsov D, Robert-Nicoud M, Gruender S, Schild L, Rossier BC. Mutational analysis of cysteine-rich domains of the epithelium sodium channel. Identification of cysteines essential for channel expression at the cell surface. J Biol Chem. 1999;274:2743–2749. doi: 10.1074/jbc.274.5.2743. [DOI] [PubMed] [Google Scholar]
- 15.McNicholas CM, Canessa CM. Diversity of channels generated by different combinations of epithelial sodium channel subunits. J Gen Physiol. 1997;109:681–692. doi: 10.1085/jgp.109.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hille, B. 1992. Ionic channels of excitable membranes. Sinauer Associates Inc. Sunderland, MA. 1–20.
- 17.Palmer LG, Frindt G. Conductance and gating of epithelial Na channels from rat cortical collecting tubule. Effects of luminal Na and Li. J Gen Physiol. 1988;92:121–138. doi: 10.1085/jgp.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams CM, Snyder PM, Welsh MJ. Interactions between subunits of the human epithelial sodium channel. J Biol Chem. 1997;272:27295–27300. doi: 10.1074/jbc.272.43.27295. [DOI] [PubMed] [Google Scholar]
- 19.Weber WM, Liebold KM, Clauss W. Amiloride-sensitive Na+ conductance in native Xenopus oocytes. Biochim Biophys Acta. 1995;1239:201–206. doi: 10.1016/0005-2736(95)00151-r. [DOI] [PubMed] [Google Scholar]
- 20.Rotin D, et al. An SH3 binding region in the epithelial Na+ channel (alpha rENaC) mediates its localization at the apical membrane. EMBO J. 1994;13:4440–4450. doi: 10.1002/j.1460-2075.1994.tb06766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldmann R, Champigny G, Lazdunski M. Functional degenerin-containing chimeras identify residues essential for amiloride-sensitive Na+ channel function. J Biol Chem. 1995;270:11735–11737. doi: 10.1074/jbc.270.20.11735. [DOI] [PubMed] [Google Scholar]
- 22.Hummler E, et al. A mouse model for the renal salt-wasting syndrome pseudohypoaldosteronism. Proc Natl Acad Sci USA. 1997;94:11710–11715. doi: 10.1073/pnas.94.21.11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerem E, et al. Respiratory disease in patients with the systemic form of pseudohypoaldosteronism type I. Pediatr Pulmonol Suppl. 1997;14:78–79. [Google Scholar]
- 24.Hogg RJ, Marks JF, Marver D, Frolich JC. Long term observations in a patient with pseudohypoaldosteronism. Pediatr Nephrol. 1991;5:205–210. doi: 10.1007/BF01095953. [DOI] [PubMed] [Google Scholar]
- 25.Hanukoglu A, Bistritzer T, Rakover Y, Mandelberg A. Pseudohypoaldosteronism with increased sweat and saliva electrolyte values and frequent lower respiratory tract infections mimicking cystic fibrosis. J Pediatr. 1994;125:752–755. doi: 10.1016/s0022-3476(94)70071-0. [DOI] [PubMed] [Google Scholar]
- 26.Marthinsen L, et al. Recurrent Pseudomonas bronchopneumonia and other symptoms as in cystic fibrosis in a child with type I pseudohypoaldosteronism. Acta Paediatr. 1998;87:472–474. [PubMed] [Google Scholar]
- 27.Bonny O, Rossier BC, Hummler E. Functional analysis of human alpha ENaC mutation (I68fr) causing PHA-1. J Am Soc Nephrol. 1997;8:M572. (Abstr.) [Google Scholar]
- 28.Bens M, et al. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol. 1999;10:923–934. doi: 10.1681/ASN.V105923. [DOI] [PubMed] [Google Scholar]