Abstract
Urogenital schistosomiasis, infection with Schistosoma haematobium, is linked to increased risk for the development of bladder cancer, but the importance of various mechanisms responsible for this association remains unclear, in part due to lack of sufficient and appropriate animal models. New advances in the study of this parasite, bladder regenerative processes, and human schistosomal bladder cancers may shed new light on the complex biological processes that connect S. haematobium infection to bladder carcinogenesis.
Keywords: urogenital schistosomiasis, bladder cancer, Schistosoma haematobium, urinary schistosomiasis, schistosomiasis haematobia
The link between Schistosoma haematobium and bladder cancer
The association between urogenital schistosomiasis and bladder cancer was documented in the early 1900s [1] and has since been corroborated by many retrospective studies of human bladder cancer in diverse regions endemic for this infection [2–4]. The World Health Organization’s International Agency for Research on Cancer (IARC) thus regards infection with Schistosoma haematobium – the causative agent for urogenital schistosomiasis—as carcinogenic to humans (Group 1, the classification reserved for suspected carcinogens with the strongest evidence) [5,6]. However, there is minimal evidence of any cancer association with infection by the related schistosomes Schistosoma mansoni and Schistosoma japonicum; these species predominantly cause hepatoenteric disease and rarely affect pelvic organs [4,5]. Analogous to S. haematobium and bladder cancer other macroparasites such as the liver flukes Opisthorchis viverrini and Clonorchis sinensis show strong associations with the onset of cholangiocarcinoma, a form of bile duct cancer [6–8]. Indirect and direct mechanisms may be responsible for the association of these parasites with their particular cancers [9]. Helminths may directly induce cancer through the activity of parasite molecules on host cells. Indirectly, they may permit co-infection by other potentially oncogenic biological species—such as viruses or bacteria [10–16])—and also elicit genetic lesions from spillover effects of host inflammatory processes directed against the parasite, such as production of reactive nitrogen and oxygen intermediates [17,18]). Additionally, the risk of bladder cancer owing to infection by S. haematobium seems to be significantly promoted by concurrent risk factors commonly associated with bladder cancer in areas of non-endemicity, including nitrosamines and other chemical exposures from industrial and agricultural sources, as well as from tobacco smoking (reviewed in [19,20]). Thus, multiple factors may intersect to confer increased risk for bladder cancer associated with S. haematobium infection in humans.
Though the association between S. haematobium infection and bladder cancer is strong, identification of the underlying mechanisms has progressed slowly and likely hampers the diagnosis and successful treatment of urogenital schistosomiasis–associated bladder cancer. The interrelated reasons for slow progress in this field include: 1) lack of a tractable animal model to study the progression of urogenital schistosomiasis; 2) until recently, a paucity of genomic information about S. haematobium; 3) few genetic tools to manipulate life stages of S. haematobium; and 4) an incomplete catalog of mutations that may be unique to human schistosomal bladder cancer compared to other bladder cancers. Over the past few years, new research efforts have directly targeted some of these key roadblocks [21–23], opening new avenues to investigate Schistosoma haematobium infection and its association with bladder cancer.
This review attempts to integrate recent insights into the regenerative pathways at work in bladder homeostasis and injury repair with the growing literature on the roles host inflammatory mechanisms may play in promoting initial neoplastic transformation and cancer progression. These findings may suggest ways to frame future experimentation on the oncogenic effects of particular S. haematobium pathogenesis mechanisms and host inflammatory pathways, as well as roles for potential co-infections in precipitating neoplastic transformation. Ultimately, such work may reveal new diagnostic and treatment modalities, with potential to test broader questions regarding the role of inflammation in epithelial cancers.
The inflammatory environment during acute and chronic S. haematobium infection of the bladder
The pathology of urogenital schistosomiasis is primarily caused by the eggs laid by S. haematobium adult worm pairs residing in the venous plexus of the bladder and other pelvic organs. On their way to exiting the body through the urinary stream, eggs transit through the bladder mucosal tissue, causing substantial tissue damage and initiating granulomatous inflammation that can progress over many years to complications including fibrosis and bladder cancer [24,25]. Temporally cross-sectional, histological observations of S. haematobium-infected human bladder tissues, especially autopsy series, combined with extrapolations from rodent hepatoenteric pathology caused by experimental S. mansoni and S. japonicum infections outline the likely timeline of changes occurring in bladder tissue in the setting of urogenital schistosomiasis [26–28]. However, the potentially unique properties of inflammatory responses in the bladder mucosa [29] imply that animal models of urogenital schistosomiasis could greatly improve our grasp of the important immune factors involved in the acute and chronic phases of this infection.
There are limitations to the available experimental models for the study of the full course of S. haematobium infection in mammalian hosts [30]. Unfortunately, transdermal infection of mice with S. haematobium cercariae, the route of natural infection of humans, results in very low rates of worm maturation and egg deposition in the pelvic organs, the key site of human pathology [31]. Infection of hamsters and non-human primates with S. haematobium cercariae leads to worm maturation and oviposition [32]. Hamsters, however, exhibit low rates of pelvic organ infection, and instead develop predominantly hepato-enteric schistosomiasis. In contrast, C. sinensis- and O. viverrini-infected hamsters make an excellent model and even acquire cholangiocarcinoma when fed a diet that is high in nitrosamines, mimicking the human scenario [33–35]. Though non-human primates develop pathology similar to human disease, ethical considerations and financial cost preclude their regular use, and a relative lack of genetic and scientific tools in general complicate testing of relevant host pathways [36].
Several years ago we sought to help overcome this impasse in animal models of urogenital schistosomiasis. By micro-injecting a bolus of S. haematobium eggs into the bladder wall of mice [37], we were able to replicate several important changes observed in human urogenital schistosomiasis, such as hematuria, increased urinary frequency, persistent and fibrotic granulomata, and systemic and regional type 2 immune activation [21]. Importantly, egg exposure also triggered persistent urothelial hyperplasia and squamous metaplasia, two potentially preneoplastic lesions of the bladder (Figure 1). Analysis of the bladder transcriptome in this model demonstrated that dramatic decreases occur in the expression of genes important for urothelial differentiation and function [38]; these processes of de-differentiation may be relevant to bladder carcinogenesis and cancer progression [39]. Thus, it is possible that helminths such as S. haematobium may have co-evolved so closely with their human hosts that they specifically modulate host epithelial (de)differentiation to benefit their own survival and reproduction (reviewed in [40]). Regardless, our animal model provides a foothold for experimental investigation of the acute (and possibly some chronic) inflammatory changes induced in the bladder by S. haematobium infection. However, our model has important limitations, given that it features administration of a single egg bolus to mice, whereas naturally infected humans experience continuous bladder oviposition by worms. Although it is unknown how this central difference in oviposition affects the pathology observed in our model, in all likelihood it results in less chronic inflammation than natural infection. Therefore, room for improvement exists; a mouse model of worm-based oviposition in pelvic tissues would be better. Ultimately, the short life span of Mus musculus (~2 years) may constrain our ability to model schistosomal bladder cancer using mice, given that these neoplasms do not arise in S. haematobium-infected humans for decades [41–44].
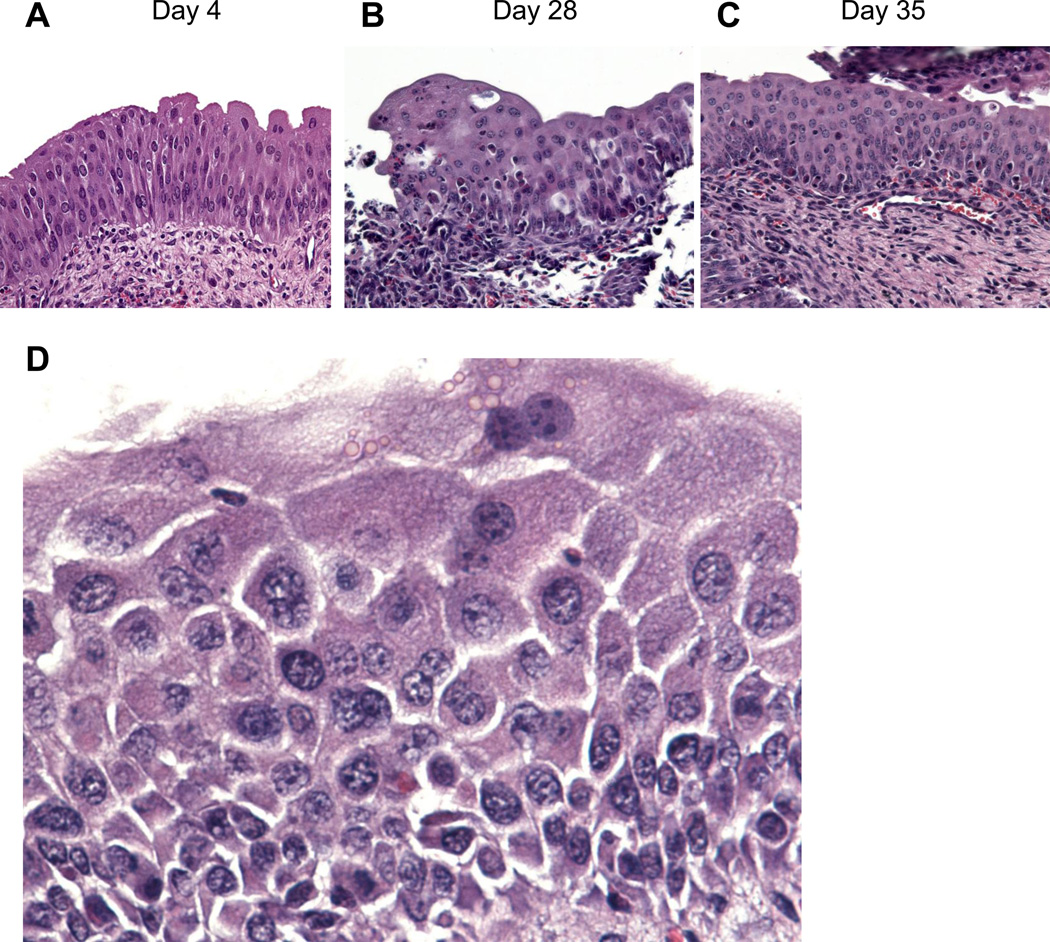
Figure 1.
Schistosoma haematobium eggs may induce mouse bladder preneoplasia. (A-C) egg injection of the mouse bladder wall induces early and sustained urothelial hyperplasia (urothelium >3 cells thick) with reactive nuclear changes. Adapted from Fu et al. [117]. (D) egg injection of the mouse bladder wall also induces squamous metaplasia, with typical signs of very fine, stratum spinosum-like spiny projections between cobblestone-resembling urothelial cells with foamy cytoplasm. Urothelial hyperplasia and squamous metaplasia may be preneoplastic lesions, in that they may be necessary but not sufficient for frank bladder carcinogenesis.
Regardless, the capacity to model the inflammatory aspects of S. haematobium infection may reveal mechanisms by which this infection facilitates bladder oncogenesis. Inflammation participates in both the initiation and progression of many cancers [45]. When taking up residence within particular host tissues, microbes and macro-organisms may initiate inflammation that can lead to eventual cancer formation [9,46,47]. While appropriate levels and forms of inflammation confer protection from pathogen dissemination during acute infection, persistent and dysregulated inflammation can create opportunities for malignant transformation of host cells. Continual tissue turnover increases risk of loss of genomic integrity in progenitor cells, and the ongoing wound healing response can construct a persistent microenvironment conducive to and immunologically tolerant of survival of cancerous cells [48–52]. The microenvironment and neoplastic cells can become fixed in positive feedback loops that perpetuate cancer and allow it to progress, invade adjacent tissue, and metastasize to distant sites.
Comparison of inflammation at different mucosal sites: the intestine and bladder
Our understanding of mechanisms involved in inflammation-associated bladder cancer significantly lags behind our understanding of inflammation-associated cancers of another epithelial organ, the intestinal tract. Given the prominence of lymphoid tissue structures found in association with the intestine and the substantial residential microbial community present in the intestinal lumen, the intestinal epithelium likely receives dramatically different inflammatory signals compared to the comparatively sterile bladder urothelium. The Peyer’s patches of the intestine are lymphoid structures which have recently been observed as preferential sites of egress for S. mansoni eggs to join the fecal stream [53], while it is arguable whether any substantial lymphoid structures exist in the human bladder save when bladder inflammation, or cystitis, is present [54]. Importantly, S. haematobium infection leads to profound and persistent aggregations of lymphoid cells in subepithelial regions of the bladder [55–57]. Thus, studying urogenital schistosomiasis may lead to a broader understanding of how chronic cystitis results in bladder carcinogenesis (reviewed in [58]).
To date, the effects of inflammatory signaling on urothelial cells remain poorly characterized. The transcriptional innate immune response of the urothelium to bacterial infection has been examined [59,60], but it remains to be seen what effects specific cytokines exert on the urothelium. This is relevant to understanding schistosomal bladder cancer considering that S. haematobium eggs are powerful inducers of host cytokine responses. STAT3 is a key transcription factor activated within cells by inflammatory signals and has been implicated in malignant transformation of the colon as a result of IL-6 signaling [61–64]. Constitutively active STAT3 in urothelial cells has been demonstrated to lead to invasive bladder cancer in a mouse model [65], but the inflammatory cues that might precipitate such signaling remain to be determined.
The effects of inflammation on epithelial regeneration and carcinogenesis are relevant to understanding epithelial neoplasms in general. Consequently, the unique structure of the intestinal crypt has been probed with lineage tracing tools to follow the contribution of various cell populations to epithelial regeneration in this tissue site. Recently, some approaches previously used to investigate the intestinal epithelium have been adapted to characterizing urothelial regeneration, resulting in important new insights. Mysorekar and colleagues demonstrated that bone morphogenetic protein 4 (Bmp4) plays an indispensable role in replenishment of bladder urothelium during bacterial injury, but not chemical injury with protamine sulfate [66]. Using inducible reporter systems to mark progenitor cells and their progeny, sonic-hedgehog (Shh) expressing basal cells were shown to reconstitute the urothelium after both bacterial infection and chemical injury [67]. We have performed lineage tracing experiments using multi-color reporter systems that suggest that clonal bladder tumors induced by chronic exposure to the compound N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN) arise exclusively from the Shh-expressing cell population [68]. In a different injury model based on use of cyclophosphamide, suprabasal ‘intermediate’ cells that form a portion of the Shh-expressing bladder cell population can contribute substantially to reconstitution of the adult urothelium [69], though it is untested whether these intermediate cells would contribute to tumor formation after BBN treatment.
Nevertheless, it is now becoming clear that the cells involved in the processes of homeostatic maintenance (turnover) and regeneration after epithelial injury can vary by tissue and type of injury (reviewed in [70]). Identifying the cell of origin involved in schistosomal bladder cancer could help answer the question of why S. haematobium infection is predominantly, though not exclusively, associated with squamous cell carcinoma (SCC) of the bladder, rather than the urothelial carcinoma type that is more prevalent worldwide and arises later in life in populations not exposed to S. haematobium 9,20]. It is not understood whether transdifferentiation of the urothelium to squamous metaplasia occurs prior to or subsequent to transformation [71–73]. Interestingly, squamous metaplasia is often observed in S. haematobium-infected bladders featuring SCC (Figure 2), providing some plausible support in favor of metaplasia being part of the neoplastic sequence (Figure 3). Defining this progression in the setting of urogenital schistosomiasis may be an important basis for improving diagnosis and treatment options.
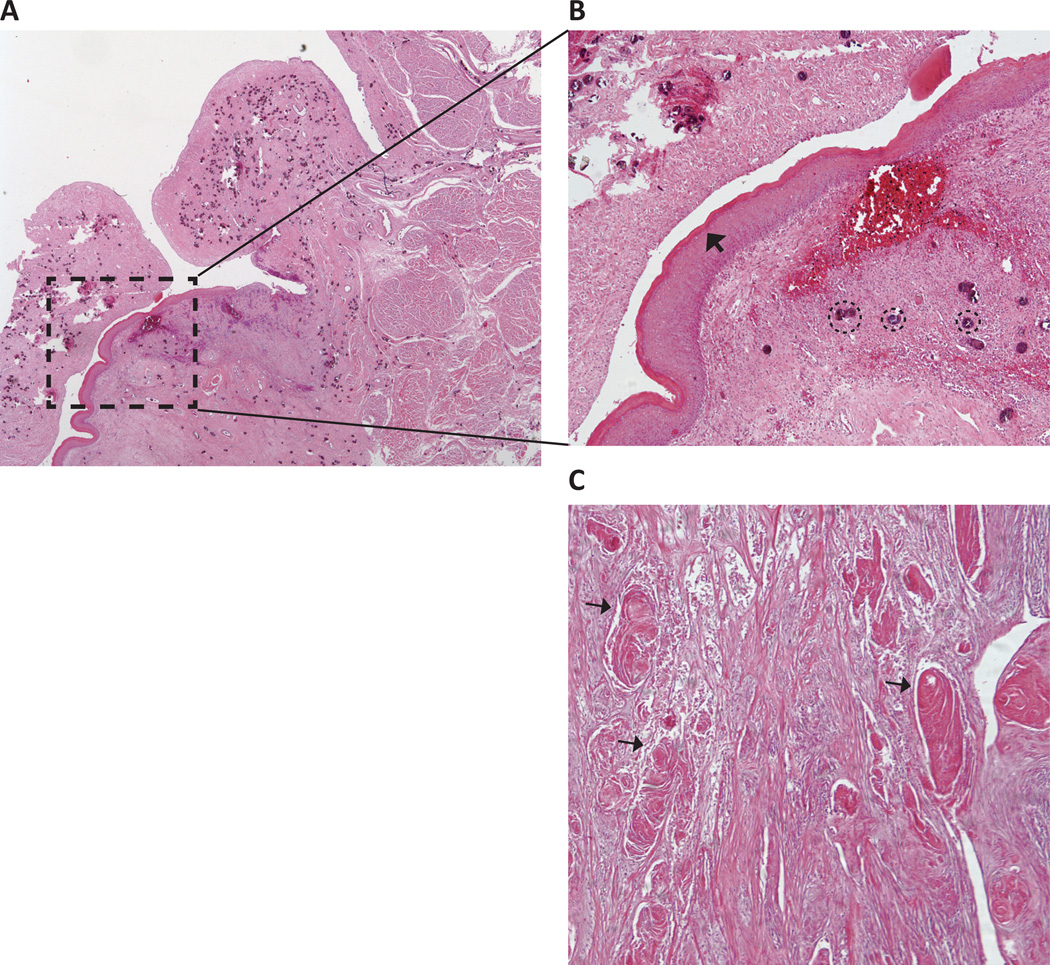
Figure 2.
Schistosoma haematobium-associated bladder squamous metaplasia and carcinoma. Micrographs from stained sections of a bladder with keratinized, moderately differentiated squamous cell carcinoma associated with urogenital schistosomiasis. (A) Low power view of bladder section. (B) Higher power view of area indicated by arrow in (A) demonstrating squamous metaplasia of the urothelium with infiltration of the lamina propria by a large number of S. haematobium ova (several eggs are circled as examples). In this specimen, the squamous metaplasia is evident as a hyperkeratotic squamous epithelium (arrowhead) lining the bladder lumen. (C) Another region of the same bladder exhibits abundant keratin pearls (examples indicated by arrows), a classic sign of squamous cell carcinoma.
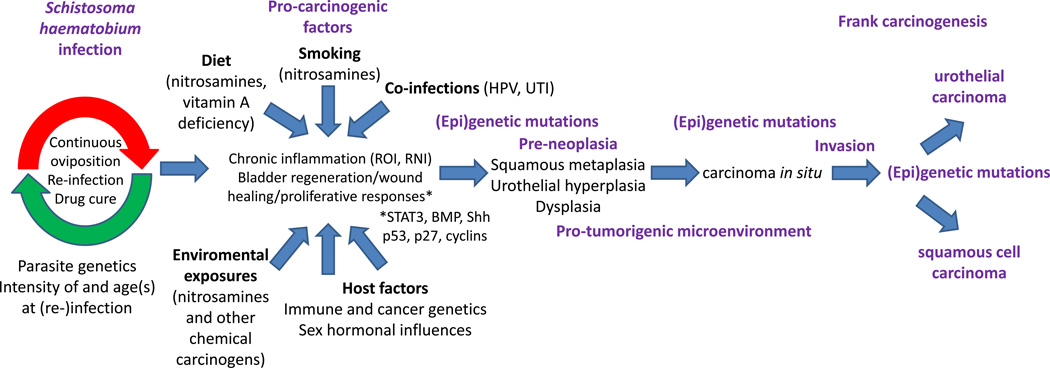
Figure 3.
Possible risks and pathways for schistosomal bladder cancer. Long-lived S. haematobium worms and re-infection of hosts by new parasites results in long-term egg deposition in the bladder. Oviposition kinetics are subject to modulation by S. haematobium strain genetics, intensity and host age(s) at parasite exposure, anthelminthic chemotherapy, and host immunity. Egg deposition in the bladder leads to chronic inflammation and host regenerative, proliferative, and wound healing responses (possibly STAT3, BMP, sonic hedgehog [Shh], p53, p27, and cyclin-mediated), all of which are modified by numerous cancer risk factors, such as diet (nitrosamines in beer and other fermented foods, micronutrient deficiencies), smoking (another source of nitrosamines and other carcinogens), co-infections by human papillomavirus (HPV) and/or bacterial uropathogens (urinary tract infections, UTI), exposure to environmental carcinogens (including nitrosamines and other occupational chemicals), and host polymorphisms in tumor suppressor genes, oncogenes, and immune function-related genes. Gender, in the form of sex hormones, may also be an important risk factor, given that males are more prone to bladder cancer in general. Genetic mutations and epigenetic alterations (i.e., DNA methylation, histone modification) accumulate in bladder urothelial cells, driving pre-neoplastic lesions such as squamous metaplasia, urothelial hyperplasia, and dysplasia. At the same time, a pro-tumorigenic microenvironment (both stromal and immunologic) arises in this context. As additional genetic and epigenetic abnormalities continue to develop, carcinoma in situ ensues, with eventual invasion and onset of urothelial or squamous cell carcinoma.
One pathway potentially involved in this progression involves retinoic acid (vitamin A), a lack of which results in squamous metaplasia of the bladder urothelium [72]. Indeed, regions endemic for schistosomiasis overlap extensively with areas known for widespread vitamin A deficiency. Thus, we speculate that many people exposed to S. haematobium may have vitamin A deficiency-associated squamous metaplasia of the bladder. The requirement for retinoic acid receptors in urothelial cells for the processes of urothelial formation and regeneration was recently demonstrated [69]. Further, the immune system deploys retinoic acid receptors for diverse, important functions such as control of trafficking of dendritic cell precursors to the intestine [74] and establishment of secondary and tertiary lymphoid organs by lymphoid tissue inducer (LTi) cells [75,76]. These diverse roles of vitamin A imply that it may be insightful to study how it shapes the relationship between the immune system and urothelial regeneration and differentiation in the context of S. haematobium infection. Thus, continued work on the dynamics of bladder urothelial regeneration may suggest experiments to explore how particular urothelial and immune cell populations interact in the context of S. haematobium egg-associated inflammation and carcinogenesis.
The role of co-infections in carcinogenesis
Akin to the challenge presented by a developing tumor, managing an active helminth infection presents the host with potential survival tradeoffs between the resistance and tolerance mechanisms available [77,78]. For example, the immunoregulatory dimension (IL-10 and TGF-β) of an established T helper 2 (Th2, type 2) response to helminths may delay or prevent clearance of viruses or bacteria that otherwise requires full initiation of T helper 1 (Th1, type 1) effector molecules and immune cells [79,80]. Schistosome infection may thus significantly alter susceptibility and progression of viral, bacterial or protozoan infections both locally and elsewhere in the body [81,82].
Viral and bacterial infections have been linked to initiation of cancers at a variety of different organ sites. Specific pathogenesis mechanisms used by these bacteria and viruses to invade or otherwise modulate host cells can facilitate transformation by destabilizing the host genome [83]. The inflammatory environment established by the presence of S. haematobium eggs in the bladder lamina propria, and especially the tolerizing influence of IL-10 and TGF-β during chronic phases of helminth infection more broadly [80], may counteract successful clearance of viruses or bacteria that take up residence in tissues such as the bladder.
In addition to the impact of viral integration on the host genome, viruses can promote cancer through expression of viral oncoproteins that may alter rates of cellular proliferation, suppress apoptosis, and increase genomic instability through interference with cell cycle regulation and DNA repair mechanisms. Several groups have investigated a possible role for human papillomavirus (HPV) involvement in schistosomal bladder cancer, with some studies finding potential associations [11,84,85] whereas samples from patient groups from other geographic regions do not show evidence for the HPV strains studied [86]. The definitive demonstration of HPV or other viral involvement in the etiology of schistosomal bladder cancer may be complicated by the possibility of “hit-and-run” scenarios where viruses transiently affect the genetic or epigenetic states of cells in a pro-tumorigenic manner, yet may be lost from subsequent tumors if viral persistence is not necessary for tumor survival [87]. Nevertheless, deep sequencing could be an unbiased approach to identifying persistent viruses that may be associated with schistosomal bladder cancers [88].
Some studies have observed an association in humans between S. haematobium infection and bacterial urinary tract co-infections, with evidence that levels of procarcinogenic N-nitrosamines in urine are increased [89]. Additional work by Hicks and colleagues suggest that sub-carcinogenic doses of nitrosamines are not sufficient to induce bladder cancer in baboons, except for those infected with S. haematobium 90]. This has implications for the potential role of nitrosamines in schistosomal bladder cancer. Specifically, if S. haematobium-bacterial uropathogen co-infection is common in humans and bacterial metabolism indeed leads to higher nitrosamine levels in the bladder, is bacterial co-infection sufficient in and of itself to induce bladder cancer in some individuals? Or do these individuals need exposure to additional nitrosamine sources, for example through tobacco products? Clearly, any application of the rodent model of nitrosamine-induced bladder cancer to animal models of urogenital schistosomiasis must be undertaken carefully. These issues underscore the potential complexity of the role of nitrosamines and microbial co-infection in schistosomal bladder cancer.
Carcinogenic effects of products from Schistosoma haematobium eggs
Schistosome eggs are well known for their ability to influence surrounding host tissues. For instance, soluble egg antigen (SEA) preparations from S. mansoni eggs have been studied for their effects on a variety of host cell types. Omega-1 protein from S. mansoni eggs shapes dendritic cell promotion of Th2 activation via protein internalization by the mannose receptor and subsequent degradation of messenger and ribosomal RNAs [91]. Another S. mansoni-derived egg protein with similar potential for entry into host cells is IPSE/alpha-1 [92], which also features other immunomodulatory properties [93]. Botelho and colleagues showed that SEA preparations from S. haematobium may have proliferative and genotoxic effects on urothelial cell cultures [94], potentially through effects on estrogen receptor activity [95]. Interpretation of these results, however, must be tempered by the knowledge that immortalized urothelial cells were used in this work, which by definition differs from normal urothelium. This work has similarities to observations made by Smout et al., who demonstrated that the carcinogenic liver fluke O. viverrini produces a homolog for granulin, a potent human growth factor implicated in cell proliferation and wound healing, that drives proliferation of host cells [96].
The recent genomic and transcriptomic characterization of S. haematobium adult worms and eggs [23] may greatly accelerate identification and characterization of S. haematobium-specific factors that may be involved in cancer induction. Ongoing improvements in the annotation of the genome will further drive a deeper understanding of procarcinogenic schistosome products. Co-culture of these parasite products with urothelial cells will facilitate screening assays for proliferative, transcriptional, epigenetic, and metabolic endpoints consistent with cancer phenotypes. Targets worthy of deeper scrutiny could then be tested for in vivo activity by injecting transgenic eggs [22] into the mouse bladder wall [21]. Physiologic validation of candidate procarcinogenic schistosome products will be essential to understanding schistosomal bladder cancer.
Mapping the genetic and transcriptional landscape of human schistosomal bladder cancer
Bladder cancers are generally understood to group into somewhat exclusive phenotypes based on FGFR3 and TP53 mutations. Whereas the former cancers tend to be recurrent but superficial and non-muscle invasive, the latter set of neoplasms exhibit a propensity for rapid development of carcinoma in situ and progression to muscle invasion [97,98]. Most of this body of research has been based on investigation of non-schistosomal bladder cancer cases in developed countries. The association of decreasing prevalence of squamous cell carcinoma (SCC) of the bladder in Egypt with falling schistosome infection rates suggests a link between urogenital schistosomiasis and bladder cancer histology [99]. Shifts in agricultural employment, pesticide and urbanization may be increasingly powerful factors in observed changes in bladder cancer risk and histology within Egypt [100], underscoring the complex interactions involved in schistosomal bladder cancer.
Our knowledge to date about the particular pathways altered in schistosomal bladder cancer has been based on immunohistochemical analyses of formalin-fixed, paraffin-embedded tumor specimens. Examination of markers involved in SCC (schistosomal and non-schistosomal) have suggested possible discriminatory power for p53, p27, and Ki67, along with various cyclins and cytokeratins [101–104].
Though informative, these immunohistochemistry approaches are low-throughput, require validated reagents, and rely on prior knowledge about potentially relevant pathways. Moreover, these analyses typically focus on well-established, end-stage cancers that have accumulated numerous mutations, with little or no evidence of causality or sequence. Significant molecular complexity may remain unobserved. The efforts to uncover this complexity has driven the formation of research consortia to apply high-throughput sequencing to analyze the exomic, transcriptomic, epigenomic, and genetic architecture of a broad range of cancers (i.e., the Cancer Genome Atlas: http://cancergenome.nih.gov/ and the International Cancer Genome Consortium: https://www.icgc.org/), including urothelial cell carcinoma of the bladder [105]. Sadly, as of this review, schistosomal bladder cancer is not currently one of the diseases targeted by these consortia. Applying high-throughput sequencing to non-schistosomal urothelial carcinomas of the bladder has already suggested new markers that may aid in prognosis and guide treatment [106–109]. Comparing squamous and urothelial forms of schistosomal bladder cancer with bladder cancers of other etiologies could provide important new insights into the relative contributions of S. haematobium products, possible viral or bacterial co-infections, and inflammation on cancer induction or progression. Further, genomic sequences of schistosomal bladder cancers could be compared to squamous cell carcinomas from other tissues [110–112], which may suggest common and unique pathways at work in these aggressive cancers. Earlier sequencing technologies were applied to cholangiocarcinomas associated with O. viverrini infection and have documented many previously unrecognized mutations in this form of cancer [7]. With new sequencing and analysis approaches allowing comprehensive measurements of complex genetic rearrangements [113], it is now possible to gain useful insights into the myriad changes occurring in tumors such as schistosomal bladder cancers [114].
Concluding remarks and future perspectives
The initiation and progression of schistosomal bladder cancer is almost certainly a complex and multi-step process, involving the actions of the parasite, the host immune and tissue regenerative response, and possible participation of co-infections, micronutrients (e.g., vitamin A), and environmental exposures to carcinogens. Superimposed on these processes are background factors consisting of parasite genetic diversity, individual variations in intensity and recurrence of infection, host genetic diversity (including polymorphic loci that affect immune responses or susceptibility to cancer), and sex differences in immune and urogenital biology. Though animal models will never recapitulate all aspects of these complex processes, appropriate application of such models will play an important role in efforts to isolate and characterize the pathways involved in schistosomal bladder cancer.
Unbiased molecular approaches will be critical to generating hypotheses about the transformation processes that gives rise to human bladder cancers associated with urogenital schistosomiasis. The sequencing approaches described above, while useful in addressing this need, face the ongoing challenges of reliably reducing false positives and correctly identifying driver versus passenger mutations [115]. Again, improved animal models of urogenital schistosomiasis will play an important role in demonstrating the relevance of candidate cancer mechanisms.
Further investigation into the immunological properties of the mammalian bladder will clarify its shared and unique properties with the immunologically distinct epithelial sites of the skin, gut, and lung. By investigating the behavior of key morphogenetic pathways (Wnt, Notch, Shh) in progenitor cell populations during inflammatory responses in the bladder, we may begin to characterize how urothelial regeneration becomes neoplastic during chronic S. haematobium infection, with implications for our understanding of other inflammation-associated cancers that arise from epithelial cells.
In contrast to the perception of urogenital schistosomiasis being an exotic infectious disease of marginal importance, Schistosoma haematobium may infect nearly 200 million people worldwide [25]. Given that bladder cancer may be the most expensive neoplasm to manage from diagnosis to death, in large part due to its recurrent nature [116], it makes sense from both the economic and human perspectives to invest significant, further effort in understanding schistosomal bladder cancer in order to improve diagnostic and therapeutic approaches.
Highlights.
The causal link between S. haematobium infection and bladder cancer is accepted
The mechanisms of schistosomal bladder cancer (SBC) are poorly understood
Newly identified bladder regeneration pathways may be relevant mechanisms to SBC
New mouse models and genomics methods may expand knowledge of SBC
Acknowledgements
We gratefully acknowledge the assistance of Justin Odegaard with micrograph preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferguson AR. Associated bilharziosis and primary malignant disease of the urinary bladder, with observations on a series of forty cases. J. Pathol. Bacteriol. 1911;16:76–94. [Google Scholar]
- 2.Gelfand M, et al. Relation between carcinoma of the bladder and infestation with Schistosoma hæmatobium . Lancet. 1967;289:1249–1251. doi: 10.1016/s0140-6736(67)92714-6. [DOI] [PubMed] [Google Scholar]
- 3.Thomas JE, et al. Relationship between bladder cancer incidence, Schistosoma haematobium infection, and geographical region in Zimbabwe. TransRSoc. Trop. Med. Hyg. 1990;84:551–553. doi: 10.1016/0035-9203(90)90036-e. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo E. Association Between Schistosomiasis and Cancer: A Review. Infect. Dis. Clin. Pract. 2007;15:145–148. [Google Scholar]
- 5.Organization WH. Evaluation of carcinogenic risk to humans. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr. 61: 45–119. IARC Monogr. 1994;61 [PMC free article] [PubMed] [Google Scholar]
- 6.Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 2012;100:1–441. [PMC free article] [PubMed] [Google Scholar]
- 7.Ong CK, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat. Genet. 2012;44:690–693. doi: 10.1038/ng.2273. [DOI] [PubMed] [Google Scholar]
- 8.Sripa B, et al. The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends Parasitol. 2012;28:395–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vennervald BJ, Polman K. Helminths and malignancy. Parasite Immunol. 2009;31:686–696. doi: 10.1111/j.1365-3024.2009.01163.x. [DOI] [PubMed] [Google Scholar]
- 10.Plieskatt JL, et al. Infection with the carcinogenic liver fluke Opisthorchis viverrini modifies intestinal and biliary microbiome. FASEB J. 2013;27:4572–4584. doi: 10.1096/fj.13-232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khaled HM, et al. Human papilloma virus infection and overexpression of p53 protein in bilharzial bladder cancer. Tumori. 2001;87:256–261. doi: 10.1177/030089160108700409. [DOI] [PubMed] [Google Scholar]
- 12.Helal TEa, et al. Human papilloma virus and p53 expression in bladder cancer in Egypt: relationship to schistosomiasis and clinicopathologic factors. Pathol. Oncol. Res. 2006;12:173–178. doi: 10.1007/BF02893365. [DOI] [PubMed] [Google Scholar]
- 13.Adeyeba OA, Ojeaga SGT. Urinary schistosomiasis and concomitant urinary tract pathogens among school children in metropolitan Ibadan. Afr J Biomed Res. 2002;5:103–108. [Google Scholar]
- 14.Laughlin LW, et al. Bacteriuria in urinary schistosomiasis in Egypt a prevalence survey. AmJTrop. Med. Hyg. 1978;27:916–918. doi: 10.4269/ajtmh.1978.27.916. [DOI] [PubMed] [Google Scholar]
- 15.Uneke CJ, et al. An Assessment of Schistosoma haematobium infection and urinary tract bacterial infection amongst school children in rural eastern Nigeria - ISPUB. [Online] Available: http://archive.ispub.com/journal/the-internet-journal-of-laboratory-medicine/volume-4-number-1/an-assessment-of-Schistosoma-haematobium-infection-and-urinary-tract-bacterial-infectionamongst-school-children-in-rural-eastern-nigeria-2.html#sthash.VyTbYQ5c.dpbs. [Google Scholar]
- 16.Stelekati E, Wherry EJ. Chronic bystander infections and immunity to unrelated antigens. Cell Host Microbe. 2012;12:458–469. doi: 10.1016/j.chom.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma N, et al. Nitrative DNA damage and Oct3/4 expression in urinary bladder cancer with Schistosoma haematobium infection. Biochem. Biophys. Res. Commun. 2011;414:344–349. doi: 10.1016/j.bbrc.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 18.Vennervald BJ, Polman K. Helminths and malignancy. Parasite Immunol. 2009;31:686–696. doi: 10.1111/j.1365-3024.2009.01163.x. [DOI] [PubMed] [Google Scholar]
- 19.Bedwani R, et al. Epidemiology of bladder cancer in Alexandria, Egypt: Tobacco smoking. IntJCancer. 1997;73:64–67. doi: 10.1002/(sici)1097-0215(19970926)73:1<64::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Mostafa MH, et al. Relationship between Schistosomiasis and Bladder Cancer. Clin. Microbiol. Rev. 1999;12:97–111. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu C-L, et al. A Novel Mouse Model of Schistosoma haematobium Egg-Induced Immunopathology. PLoS Pathog. 2012;8:e1002605. doi: 10.1371/journal.ppat.1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinaldi G, et al. Genetic Manipulation of Schistosoma haematobium the Neglected Schistosome. PLoS Negl Trop Dis. 2011;5:e1348. doi: 10.1371/journal.pntd.0001348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young ND, et al. Whole-genome sequence of Schistosoma haematobium . Nat. Genet. 2012;44:221–225. doi: 10.1038/ng.1065. [DOI] [PubMed] [Google Scholar]
- 24.Barsoum RS, et al. Human Schistosomiasis: Clinical Perspective: Review. J. Adv. Res. 2013;4:433–444. doi: 10.1016/j.jare.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gryseels B, et al. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 26.Burke ML, et al. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31:163–176. doi: 10.1111/j.1365-3024.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 27.Cheever AW, et al. The Relation of Worm Burden to Passage of Schistosoma haematobium Eggs in the Urine of Infected Patients. AmJTrop. Med. Hyg. 1975;24:284–288. doi: 10.4269/ajtmh.1975.24.284. [DOI] [PubMed] [Google Scholar]
- 28.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 29.Chan CY, et al. Mast Cell Interleukin-10 Drives Localized Tolerance in Chronic Bladder Infection. Immunity. 2013;38:349–359. doi: 10.1016/j.immuni.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheever AW. Schistosoma haematobium: The pathology of experimental infection. Exp. Parasitol. 1985;59:131–138. doi: 10.1016/0014-4894(85)90065-7. [DOI] [PubMed] [Google Scholar]
- 31.Rheinberg CE, et al. Schistosoma haematobium, S. intercalatum, S. japonicum, S. mansoni and S. rodhaini in mice: relationship between patterns of lung migration by schistosomula and perfusion recovery of adult worms. Parasitol. Res. 1998;84:338–342. doi: 10.1007/s004360050407. [DOI] [PubMed] [Google Scholar]
- 32.Vuong PN, et al. Histopathological observations in new and classic models of experimental Schistosoma haematobiurn infections. Trop. Med. Int. Heal. 1996;1:348–358. doi: 10.1046/j.1365-3156.1996.d01-52.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, et al. Effect of Clonorchis sinensis infection and dimethylnitrosamine administration on the induction of cholangiocarcinoma in Syrian golden hamsters. Korean J. Parasitol. 1993;31:21–30. doi: 10.3347/kjp.1993.31.1.21. [DOI] [PubMed] [Google Scholar]
- 34.Thamavit W, et al. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978;38:4634–4639. [PubMed] [Google Scholar]
- 35.Thamavit W, et al. Level of Opisthorchis infestation and carcinogen dose-dependence of cholangiocarcinoma induction in Syrian golden hamsters. Virchows ArchBCell Pathol. Incl. Mol. Pathol. 1987;54:52–58. doi: 10.1007/BF02899196. [DOI] [PubMed] [Google Scholar]
- 36.Sauer UG, et al. Ethical review of projects involving non-human primates funded under the European Union’s 7th Research Framework Programme. ATLA Altern. to Lab. Anim. 2013;41:271–306. doi: 10.1177/026119291304100405. [DOI] [PubMed] [Google Scholar]
- 37.Fu C-LL, et al. Mouse bladder wall injection. J. Vis. Exp. 2011 doi: 10.3791/2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray D, et al. Transcriptional Profiling of the Bladder in Urogenital Schistosomiasis Reveals Pathways of Inflammatory Fibrosis and Urothelial Compromise. PLoS Negl Trop Dis. 2012;6:e1912. doi: 10.1371/journal.pntd.0001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khandelwal P, et al. Cell biology and physiology of the uroepithelium. AmJPhysiol. Renal Physiol. 2009;297:F1477–F1501. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyett D, Hsieh MH. Wormholes in Host Defense: How Helminths Manipulate Host Tissues to Survive and Reproduce. PLoS Pathog. 2014;10:4. doi: 10.1371/journal.ppat.1004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas JE, et al. Relationship between bladder cancer incidence, Schistosoma haematobium infection, and geographical region in Zimbabwe. TransRSoc. Trop. Med. Hyg. 1990;84:551–553. doi: 10.1016/0035-9203(90)90036-e. [DOI] [PubMed] [Google Scholar]
- 42.Fukushima S, et al. Comparative study of urinary bladder carcinomas in Japanese and Egyptians. Acta Pathol. Jpn. 1989;39:176–179. doi: 10.1111/j.1440-1827.1989.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz DA. Helminths in the induction of cancer II. Schistosoma haematobium and bladder cancer. Trop. Geogr. Med. 1981;33:1–7. [PubMed] [Google Scholar]
- 44.Bhagwandeen SB. Schistosomiasis and carcinoma of the bladder in Zambia. S. Afr. Med. J. 1976;50:1616–1620. [PubMed] [Google Scholar]
- 45.Grivennikov SI, et al. Immunity, Inflammation, and Cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porta C, et al. Mechanisms linking pathogen-associated inflammation and cancer. Cancer Lett. 2011;305:250–262. doi: 10.1016/j.canlet.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Cleary AS, et al. Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature. 2014;508:113–117. doi: 10.1038/nature13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanahan D, Coussens LM. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 50.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 51.Horst Gvan der, et al. Epithelial Plasticity, Cancer Stem Cells, and the Tumor-Supportive Stroma in Bladder Carcinoma. Mol. Cancer Res. 2012;10:995–1009. doi: 10.1158/1541-7786.MCR-12-0274. [DOI] [PubMed] [Google Scholar]
- 52.Rosin MP, et al. Inflammation, Chromosomal Instability, and Cancer: The Schistosomiasis Model. Cancer Res. 1994;54:1929s–1933s. [PubMed] [Google Scholar]
- 53.Turner JD, et al. Blood Flukes Exploit Peyer’s Patch Lymphoid Tissue to Facilitate Transmission from the Mammalian Host. PLoS Pathog. 2012;8:e1003063. doi: 10.1371/journal.ppat.1003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng L, et al. Bladder Pathology. John Wiley & Sons, Inc; 2012. Inflammatory and Infectious Conditions; pp. 17–44. [Google Scholar]
- 55.Kehinde EO, et al. Parasites of urological importance. Urol. Int. 2008;81:1–13. doi: 10.1159/000137633. [DOI] [PubMed] [Google Scholar]
- 56.Elliott DE. Schistosomiasis. Pathophysiology, diagnosis, and treatment. Gastroenterol. Clin. North Am. 1996;25:599–625. doi: 10.1016/s0889-8553(05)70265-x. [DOI] [PubMed] [Google Scholar]
- 57.Ghoneim Ma. Bilharziasis of the genitourinary tract. BJU Int. 2002;89 Suppl 1:22–30. doi: 10.1046/j.1464-4096.2001.138.138.x. [DOI] [PubMed] [Google Scholar]
- 58.Payne R, Hsieh M. Reinforcements arrive for the war against chronic cystitis and bladder cancer. BJU Int. 2012;110:1223–1224. doi: 10.1111/j.1464-410X.2012.11417.x. [DOI] [PubMed] [Google Scholar]
- 59.Billips BK, et al. Modulation of Host Innate Immune Response in the Bladder by Uropathogenic Escherichia coli . Infect. Immun. 2007;75:5353–5360. doi: 10.1128/IAI.00922-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duell BL, et al. Innate Transcriptional Networks Activated in Bladder in Response to Uropathogenic Escherichia coli Drive Diverse Biological Pathways and Rapid Synthesis of IL-10 for Defense against Bacterial Urinary Tract Infection. J. Immunol. 2012;188:781–792. doi: 10.4049/jimmunol.1101231. [DOI] [PubMed] [Google Scholar]
- 61.Grivennikov S, et al. IL-6 and Stat3 Are Required for Survival of Intestinal Epithelial Cells and Development of Colitis-Associated Cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H, et al. Persistently Activated Stat3 Maintains Constitutive NF-κB Activity in Tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, et al. IL-17 can promote tumor growth through an IL-6–Stat3 signaling pathway. J. Exp. Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu H, et al. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ho PL, et al. Stat3 Activation in Urothelial Stem Cells Leads to Direct Progression to Invasive Bladder Cancer. Cancer Res. 2012;72:3135–3142. doi: 10.1158/0008-5472.CAN-11-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mysorekar IU, et al. Bone Morphogenetic Protein 4 Signaling Regulates Epithelial Renewal in the Urinary Tract in Response to Uropathogenic Infection. Cell Host Microbe. 2009;5:463–475. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin K, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shin K, et al. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat. Cell Biol. 2014 doi: 10.1038/ncb2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gandhi D, et al. Retinoid Signaling in Progenitors Controls Specification and Regeneration of the Urothelium. Dev. Cell. 2013;26:469–482. doi: 10.1016/j.devcel.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doupé DP, Jones PH. Cycling progenitors maintain epithelia while diverse cell types contribute to repair. BioEssays. 2013;35:443–451. doi: 10.1002/bies.201200166. [DOI] [PubMed] [Google Scholar]
- 71.Baithun S, et al. Squamous change in bladder cancer and its relevance to understanding clonal evolution in development of bladder cancer. Cancer Surv. 1998;31:17–27. [PubMed] [Google Scholar]
- 72.Liang F-X, et al. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J. Cell Biol. 2005;171:835–844. doi: 10.1083/jcb.200505035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu R-L, et al. Uroplakin II Gene Is Expressed in Transitional Cell Carcinoma But Not in Bilharzial Bladder Squamous Cell Carcinoma: Alternative Pathways of Bladder Epithelial Differentiation and Tumor Formation. Cancer Res. 1998;58:1291–1297. [PubMed] [Google Scholar]
- 74.Zeng R, et al. Retinoic acid regulates the development of a gut-homing precursor for intestinal dendritic cells. Mucosal Immunol. 2013;6:847–856. doi: 10.1038/mi.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 76.Van de Pavert SA, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ayres JS, Schneider DS. Tolerance of Infections. Annu. Rev. Immunol. 2012;30:271–294. doi: 10.1146/annurev-immunol-020711-075030. [DOI] [PubMed] [Google Scholar]
- 78.Goldszmid RS, et al. Host Immune Response to Infection and Cancer: Unexpected Commonalities. Cell Host Microbe. 2014;15:295–305. doi: 10.1016/j.chom.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Potian JA, et al. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J. Exp. Med. 2011;208:1863–1874. doi: 10.1084/jem.20091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salgame P, et al. Effect of helminth-induced immunity on infections with microbial pathogens. Nat. Immunol. 2013;14:1118–1126. doi: 10.1038/ni.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abruzzi A, Fried B. Chapter 1 - Coinfection of Schistosoma (Trematoda) with Bacteria, Protozoa and Helminths. In: Hay DR, S.I., editors. Advances in Parasitology. Volume 77. Academic Press; 2011. pp. 1–85. [DOI] [PubMed] [Google Scholar]
- 82.Brindley PJ, Hotez PJ. Break Out: urogenital schistosomiasis and Schistosoma haematobium infection in the post-genomic era. PLoS Negl. Trop. Dis. 2013;7:e1961. doi: 10.1371/journal.pntd.0001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weitzman MD, Weitzman JB. What’s the Damage? The Impact of Pathogens on Pathways that Maintain Host Genome Integrity. Cell Host Microbe. 2014;15:283–294. doi: 10.1016/j.chom.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khaled HM, et al. Correlation between p53 mutations and HPV in bilharzial bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2003;21:334–341. doi: 10.1016/s1078-1439(03)00014-0. [DOI] [PubMed] [Google Scholar]
- 85.Shaker OG, et al. Is there a correlation between HPV and urinary bladder carcinoma? Biomed. Pharmacother. 2013;67:183–191. doi: 10.1016/j.biopha.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 86.Cooper K, et al. Human papillomavirus and schistosomiasis associated bladder cancer. Mol. Pathol. 1997;50:145–148. doi: 10.1136/mp.50.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Niller HH, et al. Viral hit and run-oncogenesis: Genetic and epigenetic scenarios. Cancer Lett. 2011;305:200–217. doi: 10.1016/j.canlet.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 88.Ding D, et al. Recurrent Targeted Genes of Hepatitis B Virus in the Liver Cancer Genomes Identified by a Next-Generation Sequencing–Based Approach. PLoS Genet. 2012;8:e1003065. doi: 10.1371/journal.pgen.1003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hicks RM, et al. Association of bacteriuria and urinary nitrosamine formation with Schistosoma haematobium infection in the Qalyub area of Egypt. Trans R Soc Trop Med Hyg. 1982;76:519–527. doi: 10.1016/0035-9203(82)90153-5. [DOI] [PubMed] [Google Scholar]
- 90.Hicks RM, et al. Effect of Schistosoma haematobium and N-butyl-N-(4-hydroxybutyl)nitrosamine on the development of urothelial neoplasia in the baboon. Br J Cancer. 1980;42:730–755. doi: 10.1038/bjc.1980.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Everts B, et al. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J. Exp. Med. 2012;209:1753–1767. S1. doi: 10.1084/jem.20111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaur I, et al. Interleukin-4-Inducing Principle from Schistosoma mansoni Eggs Contains a Functional C-Terminal Nuclear Localization Signal Necessary for Nuclear Translocation in Mammalian Cells but Not for Its Uptake. Infect. Immun. 2011;79:1779–1788. doi: 10.1128/IAI.01048-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schramm G, et al. IPSE/alpha-1: a major immunogenic component secreted from Schistosoma mansoni eggs. Mol. Biochem. Parasitol. 2006;147:9–19. doi: 10.1016/j.molbiopara.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Botelho MC, et al. Tumour-like phenotypes in urothelial cells after exposure to antigens from eggs of Schistosoma haematobium: An oestrogen–DNA adducts mediated pathway? IntJParasitol. 2013;43:17–26. doi: 10.1016/j.ijpara.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 95.Botelho M, et al. Inactivation of estrogen receptor by Schistosoma haematobium total antigen in bladder urothelial cells. Oncology Reports. 2011 doi: 10.3892/or.2011.1552. [DOI] [PubMed] [Google Scholar]
- 96.Smout MJ, et al. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini promotes proliferation of host cells. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neuzillet Y, et al. A Meta-Analysis of the Relationship between FGFR3 and TP53 Mutations in Bladder Cancer. PLoS One. 2012;7:e48993. doi: 10.1371/journal.pone.0048993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rhijn BWG. van, et al. FGFR3 and P53 Characterize Alternative Genetic Pathways in the Pathogenesis of Urothelial Cell Carcinoma. Cancer Res. 2004;64:1911–1914. doi: 10.1158/0008-5472.can-03-2421. [DOI] [PubMed] [Google Scholar]
- 99.Salem HK, Mahfouz S. Changing Patterns (Age, Incidence, and Pathologic Types) of Schistosoma-associated Bladder Cancer in Egypt in the Past Decade. Urology. 2012;79:379–383. doi: 10.1016/j.urology.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 100.Zarzour AH, et al. Muscle invasive bladder cancer in Upper Egypt: the shift in risk factors and tumor characteristics. BMC Cancer. 2008;8:250. doi: 10.1186/1471-2407-8-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abdulamir AS, et al. Tumor markers of bladder cancer: the schistosomal bladder tumors versus non-schistosomal bladder tumors. J. Exp. Clin. Cancer Res. 2009;28:27. doi: 10.1186/1756-9966-28-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gaisa NT, et al. Different immunohistochemical and ultrastructural phenotypes of squamous differentiation in bladder cancer. Virchows Arch. 2011;458:301–312. doi: 10.1007/s00428-010-1017-2. [DOI] [PubMed] [Google Scholar]
- 103.Rausch S, et al. Squamous cell carcinogenesis and squamous cell carcinoma of the urinary bladder: A contemporary review with focus on nonbilharzial squamous cell carcinoma. Urol. Oncol. 2013 doi: 10.1016/j.urolonc.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 104.Youssef RF, et al. Expression of cell cycle–related molecular markers in patients treated with radical cystectomy for squamous cell carcinoma of the bladder. Hum. Pathol. 2011;42:347–355. doi: 10.1016/j.humpath.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 105.Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Balbás-Martínez C, et al. Recurrent inactivation of STAG2 in bladder cancer is not associated with aneuploidy. Nat. Genet. 2013;45:1464–1469. doi: 10.1038/ng.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choi W, et al. Identification of Distinct Basal and Luminal Subtypes of Muscle-Invasive Bladder Cancer with Different Sensitivities to Frontline Chemotherapy. Cancer Cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gui Y, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat. Genet. 2011;43:875–878. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Solomon DA, et al. Frequent truncating mutations of STAG2 in bladder cancer. Nat. Genet. 2013;45:1428–1430. doi: 10.1038/ng.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Justilien V, et al. The PRKCI and SOX2 Oncogenes Are Coamplified and Cooperate to Activate Hedgehog Signaling in Lung Squamous Cell Carcinoma. Cancer Cell. 2014;25:139–151. doi: 10.1016/j.ccr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91–95. doi: 10.1038/nature13176. [DOI] [PubMed] [Google Scholar]
- 112.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McPherson A, et al. nFuse: Discovery of complex genomic rearrangements in cancer using high-throughput sequencing. Genome Res. 2012;22:2250–2261. doi: 10.1101/gr.136572.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meyerson M, et al. Advances in understanding cancer genomes through second-generation sequencing. Nat. Rev. Genet. 2010;11:685–696. doi: 10.1038/nrg2841. [DOI] [PubMed] [Google Scholar]
- 115.Marx V. Cancer genomes: discerning drivers from passengers. Nat. Methods. 2014;11:375–379. doi: 10.1038/nmeth.2891. [DOI] [PubMed] [Google Scholar]
- 116.Kaufman DS, et al. Bladder cancer. Lancet. 2009;374:239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 117.Fu C-L, et al. A Novel Mouse Model of Schistosoma haematobium Egg-Induced Immunopathology. PLoS Pathog. 2012;8:e1002605. doi: 10.1371/journal.ppat.1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]