Abstract
This contribution addresses four potential misconceptions associated with high-resolution dynamic nuclear polarization/magic angle spinning (DNP/MAS) experiments. First, spectral resolution is not generally compromised at the cryogenic temperatures at which DNP experiments are performed. As we demonstrate at a modest field of 9 T (380 MHz 1H), 1 ppm linewidths are observed in DNP/MAS spectra of a membrane protein in its native lipid bilayer, and <0.4 ppm linewidths are reported in a crystalline peptide at 85 K. Second, we address the concerns about paramagnetic broadening in DNP/MAS spectra of proteins by demonstrating that the exogenous radical polarizing agents utilized for DNP are distributed in the sample in such a manner as to avoid paramagnetic broadening and thus maintain full spectral resolution. Third, the enhanced polarization is not localized around the polarizing agent, but rather is effectively and uniformly dispersed throughout the sample, even in the case of membrane proteins. Fourth, the distribution of polarization from the electron spins mediated via spin diffusion between 1H–1H strongly dipolar coupled spins is so rapid that shorter magnetization recovery periods between signal averaging transients can be utilized in DNP/MAS experiments than in typical experiments performed at ambient temperature.
Introduction
Dynamic nuclear polarization (DNP) increases the polarization in the bulk nuclear spin bath by 2–3 orders of magnitude,1–3 and thus results in a significant gain in the sensitivity of NMR experiments. Concurrently, the excellent resolution available with contemporary magic angle spinning (MAS) instrumentation and methods is not compromised by DNP, establishing DNP/MAS as a sensitive and high-resolution spectroscopic technique of great utility for structural characterization of membrane proteins in lipid bilayers,2, 4 amyloid fibrils,5 and crystalline peptides and proteins.6 The enhanced polarization can be exploited synergistically with recent advances in solidstate NMR methodology that has made possible assignments of membrane proteins7 and high-quality structure determination of amyloid fibrils8 and microcrystalline protein.9
Generally, unpaired electrons in stable, soluble, exogenously introduced free radicals are the source of the high electron Boltzman polarization for DNP experiments.10 In particular, biradicals are used as polarizing agents at high field because they can take full advantage of the cross effect (CE), an electron-nuclear polarization transfer mechanism that performs well at high frequencies.11 These polarizing agents can be used at sufficiently low concentration to be statistically separated from the spins of interest, and are therefore not in direct dipolar contact with the bulk nuclei detected in MAS NMR experiments. Nevertheless, they still perform as effective sources of polarization. On the other hand, high concentrations of radicals can be used when they are sterically excluded from the sites of interest.6 In either case, the absence of direct electron-nuclear dipolar couplings between the unpaired electron spins and the detected nuclear spins has important implications.
First, an efficient transfer scheme is required to distribute the high polarization from the shell of directly electron-coupled nuclear spins to the bulk of the sample. In the case of protonated biological samples, the ~100 M concentration of 1H spins with their high gyromagnetic ratio provide a sufficiently strongly coupled spin bath to uniformly distribute polarization throughout the medium, even if the sample is a heterogenous mixture comprised of cryoprotectant and membrane protein embedded in native lipid bilayers.12 Proton spin diffusion distributes the polarization originating from the electrons to the entirety of the sample with a timescale of hundreds ofmilliseconds, thereby permitting short magnetization recovery delays between signal averaging transients.
Also, because the 1H spin diffusion and subsequent cross polarization (CP) polarize nuclei that are well separated from the biradicals, spectral resolution is not compromised by the presence of the paramagnetic polarizing agents. Due to the relatively low gyromagnetic ratio of the 15N and 13C, the nuclei most commonly detected in biomolecular MAS experiments, the paramagnetic electron spin must be relatively close (~10 Å) to induce pseudo-contact shifts and line broadening. In fact, Nadaud and colleagues recently showed that even when a nitroxide label is covalently bound to a small globular protein, only spins in relatively close proximity are affected by the spin label.13 In the case of most DNP experiments, the biradicals are simply present in the matrix, not covalently bound to the protein of interest, and thus not in close spatial proximity to the detected spins.
However, even with a very efficient spin-diffusion process, the temperature must still be lowered to ≤100 K to lengthen the 1H T1 relaxation time sufficiently for relayed transfer of enhanced magnetization throughout the sample and also extend the relaxation properties of the electron spins participating in the initial polarization transfer. The resolution of MAS spectra at these temperatures is a point of concern in the solid state NMR community. However, although the resolution of some spectra degrade at low temperatures, either due to improper cryoprotection or trapped conformational heterogeneity, we demonstrate here that high-resolution spectra of a membrane protein and crystalline peptides can be acquired at temperatures below 100 K. We also show spectra of a peptide in which some of the resonances heterogenously broaden at lower temperature, while other resonances remain narrow.
Experimental
Membrane preparation
[14–13C]retinal, [ε-13C]lysine-labeled bacteriorhodopsin (bR) was obtained as indicated by Griffiths et al.14 [ζ-15N]lysine-labeled bR was obtained as described by Argade et al.15 Uniformly 13C, 15N-labeled bR was obtained as set out by Bajaj et al.16 The purple membranes were isolated according to the method of Oesterhelt and Stoeckenius,17 washed in 0.3 M guanidinium hydrochloride at pH 10, until the supernatant had the same pH, and then washed further with 60 vol% d8-glycerol, 40 vol% aqueous 0.3 M guanadine.HCl (pH = 10), which acts as a cryoprotectant. The concentration of the nonperturbing biradical polarizing agent, TOTAPOL18 was 15–20 mM in the [14–13C]retinal, [ε-13C]lysine-labeled and uniformly 13C, 15N-labeled samples, and 50 mM in the [ζ-15N]lysine-labeled sample. Membranes were collected in a pellet by ultracentrifugation (5 h at 323 000 g) and packed into a 4 mm outside diameter, single-crystal sapphire rotor that is transparent at both optical and microwave wavelengths.
Peptide preparation
N-formyl-Met-Leu-Phe-OH (N-f-MLF-OH) was obtained from Bachem (King of Prussia, PA). Uniformly 13C, 15N-labeled N-f-MLF-OH was synthesized by CS Bio Inc. (Menlo Park, CA) and diluted with natural abundance compound. In each case, the peptide was recrystallized from isopropanol. Small, needlelike crystals were obtained after dissolution in warm solvent and subsequent drying.
Uniformly 13C, 15N-labeled alanyl-prolyl-glycine (APG) was also diluted to 10% with the corresponding natural abundance tripeptide. The tripeptide was synthesized by CS Bio (Menlo Park,CA) with labeled amino acids from Cambridge Isotopes (Andover, MA) and BaChem (Switzerland). APG was recrystallized from water. The peptide, dissolved in a minimal amount of water (the solubility was approximately 45 mg ml−1), produced crystals within a week of being placed in a desiccator next to a container of excess ethylene glycol.
All peptide crystals were crushed and packed in 4 mm zirconia rotors. The drive-tips on all of the rotors were bonded to the rotor sleeve with a low-temperature epoxy as described by Barnes et al.19
Instrumentation
All spectra were collected on a custom-built 250 GHz, 380 MHz, 95 MHz, 38 MHz (e−, 1H, 13C, 15N) frequency spectrometer (courtesy of D. J. Ruben). The quadruple resonance MAS probe is equipped with a cryogenic sample exchange system, microwave transmission circuit, and a fiber-optic for in situ optical illumination of the sample described by Barnes et al.19 Details of the 250 GHz gyrotron, corrugated microwave transmission line, and heat exchanger can be found elsewhere.20–24
Accumulation of bR photointermediates
Accumulation of bR photointermediates was performed in situ, via optical irradiation from a fiber-optic while the rotor was spinning in the MAS module. In this manner, access to the full temperature range of the probe can be utilized to both accumulate photointermediates at the appropriate temperature and cryogenically trap them at ~90 K for data acquisition in the dark. At such low temperatures the lifetimes of the photointermediates can be extended indefinitely. For example, experiments were performed on theMo intermediate for over a month without the protein relaxing to intermediates further along the photocycle.
bR568 was accumulated by irradiating dark-adapted bR (a thermally equilibrated mixture of bR555 and bR568) at 273 K with 1 W of 532 nm irradiation for 90 min. The Mo photocycleintermediate was accumulated by irradiating light adapted bR at 210 K with 1 W of 532 nm light for 120 min.
Results and discussion
High resolution spectra of the active site of a membrane protein
Fig. 1 shows well-resolved correlation spectra of bR embedded in its native lipid bilayer. We emphasize that we acquired these spectra with samples containing 15–20 mM TOTAPOL (i.e., 30–40 mM electrons) and that they do not show signs of paramagnetic broadening. The high signal-to-noise ratio available with the boost in nuclear polarization from DNP enables otherwise inaccessible quantitative measurements.19 Here we discuss the narrow linewidths and excellent site-resolution seen in the spectra. We emphasize two strategies, selective labeling and utilizing unique chemical shifts, employed to acquire the well-resolved spectra of this large (26 kD) membrane protein at a modest field of 9 T, as shown in Fig. 1.
Fig. 1.
High resolution DNP spectra of bR. (a) RFDR20 correlation spectrum of 14 mg of [14–13C]retinal, [ε-13C]lysine-labeled bR in the dark-adapted state with 1D direct 13C dimension slices overlaid on the 2D contours. 12.5 days worth of acquisition time from mixing times between 8 and 28 ms were averaged together. The DNP enhancement is ~40, relative to the signal recorded in the absence of microwaves. ωr/2π = 8000 Hz and the temperature is 93 K. The resulting signal-to-noise negated the need for any line-broadening. The bR568 correlation in the upper left has a linewidth of 1.2 ppm (115 Hz). (b) Expansion of the lower right portion of the spectrum in (a) demonstrating the narrow linewidths achievable with MAS DNP. Whereas the bR555 correlation is slightly broader due to the presence of two conformations of bR555, the bR568 resonance is extremely narrow with a linewidth of 1.0 ppm (95 Hz). (c) Carbon–nitrogen 2D spectrum of uniformly 13C, 15N-labeled bR568,16 ωr/2π = 6000 Hz. After an indirect chemical shift evolution on the resolved Schiff base (165 ppm in the 15N dimension), the magnetization is transferred along the retinal chain all the way to C11, and also in the other direction directly to Cε. The carbon resonances show relatively narrow linewidths (1.2–2.2 ppm).
Fig. 1a and b show resolved 13C–13C correlation spectra of [14–13C]-labeled retinal covalently bound to [ε-13C]-lysine in bacteriorhodopsin.14 Strategically labeling sites of interest is a well-established technique to reduce spectral crowding and to acquire high-quality distance constraints, although we also note that this strategy reduces the amount of structural information that can be extracted from a given sample. In the present example, crosspeaks arising from the two states in the dark-adapted mixture are clearly resolved and exhibit narrow resonances. The slightly larger linewidth of the bR555 resonances is a manifestation of the previously characterized heterogeneity of bR555.21 The bR568 cross peak has a linewidth of 1.2 ppm in the C14 direct dimension (Fig. 1a) and 1 ppm (95 Hz) in the Cε direct dimension (Fig. 1b). These are the narrowest resonances of a membrane protein from DNP/MAS experiments reported thus far in the literature. Any static disorder frozen out at these cryogenic temperatures does not degrade the resolution of the spectra significantly. However, we expect optimization of the lipid environment and cryoprotectant as well as extension of DNP to higher magnetic fields to lead to still higher resolution.
The spectrum in Fig. 1c utilizes the single site resolution available in the spectrum of uniformly 13C, 15N-labeled bR. The Schiff base, situated in the active site of the protein, has a unique chemical environment that manifests itself as a 15N chemical shift well-resolved from the other amide and side-chain nitrogen resonances. Therefore, experiments that first evolve an indirect 15N chemical shift evolution will yield single site resolution from the Schiff base and result in resolved, high-quality spectra. Reducing the spectral crowding in the 13C dimension by limiting the observed resonances to spins in close proximity to a single resolved nitrogen resonance yields excellent resolution and the capability to simultaneously assign multiple carbon resonances in the active site of the protein. This strategy of transferring polarization from a site which is resolved in the indirect dimension, either by existing in a unique chemical environment, like the Schiff base in bR, or by selective isotopic labeling, yields resolved spectra of high molecular weight systems even at relatively low magnetic fields (9 T).
High resolution MAS spectra of peptides from 75 to 273 K
The absence of appropriate instrumentation capable of acquiring high-resolution spectra at cryogenic temperatures has contributed to the misconception that MAS spectra are always broader at cryogenic temperatures. The recent development of a cryogenic sample exchange DNP probe demonstrated the resolution achievable at 90 K (0.3 ppm, 30 Hz).19 Here, we elaborate further on the high resolution available in MAS spectra between 75 and 273 K. We focus on crystalline tripeptides at low temperature, because they do not contain bulk water that necessitates the use of a cryoprotectant, such as glycerol, to maintain long-range homogeneity at low temperatures.
(i) N-formyl-Met-Leu-Phe-OH (N-f-MLF-OH)
Fig. 2 shows high-resolution 13C MAS spectra of crystalline natural abundance N-f-MLF-OH recorded from 273 to 75 K and 15N spectra of uniformly 13C, 15N-labeled peptide at 80 K. The resonances are narrow (<0.6 ppm, 60 Hz) over the entire temperature range. An expansion of the aromatic region is shown in Fig. 2b. The top spectrum at 273 K shows only the Fζ resonance because the 2-fold flipping of the phenylalanine ring interferes with the 1H decoupling field, broadening the δ, δ′ and ε, ε′ aromatic resonances beyond the detection limit.22 However, the dynamics slow down at 190 K revealing resonances arising from two coexisting forms of the peptide. Without proper optimization of the B0 field homogeneity and the magic angle, this spectral crowding leads to an apparent broadening. However, the spectrum in Fig. 2b at 190 K clearly shows that nearly all of the single aromatic resonances are resolved from one another. Each single resonance is not by itself appreciably broader than resonances at room temperature.
Fig. 2.
Temperature dependent high-resolution MAS spectra of N-f-MLF-OH. The spinning frequency is between 4000 and 5500 Hz, with −5 Hz stability. (a) Temperature dependent 13C spectra from 273 to 75 K showing that excellent resolution is maintained throughout the 200 K temperature range. (b) Expansion of the aromatic regions at 273, 190, and 82 K showing resolved resonances, even with coexisting conformations at 190 K. (c) Expansion of the alpha carbon region showing the resolved resonances of the two conformations present at 75 K, each different from the resonances of the single conformation observed at 273 K. (d) 15N spectrum of [U-13C, 15N]-N-f-MLF-OH. The shoulder on the resonances is a manifestation of the two conformations present at 75 K. Nonetheless, excellent resolution (linewidth of 39 Hz) is still maintained at 75 K.
An expansion of the Cα region is shown in Fig. 1c revealing two backbone conformations present at 75 K. The two coexistent forms manifest themselves as a doubling of the resonances, while each of the lines comprising the doublet however remains narrow. We attribute this to a small change in torsion angles rather than a globally different structure because the Mε resonance is still extremely sharp <30 Hz at 75 K, indicating lack of heterogeneous broadening due to cryogenically freezing the peptide. The two low temperature conformations are also apparent in the asymmetric lineshapes of the 15N resonances (Fig. 1d). In a similar ratio to the two conformations seen in the Cα region of the 13C spectrum, the two conformations manifest themselves as a single 15N resonance with a shoulder; the dominant resonances however remain narrow (only 39 Hz for the amide of Met). We emphasize that the 15N spectrum was acquired on a uniformly 13C, 15N-labeled sample, thus the 15N–13C J-coupling contributes to the linewidths in the spectrum.
(ii) Alanyl-proyl-glycine (APG)
To demonstrate that the resolution seen in the N-f-MLF-OH spectra is not unique to a single system, we also show comparable resolution in cryogenic MAS spectra of another tripeptide, APG. APG is different from N-f-MLF-OH in two important aspects. First, the crystal lattice contains one water molecule per unit cell that we show does not lead to significant broadening of resonances at low temperatures. Second, the proline ring samples two puckered conformations, a structural feature of five-membered rings that has been well characterized in the literature23, 24 and has also been observed in both the X-ray and solid state NMR structures of APG.25 We emphasize that in contrast to the 13C spectra of natural abundance N-f-MLF-OH in Fig. 2, the 13C spectra in Fig. 3 are taken of uniformly labeled 13C, 15N-labeled APG.
Fig. 3.
High-resolution spectra of APG showing low temperature heterogeneous broadening for only the 3 carbon resonances that sample two puckered conformations, ωr/2π = 4831 Hz. (a) 13C spectra of APG at 293 and 75 K. (b) The carbon aliphatic region of a 15N–13C correlation spectrum with 1D direct 13C dimension slices overlaid on the 2D contours. (c) X-Ray and solid state NMR structures of APG from Barnes et al.25
Examination of the linewidths at 293 and 75 K in Fig. 3a reveals that most of the 13C lines remain narrow (broadening <17 Hz) at 75 K, including the Pα, Gα, and Aβ resonances. In fact, a 13C–13C J-coupling is resolved on the Gα resonance in the 15N–13C correlation spectrum (Fig. 3b). The three sites that do show broadening are on the proline ring; the Pβ and Pγ resonances (Fig. 3a) and the Pδ resonance (Fig. 3b) broaden by ~40 Hz. We attribute this to the conformational heterogeneity of the five-membered ring (Fig. 3c) which is currently under investigation.
Efficient and uniform polarization distribution via proton spin diffusion
The 15N 1D DNP CPMAS spectrum of light-adapted [U–13C, 15N]-bR is shown in Fig. 4. The buried Schiff base at 165 ppm is well resolved from the amide, arginine and other lysine sidechain nitrogen resonances. To address whether the enhanced polarization originating from the electron spins and DNP is uniformly distributed across the membrane, and the protein embedded therein, we integrated the intensity of the four separated lineshapes in the 15N spectrum in Fig. 4. If the polarization is uniformly distributed, the integral of each resonance should be proportional to the number of contributing nuclei. As shown in Table 1, the relative intensities of the resonances correspond very well to this expectation, providing clear evidence that the polarization propagates to the buried active site as thoroughly as to the rest of the protein embedded in its native membrane. Proton spin diffusion thus effectively transfers the DNP enhanced polarization throughout the heterogenous sample of cryoprotectant and membrane protein in lipid bilayers.
Fig. 4.
1D 15N spectrum of U-[13C, 15N]-bR showing the 15N Schiff base resonance adjacent to the peptide backbone signal, ωr/2π = 6000 Hz.
Table 1.
Relative experimental intensities (peak areas) of a DNP enhanced 15N spectrum of bR vs. number of 15N sites present
| Peak assignment | Peak area | Number of sites |
|---|---|---|
| Schiff base | 1 | 1 |
| Amides | 240 ± 13 | 248 |
| Arginines | 25 ± 2 | 21 |
| Lysines | 6 ± 1 | 6 |
Accelerated data acquisition
In the original expression for the sensitivity enhancement from the classical CP experiment, the ratio of the spin–lattice relaxation times of the abundant and rare spins (T1S/T1I) plays an important role.26 This term represents the gain in time savings from utilizing the shorter spin–lattice relaxation time of 1H magnetization versus 13C or 15N. This is important as the experimental gain in sensitivity between a Bloch decay and CP experiment is typically only a factor of ~2.5 versus the theoretical maximum of 4. Thus much of the improvement in sensitivity from using CP actually comes from the reduced time needed to recover longitudinal magnetization between signal averaging transients. The situation is similar in DNP experiments, although the polarization enhancements are of course much larger.
The radical concentration, initial electron-to-nuclear polarization transfer rate, and proton spin diffusion efficiency govern the rate of longitudinal build up of magnetization during the polarization period between DNP/MAS signal averaging transients. This time constant differs from classical NMR experiments in which the rate of recovery of longitudinal magnetization is dependent on the spectral density function evaluated at the nuclear Larmor frequency. Optimal repetition rates of DNP/MAS experiments at cryogenic temperature are thus much higher than for samples without paramagnetic dopants. This effect has recently been exploited at ambient temperatures to reduce the proton T1, which leads to much improved repetition rates and sensitivity.27 Here we show that similar improvements in data acquisition rates are seen by increasing the concentration of polarizing agents in DNP experiments.
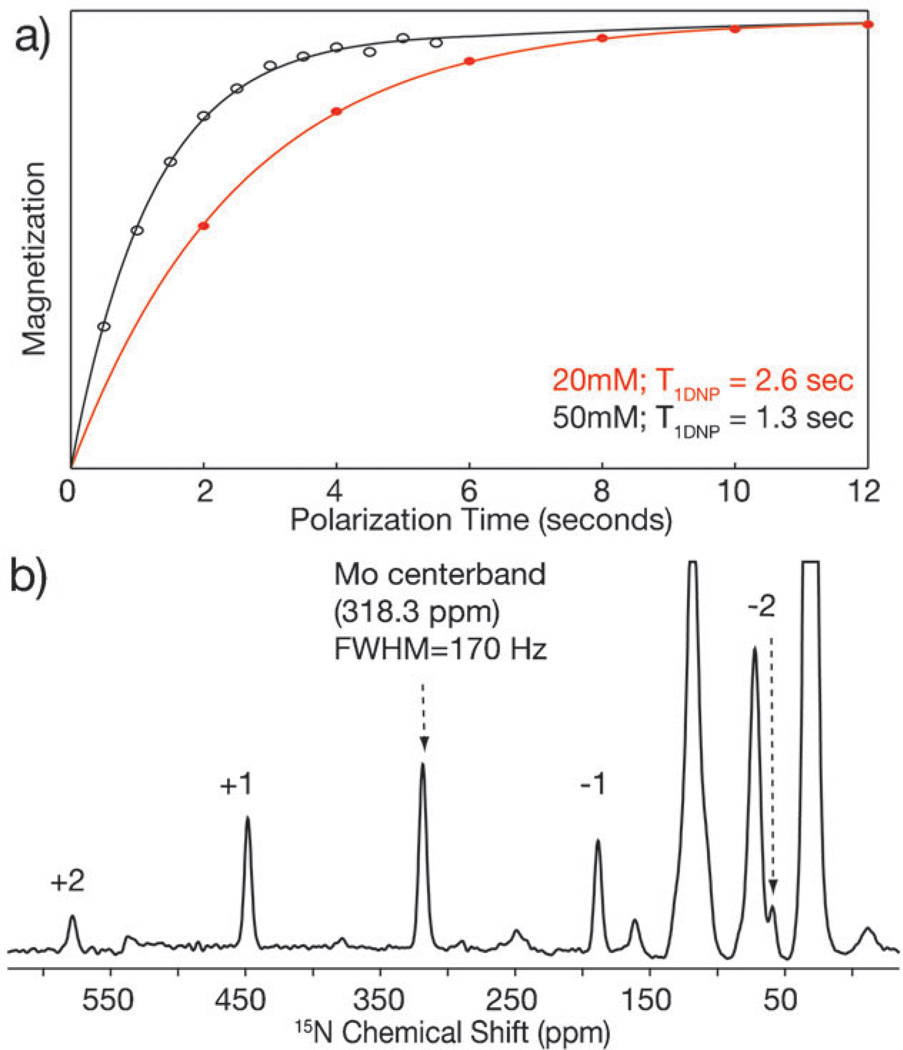
As shown in Fig. 5a, increasing the biradical concentration from 20 to 50 mM reduces the longitudinal proton magnetization build-up time constant (T1 DNP) by a factor of two, thus doubling the rate of signal averaging. Relatively short recycle delays of 1.6 s (1.26*T1 DNP) for experiments on a sample of [ζ-15N]lysine-labeled bR were used to exploit the short proton T1. Due to the cryogenic temperatures, extensive cooling power from the spinning gases, and the use of thermally conductive sapphire rotors, the sample is not at risk of detrimental heating that can arise from high RF powers and short delays between transients at ambient temperatures.
Fig. 5.
Accelerated DNP (a) difference in longitudinal relaxation time, T1DNP, under microwave irradiation between 20 and 50 mM TOTAPOL. The magnetization of the bulk protons is measured via CP to the carbonyls of [U-15N, 13C]-bR. (b) DNP spectrum of [ζ-15N]lysine-labeled bR trapped in the Mo photocycle intermediate. 81 920 Transients, 1.6 s recycle delay, 36.4 h acquisition time, ωr/2π = 5000 Hz, T = 89 K. The isotropic shift is 318.3 ppm, the span is 620 ppm, the skew is −0.034, and the tensor elements are: δ11 = 618, δ22 = 340, and δ33 = −2.5 ppm.
Fig. 5b shows spectra of the Mo photocycle intermediate after only 1.5 days of signal averaging. There is sufficient signal-to-noise to complete a Herzfeld–Berger analysis of the spinning sideband intensities28 and extract the chemical shift anisotropy parameters, which are given in the figure caption. Even the +2 sideband with only a small fraction of the intensity of the centerband has sufficient signal-to-noise to be fitted and used in the chemical shift analysis.
The 15N resonance in the active site is not broadened even when the electron concentration is increased to 100 mM. The linewidth of the Schiff base resonance is the same in samples containing 15 and 50 mM TOTAPOL. The biradical is excluded from the center of the protein by steric hindrance and paramagnetic electrons are not close enough to the active site to result in pseudo-contact shifts and broadening.
Conclusions
Excellent resolution and sensitivity are available in cryogenic MAS DNP spectra of membrane proteins and crystalline peptides. With proper cryoprotection, similar linewidths to those reported here should be achievable on crystalline proteins and other integral membrane proteins.
Acknowledgements
This research was supported by the National Institutes of Health through grants EB002804, EB003151 and EB002026 to R.G.G., EB001035 to J.H., and grants EB001965 and EB004866 to R.J.T. A.B.B. and L.B.A. were partially supported by graduate research fellowships from the National Science Foundation. B.C. was funded by the Deutsche Forschungsgemeinschaft (research fellowship CO 802/1-1). Stimulating conversations with T. Maly and M. Bayro are gratefully acknowledged.
References
- 1.Carver TR, Slichter CP. Phys. Rev. 1956;102:975–980. [Google Scholar]
- 2.Rosay M, Lansing JC, Haddad KC, Bachovchin WW, Herzfeld J, Temkin RJ, Griffin RG. J. Am. Chem. Soc. 2003;125:13626–13627. doi: 10.1021/ja036898k. [DOI] [PubMed] [Google Scholar]
- 3.Barnes AB, De Paëpe G, van der Wel P, Hu K, Joo C, Bajaj VS, Mak-Jurkauskas M, Sirigiri JR, Herzfeld J, Temkin R, Griffin RG. Appl. Magn. Reson. 2008;34:237–263. doi: 10.1007/s00723-008-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mak-Jurkauskas ML, Bajaj VS, Hornstein MK, Belenky M, Temkin RJ, Griffin RG, Herzfeld J. Proc. Natl. Acad. Sci. U. S. A. 2008;105:883–888. doi: 10.1073/pnas.0706156105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debelouchina GT, Bayro MJ, van der Wel PCA, Caproini M, Barnes AB, Rosay M, Werner M, Griffin RG. Phys. Chem. Chem. Phys. 2010 doi: 10.1039/c003661g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Wel PCA, Hu K-N, Lewandowski JR, Griffin RG. J. Am. Chem. Soc. 2006;128:10840–10846. doi: 10.1021/ja0626685. [DOI] [PubMed] [Google Scholar]
- 7.Shi L, Ahmed M, Zhang W, Whited G, Brown L, Ladizhansky V. J. Mol. Biol. 2009;386:1078–1093. doi: 10.1016/j.jmb.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Wasmer C, Lange A, Van Melckebeke H, Siemer A, Riek R, Meier B. Science. 2008;319:1523. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- 9.Franks W, Wylie B, Schmidt H, Nieuwkoop A, Mayrhofer R, Shah G, Graesser D, Rienstra C. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4621. doi: 10.1073/pnas.0712393105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song K-N, Hu C, Swager TM, Griffin RG. J. Am. Chem. Soc. 2006;128:11385–11390. doi: 10.1021/ja061284b. [DOI] [PubMed] [Google Scholar]
- 11.Hu K-N, Song H-h, Yu C, Swager TM, Griffin RG. J. Chem. Phys. 2008;128:052302. doi: 10.1063/1.2816783. [DOI] [PubMed] [Google Scholar]
- 12.Becerra LR, Gerfen GJ, Temkin RJ, Singel DJ, Griffin RG. Phys. Rev. Lett. 1993;71:3561–3564. doi: 10.1103/PhysRevLett.71.3561. [DOI] [PubMed] [Google Scholar]
- 13.Nadaud PS, Helmus JJ, Höfer N, Jaroniec CP. J. Am. Chem. Soc. 2007;129:7502–7503. doi: 10.1021/ja072349t. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths JM, Lakshmi KV, Bennett AE, Raap J, Vanderwielen CM, Lugtenburg J, Herzfeld J, Griffin RG. J. Am. Chem. Soc. 1994;116:10178–10181. [Google Scholar]
- 15.Argade P, Rothschild K, Kawamoto A, Herzfeld J, Herlihy W. Proc. Natl. Acad. Sci. U. S. A. 1981;78:1643. doi: 10.1073/pnas.78.3.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajaj VS, Mak-Jurkauskas M, Belenky M, Herzfeld J, Griffin RG. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9244. doi: 10.1073/pnas.0900908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oesterhelt D, Stoeckenius W. Proc. Natl. Acad. Sci. U. S. A. 1973;70:2853. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu K-N, Yu H-h, Swager TM, Griffin RG. J. Am. Chem. Soc. 2004;126:10844–10845. doi: 10.1021/ja039749a. [DOI] [PubMed] [Google Scholar]
- 19.Barnes AB, Mak-Jurkauskas ML, Matsuki Y, Bajaj VS, van der Wel PCA, DeRocher R, Bryant J, Sirigiri JR, Temkin RJ, Lugtenburg J, Herzfeld J, Griffin RG. J. Magn. Reson. 2009;198:261–270. doi: 10.1016/j.jmr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett AE, Ok JH, Griffin RG, Vega S. J. Chem. Phys. 1992;96:8624–8627. [Google Scholar]
- 21.Bajaj VS, Hornstein MK, Kreischer KE, Sirigiri JR, Woskov PP, Mak M, Herzfeld J, Temkin RJ, Griffin RG. J. Magn. Reson. 2007;190:86–114. doi: 10.1016/j.jmr.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajaj VS, van der Wel PCA, Griffin RG. J. Am. Chem. Soc. 2009;131:118–128. doi: 10.1021/ja8045926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poupko R, Luz Z, Zimmermann H. J. Am. Chem. Soc. 1982;104:5307–5314. [Google Scholar]
- 24.Wells E, Ferguson R, Hallett J, Peterson L. Can. J. Chem. 1968;46:2733–2742. [Google Scholar]
- 25.Barnes AB, Andreas LB, Huber M, Ramachandran R, van der Wel PCA, Veshtort M, Griffin RG, Mehta MA. J. Magn. Reson. 2009;200:95–100. doi: 10.1016/j.jmr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pines A, Gibby MG, Waugh JS. J. Chem. Phys. 1973;59:569–590. [Google Scholar]
- 27.Wickramasinghe N, Parthasarathy S, Jones C, Bhardwaj C, Long F, Kotecha M, Mehboob S, Fung L, Past J, Samoson A. Nat. Methods. 2009;6:215. doi: 10.1038/nmeth.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herzfeld J, Berger AE. J. Chem. Phys. 1980;73:6021–6030. [Google Scholar]