Summary
Background
Recent studies have shown that factor VIIa binds to endothelial cell protein C receptor (EPCR), a cellular receptor for protein C and activated protein C. At present, the physiologic significance of FVIIa interaction with EPCR in vivo remains unclear. Objective: To investigate whether exogenously administered FVIIa, by binding to EPCR, induces a barrier protective effect in vivo.
Methods
Lipopolysaccharide (LPS)-induced vascular leakage in the lung and kidney, and vascular endothelial growth factor (VEGF)-induced vascular leakage in the skin, were used to evaluate the FVIIa-induced barrier protective effect. Wild-type, EPCR-deficient, EPCR-overexpressing and hemophilia A mice were used in the studies.
Results
Administration of FVIIa reduced LPS-induced vascular leakage in the lung and kidney; the FVIIa-induced barrier protective effect was attenuated in EPCR-deficient mice. The extent of VEGF-induced vascular leakage in the skin was highly dependent on EPCR expression levels. Therapeutic concentrations of FVIIa attenuated VEGF-induced vascular leakage in control mice but not in EPCR-deficient mice. Blockade of FVIIa binding to EPCR with a blocking mAb completely attenuated the FVIIa-induced barrier protective effect. Similarly, administration of protease-activated receptor 1 antagonist blocked the FVIIa-induced barrier protective effect. Hemophilic mice showed increased vascular permeability, and administration of therapeutic concentrations of FVIIa improved barrier integrity in these mice.
Conclusions
This is the first study to demonstrate that FVIIa binding to EPCR leads to a barrier protective effect in vivo. This finding may have clinical relevance, as it indicates additional advantages of using FVIIa in treating hemophilic patients.
Keywords: activated protein C receptor, capillary permeability, factor VIIa, hemophilia A, protein C
Introduction
Recent studies from our laboratory [1,2] and others [3,4] have established that, upon vascular injury, factor VIIa, the protease that activates the coagulation cascade following its binding to its cofactor, tissue factor (TF), also binds to endothelial cell protein C receptor (EPCR) on the endothelium. EPCR is the key component in the protein C (PC)/activated protein C (APC) anticoagulant pathway. EPCR also serves as the ‘signaling’ receptor for APC by enabling EPCR-bound APC to cleave protease-activated receptor (PAR) 1 [5]. Recent studies have shown that EPCR–APC also activates PAR3 [6]. EPCR–APC-mediated cell signaling was shown to play a key role in suppressing injury-induced vascular permeability [7–10].
The discovery that FVIIa binds to EPCR in true ligand fashion, with a similar affinity as that of PC/APC, raises the possibility of hitherto unknown functions of FVIIa and/or EPCR. Although our earlier studies showed that FVIIa binding to EPCR on the endothelial cell surface had no influence on FVIIa's coagulant activity [1], others have shown that FVIIa binding to EPCR inhibits the procoagulant activity of TF–FVIIa [4]. Recent studies from our laboratory suggest that EPCR mediates the internalization of FVII/FVIIa [2], and facilitates the transport of FVII/FVIIa from the circulation to extravascular tissues [11]. Although our studies involving a heterologous cell model system failed to provide evidence that FVIIa–EPCR, like APC–EPCR, is capable of activating PAR-mediated cell signaling [1], our subsequent studies have shown that FVIIa–EPCR on endothelial cells can activate endogenous PAR1, and FVIIa–EPCR-mediated cell signaling confers a barrier protective effect in vitro [8]. in vivo studies in mice showed that the administration of high doses of FVIIa prior to lipopolysaccharide (LPS) treatment attenuated LPS-induced vascular leakage in the lung and kidney [8]. However, the involvement of EPCR in the FVIIa-induced barrier protective effect in vivo has yet to be shown.
The present study was carried out to investigate whether exogenously administered FVIIa provides the barrier protective effect in vivo, and the role of EPCR and PAR1 in mediating the FVIIa-induced barrier protective effect. Both LPS-induced vascular leakage in the lung and kidney and vascular endothelial growth factor (VEGF)-induced vascular leakage in the skin were used as model systems. The data obtained clearly show that FVIIa reduces LPS-induced and VEGF-induced vascular permeability in an EPCR-dependent fashion. Our data also show that therapeutic concentrations of FVIIa are sufficient to markedly reduce VEGF-induced vascular permeability, and that the FVIIa-induced barrier protective effect involves PAR1 activation. This is the first study to demonstrate that FVIIa binding to EPCR leads to a barrier protective effect in vivo.
Materials and methods
Reagents
Recombinant human FVIIa was from Novo Nordisk A/S (Maaloev, Denmark), recombinant APC (Xigris) was from Eli Lilly (Indianapolis, IN, USA), and recombinant FVIII (Kogenate FS) was from Bayer Healthcare Pharmaceuticals (Berkeley, CA, USA). Thrombin was from Haematologic Technologies (Essex Junction, VT, USA). The non-peptide selective PAR1 receptor antagonist SCH79797 was from R&D Systems (Minneapolis, MN, USA). The mAbs against mouse EPCR (mAb 1560, blocking mAb for EPCR; and mAb 1567, non-blocking mAb for EPCR) were prepared by immunizing rats with recombinant mouse soluble EPCR [12]. Recombinant mouse VEGF (VEGF164) was from R&D Systems.
Cell culture
Mouse brain microvascular endothelial cells (bEND3) were obtained from (American Type Culture Collection, Manassas, VA, USA). bEND3 cells were grown in Dulbecco's modified Eagle's medium (Life Technologies, Grand Island, NY, USA) containing 1% penicillin/streptomycin, supplemented with 10% heat-inactivated fetal bovine serum.
Endothelial barrier permeability in vitro assay
Endothelial cell permeability was analyzed in a dual-chamber system with Evans blue–labeled bovine serum albumin (BSA), as described in our earlier publication [8].
Mice
All animal procedures were approved by the Institutional Animal Use and Care Committee. The generation of EPCR-deficient mice (Procr−/−) and EPCR-overexpressing mice (Tie2-EPCR) has been described previously [12,13]. Both EPCR-deficient and EPCR-overexpressing mice were backcrossed into C57BL/6 mice for at least 10 generations. FVIII−/− breeding pairs were obtained from Jackson Laboratory (Bar Harbor, ME, USA), and FVIII−/− mice were generated in-house by homozygous breeding. Wild-type C57BL/6 mice were obtained from Jackson Laboratory, or from the breeding colonies maintained in-house. Mice aged 6–10 weeks, both male and female, were used.
Vascular permeability in vivo assay
LPS-induced injury
LPS-induced vascular permeability in the lung and kidney was measured as described previously [8].
VEGF-induced vascular permeability in skin
One day before the experiment, abdominal hair of the mice was removed with Nair hair remover. Mice were injected with saline or human FVIIa (50–400 μg kg−1 body weight in 100 μL) intravenously via the tail vein. Immediately following FVIIa injection, 100 μL of 1% Evans blue dye was injected intravenously via the tail vein. Thirty minutes later, after 100 μL of blood had been obtained via the submandibular vein into citrate anticoagulant, 50 ng of VEGF (in 25 μL, dissolved in phosphate-buffered saline [PBS] containing 1 mg mL−1 BSA) per site was injected intradermally into the preshaven abdomen at four different sites. As a control, 25 μL of PBS (containing 1 mg mL−1 BSA) per site was injected into the same mouse at two different sites. Thirty minutes following VEGF administration, the mice were killed, and the sites of injection in the skin were removed by dermal biopsy. The skin samples were incubated in 200 lL of formamide at 60 °C for 24 h, and the extracted Evans blue content was quantified by dual-wavelength spectrophotometric analysis at 620 nm and 740 nm. When blocking EPCR mAb or non-blocking EPCR mAb was given to mice (4 mg kg−1 body weight), they were administered intraperitoneally 30 min before the injection of FVIIa, FXa, or thrombin. Statistical analyses were performed with an unpaired Student's t-test or oneway anova as appropriate.
Histological examination
Tissues were fixed in Excel fixative (American Master Tech Scientific, Lodi, CA, USA) and processed with paraffin-embedding procedures. Sections (5 μm in thickness) were cut and stained with hematoxylin and eosin, and the stained sections were observed under a light microscope (Olympus BX41, Center Valley, PA, USA) to assess extravasation of blood cells. Images were captured with an Olympus DP25 camera.
ELISA for thrombin–antithrombin (TAT) complexes
Mouse TAT complex levels were measured with the mouse TAT Complex ELISA Kit (Assaypro, St Charles, MO, USA).
Results
Administration of FVIIa reduces LPS-induced vascular leakage in wild-type but not in EPCR-deficient mice
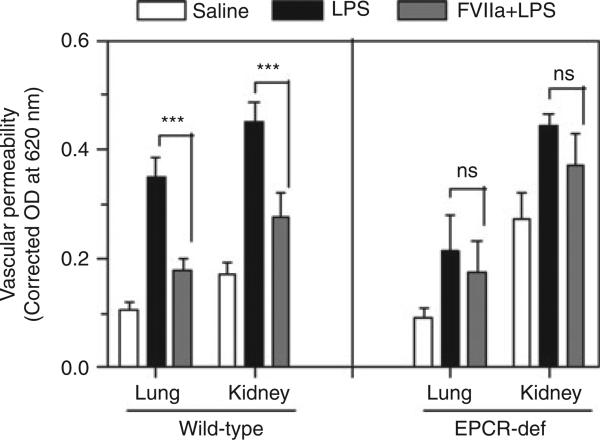
As shown in our earlier study [8], LPS administration markedly increased vascular leakage in the lung and kidney in wild-type mice, and administration of recombinant FVIIa prior to LPS treatment significantly reduced vascular leakage (Fig. 1). LPS treatment also increased vascular leakage in the lung and kidney in EPCR-deficient mice. The extent of LPS-induced leakage in the lung in EPCR-deficient mice was slightly lower than that observed in wild-type mice, but the difference between them did not reach statistical significance. More importantly, in contrast to what was observed in wild-type mice, administration of FVIIa had no significant effect on LPS-induced vascular leakage in EPCR-deficient mice, indicating an essential role of EPCR in mediating the FVIIa-induced barrier protective effect.
Fig. 1.
FVIIa protects mice from increased vascular permeability upon lipopolysaccharide (LPS) challenge in an endothelial cell protein C receptor (EPCR)-dependent fashion. Wild-type mice (13–17 mice per group) or EPCR-deficient mice (EPCR-def) (8–12 mice per group) were injected with saline (open bars) or LPS (5 mg kg−1) (black bars) intraperitoneally In a third group, FVIIa (250 μg per mouse) was administered via the tail vein to mice 2 h before the LPS challenge (gray bars). Vascular permeability in the lung and kidney was measured at 18 h after LPS challenge by injecting 0.1 mL of 1% Evans blue dye via the tail vein 45 min before the termination of the experiment, and measuring the extravasation of dye into the lung and kidney. ***Statistically significant difference (P < 0.001). NS, no statistically significant difference between the groups. (Note that the data shown for the wild-type mice in the left panel are very similar to those was reported in our earlier study [8], and represent cumulative data from wild-type mice used in that study and additional wild-type mice used along with EPCR-def mice.)
FVIIa-induced barrier protection in the skin requires FVIIa binding to EPCR and FVIIa protease activity
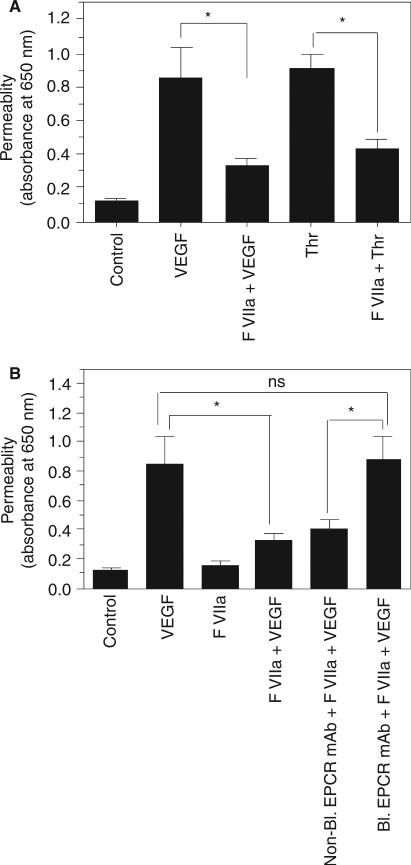
To evaluate whether therapeutic concentrations of FVIIa can protect vascular barrier integrity, and the role of EPCR in mediating the FVIIa-induced barrier protective effect, we employed VEGF-induced hyperpermeability in the skin as a model system. First, we examined in vitro whether FVIIa attenuates the VEGF-induced increase in permeability in mouse endothelial cells cultured to confluence in a Transwell system. As shown in Fig. 2A, VEGF treatment markedly increased mouse endothelial cell permeability, and pretreatment of cells with FVIIa (10 nm) significantly reduced VEGF-induced permeability. The FVIIa-mediated decrease in VEGF-induced endothelial cell permeability was attenuated if the endothelial cells were treated with the blocking EPCR mAb but not if they were treated with the non-blocking EPCR mAb prior to the addition of FVIIa, indicating that the FVIIa-induced barrier protective effect is dependent on EPCR (Fig. 2B).
Fig. 2.
FVIIa treatment reduces vascular endothelial growth factor (VEGF)-induced barrier disruption in mouse endothelial cells. (A) Mouse endothelial cells (bEND3) were plated on 12-transwell chambers and cultured for 4 days. Confluent monolayers were pretreated with FVIIa (10 nm) for 2 h before they were treated with VEGF (50 ng mL−1) or thrombin (Thr) (5 nm) for 30 min. After 30 min, the cells were washed with phosphate-buffered saline, Evans blue-labeled bovine serum albumin was added to the upper chamber, and the amount of dye that had leaked into the bottom chamber after 30 min was determined by measuring the absorbance at 650 nm (n = 7). (B) Mouse endothelial cells cultured to confluency in tran-swells, as described in (A), were treated with control vehicle, nonblocking endothelial cell protein C receptor (EPCR) mAb or blocking EPCR mAb (25 μg mL−1), followed by FVIIa (10 nm). After 2 h of treatment with FVIIa, VEGF (50 ng mL−1) was added to the cells, and cell permeability following VEGF treatment was determined as described in (A) (n = 4). *P < 0.05. NS, no statistically significant difference.
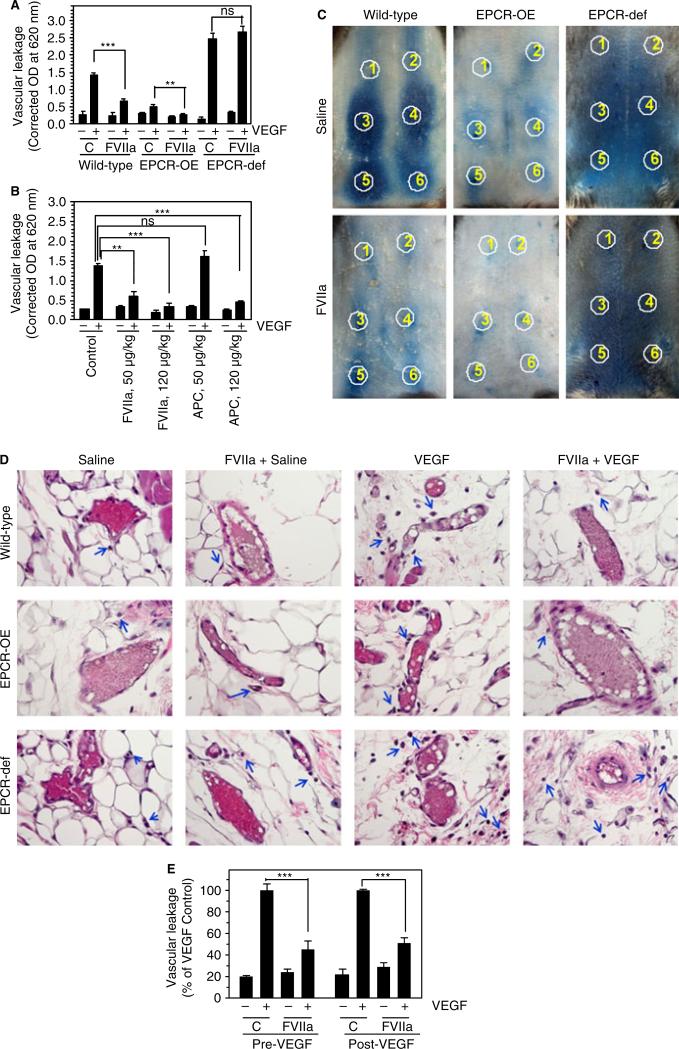
Next, to test whether exogenously administered FVIIa can protect vascular barrier integrity in vivo, where endogenous ligands for EPCR are present in circulating blood, mice were treated with FVIIa before an intradermal injection of VEGF into the skin. As shown in Fig. 3A, injection of VEGF into the skin increased vascular leakage around the injection sites, and the extent of VEGF-induced permeability in the skin inversely correlated with EPCR expression levels in mice, i.e. highest in EPCR-deficient mice, moderate in wild-type mice, and lowest in EPCR-overexpressing mice. More importantly, administration of FVIIa (400 μg kg−1) markedly attenuated VEGF-induced hyperpermeability in wild-type mice, whereas it had no effect in EPCR-deficient mice (Fig. 3A). Administration of FVIIa further reduced an already low level of VEGF-induced permeability in EPCR-overexpressing mice (Fig. 3A). Next, to evaluate the relative effectiveness of FVIIa and APC in providing a barrier protective effect, different doses of FVIIa or APC (50 and 120 μg kg−1 body weight) were administered to wild-type mice before injection of VEGF. Surprisingly, FVIIa appeared to be more effective than APC in reducing VEGF-induced hyperpermeability in the skin (Fig. 3B). FVIIa, even at the dose of 50 μg kg−1, provided effective and statistically significant protection against VEGF-induced barrier disruption (Fig. 3B). No significant protection was observed with APC at this low dose. However, at 120 μg kg−1, both FVIIa and APC showed similar degrees of barrier protective effect against VEGF-induced hyperpermeability (Fig. 3B).
Fig. 3.
FVIIa suppression of vascular endothelial growth factor (VEGF)-induced vascular permeability requires endothelial cell protein C receptor (EPCR). (A) Wild-type EPCR-overexpressing (EPCR-OE) or EPCR-deficient (EPCR-def) mice were injected with saline or FVIIa (400 μg kg−1 body weight), followed by Evans blue dye (1%, 100 μL), intravenously via the tail vein. After 30 min, saline or VEGF (50 ng) was injected intradermally at six different sites (two with saline, and four with VEGF). Thirty minutes after injection of VEGF, dermal tissues around the injection sites were removed by biopsy, the dye in the skin biopsies was eluted, and the absorbance of the dye was measured as described in Materials and methods (five mice per group). (B) Wild-type mice were injected with saline or varying doses of FVIIa or activated protein C (APC) (50 or 120 μg kg−1), followed by Evans blue dye, and VEGF was then injected intradermally, as described in (A) (six mice per group). (C) Wild-type EPCR-OE or EPCR-def mice were injected with saline or FVIIa (120 μg kg−1 body weight), followed by Evans blue dye, and VEGF was then injected intradermally. Photographs were taken 30 min after VEGF administration. Circles indicate injection sites; 1 and 2 indicate saline-injected sites, and 3–6 indicate VEGF-injected sites. The photographs shown are representative of five mice per group. (D) Histological examination of skin tissue sections of wild-type, EPCR-OE and EPCR-def mice injected with saline or FVIIa (120 μg kg−1 body weight), and then intradermally injected with saline or VEGF. The blue arrows indicate extravasated leukocytes. The images shown in the figure are representative micrographs from > 20 images obtained from skin tissue sections by two different investigators (six mice per group). (E) Wild-type mice were first injected with saline or FVIIa (120 μg kg−1), and then with Evans blue dye via the tail vein, and saline or VEGF intradermally (Pre-VEGF). In the Post-VEGF group, wild-type mice were first injected intradermally with saline or VEGF, and 30 min later, FVIIa (120 μg kg−1) or saline was given intravenously. Thirty minutes after FVIIa administration, Evans blue dye was injected into the mice. Leakage of the dye into skin tissues was measured 30 min after VEGF (Pre-VEGF) or Evans blue dye (Post-VEGF) administration (six mice per group). Values were normalized to the value obtained in the VEGF-injected mice that were not treated with FVIIa within the specific experimental group. **P < 0.01; ***P < 0.001. C, control; NS, no statistically significant difference.
VEGF-induced vascular leakage and the EPCR-dependent FVIIa-induced barrier protective effect were clearly evident on visual examination of the spread of Evans blue dye surrounding VEGF-injected sites (Fig. 3C), as well as by microscopic examination of skin tissue sections (Fig. 3D). Microscopic examination of hematoxylin and eosin-stained skin tissue sections from saline-injected or VEGF-injected sites showed that VEGF treatment induced extravasation of leukocytes into peripheral tissues in both wild-type and EPCR-deficient mice, but more so in the latter. In addition to leukocytes, we also found extravasation of a few red blood cells, which moved much further than leukocytes from blood vessels (data not shown). FVIIa treatment prevented VEGF-induced extravasation of leukocytes in wild-type mice but not in EPCR-deficient mice (Fig. 3D). VEGF-induced extravasation of leukocytes into peripheral tissue was minimal in both control and FVIIa-treated EPCR-overexpressing mice (Fig. 3D).
To investigate whether FVIIa restores the barrier integrity disrupted by VEGF, FVIIa was administered to mice 30 min after intradermal injections of VEGF. As shown in Fig. 3E, FVIIa administered following VEGF injury was capable of reducing VEGF-induced vascular leakage. Thrombin is known to have a bimodal effect on endothelial cell permeability, at least in in vitro model systems, with a low (picomolar) concentration eliciting a barrier protective effect, but a high (nanomolar) concentration eliciting a barrier disruptive effect [14]. Although it is unlikely that the administration of low doses of FVIIa generated such widely varying amounts of thrombin in wild-type, EPCR-deficient and EPCR-overexpressing mice, we measured thrombin generation in these mice by measuring the TAT complexes in blood. No significant differences were found in TAT complex levels among all groups of mice (Table 1).
Table 1.
Thrombin-antithrombin (TAT) complex levels in wild-type, endothelial cell protein C receptor (EPCR)-overexpressing and EPCR-deficient mice before and after administration of FVIIa (120 μg kg–1 body weight); blood was drawn prior to FVIIa administration and 1 h after FVIIa administration (n = 10 mice per group)
| Genotype | TAT complex levels (ng mL –1) | |
|---|---|---|
| Before FVIIa | After FVIIa | |
| Wild type | 11.5 ± 0.56 | 13.7 ± 1.42 |
| EPCR-overexpressing | 11.8 ± 1.10 | 13.2 ± 1.16 |
| EPCR-deficient | 11.6 ± 0.37 | 12.7 ± 0.82 |
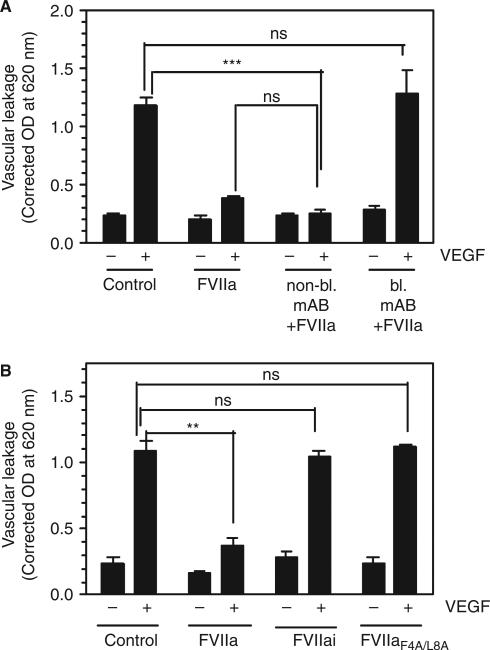
The failure of FVIIa to provide a barrier protective effect in EPCR-deficient mice, in contrast to that observed in wild-type mice, suggests that FVIIa binding to EPCR is necessary for the barrier protective effect. To confirm this observation, we investigated whether blocking FVIIa binding to EPCR with a blocking EPCR mAb attenuated the FVIIa-induced barrier protective effect in wild-type mice. Administration of blocking EPCR mAb prior to FVIIa treatment completely blocked the FVIIa-induced barrier protective effect (Fig. 4A). In contrast, non-blocking EPCR mAb had no noticeable effect on the FVIIa-induced barrier protective effect. The requirement for FVIIa binding to EPCR to mediate the barrier protective effect was further confirmed by the observation that an FVIIa mutant, FVIIaF4A/L8A, which does not bind to EPCR, failed to provide the barrier protective effect (Fig. 4B). To determine whether FVIIa binding to EPCR alone is sufficient to induce the barrier protective effect or whether it requires, in addition to EPCR binding, protease activity, active site-inhibited FVIIa (FVIIai) was administered to the mice in place of FVIIa. FVIIai, which binds EPCR as effectively as FVIIa [2], failed to provide the barrier protective effect (Fig. 4B), indicating that FVIIa protease activity is required for the EPCR–FVIIa-induced barrier protective effect.
Fig. 4.
Blockade of FVIIa binding to endothelial cell protein C receptor (EPCR) attenuates the FVIIa-induced barrier protective effect. (A) Wild-type mice were injected with saline, non-blocking EPCR mAb or blocking EPCR mAb intraperitoneally (4 mg kg−1 body weight) before being injected with FVIIa (120 μg kg−1 body weight) and vascular endothelial growth factor (VEGF) (six mice per group). (B) Wild-type mice were injected with saline, FVIIa, active site-inhibited FVIIa (FVIIai) or FVIIaF4A/L8A mutant (120 μg kg−1 body weight) before VEGF was injected (six mice per group). Vascular leakage in the skin was measured as described in Fig. 3. **P < 0.01; ***P < 0.001. NS, no statistically significant difference.
The EPCR–FVIIa-induced barrier protective effect involves PAR1 activation
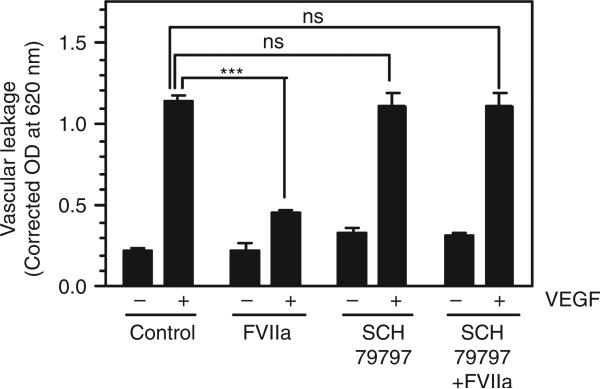
To investigate the role of PAR1 in the FVIIa-induced barrier protective effect in vivo, a PAR1-selective antagonist (SCH79797) was administered to wild-type mice 30 min prior to FVIIa administration. The PAR1 antagonist had no effect on VEGF-induced vascular permeability as such (Fig. 5). However, the PAR1 antagonist fully blocked the barrier protective effect of FVIIa (Fig. 5). It may be pertinent to note here that, although SCH79797 is frequently used as a PAR1 antagonist, it was shown in in vitro cell proliferation and survival studies that it could act independent of PAR1 [15]. However, it is unlikely that a potential PAR1-independent effect of SCH79797 is responsible for the attenuation of the FVIIa-induced barrier protective effect, as SCH79797 had no effect on basal or VEGF-induced permeability in the absence of FVIIa.
Fig. 5.
Protease-activated receptor 1 (PAR1) antagonist blocks the FVIIa-induced barrier protective effect. Wild-type mice were injected with a control vehicle or the PAR1 antagonist SCH79797 (1.7 mg kg−1 body weight) via the tail vein. Thirty minutes later, saline or FVIIa (120 μg kg−1 body weight) was injected into mice via the tail vein, and this was followed by intradermal injection of vascular endothelial growth factor (VEGF) or saline. Vascular leakage into the skin tissue was measured as described in Materials and methods (six mice per group). ***P < 0.001. NS, no statistically significant difference.
FXa and thrombin provide a barrier protective effect independently of EPCR
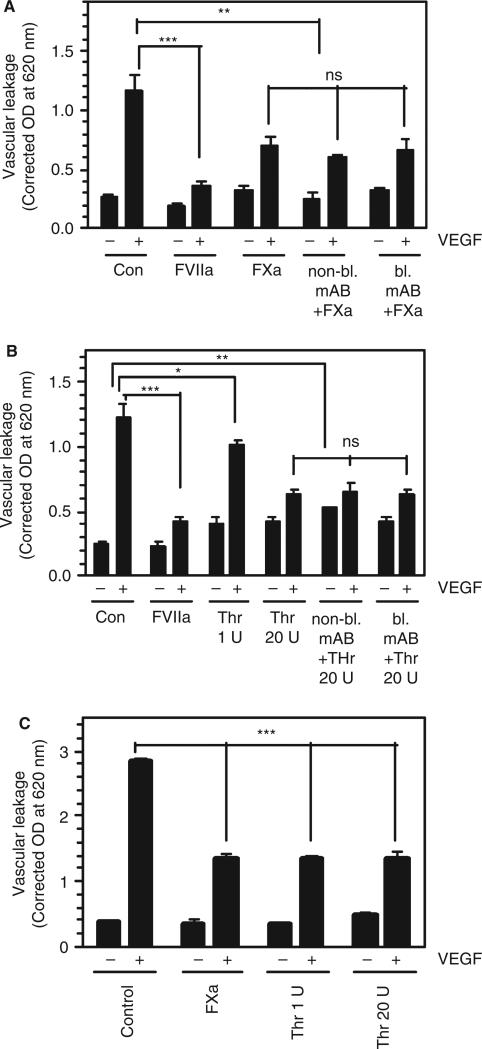
Next, we examined whether FXa or thrombin provides a barrier protective effect in vivo, and the involvement of EPCR in this process. As shown in Fig. 6A,B, both FXa and thrombin partly attenuated VEGF-induced vascular leakage. A lower concentration of thrombin (1 U kg−1 body weight) reduced VEGF-induced vascular leakage minimally, but in a statistically significant fashion, whereas a higher concentration of thrombin (20 U kg−1 body weight) substantially reduced VEGF-induced vascular leakage. Nonetheless, the barrier protective effects against VEGF-induced vascular leakage provided by both FXa and thrombin were lower than that the effect obtained with FVIIa. More importantly, administration of either non-blocking or blocking EPCR mAb had no significant effect on the partial protection conferred by FXa or thrombin (Fig. 6A,B), indicating that EPCR was not involved in mediating the barrier protective effect of FXa or thrombin. This notion was further confirmed by the observation that both FXa and thrombin also reduced VEGF-induced vascular leakage in EPCR-deficient mice (Fig. 6C).
Fig. 6.
FXa and thrombin provide a partial barrier protective effect against vascular endothelial growth factor (VEGF)-induced hyper-permeability independently of endothelial cell protein C receptor (EPCR). (A) Wild-type mice were injected with a control vehicle (Con), FVIIa (120 μg kg−1 body weight), FXa (120 μg kg−1 body weight), non-bocking EPCR mAb, or blocking EPCR mAb, followed by FXa. The mAbs were administered intraperitoneally at 4 mg kg−1 body weight 30 min before FXa (three mice per group for the EPCR mAb group; otherwise, six mice per group). (B) Wild-type mice were injected with a control vehicle (Con), FVIIa, thrombin (Thr) (1 U or 20 U kg−1 body weight [0.25 or 5 μg kg−1 body weight]), blocking EPCR mAb, or non-blocking EPCR mAb, followed by Thr (20 U kg−1 body weight) (three mice per group for the EPCR mAb group; otherwise, six mice per group). (C) EPCR-deficient mice were injected with a control vehicle, FXa (120 μg kg−1 body weight), or Thr (1 U or 20 U kg−1 body weight [0.25 or 5 μg kg−1 body weight]) (five mice per group). In all experimental groups, 30 min after FVIIa, FXa or Thr injection, VEGF or saline was injected intradermally, and vascular leakage into the surrounding skin tissue was measured as described in Materials and methods. *P < 0.05; **P < 0.01; ***P < 0.001. NS, no statistically significant difference.
Increased vascular leakage in hemophilic mice and correction with FVIIa treatment
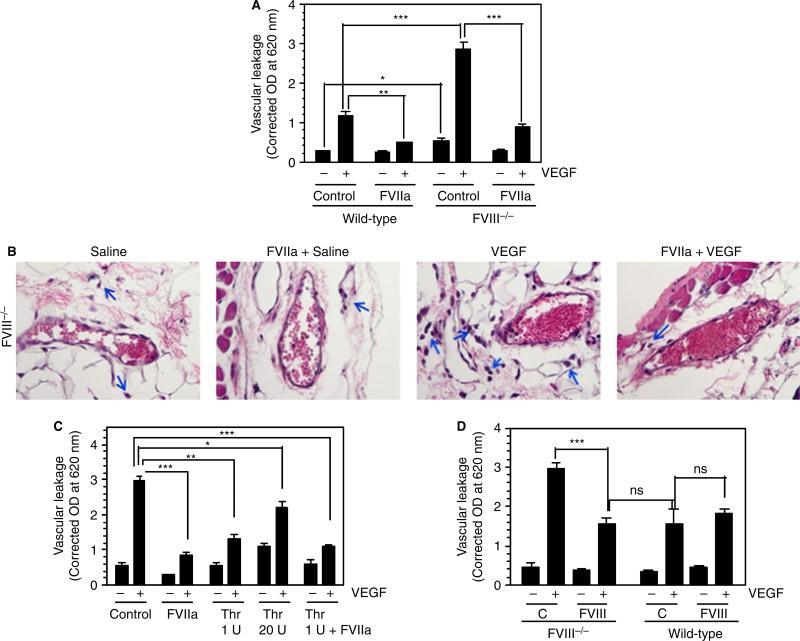
Evaluation of vascular leakage in FVIII−/− mice injected with saline or VEGF into the skin showed that vascular leakage was much greater in FVIII−/− mice than in wild-type mice, even under basal conditions (saline-injected), and that VEGF markedly increased vascular leakage in FVIII−/− mice as compared with wild-type mice (Fig. 7A,B). Similarly to what was observed in wild-type mice, administration of FVIIa markedly reduced VEGF-induced vascular leakage in hemophilic mice (Fig. 7A,B). Measurement of TAT complex levels in the plasma of hemophilic mice showed that they were significantly lower than in wild-type mice (7.5 ± 1.0 vs. 11.5 ± 0.55 ng mL−1), and that FVIIa treatment increased TAT complex levels (16.1 0.21 ng mL−1). Pretreatment of FVIII−/− mice with ±blocking EPCR mAb prior to FVIIa administration slightly, but not statistically significantly, increased TAT complex levels (21.2 ± 3.6 ng mL−1). To investigate the contribution of FVIIa-induced thrombin generation in reducing VEGF-induced barrier disruption, we determined the effect of exogenously administered thrombin on VEGF-induced vascular leakage in FVIII−/− mice. As shown in Fig. 7C, thrombin only partially reduced VEGF-induced vascular leakage in hemophilic mice. It is interesting to note that a low concentration of thrombin (1 U kg−1 body weight) was slightly more effective than a higher concentration of thrombin (20 U kg−1 body weight). In both cases, thrombin's protective effect was much lower than that of FVIIa (Fig. 7C). Administration of thrombin along with FVIIa did not further reduce VEGF-induced vascular permeability in the group that received FVIIa alone.
Fig. 7.
Increased vascular leakage in hemophilia is corrected by administration of therapeutic concentrations of FVIIa. (A) Wild-type or FVIII−/− mice were treated with saline (control) or FVIIa (120 μg kg−1 body weight) by injection via the tail vein. Thirty minutes later, saline or vascular endothelial growth factor (VEGF) (50 ng) was injected intradermally, and VEGF-induced vascular leakage was measured as described in Materials and methods (n = 8 mice/group). (B) FVIII−/− mice were treated with saline or FVIIa, and then injected with VEGF as in (A). Skin tissue sections were stained with hematoxylin and eosin. Arrows indicate leukocytes that leaked into the tissue. The sections shown are representative of six mice per group. (C) FVIII−/− mice were treated with saline (control), FVIIa (120 μg kg−1 body weight), thrombin (Thr) (1 U or 20 U kg−1 body weight [0.25 or 5 μg kg−1 body weight]), or FVIIa + Thr (120 μg + 1 U kg−1 body weight), and then injected with VEGF (three to seven mice per group). (D) FVIII−/− or wild-type mice were injected via the tail vein with saline or FVIII (200 U kg−1 body weight). Thirty minutes later, saline or VEGF (50 ng per site) was injected intradermally (five mice per group). VEGF-induced vascular leakage was measured as described in Materials and methods. *P < 0.05; **P < 0.01; ***P < 0.001. C, control; NS, no statistically significant difference.
In additional experiments, we investigated whether administration of FVIII to FVIII−/− mice would correct the hyperpermeability observed in these mice. As shown in Fig. 7D, administration of FVIII (200 U kg−1 body weight) to FVIII−/− mice corrected VEGF-induced hyper-permeability in these mice. The extent of vascular leakage in FVIII−/− mice treated with FVIII was very similar to that observed in wild-type mice. Administration of the same dose of FVIII to wild-type mice had no effect on VEGF-induced permeability. Similar results were obtained when FVIIIa was administered in place of FVIII (data not shown).
Discussion
Although recent studies by us and other investigators have established that FVIIa binds to EPCR, the relevance of this interaction in vivo is not completely understood. In vivo studies have shown that administration of FVIIa attenuates LPS-induced vascular leakage in the lung and kidney [8]. In the present study, we provide compelling evidence, for the first time, that FVIIa provides barrier protective effects in vivo through binding to EPCR, and the subsequent activation of PAR1-mediated signaling. The data presented here also show that hemophilic conditions increase the susceptibility to barrier dysfunction and that administration of a therapeutic concentration of FVIIa restores barrier integrity.
Recently, von Drygalski et al. [16] demonstrated increased vascular leakage in the lung and kidney in EPCRlow mice as compared with control mice in response to LPS, indicating that EPCR is required to maintain vascular integrity in vivo during endotoxemia. It was somewhat surprising for us to find in the present study that there were no significant differences between EPCR-deficient and control mice in vascular leakage in the lung and kidney in basal conditions or in response to LPS. Unknown genotypic differences between the EPCRlow and EPCR-deficient mice used in these studies, possible differences in the LPS product used and its potency in inducing leakage and/or differences in the methodology employed to assess vascular leakage could have contributed to this result. Nonetheless, our data show that the protective effect of exogenously administered FVIIa in reducing vascular leakage in endotoxemia is dependent on EPCR, as FVIIa failed to reduce LPS-induced vascular leakage in EPCR-deficient mice.
More importantly, a clear role of EPCR in maintaining vascular barrier integrity in vivo is clearly evident in the VEGF-induced vascular hyperpermeability model, as the extent of VEGF-induced vascular leakage in the skin was markedly higher in EPCR-deficient mice than in wild-type mice, whereas overexpression of EPCR markedly attenuated VEGF-induced vascular leakage. The observation that administration of therapeutic concentrations of FVIIa significantly reduced VEGF-induced vascular leakage in wild-type but not in EPCR-deficient mice confirms the conclusion reached from the endotoxemia model, i.e. that FVIIa provides a barrier protective effect, and that the FVIIa-induced protective effect is mediated through EPCR. At present, it is not entirely clear why EPCR-overexpressing mice were protected from VEGF-induced vascular permeability even in the absence of FVIIa treatment. It was shown previously that EPCR-overexpressing mice had a survival advantage over wild-type mice after a lethal dose of LPS, presumably by generating more APC in response to LPS challenge [12]. It is unlikely that VEGF administration leads to more APC generation, particularly in the short duration of our experimental period. It is possible that increased vascular compartmentalization of PC in EPCR-overexpressing mice [12] may have a role in protecting EPCR-overexpressing mice from VEGF-induced vascular permeability.
It is interesting to note that FVIIa appears to be more efficient than APC in providing the barrier protective effect in our model system, but the reasons for this are unknown at present. It may be pertinent to note here that FVIIa and APC have similar molecular masses (50 kDa and 55 kDa, respectively) and circulatory half-lives in mice (~ 20–25 min) [2,17,18]. Therefore, it is unlikely that similar doses of these proteins would result in widely differing in vivo concentrations. Earlier in vitro studies using human endothelial cells also showed that FVIIa and APC bound EPCR with similar affinities [1] and cleaved endogenous PAR1 with similar efficiencies [8]. The lack of specific antibodies against murine PAR1 with which to measure the cleavage of endogenous murine PAR1 prevented us from evaluating FVIIa and APC cleavage of murine PAR1. It is unlikely that traces of FXa or thrombin generated in response to FVIIa administration partly contribute to the FVIIa-induced barrier protective effect, as an FVIIa mutant that is capable of activating coagulation but lacks an EPCR-binding site was completely unable to restore barrier integrity in wild-type mice.
Exogenous administration of both FXa and thrombin also reduced VEGF-induced vascular leakage. These data were consistent with in vitro findings showing that thrombin (at low concentrations) [19] and FXa [10,20] provide a barrier protective effect in either an EPCR-dependent or an EPCR-independent manner. The partial protective effect that we observed with thrombin and FXa appeared to be independent of EPCR, as we observed a similar protective effect in EPCR-deficient mice. It has been suggested that FXa could have an EPCR-independent barrier protective effect via its interaction with a putative binding site on endothelial cells and the subsequent activation of PAR2 [10,20]. At present, it is unknown whether such a mechanism is responsible for the FXa-induced barrier protective effect observed in the present study.
Our earlier in vitro studies suggested that FVIIa–EPCR-induced PAR1 activation is responsible for the FVIIa-induced barrier protective effect against thrombin-induced leakage [8]. Consistent with the in vitro finding, blockade of PAR1 activation by the administration of a PAR1 antagonist (SCH79797) attenuated the FVIIa-induced barrier protective effect against VEGF-induced vascular permeability. At present, it is unknown whether the FVIIa-induced barrier protective effect is solely mediated through FVIIa–EPCR activation of PAR1, or whether it includes the activation of other PARs. Recent studies have shown that APC–EPCR can cleave PAR1 and PAR3 at non-canonical cleavage sites, generating novel N-terminal tethered ligands, and that the activation of PAR1 and PAR3 by these novel tethered ligands is responsible for APC-mediated cytoprotective effects [6,21]. At present, it is unknown whether FVIIa–EPCR can cleave PAR1 and PAR3 at these non-canonical cleavage sites, and, if so, whether they are responsible for the FVIIa-induced barrier protective effect.
Similarly to EPCR-deficient mice, hemophilic mice showed increased susceptibility to VEGF-induced vascular leakage. It is noteworthy that increased vascular leakage was also observed in hemophilic mice, as compared with controls, even under basal conditions. These data suggest that traces of thrombin generated in basal conditions may play an important role in vascular endothelial barrier stabilization in vivo. This notion was supported by the observation that infusion of FVIII reduced the hyper-vascular permeability of FVIII−/− mice to the same level as that found in wild-type mice. More importantly, administration of a therapeutic concentration of FVIIa markedly reduced VEGF-induced vascular leakage in hemophilic mice. It is unlikely that traces of thrombin generated by FVIIa are responsible for this protective effect, as the FVIIa-induced barrier protective effective is more pronounced than the thrombin effect.
Although we are not aware of any reports that have linked bleeding in hemophiliacs to increased vascular permeability, it is interesting to note that increased capillary permeability and lung epithelial permeability have been reported in hemophilic patients [22,23]. Our present studies with hemophilic mice clearly establish that vascular barrier integrity is compromised in hemophilia. Disruption of barrier integrity in vivo with VE-cadherin antibodies or platelet-activating factor was shown to result in plasma leakage and bleeding [24,25]. On the basis of our finding that a therapeutic concentration of FVIIa is capable of restoring barrier integrity in hemophilic mice, we speculate that prophylactic administration of FVIIa to hemophiliacs would prevent joint bleeding and joint destruction, not only by stopping early bleeding by generating thrombin, but also by protecting the integrity of the vascular endothelium. However, further in vivo studies with appropriate model systems are required to test this possibility.
Recent evidence indicates that the breakdown in endothelial barrier function plays a crucial role in the pathogenesis of sepsis, and that prevention of vascular leakage reduces the mortality resulting from sepsis [26]. Vascular permeability induced by VEGF secreted by ischemic tissue was shown to expand the area of tissue damage in myocardial infarction and stroke [27]. If the administration of FVIIa can prevent the vascular permeability associated with inflammation and other injuries, it should be possible to use FVIIa as a therapeutic agent to reduce vascular leakage in specific disease conditions. However, the risk of adverse effects of FVIIa, such as, rarely, thromboembolism [28–30], should be taken account of in such considerations. This is particularly important because this risk may increase with increased exposure to TF in injury. It may be pertinent to note here that low doses of FVIIa have been used successfully for treating trauma patients, who may have elevated levels of TF [31]. Our present data serve as a proof-of-concept that FVIIa could protect vascular barrier integrity in vivo involving EPCR, and highlights the need for additional studies in this area.
Addendum
J. Sundaram performed most of the experiments described in the manuscript. R. Goplalakrishnan performed endotoxemia experiments. S. Keshava performed histochemical studies. C. T. Esmon provided crucial reagents and EPCR transgenic mice. U. R. Pendurthi contributed to study design and data analysis. L. V. M. Rao designed the research, analyzed the data, and wrote the manuscript. All authors contributed to the preparation of the final version of the manuscript.
Acknowledgements
The authors thank C. Clark for providing technical support with the LPS-induced vascular permeability experiments, and E. Persson (Novo Nordisk, Denmark) for providing the FVIIaF4A/L8A mutant. U. R. Pendurthi and L. V. M. Rao appreciate thank U. Hedner (University of Lund, Sweden) for discussions on the subject matter. This work was supported by National Institutes of Health grant HL1074830 (LVMR) and UM1HL120877 (CTE).
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Ghosh S, Pendurthi UR, Steinoe A, Esmon CT, Rao LV. Endothelial cell protein C receptor acts as a cellular receptor for factor VIIa on endothelium. J Biol Chem. 2007;282:11849–57. doi: 10.1074/jbc.M609283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nayak RC, Sen P, Ghosh S, Gopalakrishnan R, Esmon CT, Pendurthi UR, Rao LVM. Endothelial cell protein C receptor cellular localization and trafficking. Blood. 2009;114:1974–86. doi: 10.1182/blood-2009-03-208900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preston RJ, Ajzner E, Razzari C, Karageorgi S, Dua S, Dahlback B, Lane DA. Multifunctional specificity of the protein C/activated protein C Gla domain. J Biol Chem. 2006;281:28850–7. doi: 10.1074/jbc.M604966200. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Sagaseta J, Montes R, Puy C, Diez N, Fukudome K, Hermida J. Binding of factor VIIa to the endothelial cell protein C receptor reduces its coagulant activity. J Thromb Haemost. 2007;5:1817–24. doi: 10.1111/j.1538-7836.2007.02648.x. [DOI] [PubMed] [Google Scholar]
- 5.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by protein C pathway. Science. 2002;296:1880–2. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 6.Burnier L, Mosnier LO. Novel mechanisms for activated protein C cytoprotective activities involving noncanonical activation of protease-activated receptor 3. Blood. 2013;122:807–16. doi: 10.1182/blood-2013-03-488957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–84. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 8.Sen P, Gopalakrishan R, Kothari H, Keshava S, Clark C, Esmon CT, Pendurthi UR, Rao LVM. Factor VIIa bound to endothelial cell protein C receptor activates protease activated receptor-1 and mediates cell signaling and barrier protection. Blood. 2011;117:3199–208. doi: 10.1182/blood-2010-09-310706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuepbach RA, Feistritzer C, Fernandez JA, Griffin JH, Riewald M. Protection of vascular barrier integrity by activated protein C in murine models depends on protease-activated receptor-1. Thromb Haemost. 2009;101:724–33. doi: 10.1160/th08-10-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuepbach RA, Riewald M. Coagulation factor Xa cleaves PAR1 and mediates signaling dependent on binding to the endothelial protein C receptor. J Thromb Haemost. 2010;8:379–88. doi: 10.1111/j.1538-7836.2009.03682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark CA, Vatsyayan R, Hedner U, Esmon CT, Pendurthi UR, Rao LV. Endothelial cell protein C receptor-mediated redistribution and tissue-level accumulation of factor VIIa. J Thromb Hae-most. 2012;10:2383–91. doi: 10.1111/j.1538-7836.2012.04917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Zheng X, Gu J, Hunter J, Ferrell GL, Lupu F, Esmon NL, Esmon CT. Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J Thromb Haemost. 2005;3:1351–9. doi: 10.1111/j.1538-7836.2005.01385.x. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Zheng X, Gu JM, Ferrell GL, Brady M, Esmon NL, Esmon CT. Extraembryonic expression of EPCR is essential for embryonic viability. Blood. 2005;106:2716–22. doi: 10.1182/blood-2005-01-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae JS, Kim YU, Park MK, Rezaie AR. Concentration dependent dual effect of thrombin in endothelial cells via Par-1 and Pi3 Kinase. J Cell Physiol. 2009;219:744–51. doi: 10.1002/jcp.21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Serio C, Pellerito S, Duarte M, Massi D, Naldini A, Cirino G, Prudovsky I, Santucci M, Geppetti P, Marchionni N, Masotti G, Tarantini F. Protease-activated receptor 1-selective antagonist SCH79797 inhibits cell proliferation and induces apoptosis by a protease-activated receptor 1-independent mechanism. Basic Clin Pharmacol Toxicol. 2007;101:63–9. doi: 10.1111/j.1742-7843.2007.00078.x. [DOI] [PubMed] [Google Scholar]
- 16.von Drygalski A, Furlan-Freguia C, Ruf W, Griffin JH, Mosnier LO. Organ-specific protection against lipopolysaccharide-induced vascular leak is dependent on the endothelial protein C receptor. Arterioscler Thromb Vasc Biol. 2013;33:769–76. doi: 10.1161/ATVBAHA.112.301082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen LC, Elm T, Ezban M, Krogh TN, Karpf DM, Steino A, Olsen EH, Sorensen BB. Plasma elimination kinetics for factor VII are independent of its activation to factor VIIa and complex formation with plasma inhibitors. Thromb Haemost. 2009;101:818–26. [PubMed] [Google Scholar]
- 18.Zhong Z, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, Thiyagarajan M, Deane R, Fernandez JA, Lane S, Zlokovic AB, Liu T, Griffin JH, Chow N, Castellino FJ, Stojanovic K, Cleveland DW, Zlokovic BV. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119:3437–49. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae JS, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–16. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae JS, Yang L, Rezaie AR. Factor X/Xa elicits protective signaling responses in endothelial cells directly via PAR-2 and indirectly via endothelial protein C receptor-dependent recruitment of PAR-1. J Biol Chem. 2010;285:34803–12. doi: 10.1074/jbc.M110.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosnier LO, Sinha RK, Burnier L, Bouwens EA, Griffin JH. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood. 2012;120:5237–46. doi: 10.1182/blood-2012-08-452169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neri Serneri GG, Gensini GF, Gensini RA. Increased capillary permeability in haemophilia and afibrinogenaemia. Acta Haematol. 1974;52:336–44. doi: 10.1159/000208261. [DOI] [PubMed] [Google Scholar]
- 23.O'Doherty MJ, Page CJ, Harrington C, Nunan T, Savidge G. Haemophilia, AIDS and lung epithelial permeability. Eur J Haematol. 1990;44:252–6. doi: 10.1111/j.1600-0609.1990.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 24.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, Mcdonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA. 1999;96:9815–20. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatakeyama K, Yano S, Watanabe K. Gastric bleeding and increased gastric vascular permeability induced by platelet activating factor (PAF): effect of drugs that affect arachidonate metabolism. Jpn J Pharmacol. 1991;56:271–7. doi: 10.1254/jjp.56.271. [DOI] [PubMed] [Google Scholar]
- 26.Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med. 2011;3:88ps25. doi: 10.1126/scitranslmed.3002011. [DOI] [PubMed] [Google Scholar]
- 27.Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol. 2000;279:L419–22. doi: 10.1152/ajplung.2000.279.3.L419. [DOI] [PubMed] [Google Scholar]
- 28.Hedner U. Factor VIIa and its potential therapeutic use in bleeding-associated pathologies. Thromb Haemost. 2008;100:557–62. [PubMed] [Google Scholar]
- 29.Logan AC, Goodnough LT. Recombinant factor VIIa: an assessment of evidence regarding its efficacy and safety in the off-label setting. Hematology Am Soc Hematol Educ Program. 2010;2010:153–9. doi: 10.1182/asheducation-2010.1.153. [DOI] [PubMed] [Google Scholar]
- 30.Yank V, Tuohy CV, Logan AC, Bravata DM, Staudenmayer K, Eisenhut R, Sundaram V, McMahon D, Olkin I, McDonald KM, Owens DK, Stafford RS. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med. 2011;154:529–40. doi: 10.7326/0003-4819-154-8-201104190-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein DM, Dutton RP, Hess JR, Scalea TM. Low-dose recombinant factor VIIa for trauma patients with coagulopathy. Injury. 2008;39:1054–61. doi: 10.1016/j.injury.2008.03.032. [DOI] [PubMed] [Google Scholar]