Abstract
Professional recommendations for the return of results from exome and whole-genome sequencing (ES/WGS) have been controversial. The lack of clear guidance about whether and, if so, how to return ES/WGS incidental results limits the extent to which individuals and families might benefit from ES/WGS. The perspectives of genetics professionals, particularly those at the forefront of using ES/WGS in clinics, are largely unknown. Data on stakeholder perspectives could help clarify how to weigh expert positions and recommendations. We conducted an online survey of 9,857 genetics professionals to learn their attitudes on the return of incidental results from ES/WGS and the recent American College of Medical Genetic and Genomics Recommendations for Reporting of Incidental Findings in Clinical Exome and Genome Sequencing. Of the 847 respondents, 760 completed the survey. The overwhelming majority of respondents thought that incidental ES/WGS results should be offered to adult patients (85%), healthy adults (75%), and the parents of a child with a medical condition (74%). The majority thought that incidental results about adult-onset conditions (62%) and carrier status (62%) should be offered to the parents of a child with a medical condition. About half thought that offered results should not be limited to those deemed clinically actionable. The vast majority (81%) thought that individual preferences should guide return. Genetics professionals’ perspectives on the return of ES/WGS results differed substantially from current recommendations, underscoring the need to establish clear purpose for recommendations on the return of incidental ES/WGS results as professional societies grapple with developing and updating recommendations.
Introduction
The unprecedented clinical diagnostic power of exome and whole-genome sequencing (ES/WGS) has motivated rapid commercialization of ES/WGS for research and clinical service. Because ES/WGS can assess virtually every gene in the human genome for disease-predisposing variants, it can provide information on putatively every allele that influences risk of disease. Accordingly, even if no primary result (i.e., the reason for which the test was ordered) is found via ES/WGS, there are multiple, and potentially hundreds, of so-called secondary or incidental results that can be offered for return to individuals and families.1,2 Whether to return all or some of these incidental results and, if in part, which incidental results to return, how to most effectively return them, and how best to use incidental results to improve health is unclear but has dominated professional discourse about the potential benefits and risks of using ES/WGS.3–5 The lack of an informed approach for returning incidental results from ES/WGS is compounded by existing challenges to the traditional process of communicating and disseminating genetic results.
The lack of clear guidance about whether and, if so, how to return incidental results from ES/WGS limits the extent to which individuals and families are empowered to translate genomic information into improved lifestyles, medical care, and ultimately long-term health. Recently, the American College of Medical Genetics and Genomics (ACMG) issued recommendations on the return of incidental results from ES/WGS.6,7 These recommendations have proven to be highly controversial; a relatively small but outspoken group of researchers and ethicists are expressing widely divergent views on (1) whether and, if so, what incidental results should be offered for return, (2) issues of informed consent, and (3) providing results for adult-onset conditions to the parents of children who undergo ES/WGS.8–14 Despite the debate, the perspectives of genetics professionals, particularly those at the forefront of using ES/WGS in clinical settings (i.e., medical geneticists and genetic counselors), on the return of incidental results from ES/WGS or the ACMG recommendations are largely unknown. Empirical data on such stakeholder perspectives could help clarify how to weigh various expert positions and recommendations. To this end, we developed a web-based survey and queried nearly 10,000 genetics professionals about the return of incidental results from ES/WGS.
Subjects and Methods
We sought to characterize genetics professionals’ attitudes toward (1) the return of clinical ES/WGS results (12 items), (2) the process of returning results (five items), and (3) the ACMG recommendations for reporting incidental findings from clinical ES/WGS (nine items). Personal and professional demographics were also collected (nine items). A five-point Likert-type scale was used to rate the level of agreement in the majority of items assessing attitudes. Six items utilized dichotomous responses (“yes” or “no”) and a “don’t know” option. Six items used pregiven categorical response options without restriction (e.g., indicate all that apply). Each page of items included a space for comments. A complete list of survey questions is included in Table S1, available online.
We developed survey items from a review of the medical literature, including previously published surveys on the return of genetic results and interviews with genetics professionals on the return of results, to identify key topics and examples. We programmed a draft of a 35-item survey with SurveyMonkey. This draft survey was pilot tested with five individuals who are medical geneticists, genetic counselors, and or other genetics professionals. After editing survey items per their feedback, we tested the survey again on ten individuals to identify typographical errors, problems in question logic, and instructions that needed further clarification.
To identify potential respondents, we collected e-mail addresses, professional degrees, and states of residence from the publically available web-based directories of three different societies of genetics professionals: the American Society of Human Genetics (ASHG), the ACMG, and the National Society of Genetic Counselors (NSGC). Although nongenetics health professionals may order ES/WGS, we chose to focus our questions on genetics professionals because collectively they are at the forefront of developing and utilizing clinical ES/WGS in clinical settings. Available contact information was collected in the fall of 2012 and was combined to form a single nonredundant e-mail distribution list. Individuals without an e-mail address were excluded. The University of Washington Human Subjects Division reviewed the study protocol and determined it to be nonhuman subjects research because respondents were anonymous.
On August 19, 2013, an e-mail was sent to 9,857 addresses to invite genetics professionals to complete the online survey. Thirty invitations were returned automatically as a result of invalid addresses. About half (∼50%) of survey responses were received within the first 24 hours. Two reminders to respond to the survey were sent by e-mail over the next 4 weeks. The survey was made accessible in SurveyMonkey until September 17, 2013.
The survey provided a brief introduction explaining its purpose; definitions of relevant terms, such as “actionable result,” “primary result,” and “incidental finding”; and the following description of the ACMG recommendations: “In March 2013, the ACMG released their recommendations for reporting of incidental findings in clinical exome and genome sequencing. They recommended that laboratories performing clinical sequencing seek and report mutations known or expected to be pathogenic in 56 genes (i.e., the minimum list) in all patients, irrespective of age, who undergo germline (or constitutional) exome/genome sequencing.” Also included was a direct electronic link to the ACMG recommendations as a reference for respondents.
Survey response data were downloaded from SurveyMonkey and analyzed with Stata/IC (Intercooled Stata) version 12. We used descriptive statistics to summarize responses to each survey item. To facilitate analysis and interpretation, we collapsed response categories for Likert-scale-type items to construct an “agree” category combining both “strongly agree” and “agree” and a corresponding “disagree” category. “Neither agree nor disagree” responses were tabulated. Because respondents were permitted to skip items, the sample size varied by item.
To assess response bias, we compared our respondents’ demographic data to data available on nonrespondents from our initial e-mail invitation list and to reported estimates of demographic characteristics from studies conducted among the membership of the NSGC,15 medical geneticists certified by the American Board of Medical Genetics (ABMG),16 and ASHG members.17 Additional data on common demographic variables among members of ASHG and ACMG were unavailable. We used chi-square (χ2) goodness-of-fit tests to evaluate categorical demographic variables and evaluated mean age by a two-sample t test with unequal variances.
Results
The survey response rate was 9% (n = 847/9,857), and the majority of respondents finished the survey so that the overall completion rate was 8% (n = 760/9,857). The majority of respondents were female (58%), white (89.8%), and of non-Hispanic ethnicity (96%) and resided in the United States (81%) (Table S2). The mean age of respondents was 46.2 years and ranged from 23 to 85 years. Ninety-nine percent of respondents had a master’s degree, Ph.D., medical degree, or a combination of Ph.D. and medical degree. Several professions, including clinical geneticist (24%) genetic counselor (22%), and human geneticist (19%), were well represented among respondents. Three quarters of respondents, including those in clinical genetics research (79%), basic science research (67%), patient care (64%) and Ethical, Legal, and Social Implications (ELSI) research (49%), were engaged in multiple types of work. The majority of respondents (73%) worked in academia.
Comparison to other sources of demographic data for genetics professionals suggested that nonresponse was not a substantial source of bias (Table S3). Self-identified genetic counselors who responded did not differ significantly in gender, age, race, or education from those who responded to the NSGC’s 2012 professional survey.15 The only assessed variable that differed was work environment (χ2 = 68.04, 3 degrees of freedom [df], p = 1.1 × 10−14); specifically, the proportion of academics was greater in the survey respondents than in the overall field of genetic counselors. Self-identified medical geneticists (including clinical geneticists) who responded did not differ significantly in gender, age, education, or work environment from those reported in a 2003 ABMG workforce survey.16 A comparison of age, education, and work environment between survey respondents and results from a 1989 ASHG member survey produced mixed results.17 Although no difference was observed in work environment, the distribution of age (two-sample t test unequal variance t = 8.5318, p < 0.0001) and education (χ2 = 44.38, 3 df, p = 1.3 × 10−9) differed significantly. As might be expected from such a heterogeneous sample of genetics professionals and a gap of nearly a quarter century, the trend was for a higher proportion of younger professionals and individuals with either a master’s of science or a doctorate in both medicine (i.e., M.D.) and philosophy (i.e., Ph.D.) in our survey. Finally, a comparison of educational degrees and geographical locations (i.e., the Northeast, Midwest, South, and West) between survey respondents and nonrespondents revealed no significant differences in education or geographic region (Table S3).
Overall Attitudes toward the Return of Incidental Results from Clinical ES/WGS
The overwhelming majority of genetics professionals (85%, n = 648/763) agreed that incidental results from ES/WGS should be offered to adult patients, and three-quarters of professionals agreed that incidental ES/WGS results should be offered to healthy adults (75%, n = 567/760) and the parents of a child with a medical condition (74%, n = 562/762). Genetics professionals were split almost evenly as to whether only actionable results from ES/WGS should be offered for return to adult patients, parents of a child with a medical condition, or healthy adults (Figure 1A). Almost all respondents (88%, n = 665/755) thought that results about childhood-onset conditions should be offered to the parents of a child who has a medical condition and who has undergone clinical ES/WGS. The majority of respondents also agreed with offering parents their child’s results for adult-onset conditions (62%, n = 467/753) and carrier status (62%, n = 471/757).
Figure 1.
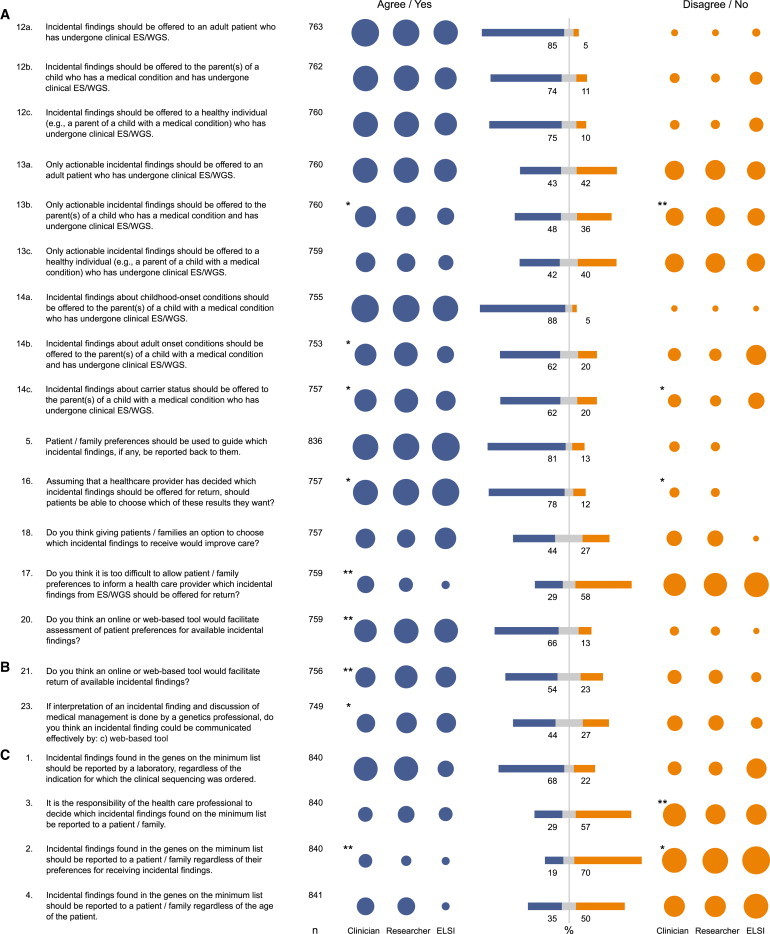
Attitudes of Genetics Professionals toward the Return of Incidental Results
This figure illustrates genetics professionals’ (A) overall attitudes toward the return of incidental results from clinical ES/WGS, (B) attitudes about how to offer incidental results for return, and (C) attitudes toward the ACMG recommendations on incidental results from ES/WGS. Question number (e.g., 1) corresponds to the original question number on the online survey. Sample size (n) is presented and varies per question because respondents were allowed to skip questions. Blue bars indicate the proportion of respondents who agreed (including both “strongly agree” and “agree”) with corresponding statements or answered “yes” to corresponding questions. Orange bars indicate the proportion of respondents who disagreed (including both “strongly disagree” and “disagree”) with corresponding statements or answered “no” to corresponding questions. Grey bars indicate the proportion of respondents who selected “neither agree nor disagree” for corresponding statements or answered “don’t know” to corresponding questions. All bar values are rounded percentages calculated with the total number of respondents for each corresponding statement or question (n) as the denominator. Because small SEs ranged between 1% and 2%, bars are not shown. Colored circles indicate the proportion of respondents who agreed (blue) or disagreed (orange) by grouped categories of self-reported profession: clinician (n = 380, including clinical geneticist, medical geneticist, and genetic counselor), researcher (n = 207, including human geneticist, population geneticist, and genetic epidemiologist), and ELSI researcher (n = 26). A two-sample test of proportion was used for assessing significant differences in agreement or disagreement between clinicians and researchers. One asterisk indicates a significant difference with a p value < 0.05; two asterisks indicate a significant difference with a p value < 0.01.
The vast majority of genetics professionals (81%, n = 673/836) agreed that the preferences of a patient or family should guide which incidental results are offered for return. The majority of professionals (78%, n = 593/757) thought that patients should be able to choose which incidental results to receive after a healthcare provider has decided which results should be offered for return, even though less than half of respondents (44%, n = 330/757) thought that giving patients and families the option to choose which results to receive would improve care. Twice as many respondents (58%, n = 442/759 versus 29%, n = 219/759) thought it not “too difficult to allow patient/family preferences to inform a healthcare provider which incidental findings from ES/WGS should be offered for return.” To this end, the majority of genetics professionals (66%, n = 503/759) thought that a web-based tool could facilitate assessment of patient preferences for the return of incidental findings.
Genetics professionals were asked to complete the statement “Healthcare providers have an obligation to offer return of positive incidental findings from clinical ES/WGS to patients for [different types of conditions]” (Figure S1). Respondents could choose more than one response category. The majority completed the statement with results about Mendelian conditions (67%, n = 535/794) and results that inform the potential for adverse drug reactions (i.e., pharmacogenomic) (61%, n = 488/794). Nearly half (49%, n = 393/794) thought results about carrier status should be offered for return. Only one-fifth (20%, n = 156/794) thought there was an obligation to offer incidental results for complex traits. Twenty-five percent (n = 196/794) of respondents indicated that healthcare providers had no obligation to offer incidental results.
A total of 349 respondents provided information about ES/WGS results that they “typically” return (Figure S1). Ninety-four percent (n = 328/349) offer a primary result. Sixty-eight percent (n = 239/349) offer incidental results for Mendelian conditions. Nearly half offer incidental results for adverse drug response (47%, n = 164/349) and carrier status (45%, n = 157/349). Only twenty-five percent (n = 69/349) offer incidental results for complex conditions. Review of qualitative comments from respondents who indicated that they do not typically offer a primary result (Table S4) revealed that most of these individuals answered incorrectly and had never returned ES/WGS results in a clinical setting. Excluding these responses, virtually all respondents who conduct clinical ES/WGS offer primary results.
Attitudes about How to Offer Incidental Results for Return
Genetics professionals most frequently (79%, n = 593/755) ranked a “face-to-face meeting with a genetic counselor” as the preferred approach to returning an incidental result (Table 1). Alternatives that also ranked high included return via a “phone call with a genetic counselor” (63%, n = 476/755) and via “an interactive website with access to a genetic counselor by phone or online” (53%, n = 403/755). Fifty-four percent (n = 409/756) of respondents thought that “an online or web-based tool could facilitate return of incidental findings from ES/WGS.” The least preferred (81%, n = 610/755), or the last-ranked approach to returning incidental results, was notification via conventional mail. Consistent with these responses, most genetics professionals thought that incidental results from ES/WGS could not be communicated effectively by conventional mail (61%, n = 458/753) or e-mail (57%, n = 431/751), although 44% (n = 329/749) of respondents thought that a web-based tool could be used effectively to communicate ES/WGS results (Figure 1B).
Table 1.
Attitudes toward the Mode of Return in Response to Question 22a
| First Choice | Second Choice | Third Choice | Last Choice | |
|---|---|---|---|---|
| A report sent in the mail | 2.3% (17) | 4.0% (30) | 13.0% (98) | 80.8% (610) |
| A phone call with a genetic counselor | 5.6% (42) | 63.0% (476) | 26.5% (200) | 4.9% (37) |
| An interactive website with access to a genetic counselor by phone or online | 13.6% (103) | 23.4% (177) | 53.4% (403) | 9.5% (72) |
| A face-to-face meeting with a genetic counselor | 78.5% (593) | 9.5% (72) | 7.2% (54) | 4.8% (36) |
Italics indicate the largest proportion of responses per rank order.
Question 22: Rank the following responses to the statement in order of preference: “I think incidental findings should be communicated by …” (n = 755).
Attitudes toward the ACMG Recommendations on Incidental Results from ES/WGS
The majority of genetics professionals (68%, n = 573/840) agreed that results identified from the ACMG minimum list of genes should be reported regardless of the indication for sequencing (Figure 1C). The majority (57%, n = 482/840) disagreed that healthcare providers should decide which of these results should be returned. Genetics professionals were asked how they would respond to the situation where patients or families decline to receive incidental results from the ACMG minimum list (Figure S1). The majority of respondents (58%, n = 464/795) indicated that they would perform ES/WGS and not return incidental results per the request of the patient or family. Nearly half (47%, n = 376/795) would discuss each possible incidental result with the patient or family and offer for return only the results they opted to receive. Twenty-two percent (n = 175/795) would not perform ES/WGS if an alternative but less desirable test were available. Only 6% (n = 48/795) of respondents would decline to perform ES/WGS if no alternative test were available.
The majority of genetics professionals (65%, n = 518/799) thought that the biggest challenge in returning incidental results from ES/WGS is that most healthcare providers do not have the time and/or expertise to return incidental findings (Figure S1). Approximately half of genetics professionals considered it a challenge to not allow patients or families to select which incidental results they want to receive on the basis of their values (52%, n = 413/799) or to opt out of receiving incidental results (49%, n = 389/799). One of every two genetics professionals (48%, n = 386/799) thought returning incidental results from ES/WGS for adult-onset conditions to patients who are minors would be a major challenge. Only 23% (n = 185/799) thought identifying incidental results in ES/WGS data would be burdensome for a service lab, and one in five thought returning incidental ES/WGS results would increase the cost of healthcare.
We also asked respondents about possible concerns they might have for individuals to whom incidental results from ES/WGS were offered per the ACMG recommendations (Figure S1). Given the options provided, the overwhelming majority of genetics professionals were concerned that an individual might experience stress and anxiety (74%, n = 591/797). Only one-third were concerned that individuals might experience discrimination (31%, n = 247/797) or that their privacy would be violated (26%, n = 210/797). Only 14% (n = 113/797) of genetics professionals had no concerns.
Discussion
Several major conclusions about the attitudes of genetics professionals toward the return of incidental results from ES/WGS can be drawn from our findings. First, the overwhelming majority of respondents think that incidental results from ES/WGS should be offered to adult patients, healthy adults, and parents of a child with a medical condition. Second, although somewhat divided, the majority think that incidental results about adult-onset conditions and carrier status should be offered to the parents of a child who has a medical condition and who has undergone ES/WGS. Third, about half think that results offered for return should not be limited to those deemed clinically actionable. Finally, the vast majority think that the return of incidental results should be guided by individual preferences. Although comparisons to other professional surveys and our overall survey distribution list suggest demographic and professional similarities between respondents and nonrespondents, greater confidence in the generalizability of our results would be afforded by a study of nonrespondents.
General consensus among experts is that incidental results of clinical utility should be offered for return but that healthcare providers should proceed cautiously with disclosing results by accounting for the context of the encounter.3,18–21 Our survey results indicate that genetics professionals across a broad range of provider roles (e.g., medical geneticists and genetic counselors) overwhelmingly agree that incidental results from ES/WGS should be offered for return. However, genetics professionals express considerable openness toward the acceptable contexts in which results should be offered. Specifically, a majority of genetics professionals agreed with offering parents their child’s results for adult-onset conditions and carrier status, and nearly half of genetics professionals who return results from clinical ES/WGS “typically return” incidental results for adverse drug reactions and carrier status. These perspectives are consistent with the need for “flexibility” in policies governing the return of results22 and the need for further consideration of what types of results, beyond the “minimum floor,” should be offered for return.
Nearly half of survey respondents disagreed that only actionable results should be offered for return of incidental ES/WGS results. The importance of medical or clinical actionability as a criterion for return has been widely debated.14,23–27 At face value, actionability is a convincing reason for offering incidental results, yet defining what is actionable has proven to be challenging.28,29 Furthermore, evidence that actionability is the most important consideration among clinical genetics professionals has been inconsistent, perhaps in part because they have not been asked about the value of personal utility for their patients.19,24 Debate about individual preferences for results has focused on whether preferences should be considered in the return of results and is most often polarized around the individual’s right to refuse or opt out of receiving results.10,14,30,31 We provide clear evidence that genetics professionals value individuals’ and families’ preferences and that their preferences should guide the offer of incidental ES/WGS results.
Despite the substantial attention paid to the return of incidental ES/WGS results, little has been given to the best approaches for operationalizing the return of results. As we have articulated elsewhere, we think that conventional approaches to returning genetic results is far from optimal for the volume of incidental results expected from clinical ES/WGS.32,33 Our finding that genetics professionals consider lack of time and expertise as the most important practical challenge confirms the need for new tools to efficiently manage, if not transform, the return-of-results process for individuals and families.32 Accordingly, the openness of genetics professionals to different modes of returning incidental ES/WGS results provides an opportunity to consider innovative approaches to returning genetic and/or genomic results, in general, and incidental ES/WGS results in particular.34
Overall, our results suggest that many of the views of genetics professionals differ from those of the experts who developed the ACMG recommendations for the return of incidental ES/WGS results. It is likely that genetics professionals and experts agree on many of the issues and challenges in offering and returning incidental ES/WGS results but disagree on the relative weight each issue should be afforded in policy and practice. For instance, genetics professionals appeared to place greater weight on the value of offering results that might be of utility (e.g., carrier status and adult-onset conditions), but not necessarily actionable, suggesting that they recognize the potential benefits such results might have over an extended period of time. Genetics professionals also placed greater weight on supporting the right of individuals to selectively choose among results offered for return and to oppose disclosure altogether. Collectively, these trends suggest that genetics professionals prioritize flexibility in what incidental ES/WGS results should be offered for return and take a broader view of how to incorporate individual and family preferences about what results should be offered for return. In turn, this allows individuals and families the opportunity to fine tune, in partnership with their healthcare provider, the balance between maximizing benefits and minimizing potential harms.
Creating recommendations without consensus of the membership can result in a need for reconsideration and rapid modification. Since the release of the ACMG recommendations in March 2013,4 genetics professionals have broadly expressed concern and dissatisfaction with several major provisions. Perhaps in response to these criticisms, at least in part, the ACMG has issued one memo of clarification8 and one press release alluding to a modification allowing for an “opt-out” provision.35 Indeed, the importance of including an “opt-out” provision to the ACMG recommendations is evidenced by the 22% of respondents who might order a less useful test in lieu of ES/WGS if an individual elected not to receive incidental results. However, our results suggest that the gap between these modified recommendations on the return of incidental ES/WGS results and the views and practices of genetics professionals continues to be substantial.
Our findings raise the more general question as to what is the purpose of recommendations from a professional body such as the ACMG. Specifically, are recommendations intended to identify and standardize desirable practices across a profession or intended to reflect a general consensus among practitioners within a field such as medical genetics? Both have precedence within and beyond the field of medical genetics, but most examples (e.g., childhood vaccinations) are founded on a greater degree of certainty about the benefits and associated costs (e.g., false positives) of such policies. Given that the benefits of the return of incidental ES/WGS results per the ACMG recommendations are largely unknown, and are the focus of many studies currently underway, such a functional view of expert-driven policymaking seems premature.36 Likewise, in the presence of so many mixed perspectives about the return of incidental ES/WGS results, the ACMG recommendations clearly cannot reflect a general consensus. Either way, our survey results and the questions they raise underscore the need to establish a clear purpose for recommendations on the return of incidental ES/WGS results as other professional societies grapple with whether to consider developing recommendations or guidelines of their own.
Acknowledgments
We thank the genetics professionals who graciously completed our survey. Our work was supported in part by grants from the NIH National Human Genome Research Institute (award numbers U54HG006493 to M.J.B., Debbie Nickerson, and Jay Shendure; RC2HG005608 to M.J.B.; and R01HG006618 to H.K.T. and M.J.B.), the Life Sciences Discovery Fund (2065508 and 0905001), and the Washington Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplemental Data
References
- 1.Johnston J.J., Rubinstein W.S., Facio F.M., Ng D., Singh L.N., Teer J.K., Mullikin J.C., Biesecker L.G. Secondary variants in individuals undergoing exome sequencing: screening of 572 individuals identifies high-penetrance mutations in cancer-susceptibility genes. Am. J. Hum. Genet. 2012;91:97–108. doi: 10.1016/j.ajhg.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biesecker L.G. Incidental variants are critical for genomics. Am. J. Hum. Genet. 2013;92:648–651. doi: 10.1016/j.ajhg.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke W., Trinidad S.B., Clayton E.W. Seeking Genomic Knowledge: The Case for Clinical Restraint. Hastings Law J. 2013;64:1650–1664. [PMC free article] [PubMed] [Google Scholar]
- 4.Evans J.P., Rothschild B.B. Return of results: not that complicated? Genet. Med. 2012;14:358–360. doi: 10.1038/gim.2012.8. [DOI] [PubMed] [Google Scholar]
- 5.Grove M.E., Wolpert M.N., Cho M.K., Lee S.S., Ormond K.E. Views of Genetics Health Professionals on the Return of Genomic Results. J. Genet. Couns. 2013 doi: 10.1007/s10897-013-9611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Medical Genetics and Genomics. (2012). Policy Statement: Points to Consider in the Clinical Application of Genomic Sequencing. https://www.acmg.net/StaticContent/PPG/Clinical_Application_of_Genomic_Sequencing.pdf. [DOI] [PubMed]
- 7.Green R.C., Berg J.S., Grody W.W., Kalia S.S., Korf B.R., Martin C.L., McGuire A.L., Nussbaum R.L., O’Daniel J.M., Ormond K.E., American College of Medical Genetics and Genomics ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet. Med. 2013;15:565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American College of Medical Genetics and Genomics Incidental findings in clinical genomics: a clarification. Genet. Med. 2013;15:664–666. doi: 10.1038/gim.2013.82. [DOI] [PubMed] [Google Scholar]
- 9.Allyse M., Michie M. Not-so-incidental findings: the ACMG recommendations on the reporting of incidental findings in clinical whole genome and whole exome sequencing. Trends Biotechnol. 2013;31:439–441. doi: 10.1016/j.tibtech.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke W., Matheny Antommaria A.H., Bennett R., Botkin J., Clayton E.W., Henderson G.E., Holm I.A., Jarvik G.P., Khoury M.J., Knoppers B.M. Recommendations for returning genomic incidental findings? We need to talk! Genet. Med. 2013;15:854–859. doi: 10.1038/gim.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holtzman N.A. ACMG recommendations on incidental findings are flawed scientifically and ethically. Genet. Med. 2013;15:750–751. doi: 10.1038/gim.2013.96. [DOI] [PubMed] [Google Scholar]
- 12.McGuire A.L., Joffe S., Koenig B.A., Biesecker B.B., McCullough L.B., Blumenthal-Barby J.S., Caulfield T., Terry S.F., Green R.C. Point-counterpoint. Ethics and genomic incidental findings. Science. 2013;340:1047–1048. doi: 10.1126/science.1240156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townsend A., Adam S., Birch P.H., Friedman J.M. Paternalism and the ACMG recommendations on genomic incidental findings: patients seen but not heard. Genet. Med. 2013;15:751–752. doi: 10.1038/gim.2013.105. [DOI] [PubMed] [Google Scholar]
- 14.Wolf S.M., Annas G.J., Elias S. Point-counterpoint. Patient autonomy and incidental findings in clinical genomics. Science. 2013;340:1049–1050. doi: 10.1126/science.1239119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Society of Genetic Counselors (2012). 2012 Professional Status Survey: Executive Summary. National Society of Genetic Counselors, http://nsgc.org/p/cm/ld/fid=68.
- 16.Cooksey J.A., Forte G., Benkendorf J., Blitzer M.G. The state of the medical geneticist workforce: findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genet. Med. 2005;7:439–443. doi: 10.1097/01.gim.0000172416.35285.9f. [DOI] [PubMed] [Google Scholar]
- 17.Garver K.L., Lent K.M. American Society of Human Genetics membership survey results, 1989. Am. J. Hum. Genet. 1990;47:345–348. [PMC free article] [PubMed] [Google Scholar]
- 18.Presidential Commission for the Study of Bioethical Issues (2013). Anticipate and Communicate: Ethical Management of Incidental and Secondary Findings in the Clinical, Research, and Direct-to-Consumer Contexts. http://bioethics.gov/sites/default/files/FINALAnticipateCommunicate_PCSBI_0.pdf. [DOI] [PubMed]
- 19.Lohn Z., Adam S., Birch P., Townsend A., Friedman J. Genetics professionals’ perspectives on reporting incidental findings from clinical genome-wide sequencing. Am. J. Med. Genet. A. 2013;161A:542–549. doi: 10.1002/ajmg.a.35794. [DOI] [PubMed] [Google Scholar]
- 20.Costain G., Bassett A.S. Incomplete knowledge of the clinical context as a barrier to interpreting incidental genetic research findings. Am. J. Bioeth. 2013;13:58–60. doi: 10.1080/15265161.2012.754063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christenhusz G.M., Devriendt K., Dierickx K. To tell or not to tell? A systematic review of ethical reflections on incidental findings arising in genetics contexts. Eur. J. Hum. Genet. 2013;21:248–255. doi: 10.1038/ejhg.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Downing N.R., Williams J.K., Daack-Hirsch S., Driessnack M., Simon C.M. Genetics specialists’ perspectives on disclosure of genomic incidental findings in the clinical setting. Patient Educ. Couns. 2013;90:133–138. doi: 10.1016/j.pec.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daack-Hirsch S., Driessnack M., Hanish A., Johnson V.A., Shah L.L., Simon C.M., Williams J.K. ‘Information is information’: a public perspective on incidental findings in clinical and research genome-based testing. Clin. Genet. 2013;84:11–18. doi: 10.1111/cge.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemke A.A., Bick D., Dimmock D., Simpson P., Veith R. Perspectives of clinical genetics professionals toward genome sequencing and incidental findings: a survey study. Clin. Genet. 2013;84:230–236. doi: 10.1111/cge.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levenseller B.L., Soucier D.J., Miller V.A., Harris D., Conway L., Bernhardt B.A. Stakeholders’ Opinions on the Implementation of Pediatric Whole Exome Sequencing: Implications for Informed Consent. J. Genet. Couns. 2013 doi: 10.1007/s10897-013-9626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf S.M. Return of individual research results and incidental findings: facing the challenges of translational science. Annu. Rev. Genomics Hum. Genet. 2013;14:557–577. doi: 10.1146/annurev-genom-091212-153506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turbitt E., Wiest M.M., Halliday J.L., Amor D.J., Metcalfe S.A. Availability of treatment drives decisions of genetic health professionals about disclosure of incidental findings. Eur. J. Hum. Genet. 2014 doi: 10.1038/ejhg.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berg J.S., Amendola L.M., Eng C., Van Allen E., Gray S.W., Wagle N., Rehm H.L., DeChene E.T., Dulik M.C., Hisama F.M., Members of the CSER Actionability and Return of Results Working Group Processes and preliminary outputs for identification of actionable genes as incidental findings in genomic sequence data in the Clinical Sequencing Exploratory Research Consortium. Genet. Med. 2013;15:860–867. doi: 10.1038/gim.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills R.A., Haga S.B., Ginsburg G.S. Genetic testing: clinical and personal utility. Virtual Mentor. 2012;14:604–609. doi: 10.1001/virtualmentor.2012.14.8.ecas1-1208. [DOI] [PubMed] [Google Scholar]
- 30.Parker L.S. Returning individual research results: what role should people’s preferences play? Minn. J. Law Sci. Technol. 2012;13:449–484. [Google Scholar]
- 31.Holm I.A., Savage S.K., Green R.C., Juengst E., McGuire A., Kornetsky S., Brewster S.J., Joffe S., Taylor P. Guidelines for return of research results from pediatric genomic studies: deliberations of the Boston Children’s Hospital Gene Partnership Informed Cohort Oversight Board. Genet. Med. 2014 doi: 10.1038/gim.2013.190. [DOI] [PubMed] [Google Scholar]
- 32.Yu J.H., Jamal S.M., Tabor H.K., Bamshad M.J. Self-guided management of exome and whole-genome sequencing results: changing the results return model. Genet. Med. 2013;15:684–690. doi: 10.1038/gim.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabor H.K., Berkman B.E., Hull S.C., Bamshad M.J. Genomics really gets personal: how exome and whole genome sequencing challenge the ethical framework of human genetics research. Am. J. Med. Genet. A. 2011;155A:2916–2924. doi: 10.1002/ajmg.a.34357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meilleur K.G., Littleton-Kearney M.T. Interventions to improve patient education regarding multifactorial genetic conditions: a systematic review. Am. J. Med. Genet. A. 2009;149A:819–830. doi: 10.1002/ajmg.a.32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American College of Medical Genetics and Genomics (2014). ACMG Updates Recommendations on “Opt Out” for Genome Sequencing Return of Results. https://www.acmg.net/docs/Release_ACMGUpdatesRecommendations_final.pdf.
- 36.Ross L.F., Rothstein M.A., Clayton E.W. Premature guidance about whole-genome sequencing. Per. Med. 2013;10 doi: 10.2217/pme.13.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


