Abstract
Nitric oxide (NO) inhalation has been reported to increase the oxygen affinity of sickle cell erythrocytes. Also, proposed allosteric mechanisms for hemoglobin, based on S-nitrosation of β-chain cysteine 93, raise the possibilty of altering the pathophysiology of sickle cell disease by inhibiting polymerization or by increasing NO delivery to the tissue. We studied the effects of a 2-hour treatment, using varying concentrations of inhaled NO. Oxygen affinity, as measured by P50, did not respond to inhaled NO, either in controls or in individuals with sickle cell disease. At baseline, the arterial and venous levels of nitrosylated hemoglobin were not significantly different, but NO inhalation led to a dose-dependent increase in mean nitrosylated hemoglobin, and at the highest dosage, a significant arterial-venous difference emerged. The levels of nitrosylated hemoglobin are too low to affect overall hemoglobin oxygen affinity, but augmented NO transport to the microvasculature seems a promising strategy for improving microvascular perfusion.
Introduction
In sickle cell anemia erythrocytes, deoxygenation of sickle hemoglobin (hemoglobin S) leads to intracellular polymer formation, ultimately distorting the erythrocyte and retarding red cell transit through the microvasculature (1). Within the red cell, hemoglobin S has reduced affinity for oxygen, compared with hemoglobin A, because of competition between polymerization and oxygen binding. This reduced oxygen affinity results in an increase in the partial pressure of oxygen necessary for 50% hemoglobin saturation (P50). The P50 value is inversely related to the hemoglobin affinity for oxygen. A variety of therapeutic strategies for sickle cell disease aimed at reducing hemoglobin S polymerization have been under development in recent years. These strategies include attempts to modify the structure of hemoglobin S to reduce the hydrophobic interactions between hemoglobin β chains or to increase the affinity for oxygen, as well as attempts to decrease the concentration of hemoglobin S by increasing the percentage of fetal hemoglobin or by increasing red cell volume (2).
Nitric oxide (NO) has been recognized in the last decade to have important and diverse physiological effects. NO is synthesized in multiple cell types and tissues from the substrate L-arginine by NO synthase enzyme systems, which may be constitutive or inducible in response to physiological stimuli. In physiological solution, NO reacts rapidly with oxygen, heme groups, and various functional groups on biologic molecules, including thiols. In fact, the covalent formation of S-nitrosothiol compounds such as S-nitrosoglutathione and S-nitrosoalbumin in the lung airways, plasma, and other tissues may serve to stabilize this highly reactive molecule and deliver it to target sites of action (3–5).
Recent work by Stamler and colleagues suggests that NO, produced in the lungs, binds to the highly conserved cysteine residue 93 on the β chain of oxyhemoglobin to form S-nitrosohemoglobin (SNO-hemoglobin). NO thus carried on hemoglobin is envisioned to be transported to the microvasculature and may be released upon deoxygenation of the red cells in the tissues, resulting in vasodilation of the microvasculature (6–8). Recently, NO has also been reported to increase oxygen affinity of sickle cell erythrocytes (9). This effect was found to be dose-dependent in vitro, with a maximum effect occurring at a dose of 80 parts per million (ppm). This effect was also apparent in vivo after delivery of 80 ppm NO for 45 minutes. P50 was reduced by an average of 4.6 ± 2.0 mmHg in patients with sickle cell anemia. These effects were specific for sickle red cells and were not associated with marked increases in methemoglobin formation. The mechanism of the reported hemoglobin S–specific increase in oxygen affinity, which might be of therapeutic value, is not certain.
To test the potential effects of NO on the structure and function of normal and sickle hemoglobin, we studied the effects of a 2-hour treatment of 80 ppm inhaled NO (at a fraction of inspired oxygen [FiO2] of 0.21) on normal (hemoglobin A genotype [AA]) and sickle cell (hemoglobin S genotype [SS]) individuals. We measured effects on NO metabolism, methemoglobin formation, hemoglobin oxygen affinity, and binding of NO to the hemoglobin molecule.
The term nitrosylated hemoglobin is used herein to represent hemoglobin with a bound NO molecule, either on the sulfhydryl of β-globin chain system 93 or on the heme iron.
Methods
Subjects.
All protocols were approved by the National Heart, Lung, and Blood Institute’s Institutional Review Board. All subjects signed an informed consent. Seven clinically stable volunteers with sickle cell disease (SS) and 5 normal volunteers (AA) were selected for study (Table 1). All volunteers had hemoglobin electrophoresis to confirm hemoglobin S or A phenotype, as well as hemoglobin F levels. Inclusion criteria for the individuals with sickle cell were as follows: age between 18 and 65 years, electrophoretic diagnosis of sickle cell disease, and a hematocrit greater than 18%. Potential SS volunteers were excluded if they were clinically unstable, defined by having more than 2 pain crises that resulted either in a visit to the emergency department or in hospitalization during the preceding 2 months; undergoing therapy with hydroxyurea or butyrate any time in the preceding 12 months; being a smoker; or receiving blood transfusions within the preceding 3 months (or hemoglobin A >20%).
Table 1.
Baseline characteristics
NO inhalation in SS and AA volunteers.
Volunteers were instructed not to eat after midnight, and were given only water (orally) during the 6-hour study duration. A peripheral intravenous and radial artery catheter was placed. Venous and arterial blood samples were collected hourly; totals did not exceed 100 mL in a study day. Continuous blood pressure by cuff or arterial line, pulse oximetry, respiratory rate, temperature, and heart rate and rhythm were recorded. After an hour of baseline measurements, subjects breathed NO gas at a concentration of 80 ppm (INOvent Delivery System; Datex-Ohmeda Inc., Madison, Wisconsin, USA). A room air gas condenser with an oxygen blender was used to deliver an FiO2 of 0.21 at 40 L/min with an in-line reservoir bag. NO gas was delivered in-line, and concentrations of NO, oxygen, and nitrogen dioxide (NO2) were sampled at the mask to ensure a delivery of 80 ppm NO, an FiO2 of 0.21, and to maintain the NO2 level below 1.0 ppm. With this system, inhaled NO concentrations remained constant at extremes of minute ventilation (data not shown). The study was conducted in a negative-flow isolation room, and no further NO scavenging was required, as room NO levels remained at less than 200 parts per billion (ppb) during treatment (measured with the Model 280 NO Analyzer; Sievers Instruments, Boulder, Colorado, USA). The NO was discontinued after 2 hours, and room NO levels dropped to less than 20 ppb within 10 minutes. Blood was collected hourly for 3 more hours, at which time the study was terminated. In 3 additional SS patients, after 3 baseline blood collections, inhaled NO was delivered for 1 hour at 40 ppm, 1 hour at 60 ppm, and 1 hour at 80 ppm.
Oxygen dissociation curves.
Oxygen dissociation curves (ODCs) were obtained using the Hemox-Analyzer (TCS Scientific Corp., New Hope, Pennsylvania, USA). During studies, the Hemox-Analyzer was located at the bedside of the volunteers. A total of 60 μL of whole blood for SS individuals and 30 μL of whole blood for AA individuals was added to 3 mL of buffer (135 mM NaCl, 30 mM TES, 5 mM KCl, and NaOH adjusted to pH 7.4 ± 0.02 [TCS buffer; TCS Scientific Corp.]), 7.5 μL of antifoam solution, and 15 μL of 20% BSA. Samples were analyzed immediately upon collection from the patient. Nitrogen (100%) was bubbled through the sample at a constant rate that resulted in complete deoxygenation within 20 minutes, followed by reoxygenation with air for 15 minutes. The analyzer measured the oxygen tension with a standard Clark O2 electrode (Model 5331 Oxygen Probe; Yellow Springs Instrument Co., Yellow Springs, Ohio, USA) and simultaneously calculated the hemoglobin saturation using dual-wavelength spectrophotometry (9, 10). The ODCs were recorded during both deoxygenation and reoxygenation. During the study day, 7 hourly measurements of the deoxygenation and reoxygenation curves were obtained before, during, and after NO administration. To ensure reliable data, the oxygen electrode was cleaned and a new membrane applied 24 hours before each study day. The membrane was deoxygenated and reoxygenated in distilled water for a 12-hour period the day before all patient studies. The night before each study day, a sample of fresh AA blood was run as a standardization control.
Processing of blood.
Arterial and venous blood samples were drawn, immediately protected from light, and centrifuged at 750 g for 5 minutes at room temperature. Plasma was removed and stored at –70°C until assayed for nitrate/nitrite. The red blood cell pellet was removed and washed 2 times in 5 volumes of PBS. Cells were then lysed in a 1:4 dilution of 0.5 mM EDTA in nitrite-free molecular biology grade water (Biofluids Inc., Rockville, Maryland, USA). After lysis, 500 μL was passed through a 9.5-mL bed volume Sephadex G25 column to remove nitrite and small thiols. The hemoglobin concentration of the Sephadex G25 effluent was measured by conversion to cyanomethemoglobin (ε540 = 11 for heme). (Note that all stated hemoglobin concentrations are in terms of heme.) Hemoglobin samples (200 μL) were immediately drawn into 250-μL Hamilton syringes and reacted with I3–. All measurements were performed within an hour of sample collection.
Determination of nitrate/nitrite using the Griess reaction.
Serum samples were thawed, diluted 1:2 in HPLC-grade water, and filtered through a prewashed 30,000–molecular weight cutoff filtration unit (catalog no. UFC3LTKNB; Millipore Corp., Bedford, Massachusetts, USA). Samples and standards were reacted with and without nitrate reductase, according to the standard Griess reaction (11). In brief, nitrate present in samples is converted to nitrite using nitrate reductase, followed by stoichiometric reaction of nitrite with sulfanilamide and then N-(1-naphthyl)-ethylenediamine to form a purple azo product. The concentration of the azo product is measured in a spectrophotometer at a wavelength of 570 nM.
HPLC-electrospray ionization mass spectrometry.
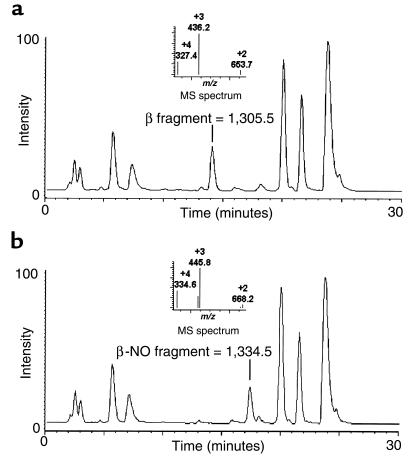
Mass spectrometry measurements of synthesized SNO-hemoglobin were performed to establish the specificity of the NO reaction with cysteine 93 and to test the operating characteristics of the I3– chemiluminescent technique (described later here). Patient samples were also run immediately upon collection to evaluate potential modification of the hemoglobin β chain with NO, glutathione, or other low-molecular-weight adducts. Liquid chromatography mass spectrometry was performed on an HP1100 integrated LC-electrospray system (Hewlett-Packard, Palo Alto, California, USA) using a 2.1 mm × 15 cm C3 Zorbax reverse-phase chromatography column at 20°C and a flow rate of 0.3 mL/min. Solvent A was 5% acetic acid in water, and solvent B was acetonitrile. Separation of the globin proteins (minus the heme groups) was achieved using a linear gradient of 33–37% solvent B over 25 minutes. The mass spectrum was scanned at 500–1,700 m/z at 0.1-m/z increments every 2 seconds while acquiring full-profile data with the fragmentor at 30 V. To determine which cysteine binds NO on the globin chains, each sample was digested with endoproteinase Glu-C (Boehringer Mannheim Biochemicals, Indianapolis, Indiana, USA) in ammonium acetate (pH 4.0) at a hemoglobin/enzyme ratio of 10:1, in the presence of 5% acetonitrile (to denature proteins), for 2 hours at 25°C. Samples were then run on a C3 column. The solvent was held isocratic (2% acetonitrile, 0.3 mL/min) for 5 minutes and then ramped to 18% acetonitrile over 15 minutes. The mass spectrometer was scanned at 200–1,700 m/z every 4 seconds. The fragment nitrosylated at β-chain cysteine 93 was identified as a mass of 1,334.5. This was 29 mass units higher than 1,305.5, the molecular weight of the protein fragment containing β-chain amino acids 91–101.
Chemiluminescent detection of SNO-hemoglobin.
The method of Zweier and colleagues for the measurement of nitrite and S-nitrosothiols by reaction with I3– to release NO gas was used (12). Briefly, 7 mL of glacial acetic acid and 2 mL of distilled water are mixed with 50 mg of KI (or NaI). A crystal of I2 is added to yield a concentration of 6–20 mM. Helium is bubbled through the reaction mixture, through 1 N NaOH, and then into the Sievers Model 280 NO analyzer. Standard curves were obtained using S-nitrosoglutathione (Sigma Chemical Co., St. Louis, Missouri, USA) and SNO-hemoglobin (synthesized as outlined later here). The chemiluminescent signal from the I3– reaction to form NO is linear at greater than 1.0 pmol (data not shown) with both nitrite ions (r2 = 0.9997, P < 0.001) and several S-nitrosothiols (S-nitrosoglutathione, S-nitrosocysteine, and S-nitroso-N-acetylcysteine; average r2 = 0.9989, P < 0.001). Nitrate ions do not react.
Standardization of measurement of SNO-hemoglobin by I3– release of NO was made by comparison of synthesized SNO-hemoglobin, by reaction of hemoglobin with S-nitrosocysteine as described by McMahon and Stamler (13), with mass spectrometry measurements. Synthesized SNO-hemoglobin had a mean 1.96 ± 0.17 moles of NO per hemoglobin tetramer (n = 15), consistent with nitrosylation of a single reactive thiol (cysteine 93) on the two β-chains of hemoglobin. There was significant correlation between the methods for quantification of SNO-hemoglobin (r = 0.63, n = 14, P < 0.05); however, measurements of SNO-hemoglobin by mass spectrometry consistently underestimated the level of nitrosylation. To determine the limits of sensitivity of the chemiluminescence assay, the SNO-hemoglobin was serially diluted in oxyhemoglobin (1 mM) from 3% to 0.001% SNO-hemoglobin/oxyhemoglobin (concentration in terms of heme subunit). Release of NO was linear over this range (r2 = 0.996, P < 0.001). Similar results were obtained from several preparations of hemoglobin S.
I3– release of NO from the heme in nitrosyl(heme)hemoglobin was measured after forming the latter by reacting NO gas with deoxyhemoglobin. Nitrosyl(heme)hemoglobin refers to hemoglobin with an NO bound to at least 1 of the heme groups (the heme, in parenthesis, is included for clarification). Briefly, purified oxyhemoglobin was deoxygenated in nitrogen for 2 hours, followed by a 45-minute exposure to 100% NO gas (bubbled through 5 N NaOH to remove NOX species, i.e., nitrate/nitrite). Formation of nitrosyl(heme)hemoglobin was confirmed by full-scan optical spectroscopy, demonstrating a Soret peak at 417 nm, the β band at 544 nm, the α band at 572 nm, and increased absorption (compared with oxyhemoglobin) at 560 nm. Mass spectrometry measurements confirmed that NO gas under these conditions did not nitrosate the globin chains. It was observed that the I3– chemiluminescence assay detects NO bound to heme (90% yield; n = 3).
During in vivo experiments, patient samples, after Sephadex G25 processing to remove nitrite contamination, were injected in duplicate (200 μL sample volume of approximately 1 mM hemoglobin) into excess I3– in the purge vessel. The residual signal represented release of NO from the S-NO bond of SNO-hemoglobin, with possible contribution from the nitrosyl(heme)hemoglobin. The concentration of NO released in nM is subtracted from the NO concentration generated by 200 μL injection of the water from the Sephadex G25 column (background control), obtained immediately before running the hemoglobin sample on the column. This value is divided by the concentration of heme subunit, and the total is multiplied by 100.
Standard laboratory tests.
Arterial and venous blood gas analysis (278 Blood Gas System; Ciba-Corning Diagnostics Corp., Medfield, Massachusetts, USA) and co-oximetry (270 Co-Oximeter; Ciba-Corning) were immediately performed (<5-minute delay) after obtaining venous and arterial samples from the volunteers to determine pH, PO2, PCO2, hemoglobin saturation, and carboxy- and methemoglobin concentrations. Methemoglobin concentrations using the Co-Oximeter were validated by comparison with measurements by absorption spectroscopy at 700, 630, 576, and 560 nm using the Winterbourn equation (14).
Statistical analysis.
The Wilcoxon test was used for experiments comparing AA and SS individuals breathing 80 ppm NO for 2 hours. Within each group (AA or SS), a signed-rank test determined significance of observed changes. For dose-titration experiments of inhaled NO, because of the high precision of the measurements, a 2-tailed paired t test was used to analyze increases in the mean values of paired samples before and after NO inhalation, as well as to analyze the significance of differences in nitrosylated hemoglobin levels between arterial and venous blood at baseline and on dose titration of inhaled NO.
Results
Baseline characteristics of the normal volunteers and the individuals with sickle cells.
Baseline characteristics of the normal volunteers and the individuals with sickle cells are outlined in Table 1. The 7 individuals with sickle cell anemia were all of hemoglobin S–only phenotype, with hemoglobin F levels in the range of 2–32.5%. Two of the individuals may have HbS-β0 thalassemia or sickle cell anemia-α thalassemia.
Clinical and laboratory parameters.
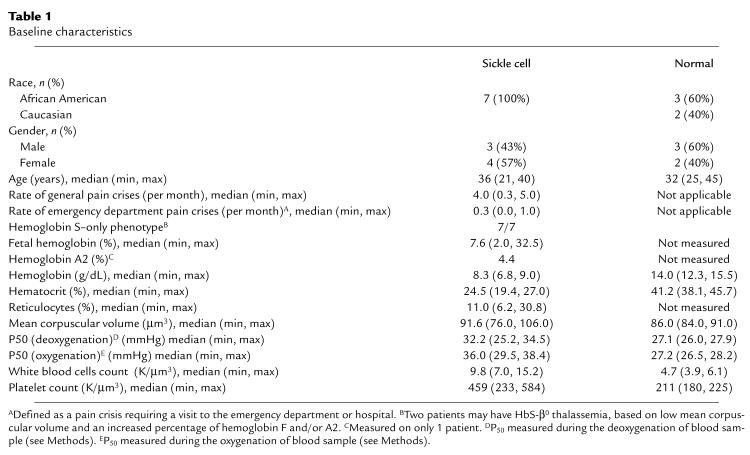
There was no observed effect of NO inhalation on vital signs, electrocardiogram, arterial or venous blood pH, PaO2, PaCO2, or oxygen saturation (data not shown). Before NO inhalation, the SS arterial pO2 was lower than the AA arterial pO2. The SS venous pO2 values are similar to those of AA individuals. This narrow difference between arterial and venous pO2 levels in SS patients is indicative of the reduced oxygen extraction or arterialization of venous blood that is characteristic of patients with sickle cell anemia. Inhaled NO did not improve the baseline arterial and venous hypoxemia that was observed in SS individuals, compared with AA individuals, when administered as 80 ppm for 2 hours (Figure 1a) or in increasing concentrations of 40, 60, and 80 ppm given each for 1 hour (Figure 1b). The FiO2 was maintained at 0.21 with an oxygen blender when delivering 80 ppm NO. There were no significant changes observed in hemoglobin concentration, hematocrit, platelet count, white blood cell count, or mean corpuscular volume after NO delivery at 80 ppm for 2 hours (data not shown).
Figure 1.
Effect of inhaled NO on arterial and venous pO2 in SS and AA individuals. (a) Before NO inhalation (hours 1 and 2), the SS arterial pO2 is lower than the AA arterial pO2. The SS venous pO2 values are similar to the AA venous pO2 values, reflecting the reduced oxygen extraction or arterialization of venous blood that is characteristic of patients with sickle cell anemia. During 2 hours of 80 ppm NO inhalation (hours 3 and 4; filled horizontal bar), there is no significant change in AA or SS pO2 for arterial and venous blood. (b) During dose escalation of inhaled NO in 3 SS individuals, there is no significant change in arterial or venous pO2 during 1-hour inhalation of 40, 60, and 80 ppm NO gas.
NO metabolism.
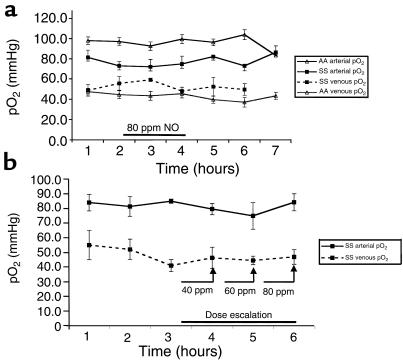
Inhaled NO at 80 ppm resulted in a substantial increase in NOX, which was predominantly nitrate. Mean baseline plasma levels of NOX in SS and AA individuals were 10 ± 8 μM and 12 ± 15 μM, respectively. At 1 and 2 hours of inhalation, mean NOX levels significantly rose to 85 ± 8 μM in the SS individuals and 60 ± 15 μM in the AA individuals (P < 0.001) (Figure 2a). Mean methemoglobin levels, obtained by the Co-Oximeter, rose from 0.3 ± 0.0 in the SS individuals and 0.24 ± 0.02% in the AA individuals to 1.2 ± 0.4% and 0.9 ± 0.09%, respectively (P = 0.001) (Figure 2b).
Figure 2.
Effect of inhaled 80 ppm inhaled NO on plasma NOX (nitrate and nitrite measured by the Griess reaction) and methemoglobin. (a) For 2 hours before NO inhalation, mean plasma levels of NOX in SS and AA individuals were 10 ± 8 μM and 12 ± 15 μM, respectively. After 2 hours of inhalation (hours 3 and 4; filled horizontal bar), mean levels rose significantly (P < 0.001) to 85 ± 8 μM in the SS individuals and 60 ± 15 μM in the AA individuals. After NO inhalation, there was a steady decrease in plasma NOX concentration. (b) During NO inhalation (hours 3 and 4; filled horizontal bar), mean methemoglobin levels rose from 0.3 ± 0.0% in the SS individuals and 0.24 ± 0.02% in the AA individuals to 1.2 ± 0.4% and 0.9 ± 0.09%, respectively (P = 0.001). After correcting for hemoglobin concentration, there was no difference in absolute methemoglobin values between AA and SS individuals. Values returned to baseline within 1–2 hours in all individuals.
ODC measurements.
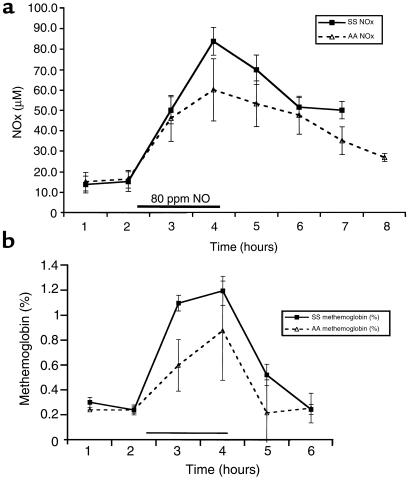
The AA ODCs at baseline before NO inhalation demonstrated minimal hysteresis when measured during deoxygenation (P50 measured before inhaled NO = 27.0 ± 0.4 mmHg) or during oxygenation (P50 measured before inhaled NO = 27.3 ± 0.3 mmHg). As expected, the baseline SS ODCs measured before NO inhalation demonstrated significant hysteresis, with P50 measurements during deoxygenation of 30 ± 1.4 mmHg and during oxygenation of 34.1 ± 1.3 mmHg. There was no significant change in any of the P50 measurements at 1 and 2 hours of 80 ppm inhaled NO in either the AA or SS individuals, measured either during deoxygenation (for SS individuals; P = 0.63) or reoxygenation (for SS individuals; P = 1.00). There was no significant change in any of the P50 measurements for up to 3 hours after NO inhalation in any of the individuals tested (Figure 3a). In 3 further studies (2 new patients and 1 previously studied) of 40, 60, and 80 ppm NO dose escalation, there was no change in the P50 (Figure 3b). No changes in ODC characteristics or partial pressure of oxygen at which 10% of the hemoglobin is saturated with oxygen (P10) were observed. There was no correlation between absolute methemoglobin formation and measured P50 (r = 0.2, P = 0.80).
Figure 3.
Effect of inhaled NO on the oxygen affinity (P50) of SS and AA erythrocytes. (a) The AA ODCs (expressed as P50) at baseline before NO inhalation (hours 1 and 2) demonstrated minimal hysteresis when measured during sample deoxygenation or during sample oxygenation. The baseline SS ODCs measured at 1 and 2 hours before NO inhalation, as expected, demonstrated significant hysteresis during sample deoxygenation and during sample oxygenation. There was no significant change in any of the P50 measurements after 1 and 2 hours of inhaled NO at 80 ppm in either the AA or SS individuals (hours 3 and 4; filled horizontal bar), measured either during sample deoxygenation (for SS individuals; P = 0.63) or reoxygenation (for SS individuals; P = 1.00). There was no significant change in any of the P50 measurements for up to 3 hours after NO inhalation in any of the individuals tested (10/10 tested in the first hour after NO; 7/10 in the second hour; and 3/10 in the third hour). (b) During dose escalation of inhaled NO in 3 SS individuals, there is no significant change in P50 measured during sample deoxygenation or reoxygenation after 1 hour inhalation each of 40, 60, and 80 ppm NO gas.
Measurement of modification of the β-chain cysteine 93 by HPLC-electrospray mass spectrometry.
Electrospray mass spectrometry of synthesized SNO-hemoglobin demonstrated a single NO adduct on the β-globin protein chains at levels in the range of 15–30%. Digestion with endoproteinase Glu-C confirms specificity of cysteine-NO reaction with hemoglobin β-chain cysteine 93. Figure 4a demonstrates the characteristic chromatogram, after enzymatic digestion of hemoglobin, of the 11–amino acid fragment (hemoglobin β-chain amino acids 91–101) that includes cysteine 93. The NO-modified fragment (Figure 4b) demonstrates 3 prominent charge states (+2, +3, +4) and an increased retention time on the column. Reaction with the remaining thiol (2 total) on the β chain and the single thiol on the α chain does not occur, unless the hemoglobin is denatured (reactions at low pH). On study days, patient samples were analyzed immediately upon collection using full-scan measurements of the β chain and proteolytic digestion with analysis of modification of the 91–101 amino acid fragment. No adducts larger than 29 mass units (consistent with glutathione or cysteine) were observed before, during, or after NO inhalation. The technique has limited sensitivity (to ∼1–2% modification by NO) owing to interference by the 22–mass unit sodium. Proteolytic digestion increases the signal/noise ratio, but loss of NO occurs before complete digestion, again limiting sensitivity. These results, however, are inconsistent with most of the mechanisms postulated to explain the reported increase in oxygen affinity with NO exposure (9).
Figure 4.
HPLC-electrospray ionization mass spectrometry of SNO-hemoglobin digested with endoproteinase Glu-C confirms specificity of S-nitrosocysteine reaction with hemoglobin β-chain cysteine 93. (a) Shown is the characteristic chromatogram, after enzymatic digestion of hemoglobin, of the 11–amino acid fragment (hemoglobin β-chain amino acids 91–101) that includes cysteine 93. The molecular weight is 1,305.5, with 3 predominant charge states (+4, +3, +2) with respective m/z of 327.4, 436.2, and 653.7 (inset). (b) Digestion of SNO-hemoglobin results in an increased retention time on the column and an increase in the molecular weight of the 11–amino acid fragment to 1,334.5, consistent with the addition of 29 mass units (NO has 30 mass units minus 1 mass unit of hydrogen lost during covalent binding). The m/z values of the 3 charge states (+4, +3, +2) increase to 334.6, 445.8, and 668.2, respectively (inset).
Measurement of nitrosylation of hemoglobin by chemiluminescent I3–reaction.
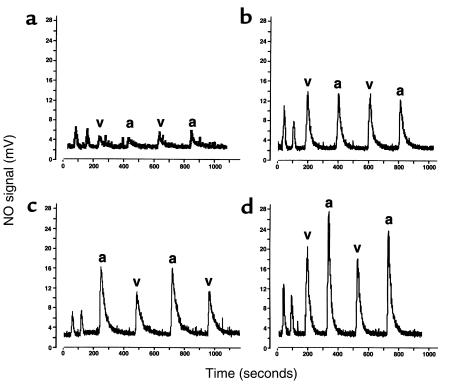
At baseline, the arterial and venous nitrosylated hemoglobin levels in the SS individuals were not significantly different. For the 3 hours before NO inhalation, the mean percentage of nitrosylated hemoglobin in the arterial blood was about 0.004%, and in the venous blood, about 0.004%. There is a dose-dependent increase in nitrosylated hemoglobin (expressed as percent moles of NO per moles of heme subunit) during NO inhalation in both arterial blood (0.011% on 40 ppm, 0.016% on 60 ppm, and 0.022% on 80 ppm) and venous blood (0.014% on 40 ppm, 0.016% on 60 ppm, and 0.016% on 80 ppm). A significant arterial-venous difference was observed on 80 ppm inhaled NO (P = 0.02). The results of experiments from 1 individual, as a representative example, are shown in Figure 5, a–d, and the mean values for all 3 SS individuals are shown in Figure 6.
Figure 5.
Chemiluminescent detection of NO released from hemoglobin reacted in I3– before and after dose escalation of inhaled NO. NO signal is measured in millivolts (y-axis), and time is measured in seconds (x-axis). (a) Sample measurements before NO inhalation are shown. The first 2 peaks are from 200 μL of water collected from the G25 column immediately before use. The following 4 peaks are from 200 μL of lysed desalted hemoglobin from the venous (v) and arterial (a) blood injected in duplicate in alternating sequence. An arterial-venous difference is not appreciated, and the signal is very low (0.0035% moles NO per moles heme subunit; calculated by dividing the area under the curve [after subtraction from G25 water] from each injection by the concentration of hemoglobin in the sample, multiplied by 100). (b) The signal obtained after breathing 40 ppm NO gas for 1 hour. The venous (0.014%) and arterial (0.011%) nitrosylated hemoglobin levels have risen. (c) The signal obtained after breathing 60 ppm NO gas for 1 hour exhibits a difference between the arterial (0.02%) nitrosylated hemoglobin level and the venous (0.014%), although this difference was not observed in all patients studied. (d) Inhalation of 80 ppm NO further increased the arterial-venous difference (arterial 0.024% and venous 0.017%). This difference was observed in all 3 individuals studied.
Figure 6.
Effect of dose escalation of inhaled NO on nitrosylated hemoglobin, expressed as the percentage moles of NO per moles of heme subunits. For 3 hours before NO inhalation, the mean percentage of nitrosylated hemoglobin in the arterial blood was 0.004% and in the venous blood, 0.004%. There is a significant stepwise increase in nitrosylated hemoglobin during NO inhalation in both arterial (0.011% on 40 ppm, 0.016% on 60 ppm, and 0.022% on 80 ppm) and venous (0.014% on 40 ppm, 0.016% on 60 ppm, and 0.016% on 80 ppm) blood (P < 0.05 for all increases except the venous sample at 40 ppm, for which P = 0.075). A significant arterial-venous difference was observed on 80 ppm inhaled NO (P = 0.02).
Therefore, inhaled NO was well tolerated and produced no changes in hemodynamics or oxygenation. NO inhalation resulted in a significant reaction with the ferrous heme of oxyhemoglobin to produce nitrate and methemoglobin and, to a lesser extent, nitrosylated the hemoglobin molecule. The specificity of the NO donor S-nitrosocysteine for reaction with the hemoglobin β-chain cysteine 93 observed in vitro using mass spectrometry suggests that the observed increase in nitrosylated hemoglobin represents SNO-hemoglobin but could also represent nitrosyl(heme)hemoglobin. The levels of nitrosylated hemoglobin observed during NO inhalation were low, and there was no increase in oxygen affinity (measured by a reduction in P50). No modification of hemoglobin with glutathione was observed during NO breathing.
Discussion
This study was designed to test recent reports that inhaled NO increases the oxygen affinity of sickle cell erythrocytes, and to test whether inhaled NO reacts with the β-chain cysteine 93 to form SNO-hemoglobin. Our data suggest that in both normal (AA) and sickle cell (SS) individuals, up to 2 hours of inhaled NO at 80 ppm is well tolerated, with minimal acute cardiovascular or hematological effects, and can be monitored by increased formation of plasma NOx compounds and small increases in methemoglobin. The metabolic fate of inhaled NO appears to be similar in SS and AA individuals, although we did observe a nonsignificant trend toward higher level NOx production in the SS individuals. We were unable to detect any change in hemoglobin oxygen affinity in either the normal patients or those with sickle cell; nor did we find any effect on arterial or venous pO2 in these individuals. We did demonstrate, however, increased levels of NO bound to hemoglobin in both arterial and venous blood from the subjects with sickle cell, and believe that this may represent the S-nitrosated cysteine 93 residue of the hemoglobin S β chain.
The lack of an observed increase in oxygen affinity (P50 reduction) is consistent with our observation that inhaled NO produces only modest increases in methemoglobin and nitrosylated hemoglobin. It has been known for more than half a century that methemoglobin in high levels produces an apparent increase in the oxygen affinity of hemoglobin, but this effect is negligible at the levels of methemoglobin observed in this study. Because of the proposed allosteric interaction of oxygen and NO on hemoglobin, SNO-hemoglobin should exhibit an increase in oxygen affinity independent of effects on polymerization. Indeed, a recent report described an increased oxygen affinity of AA SNO-hemoglobin directly proportional to the percentage of modified β-chain cysteine 93 (15). However, these data suggest that a large fraction of the hemoglobin molecules would require modification to significantly change hemoglobin oxygen affinity, and we observed only a mean 0.017% increase in the arterial hemoglobin that was nitrosylated. In previous reports, the formation of a mixed disulfide, such as glutathionyl-hemoglobin, at the highly conserved 93 cysteine on the β chain, interfered with polymerization, increased oxygen affinity, and inhibited sickling. However, this effect required a high proportion of reacted hemoglobin (in the range of 8–25% labile glutathionyl-hemoglobin and more than 95% irreversible covalent modification with thiols) (16, 17). It remains a possibility that inhaled NO catalyzes the formation of mixed disulfides of glutathione or other molecules, with subsequent sulfhydryl–mixed disulfide exchange to the β-chain cysteine 93. However, using HPLC-mass spectroscopy, we did not observe any molecular weight adducts consistent with glutathionyl-hemoglobin or any other mixed disulfide. Thus, other effects of NO adducts on oxygen affinity — directly, by changing the T→R conformational changes in hemoglobin; or indirectly (in the case of hemoglobin S), by interfering with subunit contacts in the polymer phase — are unlikely at the detected levels of nitrosylation.
There is no apparent explanation for the discrepancy between the results presented in this article and those of Head et al. (9). We calculated 95% confidence intervals, using t distributions (assuming normality), for the mean change in P50 presented here (for the first series of experiments, n = 5) and for that of Head et al., and found similar results for the AA individuals. However, there was no overlap in the confidence intervals between the SS individuals presented here (–0.86, 0.91) and those presented by Head et al. (–6.23, –2.97). This suggests that this discrepancy cannot be explained by patient sampling variability. In parallel experiments, we have found that treating AA or SS erythrocytes with NO gas and NO donor compounds only changes oxygen affinity at levels in which high levels of methemoglobin or SNO-hemoglobin are formed (B.W. Hrinczenko, A. Alayash, M.T. Gladwin, and A.N. Schechter, unpublished study). Although it is expected that higher levels of modified cysteine 93 are needed to inhibit hemoglobin S polymerization and improve oxygen affinity, it is possible that small quantities of SNO-hemoglobin would have significant peripheral physiological effects. NO, carried as SNO-hemoglobin to the microvasculature, may modulate vascular tone (6), inhibit platelet activation (18), and inhibit leukocyte P-selectin–dependent adhesion and migration (19). These actions could potentially have a favorable impact on the pathophysiology of sickle cell anemia, a disease characterized by major abnormalities in microvascular perfusion; hypercoagulability; and increased expression of erythrocyte, endothelial, and leukocyte adhesion molecules (20). Anecdotally, inhaled NO has been described to improve oxygenation and pulmonary hypertension and to shorten the course of the acute chest syndrome (21). Sickle cell anemia animal models further support the role of NO in improving cerebral vascular perfusion (22).
Recent work by Stamler and colleagues (6–8) suggests that NO binding to hemoglobin may play a role in the regulation of vascular tone, with NO binding and release tied to oxygen-induced allosteric structural transitions. In the lungs, hemoglobin is highly saturated with oxygen, and NO produced in the lungs is thought to bind to cysteine 93 on the β chain. This SNO-hemoglobin is carried by the red cells to the microvascular system, where oxygen tensions are reduced. After deoxygenation, allosteric structural changes in the hemoglobin molecule favor the release of NO, which diffuses to the arterial wall, possibly via thiol intermediaries such as glutathione, and causes vasodilation. If this model is correct, it would suggest that as a result of a direct vasodilatory effect of NO in the periphery, therapeutic delivery of NO in the form of SNO-hemoglobin may be beneficial to patients with sickle cell anemia who have impaired microvascular perfusion.
To date, this model is supported by observations in vitro using rat and human hemoglobin and whole erythrocytes (6). In vivo, increased erythrocyte SNO-hemoglobin in arterial blood compared with venous blood has been demonstrated in Sprague-Dawley rats (6), and recent studies have demonstrated an increase in SNO-hemoglobin in oxygenated blood (venous) compared with deoxygenated blood (arterial) in the human fetal-placental circulation (venous blood carries oxyhemoglobin from placenta to fetus in the fetal circulation) (23). Our observation of increased nitrosylation of human hemoglobin S upon inhalation of NO, and the formation of a significant arterial-venous difference in response to such inhalation, provides new evidence that hemoglobin stabilizes and transports NO. The specificity of the in vitro reaction of cysteine-NO with β-chain cysteine 93, evidenced by mass spectrometry experiments (Figure 4), argues that the observed in vivo nitrosylated hemoglobin represents SNO-hemoglobin. These data support the theory that human cysteine 93 participates in a covalent interaction with NO in the human lung, and that therapeutic inhalation of NO can augment S-nitrosation at this site.
It remains possible that the levels of nitrosylated hemoglobin measured before and after NO inhalation are attributable to the measurement of NO release from heme rather then from the cysteine 93 residue. However, resting measurements of nitrosyl(heme)hemoglobin by Stamler and colleagues document that basal nitrosyl(heme)hemoglobin content is actually higher in venous than arterial blood in the Sprague-Dawley rat (6). Takahashi et al. (24) performed electron paramagnetic (EPR) measurements of nitrosyl(heme)hemoglobin content of arterial and venous blood from sheep breathing 60 ppm NO and found no significant arterial-venous differences. The nitrosyl(heme)hemoglobin content of these sheep constituted less than 0.11% modification of total hemoglobin. This value is 10-fold higher than the signal measured in this current study, and it is therefore possible that EPR would be incapable of detecting the arterial-venous difference of 0.005% that we have measured. Although the weight of evidence suggests specificity for cysteine 93 S-nitrosation, it remains possible that part of our measured arterial-venous difference in nitrosylated hemoglobin is attributable to nitrosyl(heme)hemoglobin rather than SNO-hemoglobin.
It is not clear why there is no observed baseline arterial-venous difference of nitrosylated hemoglobin in SS erythrocytes, as has been previously suggested for AA erythrocytes, or why this difference widens only after inhalation of relatively high levels of NO. This may be the result of a small sample size and difficulty in measuring such low levels of bound NO. With further studies, a baseline arterial-venous difference may become apparent. Alternatively, the narrow arterial-venous difference may be unique to sickle cell disease because of the classically described reduced arterial-venous oxygen difference, which further narrows during crisis (25, 26). Note that in Figure 1a, the individuals with sickle cell have a reduced arterial pO2, whereas venous pO2 is similar to or higher than the AA values. This phenomenon is attributable to the arterialization of venous blood due to regional obstruction of flow causing shunting into larger caliber arterial-venous anastomoses. A second mechanism may be a reduced oxygen extraction secondary to an increased cardiac output from anemia or altered microvascular perfusion. It is interesting to speculate that this reduced arterial-venous oxygen difference would similarly affect the SNO-hemoglobin difference because of oxygen-NO allostery.
In conclusion, inhaled NO reacts with hemoglobin in SS and AA individuals, resulting in significant increases in NOX and mild elevations in methemoglobin. We observed no change in hemoglobin oxygen affinity or arterial and venous pO2 levels. Importantly, we report nitrosylation of hemoglobin S, possibly representing β-chain SNO-hemoglobin, during NO breathing — supporting an in vivo role for the human hemoglobin S, β-chain cysteine 93 that includes covalent binding of NO. The level of nitrosylation appears to be too low to affect overall sickle cell oxygen affinity, but may provide a mechanism to augment NO delivery to the microvasculature and possibly improve microvascular perfusion.
Acknowledgments
We thank our patients with sickle cell anemia for their support and enthusiasm for this project, and A. Suffredini and R. Danner, who helped conceive and execute important components of this study. We also acknowledge L. Keefer, D. Wink, and J. Nawrocki for providing their expertise with NO chemistry; the Critical Care Medicine Department respiratory therapy section for assistance with NO delivery and blood gas analysis; and E. Link for helping with patient recruitment and community outreach efforts.
References
- 1.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg MH. Management of sickle cell disease. N Engl J Med. 1999;340:1021–1030. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 3.Gaston B, et al. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci USA. 1993;90:10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamler JS, et al. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA. 1992;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnelle DR, Stamler JS. NO+, NO, and NO– donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch Biochem Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 6.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 7.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 8.Stamler JS, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 9.Head CA, et al. Low concentrations of nitric oxide increase oxygen affinity of sickle erythrocytes in vitro and in vivo. J Clin Invest. 1997;100:1193–1198. doi: 10.1172/JCI119631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guarnone R, Centenara E, Barosi G. Performance characteristics of Hemox-Analyzer for assessment of the hemoglobin dissociation curve. Haematologica. 1995;80:426–430. [PubMed] [Google Scholar]
- 11.Green LC, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Samouilov A, Zweier JL. Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal Biochem. 1998;258:322–330. doi: 10.1006/abio.1998.2609. [DOI] [PubMed] [Google Scholar]
- 13.McMahon TJ, Stamler JS. Concerted nitric oxide/oxygen delivery by hemoglobin. Methods Enzymol. 1999;301:99–114. doi: 10.1016/s0076-6879(99)01073-3. [DOI] [PubMed] [Google Scholar]
- 14.Winterbourn CC, Carrell RW. Oxidation of human haemoglobin by copper. Mechanism and suggested role of the thiol group of residue beta-93. Biochem J. 1977;165:141–148. doi: 10.1042/bj1650141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel RP, et al. Biochemical characterization of human S-nitrosohemoglobin. Effects on oxygen binding and transnitrosation. J Biol Chem. 1999;274:15487–15492. doi: 10.1074/jbc.274.22.15487. [DOI] [PubMed] [Google Scholar]
- 16.Garel MC, Domenget C, Galacteros F, Martin-Caburi J, Beuzard Y. Inhibition of erythrocyte sickling by thiol reagents. Mol Pharmacol. 1984;26:559–565. [PubMed] [Google Scholar]
- 17.Garel MC, et al. Covalent binding of glutathione to hemoglobin. I. Inhibition of hemoglobin S polymerization. J Biol Chem. 1986;261:14704–14709. [PubMed] [Google Scholar]
- 18.Pawloski JR, Swaminathan RV, Stamler JS. Cell-free and erythrocytic S-nitrosohemoglobin inhibits human platelet aggregation. Circulation. 1998;97:263–267. doi: 10.1161/01.cir.97.3.263. [DOI] [PubMed] [Google Scholar]
- 19.Fox-Robichaud A, et al. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998;101:2497–2505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gladwin MT, Schechter AN, Shelhamer JH, Ognibene FP. The acute chest syndrome in sickle cell disease. Possible role of nitric oxide in its pathophysiology and treatment. Am J Respir Crit Care Med. 1999;159:1368–1376. doi: 10.1164/ajrccm.159.5.9810094. [DOI] [PubMed] [Google Scholar]
- 21.Atz AM, Wessel DL. Inhaled nitric oxide in sickle cell disease with acute chest syndrome. Anesthesiology. 1997;87:988–990. doi: 10.1097/00000542-199710000-00037. [DOI] [PubMed] [Google Scholar]
- 22.French JA, II, et al. Mechanisms of stroke in sickle cell disease: sickle erythrocytes decrease cerebral blood flow in rats after nitric oxide synthase inhibition. Blood. 1997;89:4591–4599. [PubMed] [Google Scholar]
- 23.Funai EF, Davidson A, Seligman SP, Finlay TH. S-nitrosohemoglobin in the fetal circulation may represent a cycle for blood pressure regulation. Biochem Biophys Res Commun. 1997;239:875–877. doi: 10.1006/bbrc.1997.7565. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi Y, et al. Nitrosyl hemoglobin in blood of normoxic and hypoxic sheep during nitric oxide inhalation. Am J Physiol. 1998;274:H349–H357. doi: 10.1152/ajpheart.1998.274.1.H349. [DOI] [PubMed] [Google Scholar]
- 25.Rodgers GP, et al. Microcirculatory adaptations in sickle cell anemia: reactive hyperemia response. Am J Physiol. 1990;258:H113–H120. doi: 10.1152/ajpheart.1990.258.1.H113. [DOI] [PubMed] [Google Scholar]
- 26.Lonsdorfer J, et al. Cardiorespiratory adjustments in chronic sickle cell anemia. Bull Eur Physiopathol Respir. 1983;19:339–344. [PubMed] [Google Scholar]