Abstract
Many studies have investigated the individual effects of sedimentation or inundation on the performance of wetland plants, but few have examined the combined influence of these processes. Wetland plants might show greater morphological plasticity in response to inundation than to sedimentation when these processes occur simultaneously since inundation can negate the negative effects of burial on plant growth. Here, we evaluate this hypothesis by assessing growth of the emergent macrophyte Polygonum hydropiper under flooding (0 and 40 cm) and sedimentation (0, 5, and 10 cm), separately and in combination. Deep burial and high water level each led to low oxidation-reduction potential, biomass (except for 5-cm burial), and growth of thick, short roots. These characteristics were generally more significant under high water level than under deep burial conditions. More biomass was allocated to stems in the deep burial treatments, but more to leaves in the high water level treatments. Additionally, biomass accumulation was lower and leaf mass ratio was higher in the 40-cm water level + 10-cm burial depth treatment than both separate effects. Our data indicate that inundation plays a more important role than sedimentation in determining plant morphology, suggesting hierarchical effects of environmental stressors on plant growth.
Morphological plasticity refers to changes in plant morphology in response to environmental stimuli (e.g., stressors), a process that is critical for maintaining high fitness in changing environments1,2. Plant morphology is likely to adjust to the primary limiting factor when different environmental stressors occur simultaneously, if limited energy resources prevent adaptation to multiple stressors3,4,5. Therefore, environmental stressors are expected to affect plant morphological plasticity in a hierarchical manner (e.g., one factor overrules anther in determining plant morphology). However, direct experimental evidence for this hypothesis is lacking.
Inundation and sedimentation are recurring events in wetlands; these processes often occur simultaneously6,7, and can have a profound influence on vegetation dynamics8. However, most studies on morphological plasticity in plants have focused on the separate effects of inundation and sedimentation. Such studies including examinations of morphological responses to sedimentation in sand dune systems9,10, changes in root morphology in response to soil waterlogging11, and responses of shoot morphology to inundation where water depth limits light availability12,13. Studies exploring the combined effects of sedimentation and inundation on plant morphology are needed to understand the ability of wetland plants to adapt to these stressors.
Negative effects of inundation and sedimentation on plant growth include lower oxygen or light availability in submerged or buried soils13,14,15,16. Shoot elongation, high shoot mass ratio, short and thick roots, low specific root length (SRL, the length-to-mass ratio of a root fragment), and shallow root systems are examples of morphological adaptations that enable wetland plants to escape inundation or sedimentation by acquiring oxygen and light, or by diminishing root oxygen demand10,13,17,18. Wetland plants can also adapt to sedimentation by stem elongation and increased stem biomass to overcome soil mechanical resistance and grow above the sediment surface8,9,19. In contrast, more biomass may be allocated to leaves under submerged conditions, enabling growth above the water surface and facilitating photosynthesis by increasing leaf area15,20.
Inundation generally is more inhibiting to wetland plant growth than sedimentation due to more easily reduced oxygen availability in the root zones21,22, suggesting that morphological adjustments to anaerobiosis might be more pronounced under inundated conditions. Sedimentation in combination with inundation can impose strong limitations on plant growth and survival7, which might be attributed to more severe oxygen depletion11,15,22. Therefore, the morphological adjustments of wetland plants to simultaneous inundation and sedimentation are thought to be more pronounced than changes that occur under only one of these conditions2. The separate and combined effects of inundation and sedimentation may have hierarchical effects on plant morphology.
Our objective was to test the hypothesis of hierarchical adjustment of plant morphology under inundation and sedimentation. The emergent macrophyte Polygonum hydropiper was exposed to two water levels (0 and 40 cm, above the soil surface) and three burial depths (0, 5, and 10 cm). P. hydropiper is a pioneer species that commonly occurs in areas where water levers fluctuate, such as floodplains, lake beaches, or creek bottoms. This species plays an important role in soil protection and riverbank stabilization23. We hypothesized that: 1) biomass accumulation would be least affected by deep burial, intermediately affected by high water level, and would be lowest under these combined conditions; 2) roots would be shorter and thicker under deep burial and high water level alone and in combination, and this morphology would be least pronounced under deep burial, intermediate under high water level, and most pronounced under the combined conditions; 3) biomass would be preferentially allocated to stems under deep burial conditions, and preferentially allocated to leaves under high water level regardless of burial depth.
Results
Sediment oxidation-reduction potential (Eh) and biomass accumulation
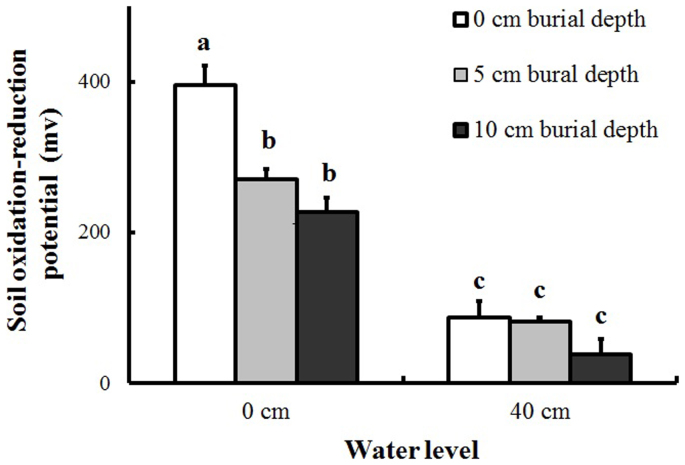
Soil Eh was significantly affected by water level, burial depth, and their interaction (Table 1). Deep burial and high water level were characterized by lower Eh, and Eh was significantly lower in the high water level than in the deep burial treatments (86.9 versus 226.8-270.8, Figure 1). However, additional burial depth did not correspond to lower Eh in the high water level treatments.
Table 1. Summary of two-way ANOVAs for biomass accumulation, biomass allocation, root morphology and soil oxidation-reduction potential at two water levels and three burial depths.
| n | Burial depth (B) | Water-level (W) | B × W | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F-values | P-values | df | F-values | P-values | df | F-values | P-values | df | ||
| Soil oxidation-reduction potential (mv) | 5 | 16.4 | <0.001 | 2 | 215.3 | <0.001 | 1 | 6.5 | 0.005 | 2 |
| Biomass accumulation (g dry wt plant−1) | 5 | 29.8 | <0.001 | 2 | 120.5 | <0.001 | 1 | 11.0 | <0.001 | 2 |
| Leaf mass ratio (%) | 5 | 6.5 | 0.005 | 2 | 78.4 | <0.001 | 1 | 5.7 | 0.009 | 2 |
| Stem mass ratio (%) | 5 | 6.0 | 0.008 | 2 | 1.4 | 0.243 | 1 | 0.8 | 0.460 | 2 |
| Rhizome mass ratio (%) | 5 | 5.8 | 0.008 | 2 | 26.9 | <0.001 | 1 | 0.5 | 0.689 | 2 |
| Root mass ratio (%) | 5 | 4.4 | 0.023 | 2 | 14.7 | <0.001 | 1 | 2.3 | 0.125 | 2 |
| Root diameter (mm) | 5 | 6.2 | 0.007 | 2 | 76.5 | <0.001 | 1 | 4.0 | 0.032 | 2 |
| Mean root length (cm) | 5 | 11.0 | <0.001 | 2 | 81.6 | <0.001 | 1 | 25.3 | <0.001 | 2 |
| Specific root length (cm dry wt mg−1) | 5 | 33.4 | <0.001 | 2 | 123.2 | <0.001 | 1 | 42.5 | <0.001 | 2 |
Figure 1. Soil oxidation-reduction potential (Eh) at the two water levels and three burial depths (mean + S.E., n = 5).
Different letters indicate significant differences among treatments. Multiple comparisons of means were performed using Tukey's test at the 0.05 significance level.
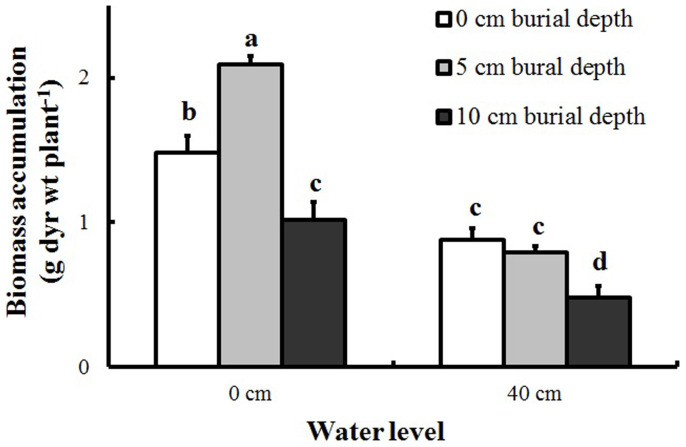
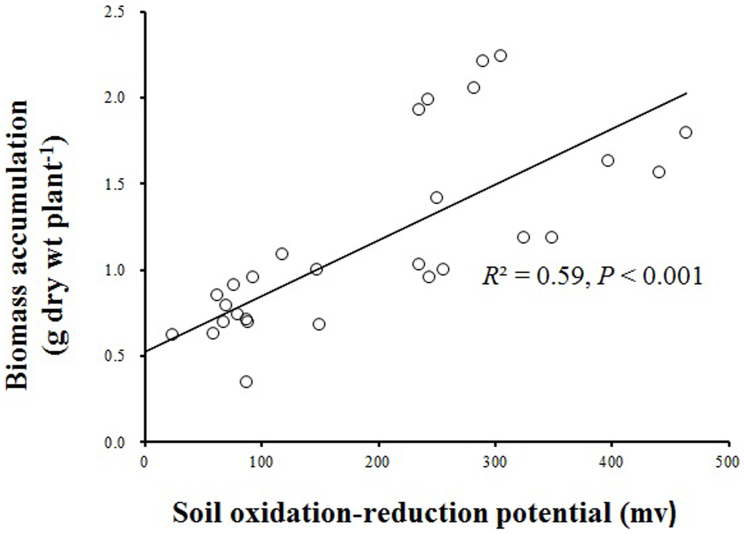
Biomass accumulation was significantly affected by water level, burial depth, and their interaction (Table 1). At the 0-cm water level, biomass accumulation was stimulated by shallow burial (2.09 versus 1.48 g plant−1) but inhibited by deep burial treatment (1.02 versus 1.48 g plant−1, Figure 2 and Figure 3). High water level alone corresponded to lower biomass accumulation (0.88 versus 1.48 g plant−1), but biomass in this treatment was not significantly different from that in the 10-cm burial treatment. The lowest biomass (0.48 g plant−1) was found in the treatment combining 40-cm water level and 10-cm burial depth. Biomass accumulation was significantly and positively correlated with Eh (Figure 4).
Figure 2. Photograph showing the growth conditions of Polygonum hydropiper ramets at two water levels and three burial depths.
LS, 0-cm water level and 0-cm burial depth; LM, 0-cm water level and 5-cm burial depth; LD, 0-cm water level and-10 cm burial depth; HS, 40-cm water level and 0-cm burial depth; HM, 40-cm water level and 5-cm burial depth; HD, 40-cm water level and 10-cm burial depth.
Figure 3. Biomass accumulation of Polygonum hydropiper ramets growing at two water levels and three burial depths (mean + S.E., n = 5).
Different letters indicate significant differences among treatments. Multiple comparisons of means were performed using Tukey's test at the 0.05 significance level.
Figure 4. Linear regression (y = 0.0032x + 0.5301) between biomass accumulation and soil oxidation-reduction potential (Eh).
Biomass allocation
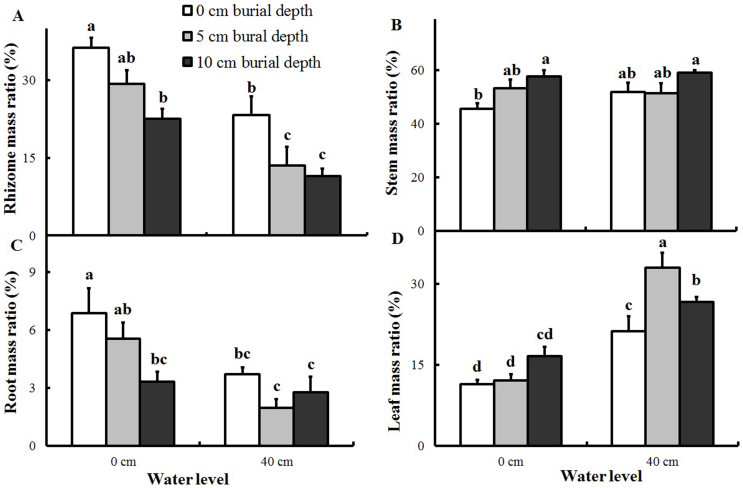
The mass ratios of leaf, rhizome, and root tissues were significantly affected by both water level and burial depth, but only the leaf mass ratio was significantly affected by the interaction between water level and burial depth (Table 1). The stem mass ratio was affected by the burial depth alone. Deep burial (10 cm) corresponded to higher stem mass ratio, and lower root and rhizome mass ratios (Figure 5A–C). Plants in the high water level treatments allocated more biomass to leaves (Figure 2 and Figure 5D) and less to roots and rhizomes regardless of burial depth. Moreover, the root and stem mass ratios were unaffected by burial depth when subjected to 40-cm inundation.
Figure 5. Biomass allocation of Polygonum hydropiper ramets growing at two water levels and three burial depths (mean + S.E., n = 5).
Different letters indicate significant differences among treatments. (A) Rhizome mass ratio. (B) Stem mass ratio. (C) Root mass ratio. (D) Leaf mass ratio. Multiple comparisons of means were performed using Tukey's test at the 0.05 significance level.
Root characteristics
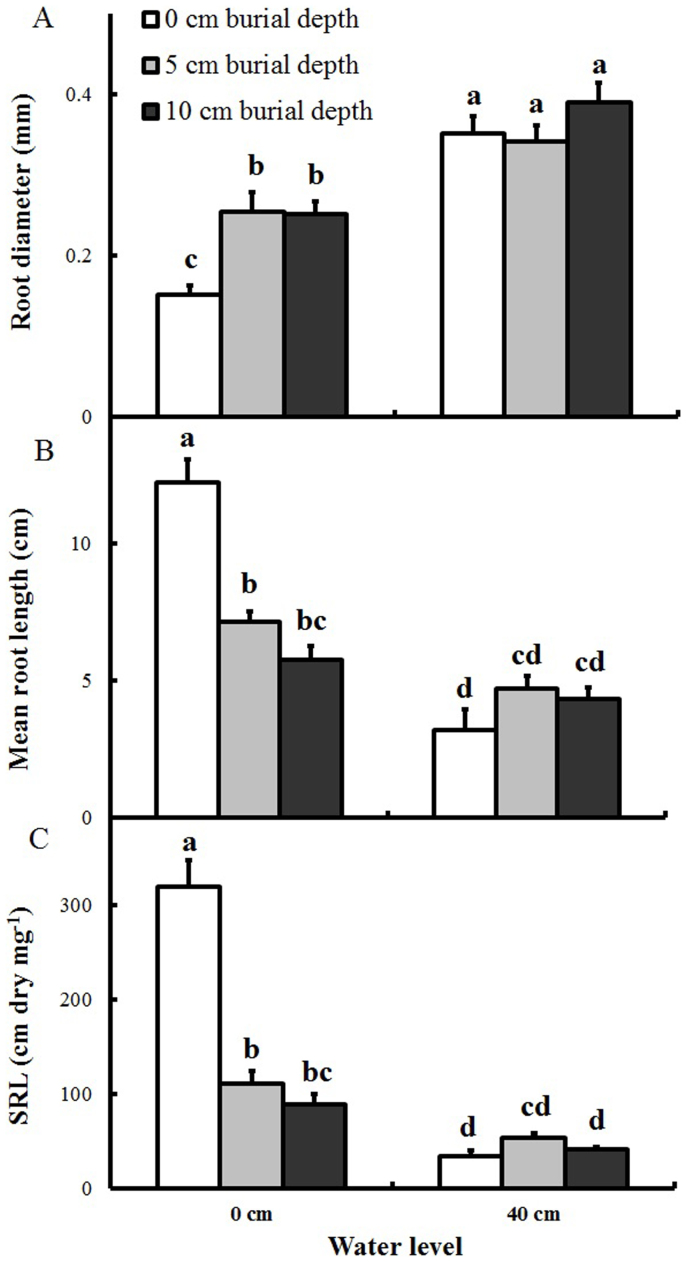
Root diameter, mean root length, and SRL were significantly affected by both water level and burial depth, with significant interactions (Table 1). At the 0-cm water level, mean root length decreased from 12.2 to 5.8 cm and SRL decreased from 319.3 to 89.4 cm mg−1 (dry wet) with increasing burial depth, and roots were thicker in plants in the deep burial treatments than in unburial plants (0.253 versus 0.152 mm, Figure 6A–B). High water level alone corresponded to lower mean root length, and SRL (Figure 6C), and to large root diameter compared to deep burial alone (P < 0.05). Root morphology in treatments combining deep burial and high water level was not significantly different from that in the 40-cm water depth treatment.
Figure 6. Root diameter, mean root length, and specific root length (SRL) of Polygonum hydropiper ramets growing at two water levels and three burial depths (mean + S.E., n = 5).
(A) Root diameter. (B) Mean root length. (C). SRL. Different letters indicate significant differences among treatments. Multiple comparisons of means were performed using Tukey's test at the 0.05 significance level.
Discussion
The main objective of this study was to investigate whether inundation may play a more important role than sedimentation in determining plant morphology. Understanding adaptations of wetland plants to simultaneous stressors is essential to predicting future changes in plant distribution and ecosystem function4,8. Our experiment showed that biomass accumulation was depressed least by deep burial, intermediately by high water level, and most by the combined effects of both stressors, with the exception of increased biomass accumulation in the 0-cm water level + 5-cm burial depth treatment. These results provide support for our first hypothesis, and are consistent with findings of some other studies4,7,24.
Oxygen deficiency is one of the main factors limiting plant growth under deep burial8 or high water level conditions25. Soil Eh can indicate the presence of anaerobic conditions15,16. Soil Eh decreased significantly under both deep burial (10 cm) and high water level conditions in this study, indicating that lower biomass accumulation under these conditions may be attributed to low oxygen availability, as demonstrated by the significant positive correlation between biomass and soil Eh. However, Eh values were unaffected by burial depth under high water level conditions, suggesting that sedimentation did not affect soil oxygen content significantly during inundation. This result is inconsistent with other reports11,15, and may related to the high proportion of large particles (sand + silt > 75%) and low organic matter content (<2%) in the soils. Soil permeability increases as the proportion of larger particles increased, while low organic matter content can limit microbial activity, thus reducing soil oxygen consumption26,27,28.
Regulation of root morphology and biomass allocation are important mechanisms by which plants can adapt to oxygen deficiency14. In this study, roots were shorter and thicker and SRL was lower in deep burial or high water level treatment, and these differences were more significant under high water level than under deep burial conditions. Additional high water level corresponded to lower root length and SRL, and to large root diameter under deep burial conditions, suggesting that inundation was the primary factor affecting root morphology in P. hydropiper. These results partly supported our second hypothesis that inundated roots would be shorter and thicker than roots subjected to sedimentation. The production of short and thick roots is an effective strategy for reducing root oxygen demand29 and radial oxygen loss to the rhizosphere15, and for increasing root porosity to facilitate oxygen transport to root tips30. However, lower SRL (thicker root or higher tissue density) suggests that this adaptation might have a high energy cost31. At 40-cm water depth, root morphology was unaffected by sediment depth because additional burial did not lead to lower Eh values. This finding indicated that plant can adapt to such anaerobiosis without additional changes in root morphology. In fact, lower root mass ratios and thicker, shorter roots might impede nutrient acquisition17.
Different patterns of biomass allocation were observed in P. hydropiper in response to deep burial compared to high water level treatments. For example, more biomass was allocated to stems than to roots under deep burial treatments, and leaves had higher biomass ratios than roots under high water level conditions regardless of burial depth, which directly supported our third hypothesis. Higher stem mass ratios facilitate rapid emergence from sediments, enabling plants to acquire oxygen and maintain normal growth8. However, at the 40-cm water level, plants were completely submerged at the beginning of the experiment, which might have affected oxygen diffusion into the soil and caused low light intensity32. Increasing leaf mass ratio might be an important strategy for adaption to inundation. This strategy can enable leaves to grow above the water surface and facilitates photosynthesis by increasing leaf surface area12,33,34. P. hydropiper was unable to acclimate to sedimentation under high water level conditions, as indicated by the lowest biomass accumulation under these combined conditions.
In summary, P. hydropiper exhibited morphological adaptations to deep burial and high water level and showed a stimulatory effect at the treatment of 0-cm water level + 5-cm burial depth. Plants subjected to waterlogged conditions may maintain the majority of their leaves and some of their roots above the water surface to acquire oxygen and light, which may help to restore aerobic metabolism and offset the costs of maintaining metabolism in inundated roots35. Sediment deposition reduces the range of daily temperature fluctuation and can provide an additional source of nutrients for plant growth8,22. Stimulatory effects of shallow burial under waterlogged conditions have been reported in other wetland macrophytes36,37. However, the high water level impeded plasticity in biomass allocation to adapt to sedimentation, which led to a further decrease in biomass, demonstrating that inundation was the primary factor affecting the growth and morphology of P. hydropiper. These results provide direct evidence support for the hypothesis of hierarchical effects of environmental stressors on plant morphology.
Plant zonation along elevation gradients is commonly observed in the Dongting Lake wetlands. For example, communities of Carex argyi, P. hydropiper and Miscanthus sacchariflorus are often distributed regularly along a gradient from low to high elevation23. Previous studies have shown that P. hydropiper is more tolerant to inundation than M. sacchariflorus and Carex argyi38,39, which explains why P. hydropiper is distributed at lower elevations than M. saccharifloru. Our findings might help to explain why P. hydropiper communities do not occur at the lowest elevations (where heavy sedimentation and prolonged inundation occur) distributed by C. argyi because P. hydropiper is unable to adapt to sedimentation under high water level conditions.
Methods
Plant materials
In March 2009, similar-size rhizomes of P. hydropiper (appropriately 3-mm thick and 4-cm long) were obtained from a stand in East Dongting Lake of Chunfeng Village (29°13′49.72″N, 113°02′32.79″E), Hunan province, China. The Dongting Lake region is characterized by a subtropical monsoon climate, with an annual mean temperature of 18.6°C and annual average precipitation of 1,454 mm. P. hydropiper is abundant in wetlands of Dongting Lake, the second largest freshwater lake in China, which experiences periodic flooding that normally occurs between May and October and is accompanied by heavy sedimentation23,40. The annual average mass of silt delivered via runoff to Dongting Lake is appropriately 1.56 × 108 t, which results in major sedimentation of wetland plants41.
Rhizomes with roots were transported to the Institute of Subtropical Agriculture, a part of the Chinese Academy of Sciences, Changsha city, Hunan province. To facilitate the development of new ramets, the rhizomes were placed in plastic buckets containing 15 cm soil (1.86% organic matter, 15.3% sand, 60.5% silt, 24.2% clay, 28.5 μg g−1 exchangeable N, 18.65 μg g−1 exchangeable P, and Eh = 428.7 mv). The soil was collected from an areas colonized by P. hydropiper in Chunfeng Village. The plants were watered as needed and placed in a net-house covered with one layer of nylon-net, which transmits appropriately 50% photosynthetically active radiation to reduce evaporation and prevent damage from strong light.
Experimental design
We transplanted 30 similar-size ramets (5–8 leaves; approximately 20-cm tall) into individual polyvinyl chloride (PVC) tubes (20-cm tall × 11-cm diameter, bottoms enclosed with nylon net) on Apirl 27, 2009. Four drainage holes (1-cm diameter) were evenly drilled 6 cm above the bottom of each tube, and the tubes were filled with 6 cm of soil. Previous studies reported that 9-cm burial or 25-cm submergence significantly inhibited growth of herbaceous plants in Dongting Lake wetlands22,42; therefore, 10-cm burial depth and 40-cm water depth (to keep plants continuously submerged) were chosen as the maximum levels for the two stressors. The experimental treatments were initiated on May 5, 2009. A two-way factorial design was applied that combined three burial depths (0, 5, and 10 cm) with two water levels (0 and 40 cm above the soil surface), resulting in six treatments (n = 5 replicates per treatment). All plant leaves remained above the sediment surface during the experiment. The 30 tubes were randomly placed into five plastic buckets (88 × 67 × 63 cm; 1 ramet per treatment per bucket). To simulate field conditions, temperature was not controlled. The soil used for burial was the same as that used for ramet growth. The water level was maintained by placing the tubes either at the bottom of the bucket or on 40-cm-high bricks. Water depth in each bucket was maintained at 46 cm using tap water (51.1 μg L−1 NH4+-N, 176 μg L−1 NO3−-N, 52.7 μg L−1 PO43+-P; pH = 7.2) and was completely replaced every 2 weeks to prevent growth of freshwater algae.
Soil oxidation-reduction potential (Eh)
Five months after the start of the experiment, soil Eh was tested at 6-cm (relative to the tube bottom) using a combined platinum-calomel electrode (FJA-4; Nanjing Zhuandi Instrument Co., China) inserted directly into the PVC tubes.
Harvest and morphological measurement
All plants were harvested after Eh measurement. The entire root system of each plant was carefully excavated from the soil and cleaned using tap water. Plants were divided into roots, rhizomes, stems, and leaves. Plant pieces were blotted with filter paper and their fresh weight was recorded (±0.0001 g). Half of the fresh roots were used for analysis of root morphology. The remaining roots and other plant tissues were dried at 85°C for 48 h and reweighed to determine a wet to dry conversion factor, and fresh weight of each sample was then converted to dry weight. Biomass accumulation was measured as the sum of the root, stem, rhizome, and leaf mass.
Four representative full-grown adventitious roots (growing from rhizomes and below the soil surface) were selected from each plant; root length and diameter were measured using a vernier caliper and microscope equipped with an ocular micrometer (Olympus BX51; Olympus, Japan), respectively. The total root length and fresh weight of the four adventitious roots (cut off laterals) were recorded for each plant, and specific root length (SRL) was calculated as total root length divided by dry root mass. Root morphological data for the individual plants were averaged by treatment for statistical analysis.
Statistical analysis
Effects of the plastic bucket on Eh, biomass accumulation, biomass allocation, and root morphology were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's post hoc tests at the 0.05 significance level; the bucket effect found to be insignificant. A general linear model, with water level and burial depth as main factors, was used to determine the main and interacting effects on Eh, biomass accumulation, leaf mass ratio, stem mass ratio, rhizome mass ratio, root mass ratio, root diameter, mean root length, and SRL. Tukey's tests (0.05 significance level) and the Bonferroni correction for multiple comparisons were applied when necessary. Normality was assessed using the Kurtosis test, and homoscedasticity was tested using Levene's test. Data on root mass ratio and SRL were log10-transformed to meet the assumptions of normality and homoscedasticity. Linear regression analysis was conducted to determine the relationship between biomass accumulation and Eh. All statistical analyses were performed using the SPSS13.0 package (SPSS Inc., USA). All data in tables and figures are presented as means + 1 SE.
Author Contributions
Y.P. and Y.H.X. wrote the main manuscript text, and designed and executed the technical assays. Y.P., Y.H.X., Z.M.D., Y.T. and D.D.P. contributed to planning the experiments, the interpretation of the data, and writing and editing the manuscript. All authors reviewed the manuscript.
Acknowledgments
This research was supported by National Key Technology Research and Development Program of China (2014BAC09B03), National Basic Research Program of China (2012CB417000) and the National Natural Science Foundation of China (30770362; 31300361).
References
- Schweitzer J. A. & Larson K. C. Greater morphological plasticity of exotic honeysuckle species may make them better invaders than native species. J. Torrey Bot. Soc. 126, 15–23 (1999). [Google Scholar]
- Davidson A. M., Jennions M. & Nicotra A. B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 14, 419–431 (2011). [DOI] [PubMed] [Google Scholar]
- Lenssen J. P. M., Menting F. B. J., Van der Putten W. H. & Blom C. W. P. M. Control of plant species richness and zonation of functional groups along a freshwater flooding gradient. Oikos 80, 523–534 (1999). [Google Scholar]
- Lenssen J. P. M., Menting F. B. J. & van der Putten W. H. Plant responses to simultaneous stress of waterlogging and shade: amplified or hierarchical effects? New Phytol. 157, 281–290 (2003). [DOI] [PubMed] [Google Scholar]
- González A. V. & Gianoli E. Morphological plasticity in response to shading in three Convolvulus species of different ecological breadth. Acta Oecol. 26, 185–190 (2004). [Google Scholar]
- Voesenek L. A. C. J., Rijnders J. H. G. M., Peeters A. J. M., van de Steeg H. M. V. & Kroon H. De. Plant hormones regulate fast shoot elongation under water: from genes to communities. Ecology 85, 16–27 (2004). [Google Scholar]
- Lowe B. J., Watts R. J., Roberts J. & Robertson A. The effect of experimental inundation and sediment deposition on the survival and growth of two herbaceous riverbank plant species. Plant Ecol. 209, 57–69 (2010). [Google Scholar]
- Maun M. A. Adaptations of plants to burial in coastal sand dunes. Can. J. Bot. 76, 713–738 (1998). [Google Scholar]
- Brown J. F. Effects of experimental burial on survival, growth and resource allocation of three species of dune plants. J. Ecol. 85, 151–158 (1997). [Google Scholar]
- Dech J. P. & Maun M. A. Adventitious root production and plastic resource allocation to biomass determine burial tolerance in woody plants from central Canadian coastal dunes. Ann. Bot. 98, 1095–1105 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong W. & Drew M. C. Root growth and metabolism under oxygen deficiency. (Marcel Dekker Press, New York, USA, 2002). [Google Scholar]
- Voesenek L. A. C. J., Colmer T. D., Pierik R., Millenaar F. F. & Peeters A. J. M. How plants cope with complete submergence. New Phytol. 170, 213–226 (2006). [DOI] [PubMed] [Google Scholar]
- Pierik R., van Aken J. M. & Voesenek L. A. C. J. Is elongation-induced leaf emergence beneficial for submerged Rumex species? Ann. Bot. 103, 353–357 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartapetian B. B. & Jackson M. B. Plant adaptations to anaerobic stress. Ann. Bot. 79, 3–20 (1997). [Google Scholar]
- Pezeshki S. R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 46, 299–312 (2001). [Google Scholar]
- Dwire K. A., Kauffman J. B. & Baham J. E. Plant species distribution in relation to water-table depth and soil redox potential in montane riparian medows. Wetlands 26, 131–146 (2006). [Google Scholar]
- Xie Y., Luo W., Ren B. & Li F. Morphological and physiological responses to sediment type and light availability in roots of the submerged plant Myriophyllum spicatum. Ann. Bot. 100, 1517–1523 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Ethylene-promoted elongation: an adaptation to submergence stress. Ann. Bot. 101, 229–248 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaço S., Santos R. & Duarte C. M. The impact of sediment burial and erosion on seagrasses: A review. Estuar. Coast. Shelf S. 79, 354–366 (2008). [Google Scholar]
- Peterson D. L. & Bazzaz F. A. Photosynthetic and growth responses of silver maple (Acer saccharinum L.) seedlings to flooding. Am. Midl. Nat. 112, 261–272 (1984). [Google Scholar]
- Pierce A. R. & King S. L. The effects of flooding and sedimentation on seed germination of two bottomland hardwood tree species. Wetlands 27, 588–594 (2007). [Google Scholar]
- Pan Y., Xie Y. H., Chen X. S. & Li F. Effects of flooding and sedimentation on the growth and physiology of two emergent macrophytes from Dongting Lake wetlands. Aquat. Bot. 100, 35–40 (2012). [Google Scholar]
- Zheng J. M., Wang L. Y., Li S. Y., Zhou J. X. & Sun Q. X. Relationship between community type of wetland plants and site elevation on sandbars of the East Dongting Lake, China. Forest. Stud. China 11, 44–48 (2009). [Google Scholar]
- Grace J. B. The factors controlling species density in herbaceous plant communities: an assessment. Perspect. Plant Ecol. Evol. Syst. 2, 1–28 (1999). [Google Scholar]
- Sorrell B. K., Mendelssohn I. A., Mackee K. L. & Wood R. A. Ecophysiology of wetland plant roots: a modeling comparison of aeration in relation to species distribution. Ann. Bot. 86, 675–685 (2000). [Google Scholar]
- Lucia F. J. Rock-fabric/petrophysical classification of carbonate pore space for reservoir characterization. AAPG. Bulletin 79, 1275–1300 (1995). [Google Scholar]
- Castelli R. M., Chambers J. C. & Tausch R. J. Soil–plant relations along a soil–water gradient in Great Basin riparian meadows. Wetlands 20, 251–266 (2000). [Google Scholar]
- Fontainea S., Mariottib A. & Abbadiea L. The priming effect of organic matter: a question of microbial competition? Soil Biol. Biochem. 35, 837–843 (2003). [Google Scholar]
- Naidoo G. & Mundree S. G. Relationship between morphological and physiological responses to waterlogging and salinity in Sporobolus virginicus (L.) Kunth. Oecologia 93, 360–366 (1993). [DOI] [PubMed] [Google Scholar]
- Armstrong W. Aeration in higher plants. (Academic Press, London, 1979). [Google Scholar]
- Eissenstat D. M. Costs and benefits of constructing roots of small diameter. J. Plant Nutr. 15, 763–782 (1992). [Google Scholar]
- Li X. L. et al. Morphological and photosynthetic responses of riparian plant Distylium chinense seedlings to simulated Autumn and Winter flooding in Three Gorges Reservoir Region of the Yangtze River, China. Acta Ecologica. Sinica. 31, 31–39 (in Chinese, 2011). [Google Scholar]
- Visser E. J. W., Bogemann G. M. & van de Streeg H. M. Flooding tolerance of Carex species in relation to field distribution and aerenchyma formation. New Phytol. 148, 93–103 (2000). [DOI] [PubMed] [Google Scholar]
- Vervuren P. J. A., Blom C. W. P. M. & Kroon H. de. Extreme flooding events on the rhine and the survival and distribution of riparian plant species. J. Ecol. 91, 135–146 (2003). [Google Scholar]
- Ferreira C. S., Piedade M. T. F., Franco A. C., Gonçalves J. F. C. & Junk W. J. Adaptive strategies to tolerate prolonged flooding in seedlings of floodplain and upland populations of Himatanthus sucuuba, a Central Amazon tree. Aquat. Bot. 90, 246–252 (2009). [Google Scholar]
- van der Putten W. H., van Dijk C. & Peters B. A. M. Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature 362, 53–55 (1993). [Google Scholar]
- Halun Z. et al. Experimental evaluation of the effects of siltation-derived changes in sediment conditions on the Philippine seagrass Cymodocea rotundata. J. Exp. Mar. Biol. Ecol. 279, 73–87 (2002). [Google Scholar]
- Li F. et al. Physiological mechanisms for plant distribution pattern: responses to flooding and drought in three wetland plants from Dongting Lake, China. Limnology 14, 71–76 (2013). [Google Scholar]
- Qin X. Y., Li F., Chen X. S. & Xie Y. H. Growth responses and non-structural carbohydrates in three wetland macrophyte species following submergence and de-submergence. Acta Physiol. Plant 35, 2069–2074 (2014). [Google Scholar]
- Li E. H., Li W., Liu G. H. & Yuan L. Y. The effect of different submerged macrophyte species and biomass on sediment resuspension in a shallow fresh-water lake. Aquat. Bot. 88, 121–126 (2008). [Google Scholar]
- Li J. B., Yin H., Chang J., Lu C. Z. & Zhou H. P. Sedimentation effects of the Dongting Lake Area. J. Geogr. Sci. 19, 287–298 (2009). [Google Scholar]
- Chen X. S., Xie Y. H., Deng Z. M., Li F. & Hou Z. Y. A change from phalanx to guerrilla growth form is an effective strategy to acclimate to sedimentation in a wetland sedge species Carex brevicuspis (Cyperaceae). Flora 206, 347–350 (2011). [Google Scholar]