Abstract
Effort of reducing CO2 emissions in developing countries may require an increasing utilization of biomass fuels. Biomass pellets seem well-suited for residential biomass markets. However, there is limited quantitative information on pollutant emissions from biomass pellets burning, especially those measured in real applications. In this study, biomass pellets and raw biomass fuels were burned in a pellet burner and a conventional stove respectively, in rural households, and metal emissions were determined. Results showed that the emission factors (EFs) ranged 3.20–5.57 (Pb), 5.20–7.58 (Cu), 0.11–0.23 (Cd), 12.67–39.00 (As), 0.59–1.31 mg/kg (Ni) for pellets, and 0.73–1.34 (Pb), 0.92–4.48 (Cu), 0.08–0.14 (Cd), 7.29–13.22 (As), 0.28–0.62 (Ni) mg/kg for raw biomass. For unit energy delivered to cooking vessels, the EFs ranged 0.42–0.77 (Pb), 0.79–1.16 (Cu), 0.01–0.03 (Cd), 1.93–5.09 (As), 0.08–0.19 mg/MJ (Ni) for pellets, and 0.30–0.56 (Pb), 0.41–1.86 (Cu), 0.04–0.06 (Cd), 3.25–5.49 (As), 0.12–0.26 (Ni) mg/MJ for raw biomass. This study found that moisture, volatile matter and modified combustion efficiency were the important factors affecting metal emissions. Comparisons of the mass-based and task-based EFs found that biomass pellets produced higher metal emissions than the same amount of raw biomass. However, metal emissions from pellets were not higher in terms of unit energy delivered.
Biomass fuels, as a sustainable alternative to fossil fuels and CO2-neutral energy source, can address concerns about climate change, reduce environmental impacts of using fossil fuels, and potentially improve energy security1,2. In addition, biomass materials can improve resource utilization or reduce waste generation, thereby adding to the productivity and profitability of industries that provide the raw materials3. Among various biomass fuels, new and upgraded options (i.e. pellets, briquettes, and powder) have become more common with several advantages to accomplish efficient and environmentally acceptable combustion conditions4. Pelletized biomass fuels, especially those made of crop residues and wood, are particularly attractive in domestic applications. Recent work has shown that reductions in the total emissions of carbon monoxide (CO), particulate matter (PM), polycyclic aromatic hydrocarbons (PAHs), and elemental carbon (EC) could be achieved by replacing the raw biomass fuels burned in traditional cooking stoves with pellets burned in modern pellet burners5.
The production and use of biomass pellets have been extensive during the past decades, mainly in Europe (e.g., Sweden, Austria, and Germany), North America and Asia4,6. For example, the delivered amount of biomass pellets to Swedish consumers increased from approximately 0.5 million tons/yr in 1997 to 1.9 million tons/yr in 20097. In China, the consumption of pelletized biomass fuels has rapidly increased from 0.2 million tons in 2008 to 2 million tons in 2010, and is projected to reach 20 million tons in 20158. However, despite the advantages, indoor biomass fuel combustion in rural areas is generally considered as one of the major anthropogenic sources of air pollution in most developing countries9, e.g. volatile organic compounds (VOCs)10, PAHs11, metals12 and PM13. Therefore, the substantially increased use of biomass fuels must be carried out with efforts to avoid air quality degradation.
During the past decades, extensive studies have been conducted on the chemical and physical processes during biomass combustion14,15. The importance of combustion-related trace element pollution and its implications to human health have been widely discussed16,17. Significant emissions of toxic heavy metals including cadmium, lead and chromium have been reported from district heating units operating on biofuels18. Investigations on the emissions of gaseous pollutants and particulate matters from the burning of biomass pellets have been conducted19,20. However, these studies are very limited in comparison of biomass pellets and the raw biomass fuels. In China, there was no field measurement on the toxic metal emissions from biomass pellet combustion, especially comparison with the raw biomass fuels. Lacking of these data prevents us from a fully assessing the environmental and health significance of replacing biomass fuels with pellets, which is critical for policy making.
The objective of the present paper was therefore to experimentally determine the emission characteristics and differences between biomass pellets and uncompressed biomass fuels burning in households, and estimate metal emission factors (EFs) for both types of fuels. In addition to provide realistic EFs under actual residential conditions, the influence of fuel properties and combustion conditions was also investigated.
Results
Metal emissions from raw and pelletized biomass fuels
EFs of copper (Cu), cadmium (Cd), lead (Pb), nickel (Ni) and arsenic (As) from combustion of biomass pellet and raw uncompressed biomass fuel were calculated, and the means and standard deviations are summarized in Table 1. The range of EFs for gaseous Pb, Cu and Ni was 0.03–0.77, 0.47–5.25 and 0.09–0.75 mg/kg, respectively. Cd and As were not detected in the gaseous phase. For particle-bound metals, EFs ranged 0.57–4.80 (Pb), 0.31–3.41 (Cu), 0.08–0.23 (Cd), 7.29–39.0 (As) and 0.19–1.00 mg/kg (Ni), respectively. The range of total EFs (particulate + gaseous) for biomass pellets was 3.20–5.57 (Pb), 5.20–7.58 (Cu), 0.11–0.23 (Cd), 12.67–39.00 (As) and 0.59–1.31 mg/kg (Ni), respectively. The total EFs for raw biomass fuels ranged 0.73–1.34 (Pb), 0.92–4.48 (Cu), 0.08–0.14 (Cd), 7.29–13.22 (As) and 0.28–0.62 mg/kg (Ni), respectively.
Table 1. EFs (mg/kg, dry basis) of Pb, Cu, Cd, Ni and As for combustion of biomass pellets and raw biomass flues. The results include gaseous phase (G), particle-bound phase (P), and total emission (T). Data are presented as mean ± standard deviation (the sample size is three for each type of fuel).
| Biomass | Pb | Cu | Cd | As | Ni | |
|---|---|---|---|---|---|---|
| Corn straw | P | 0.94 ± 0.28 | 0.70 ± 0.54 | 0.09 ± 0.01 | 8.39 ± 1.57 | 0.26 ± 0.01 |
| G | 0.05 ± 0.03 | 0.95 ± 0.50 | ND | ND | 0.14 ± 0.01 | |
| T | 0.99 ± 0.24 | 1.65 ± 1.04 | 0.09 ± 0.01 | 8.39 ± 1.57 | 0.41 ± 0.003 | |
| Corn straw pellet | P | 4.07 ± 0.59 | 2.28 ± 0.55 | 0.15 ± 0.05 | 15.81 ± 4.44 | 0.55 ± 0.21 |
| G | 0.62 ± 0.07 | 4.11 ± 1.13 | ND | ND | 0.65 ± 0.14 | |
| T | 4.69 ± 0.52 | 6.39 ± 1.69 | 0.15 ± 0.05 | 15.81 ± 4.44 | 1.20 ± 0.07 | |
| Pine wood chip | P | 0.85 ± 0.38 | 2.77 ± 0.90 | 0.12 ± 0.02 | 13.11 ± 0.15 | 0.22 ± 0.05 |
| G | 0.19 ± 0.04 | 1.41 ± 1.32 | ND | ND | 0.23 ± 0.19 | |
| T | 1.04 ± 0.43 | 4.18 ± 0.42 | 0.12 ± 0.02 | 13.11 ± 0.15 | 0.45 ± 0.24 | |
| Pine wood pellet | P | 3.71 ± 1.53 | 1.96 ± 0.20 | 0.17 ± 0.09 | 37.10 ± 2.69 | 0.70 ± 0.43 |
| G | 0.67 ± 0.14 | 5.12 ± 0.17 | ND | ND | 0.25 ± 0.08 | |
| T | 4.39 ± 1.68 | 7.08 ± 0.38 | 0.17 ± 0.09 | 37.10 ± 2.69 | 0.95 ± 0.51 |
ND: not detected.
Factors affecting metal emissions
In general, EFs of combustion-generated pollutants are affected by a number of factors including fuel properties and combustion conditions. The most critical factors could be identified to evaluate metal emissions21,22. For example, Dhammapala et al. found that PM2.5 emission from wheat stubble burning was primarily affected by combustion efficiency23. Shen et al. reported that modified combustion efficiency and fuel moisture were the most important factors affecting the EFs of PAHs for crop residues24. However, investigations regarding the impact of factors on metal emissions from biomass fuels combustion were seldom conducted. In this study, fuel properties and burning conditions that may have potential impact on metal emissions were tested, and the data were presented in Table S1 and S2 in the Supplementary Information. To quantitatively address the influence of fuel properties and combustion conditions on the metal EFs, a stepwise regression model was applied with factors, including moisture content, ash content, element content (C, H, Cl and N), modified combustion efficiency and volatile matter content as independent variables, and the metal EFs for the raw and pellet biomass fuels as dependent variables (the threshold value was 0.05). It was revealed that moisture content (M, the percentage of water in biomass), volatile matter content (VM, the percentage of volatile materials, exclusive of moisture) and modified combustion efficiency (MCE, defined as CO2/(CO2 + CO)) were included in the regression model. The regression results showed that the three factors can explain 44%–97% of the variations in the metal EFs. Therefore, the influence of these factors on the metal EFs can be evaluated based on the following equations.
 |
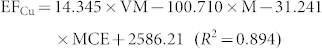 |
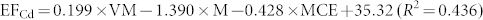 |
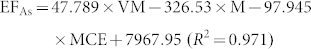 |
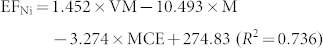 |
Where M (moisture content), VM (volatile matter) and MCE (modified combustion efficiency) are in unit of %, and EF is in unit of mg/kg.
Discussion
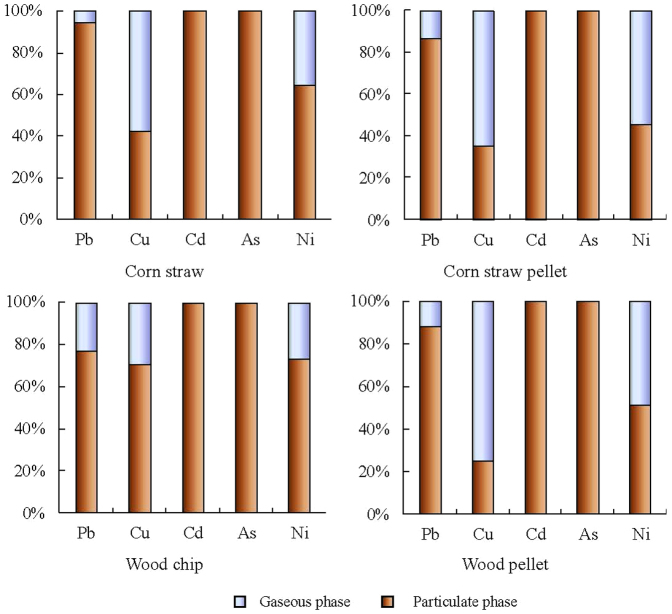
The mechanism of metal partitioning in the gaseous and particulate phases was mainly characterized by the volatility of the elements and their compounds formed during combustion. As shown in Figure 1, Cd and As were almost completely partitioned to the particulate phase. As is a metalloid with low sublimation point, and Cd has relatively low volatilization point. Previous studies on emissions from solid waste incineration found that more than 97% of Cd partitioned in the particulate phase of flue gas25. Pb was mostly (80–96%) partitioned to the particulate phase, with only about 4–20% going to the gaseous phase. It was interesting to find that the refractory-volatile heavy metal, Cu, was more than 50% distributed to the gaseous phase from burning of the biomass pellets. The volatilization temperature for Cu is highly influenced by the chlorine available during combustion4. The chlorine content in the corn straw pellet and wood pellet was 1.30% and 0.35% respectively (Table S1), much higher than that in the corn straw and wood chip (0.77% and 0.02% respectively). This can explain the high proportion of Cu partitioned to the gaseous phase from the biomass pellets. Similar behavior of Cu in the presence of chlorine has also been illustrated in some previous studies. For example, Wang et al. reported that increasing chlorine content may increase the Cu partitioned to the gaseous phase26. The enhanced percentage of Ni in the gaseous phase for pellet fuels was also regarded as due to the presence of chlorine which may promote the formation of volatile metal chlorides. These results suggest that volatilization and subsequent heterogeneous deposition to submicron particle surfaces was probably the most significant mechanism determining the partitions in the particulate phase.
Figure 1. Composition profile of metal emissions from biomass pellets and raw biomass fuels during a whole burning cycle.
Percentage in gaseous phase and particulate phase is shown as stacked bars.
The result of the stepwise regression showed that moisture content, VM and MCE have significant influence on the EFs. The relatively importance of these factors was compared by the absolute value of the standardized coefficient of the regressions (Table S3 in the Supplementary Information). Moisture content appeared to be the most important factor affecting the EFs, and volatile matter and modified combustion efficiency had similar weight on the metal emissions. Since the fuels were stored at the same condition for months prior to the experiment, the difference in moisture content was likely due to differences in fuel composition and texture. It was reported that low moisture was favorable for metal emission, as high moisture content could reduce combustion temperature27. Since the biomass fuels used in this study were in different types, it would be interesting to investigate the influence of different moisture amounts on EFs using one type of biomass fuel in further study. It should be noted that the EFs was based on the combustion experiments under the given conditions (real practice used by rural residents) in this study. Considering the possibly large variation in EFs under different conditions, influence of various fuel/stove combinations should be further studied.
Mann-Whitney U test showed that for Pb and Cu, the EFs for the biomass pellets were higher than those for the raw biomass fuels (n = 12, p = 0.021), while for Cd, As and Ni, the EFs was not statistically different between the pellets and raw biomass fuels (n = 12, p = 0.08 for Cd, 0.083 for As, and 0.05 for Ni). This result indicated that burning biomass pellets may not lead to reduction in metal emissions compared with burning the same mass amount of raw biomass fuels. Similar observations for other pollutant emissions were also reported in literature28,29. For example, Perzon found that the after-flame smoldering of wood pellets released higher concentrations of methane, alkenes and aromatic hydrocarbons than the after-flame smoldering of oats28. Bäfver et al. reported that the mass concentration of particulate material in the chimney was roughly the same for wood stoves and pellets stoves29. Kjällstrand and Olsson reported that the change from wood to pellets may not significantly decrease the emissions, and considerable differences exist between various combinations of pellet burners and boiler furnaces30.
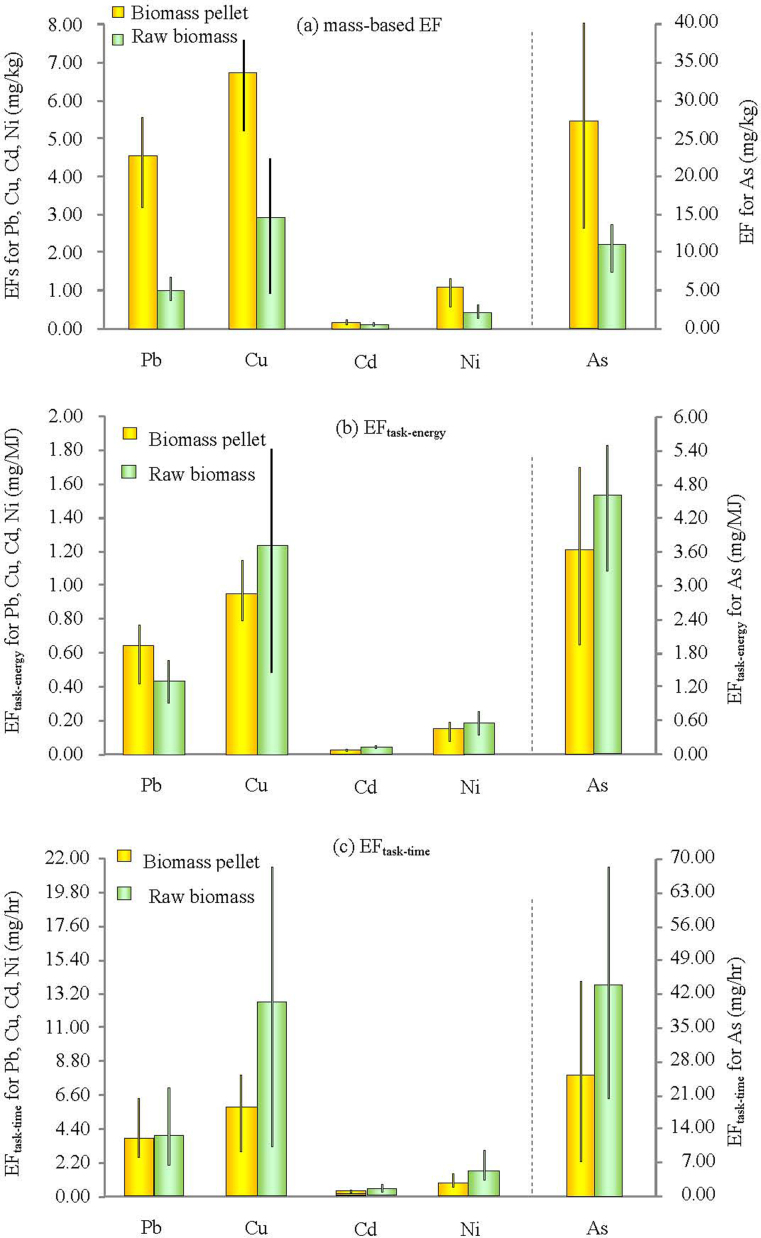
Although the mass-based EFs for biomass pellets was not lower than the raw biomass fuels, it cannot conclude that in daily household usage, burning pelletized biomass fuels will have higher metal emissions than raw biomass fuels, because different amounts of fuels are needed for a same cooking task using the different fuel/stove combinations. Therefore, emission factors based on cooking task (EFtask, in the unit of pollutant mass per cooking task) would be better to compare the potential of pollutant emissions from different fuel/stove combinations than the mass-based emission factor. The simplest task-based EF is the emission amount per unit energy delivered to the cooking vessel (EFtask-energy, in the unit of mg/MJ)31,32,33. The conversion between the mass-based EFs and task-based EFs was achieved using the fuel energy content (i.e. heat value, MJ/kg) and stove thermal efficiency (%). The calculation was described in detail in the Supplementary Information, and the calculated EFtask-energy was summarized in Table 2. Briefly, EFtask-energy for biomass pellets ranged 0.42–0.77 (Pb), 0.79–1.16 (Cu), 0.01–0.03 (Cd), 1.93–5.09 (As) and 0.08–0.19 mg/MJ (Ni), respectively. EFtask-energy for raw biomass fuels was 0.30–0.56 (Pb), 0.41–1.86 (Cu), 0.04–0.06 (Cd), 3.25–5.49 (As) and 0.12–0.26 mg/MJ (Ni), respectively. Mann-Whitney U test showed that the difference between the EFtask-energy for pellets and raw fuels was not significant (n = 12, p = 0.083 for Pb, 0.386 for Cu, 0.05 for Cd, 0.248 for As, and 0.386 for Ni).
Table 2. EFtask-energy (mg/MJ, dry basis) and EFtask-time (mg/hr, dry basis) for biomass pellets and raw biomass flues. Data are presented as mean ± standard deviation (the sample size is three for each type of fuel).
| Corn straw pellet | Pine wood pellet | Corn straw | Pine wood chip | |||||
|---|---|---|---|---|---|---|---|---|
| Metals | EFtask-energy | EFtask-time | EFtask-energy | EFtask-time | EFtask-energy | EFtask-time | EFtask-energy | EFtask-time |
| Pb | 0.72 ± 0.08 | 2.66 ± 0.17 | 0.57 ± 0.22 | 4.91 ± 2.05 | 0.44 ± 0.11 | 2.64 ± 0.79 | 0.43 ± 0.18 | 5.25 ± 2.45 |
| Cu | 0.98 ± 0.26 | 3.66 ± 1.14 | 0.92 ± 0.05 | 7.85 ± 0.12 | 0.74 ± 0.46 | 4.29 ± 2.51 | 1.74 ± 0.18 | 20.9 ± 0.89 |
| Cd | 0.02 ± 0.01 | 0.08 ± 0.02 | 0.02 ± 0.01 | 0.19 ± 0.09 | 0.04 ± 0.01 | 0.23 ± 0.02 | 0.05 ± 0.01 | 0.59 ± 0.08 |
| As | 2.41 ± 0.68 | 9.07 ± 2.97 | 4.84 ± 0.35 | 41.2 ± 4.56 | 3.74 ± 0.70 | 22.1 ± 2.89 | 5.45 ± 0.06 | 65.7 ± 4.62 |
| Ni | 0.18 ± 0.01 | 0.69 ± 0.07 | 0.12 ± 0.07 | 1.04 ± 0.53 | 0.18 ± 0.01 | 1.08 ± 0.05 | 0.19 ± 0.10 | 2.22 ± 1.08 |
Because the combustion of biomass fuels used for cooking is often a major source of indoor air pollution in developing countries, and the cooking time is relatively stable in everyday life for a household, an index based on cooking time is more preferred to measure the potential exposures resulting from daily cooking in household. Such time-based emission factors (EFtask-time, in unit of mg/hr) are particularly useful for comparing the air pollution potential of different fuel/stove combinations and assessing the impacts of fuel/stove substitutions. The calculation of EFtask-time was presented in the Supplementary Information, and the calculated EFtask-time was summarized in Table 2. EFtask-time ranged 2.54–6.35 (Pb), 2.86–7.93 (Cu), 0.07–0.25 (Cd), 6.97–44.46 (As) and 0.63–1.42 mg/hr (Ni) for biomass pellets, and 2.08–6.99 (Pb), 2.52–21.52 (Cu), 0.22–0.65 (Cd), 20.04–68.96 (As) and 1.04–2.99 mg/hr (Ni) for raw biomass fuels, respectively. Mann-Whitney U test showed that for Cd, As and Ni, the EFs for raw biomass fuels was statistically higher than biomass pellets (n = 12, p = 0.02 for Cd, 0.04 for As and Ni), while for Pb and Cu, the difference was not significant (n = 12, p = 0.77 for Pb, 0.56 for Cu). This result indicated that for Cd, As, and Ni, although combustion of pelletized biomass produced similar metal emissions with the same amount of raw uncompressed biomass fuels, performing one cooking task using the biomass pellets would produce substantially less metal emissions than using raw biomass fuels. It was noted during the experiments that combustion of the biomass pellets lasted longer than the raw biomass fuels with the same mass amount. According to the field record, the burning rate for the biomass pellets was lower than that for the raw biomass fuels, and the MCE was higher (Table S2 in the Supplementary Information). These factors may attribute to explain the lower metal emissions from biomass pellets combustion per unit cooking time.
Comparison of the mass-based EFs and task-based EFs for biomass pellets and raw biomass fuels was presented in Figure 2. The comparison of the mass-based EFs showed that burning a unit mass of biomass pellets would emit slightly higher concentration of metals (Pb, Cu) than burning the same mass amount of raw uncompressed biomass fuels. However, comparison of the task-based EFs showed that for every unit energy that was delivered to the cooking vessels, the metal emissions from pellet fuels were not higher than the raw biomass fuels. For unit time of performing one cooking task, the metal emissions of pellet fuels were even lower than the raw materials. These results illustrated that the mass-based EFs cannot completely explain the environmental effects of biomass fuels burning in real application, and task-based EFs are more useful to assess the exposure to indoor air pollution from cooking stoves in households. Considering that the raw uncompressed biomass fuels and the newly emerging biomass pellets are the main fuels used by the majority of rural residents in China, exposure risk to heavy metals emitted from biomass fuel combustion should be highlighted and arise more attention.
Figure 2. Comparison of the mass-based and task-based EFs for biomass pellets and raw biomass fuels: (a) mass-based EF; (b) EFtask-energy; (c) EFtask-time.
(The bar represents the average vale, and the upper and lower end of the short line represents the maximum and minimum value, respectively).
Methods
Fuels, stove and combustion experiment
The most popular biomass pellets used in rural China include wood pellet (made of Chinese pine, Pinus tabuliformis) and corn straw pellet (made of corn stalks, Zea mays). Samples of the two species of biomass pellets and the raw uncompressed biomass including pine wood and corn straw were collected. The physical-chemical parameters of the pelletized and raw uncompressed biomass fuels were summarized in Table S1 in the Supplementary Information.
The combustion experiments were conducted in a rural kitchen, which was representative of the common kitchens in rural China. The experimental site was located in a remote area outside Beijing with no industrial sources nearby. The biomass pellets were burned in a pellet burner that was purchased from Beijing local market. The burner has an inside brick chamber and is specifically designed to burn biomass pellets to create a source of heat for residential purposes. In this study, the pellet burner was set up under a stainless hood in the kitchen according to the real practice of the rural residents. A brick wok stove was also used in the kitchen to burn the raw uncompressed biomass fuels. This type of stove is widely applied in rural households in China, and the number was estimated to be approximately 200 million in 200034. The exhaust gas from the wok stove and the pellet burner was vented into a mixing chamber (4.5 m3) where sampling and on-line measurements were conducted. No further dilution was performed to avoid alterations in particulate mass loading. This experimental setup was successfully applied in previous studies for particulate matter and polycyclic aromatic hydrocarbons emissions from indoor crop residues burning22,24,35, and was proved to be appropriate to investigate pollutant emissions from the actual residential combustion activities. Layout of the kitchen and photos of the stoves can be found in our previous paper35.
The combustion experiments were conducted following the common way used by rural residents. Pre-weighed (approximately 1.0 kg) pellets were burned in the pellet burner. Raw pine wood and corn straw (approximately 1.0 kg, same as the pellets) were burned in the brick wok stove. The combustion experiment for each type of biomass fuel was repeated three times. Carbon dioxide and carbon monoxide were measured in the mixing chamber every 2 s over the whole burning period with an online detector equipped with nondispersive infrared sensor (GXH-3051, Junfang Technical Institute of Physical and Chemistry, China). The detector was calibrated using a span gas (CO, 1.00%; CO2, 5.00%) before each experiment. Combustion conditions, including relative humidity, smoke temperature, fire temperature, and burning duration were all recorded.
Samples collection
Multiple metals and their compounds in the exhaust gas were trapped according to the modified USEPA Method 526. Gaseous and particulate samples were collected using an active sampler (Jiangsu Eltong Electric Corp. Co., Ltd., China) at a flow rate of 1.5 L/min. The flue gas stream first flowed through a 0.4-μm glass fiber filter (99.995% collection efficiency), and particulate sample was collected on the filter. Prior to use, the filters were baked at 450°C for 6 hr. After sampling, the filters were packed with aluminum foil and stored in a desiccator for further analysis. Gaseous samples were collected through a sampling train to capture metals. The sampling train consisted of a series of four impingers following the glass fiber filter. The first impinger was empty, and the second and third impingers were filled with 100 mL combined solution of 5% HNO3 and 10% H2O2 for trapping most of the metals and their compounds in the combustion gases. The final impinger was filled with silica gel to remove the moisture content from the gases. Samples were also collected in the mixing chamber before the combustion experiment and measured for CO, CO2 and metals using the same methods. The results were used as procedure blanks and subtracted from those measured during combustion.
Laboratory analysis and quality control
The collected samples were tested for copper (Cu), cadmium (Cd), lead (Pb), nickel (Ni) and arsenic (As). Filters were digested with 10 mL hydrochloric acid (30%), 5 mL nitric acid (70%), 5 mL hydrofluoric acid (40%), and 3 ml perchloric acid (70%) solution. The digestion vessels were placed in an automatic digester (Model ED36S, LabTech Ltd., Beijing, China) and heated at 170°C for about 1 hr until the evaporation of the acid solution. The procedure was repeated and continued to heat until the residue was barely dry. Upon cooling, 50 mL ultra pure water was added and agitated carefully. The solution was transferred to a 100 mL volumetric flask and diluted to the mark with ultra pure water. Preparation and digestion of the adsorption solutions were followed the same procedure. An atomic absorption spectrophotometer (Model Z-5000, HITACH, Japan) with graphite furnace atomic absorption spectroscopy was used to determine the analytes. The result for a sample is the average of triplicate analysis. Blank samples were prepared by the same procedure for corrections. Calibration curves were prepared for each metal with standard solutions at different concentrations. Standard working solutions for Cu, Cd, Pb, Ni and As were freshly prepared by diluting the stock solutions (AccuTrace™ Reference Standard, AccuStandard, USA) with ultra pure water (Millipore, MA, USA). Detection limits were 0.042 μg/L for Cu, 0.025 μg/L for Cd, 0.075 μg/L for Pb, 0.12 μg/L for Ni and 5.1 μg/L for As. Recoveries of the spiked standards were 91.2–102.6% for Cu, 99.0–100.3% for Cd, 91.9–101.3% for Pb, 92.5–106.7% for Ni, and 101.8–106.3% for As.
Data analysis
Data were processed using Microsoft Excel 2003, and statistical analysis was performed using SPSS 11.0. The significance level of all reported statistics was p = 0.05 unless otherwise noted. Where applicable, values are reported as mean ± standard deviation (SD) except as otherwise noted.
Emission factors (EFs) were calculated based on the carbon mass balance method35. The advantage of the method is that it is not necessary to collect one hundred percent of the target species, and it was widely used in many EF measurements, especially in field studies36,37,38. Briefly, EFs were calculated as:
 |
Where Ci and CCO2 are mass concentrations of metal i and CO2, respectively. EFCO2 is the emission factor of CO2, defined as:
 |
Where Cf and Ca are carbon content in biomass fuel and ash, respectively. fCO2 is the factor to convert carbon mass into CO2. M is the mass of biomass fuels burnt. PIC is the product of incomplete combustion, and calculated as:
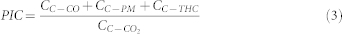 |
Where CC-CO2, CC-CO, CC-THC and CC-PM are carbon released as CO2, CO, total hydrocarbon (THC) and in particle matter (PM), respectively.
EFs reported here were emissions from the whole burning cycle as sampling lasted over the whole burning process instead of a short time interval. Modified combustion efficiencies (MCE) were calculated as CO2/(CO2 + CO) ratios. EFs were reported on a dry fuel mass basis.
Author Contributions
All authors contributed to project led by X.W. W.Z. performed the data analysis and wrote the manuscript. Y.T., H.W., L.C. and L.O. collected the samples and carried out the experiments. X.W. supervised the project and revised the manuscript. G.L. advised on laboratory experiment and commented on the contents of the manuscript. Y.Z. assisted in data analysis. All authors reviewed the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
We would like to thank Zhigang Liu, Wen Wei, Dan Hu, and Guofeng Shen for the help in sample collection and laboratory experiment. This project was funded by the National Natural Science Foundation of China (41130535).
References
- Chen Q., Zhang X., Bradford D., Sharifi V. & Swithenbank J. Comparison of emission characteristics of small-scale heating systems using biomass instead of coal. Energy Fuels 24, 4255–4265 (2010). [Google Scholar]
- Bessou C., Ferchaud F., Gabrielle B. & Mary B. Biofuels, greenhouse gases and climate change. A review. Agron. Sustain. Dev. 31, 1–79 (2011). [Google Scholar]
- International Energy Agency (IEA), Renewable Energy: Markets and Prospects by Technology, 2011. http://www.iea.org/papers/2011/Renew_Tech.pdf. Accessed on March 26, 2014.
- Boman C., Öhman M. & Nordin A. Trace element enrichment and behavior in wood pellet production and combustion processes. Energy Fuels 20, 993–1000 (2006). [Google Scholar]
- Guofeng S. et al. Reductions in emissions of carbonaceous particulate matter and polycyclic aromatic hydrocarbons from combustion of biomass pellets in comparison with raw fuel burning. Environ. Sci. Technol. 46, 6409–6416 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler F. The state of the art of small-scale pellet-based heating systems and relevant regulations in Sweden, Austria and Germany. Renew Sustain. Energ. Rev. 8, 201–221 (2004). [Google Scholar]
- Lindström E., Larsson S. H., Boström D. & Öhman M. Slagging characteristics during combustion of woody biomass pellets made from a range of different forestry assortments. Energy Fuels 24, 3456–3461 (2010). [Google Scholar]
- Yuan H. R. et al. Ignition and emission characteristics of ignition-assisting agents for densified corn stover briquette fuel. Chin. J. Chem. Eng. 18, 687–694 (2010). [Google Scholar]
- Boman C., Nordin A. & Thaning L. Effects of increased biomass pellet combustion on ambient air quality in residential areas-a parametric dispersion modeling study. Biomass Bioenerg 24, 465–474 (2003). [Google Scholar]
- Black R. R. et al. Emissions of PCDD and PCDF from combustion of forest fuels and sugarcane: A comparison between field measurements and simulations in a laboratory burn facility. Chemosphere 83, 1331–1338 (2011). [DOI] [PubMed] [Google Scholar]
- Shen G. F. et al. Emission factors and particulate matter size distribution of polycyclic aromatic hydrocarbons from residential coal combustions in rural Northern China. Atmos. Environ. 44, 5237–5243 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M., Salam A. & Alam A. M. Identification and characterization of trace metals in black solid materials deposited from biomass burning at the cooking stoves in Bangladesh. Biomass Bioenerg 33, 1376–1380 (2009). [Google Scholar]
- Watson J. G. et al. Particulate emission factors for mobile fossil fuel and biomass combustion sources. Sci. Total Environ. 409, 2384–2396 (2011). [DOI] [PubMed] [Google Scholar]
- Johansson L. S., Tullin C., Leckner B. & Sjövall P. Particle emissions from biomass combustion in small combustors. Biomass Bioenerg 25, 435–446 (2003). [Google Scholar]
- Boman C., Nordin A., Boström D. & Öhman M. Characterization of inorganic particulate matter from residential combustion of pelletized biomass fuels. Energ. Fuel 18, 338–348 (2004). [Google Scholar]
- Lighty J. S., Veranth J. M. & Sarofim A. F. J. Combustion aerosols: Factors governing their size and composition and implications to human health. Air Waste Manage. Assoc. 50, 1565–1618 (2000). [DOI] [PubMed] [Google Scholar]
- Avakian M. D. et al. The origin, fate, and health effects of combustion by-products: A research framework. Environ. Health Perspect. 110, 1155–1162 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka A. et al. Particle emissions from district heating units operating on three commonly used biofuels. Atmos. Environ. 39, 139–150 (2005). [Google Scholar]
- Johansson L. S. et al. Emission characteristics of modern and old-type residential boilers fired with wood logs and wood pellets. Atmos. Environ. 38, 4183–4195 (2004). [Google Scholar]
- Boman C., Pettersson E., Westerhol R., Boström D. & Nordin A. Stove performance and emission characteristics in residential wood log and pellet combustion, Part 1: pellet stoves. Energy Fuels. 25, 307–314 (2011). [Google Scholar]
- Chen Y. et al. Emission factors for carbonaceous particles and polycyclic aromatic hydrocarbons from residential coal combustion in China. Environ. Sci. Technol. 39, 1861–1867 (2005). [DOI] [PubMed] [Google Scholar]
- Shen G. et al. Emission of oxygenated polycyclic aromatic hydrocarbons from indoor solid fuel combustion. Environ Sci Technol. 45, 3459–3465 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhammapala R., Claiborn C., Corkill J. & Gullett B. Particulate emissions from wheat and Kentucky bluegrass stubble burning in eastern Washington and northern Idaho. Atmos. Environ. 40, 1007–1015 (2006). [Google Scholar]
- Shen G. F. et al. Emissions of PAHs from indoor crop residue burning in a typical rural stove: Emission factors, size distributions, and gas-particle partitioning. Environ. Sci. Technol. 45, 1206–1212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Meng A., Jia J. & Zhang Y. Investigation of heavy metal partitioning influenced by flue gas moisture and chlorine content during waste incineration. J. Environ. Sci. 22, 760–768 (2010). [DOI] [PubMed] [Google Scholar]
- Wang K. S. et al. The effects of FeCl3 on the distribution of the heavy metals Cd, Cu, Cr, and Zn in a simulated multimetal incineration system. Environ. Int. 26, 257–263 (2001). [DOI] [PubMed] [Google Scholar]
- Simoneit B. R. T. Biomass burning-a review of organic tracers for smoke from incomplete combustion. Appl. Geochem. 17, 129–162 (2002). [Google Scholar]
- Perzon M. Emissions of organic compounds from the combustion of oats – a comparison with softwood pellets. Biomass Bioenerg. 34, 828–837 (2010). [Google Scholar]
- Bäfver L. S., Leckner B., Tullin C. & Berntsen M. Particle emissions from pellets stoves and modern and old-type wood stoves. Biomass Bioenerg. 35, 3648–3655 (2011). [Google Scholar]
- Kjällstrand J. & Olsson M. Chimney emissions from small-scale burning of pellets and fuelwood-examples referring to different combustion appliances. Biomass Bioenerg. 27, 557–561 (2004). [Google Scholar]
- Zhang J. & Smith K. R. Emissions of carbonyl compounds from various cookstoves in China. Environ. Sci. Technol. 33, 2311–2320 (1999). [Google Scholar]
- Joshi V., Venkataraman C. & Ahuja D. R. Emissions from burning biofuels in metal cookstoves. Environ. Manage. 13, 763–772 (1989). [Google Scholar]
- Zhang J. & Smith K. R. Hydrocarbon emissions and health risks from cookstoves in developing countries. J. Expo. Anal. Environ. Epidemiol. 6, 147–161 (1996). [PubMed] [Google Scholar]
- Chen X., Zhang W., Liu G. & Liu X. The development of household biomass stove in China. Renew Energ. Resour. 11(2), 118–122 (2010). (in Chinese with English abstract). [Google Scholar]
- Zhang W., Wei W., Hu D., Zhu Y. & Wang X. J. Emission of speciated mercury from residential biomass fuel combustion in China. Energ. Fuels 27, 6792–6800 (2014). [Google Scholar]
- Zhan J. et al. Greenhouse gases and other airborne pollutants from household stoves in China: a database for emission factors. Atmos. Environ. 34, 4537–4549 (2000). [Google Scholar]
- Roden C. A., Bond T. C., Conway S. & Pinel A. B. O. Emission factors and realtime optical properties of particles emitted from traditional wood burning cookstoves. Environ. Sci. Technol. 40, 6750–6757 (2006). [DOI] [PubMed] [Google Scholar]
- Dhammapala R., Claiborn C., Simpson C. & Jimenez J. Emission factor from wheat and Kentucky bluegrass stubble burning: comparison of field and simulated burn experiments. Atmos. Environ. 41, 1512–1520 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information