Abstract
Aldosterone stimulates sodium transport in the renal collecting duct by activating the epithelial sodium channel (ENaC). To investigate the basis of this effect, we have developed a novel set of rabbit polyclonal antibodies to the 3 subunits of ENaC and have determined the abundance and distribution of ENaC subunits in the principal cells of the rat renal collecting duct. Elevated circulating aldosterone (due to either dietary NaCl restriction or aldosterone infusion) markedly increased the abundance of αENaC protein without increasing the abundance of the β and γ subunits. Thus, αENaC is selectively induced by aldosterone. In addition, immunofluorescence immunolocalization showed a striking redistribution in ENaC labeling to the apical region of the collecting duct principal cells. Finally, aldosterone induced a shift in molecular weight of γENaC from 85 kDa to 70 kDa, consistent with physiological proteolytic clipping of the extracellular loop as postulated previously. Thus, at the protein level, the response of ENaC to aldosterone stimulation is heterogenous, with both quantitative and qualitative changes that can explain observed increases in ENaC-mediated sodium transport.
Introduction
Renal regulation of sodium excretion is central to the control of blood pressure and extracellular fluid volume. Although sodium transport occurs throughout the length of the renal tubule, the fine regulation of sodium excretion occurs principally in the renal collecting duct. This regulation is mediated chiefly by the adrenal mineralocorticoid hormone, aldosterone, via effects on sodium channel activity. Physiological studies have demonstrated that these effects are heterogeneous, involving changes both in the number of functional sodium channels on the cell surface and in the open probability of individual channels (1). The molecular target for this regulation is the amiloride-sensitive epithelial sodium channel (ENaC), a heteromultimer consisting of α, β, and γ subunits (2, 3). Regulation of ENaC has been studied extensively in heterologous expression systems, but relatively little information exists regarding the regulation of ENaC subunit proteins in tissues where they are normally expressed. This situation is due in part to a lack of antibodies to the ENaC subunits that have sufficient specificity and sensitivity to allow routine immunoblotting of native tissues. Antibodies to the 3 ENaC subunits have been developed (4, 5), and although these antibodies have been useful for immunocytochemistry, they have not allowed facile immunoblotting of native tissues. Here, we have used computer-based techniques to design a novel set of peptide-directed rabbit polyclonal antibodies to the 3 subunits of rat ENaC, and have used these antibodies to provide the first information, to our knowledge, on the effects of aldosterone on ENaC subunit protein abundance and distribution in the principal cells of the renal collecting ducts of intact rats.
Methods
Antibodies.
To produce polyclonal antibodies against the α, β, and γ subunits of the rat ENaC, short peptide sequences were synthesized as follows, based on predicted amino acid sequences in rat (2, 3): αENaC (amino acids 46–68), NH2-LGKGDKREEQGLGPEPSAPRQPTC-COOH; βENaC (amino acids 617–638), NH2-CNYDSLRLQPLDTMESDSEVEAI-COOH; and γENaC (amino acids 629–650), NH2-CNTLRLDRAFSSQLTDTQLTNEL-COOH. The sequences were chosen for specificity, antigenicity, and absence of likely posttranslational modifications using computer analysis as described previously (6–8). Analysis using the BLAST computer program (National Library of Medicine, Bethesda, Maryland, USA) showed no significant overlap of the immunizing peptides with any known eukaryotic proteins, including the other ENaC subunits. The peptides were HPLC-purified, conjugated to keyhole limpet hemocyanin, and used for immunization of rabbits using standard immunization protocols. The resulting antisera were affinity-purified against the immunizing peptides as described previously (7, 8). We have previously characterized rabbit polyclonal antibodies to the other major apical sodium transporters expressed along the nephron, namely, the type 3 Na/H exchanger (NHE3) of the proximal tubule (9), the Na/K/2Cl cotransporter (BSC1/NKCC2) of the thick ascending limb (8), and the thiazide-sensitive Na/Cl cotransporter (TSC/NCC) of the distal convoluted tubule (7). In addition, we used a commercial rabbit polyclonal antibody to the α1 subunit of the Na/K/ATPase (Upstate Biotechnology Inc., Lake Placid, New York, USA). A polyclonal antibody raised to the COOH-terminal 15 amino acids of aquaporin-2 in chickens (LC54) was used for double labeling in immunofluorescence studies. This antibody labels collecting duct principal cells in a pattern identical to that of rabbit polyclonal antibodies described previously (J. Wade, unpublished study).
Animals and experimental protocols.
Experiments were conducted in male Sprague-Dawley rats (230–260 g) from Taconic Farms (Germantown, New York, USA).
Dietary NaCl restriction study.
NaCl-restricted rats received 0.2 mEq/200 g body weight per day of sodium for 10 days, and the control rats received 2.2 mEq/200 g body weight per day for 10 days, administered by ration feeding of gelled diets as described previously (7). The kidneys were harvested for semiquantitative immunoblotting and immunofluorescence immunocytochemistry as described previously (7). A final serum sample was collected for analysis of serum aldosterone concentration by RIA (Coat-a-Count; Diagnostic Products Corp., Los Angeles, California, USA).
Aldosterone infusion study.
Methoxyflurane (Metofane; Pitman-Moore, Mundelein, Illinois, USA) was used to anesthetize rats before subcutaneous implantation of osmotic minipumps (model 2ML2; ALZA Corp., Palo Alto, California, USA) that delivered 200 μg aldosterone per day (Sigma Chemical Co., St. Louis, Missouri, USA). Aldosterone was dissolved in DMSO and diluted with isotonic saline. Control rats received vehicle alone. Infusion of aldosterone or vehicle continued for 10 days. All rats received 1.0 mEq/200 g body weight per day of sodium. The kidneys were harvested for semiquantitative immunoblotting as described next.
Semiquantitative immunoblotting.
Semiquantitative immunoblotting was used to compare sodium transporter expression between groups of rats. The procedure has been described in detail previously (7, 10), and is summarized briefly as follows. The left kidneys were homogenized intact. The right kidneys were dissected to obtain cortex and inner stripe of outer medulla. These tissues were homogenized using a tissue homogenizer (Omni 1000, fitted with a microsawtooth generator) in ice-cold isolation solution containing 250 mM sucrose/10 mM triethanolamine (Calbiochem, La Jolla, California, USA) with 1 μg/mL leupeptin (Bachem California, Torrance, California, USA) and 0.1 mg/mL phenylmethyl sulfonyl fluoride (United States Biochemical Corp., Toledo, Ohio, USA). After measurement of total protein (BCA kit; Pierce Chemical Co., Rockford, Illinois, USA), the samples were solubilized at 60°C for 15 minutes in Laemmli sample buffer. SDS-PAGE was performed on 7.5%, 10%, or 12% polyacrylamide gels. An initial gel was stained with Coomassie blue as described previously to confirm equal loading among samples (10). For immunoblotting, the proteins were transferred electrophoretically from unstained gels to nitrocellulose membranes. After being blocked with 5 g/dL nonfat dry milk for 30 minutes, the blots were probed with the respective primary antibodies overnight at 4°C, followed by secondary antibody (donkey anti-rabbit IgG–conjugated with horseradish peroxidase [Pierce catalog no. 31458], diluted to 1:5,000) for 1 hour at room temperature. Sites of antibody-antigen reaction were viewed using enhanced chemiluminescence substrate (LumiGLO for Western blotting; catalog no. VC110; Kirkegaard and Perry Laboratories Inc., Gaithersburg, Maryland, USA) before exposure to x-ray film (catalog no. 165-1579; Eastman Kodak Co., Rochester, New York, USA). The band densities were quantitated by laser densitometry (model PDS1-P90; Molecular Dynamics, Sunnyvale, California, USA). To facilitate comparisons, we normalized the densitometry values, defining the mean for the control group as 100%. P < 0.05 was considered statistically significant (using unpaired t test or Welch t test).
Dot blotting.
Samples were applied directly onto the nitrocellulose membrane using a microfiltration apparatus (Bio-Dot microfiltration apparatus; Bio-Rad Laboratories Inc., Hercules, California, USA), and the resulting blots were processed as for SDS-PAGE–based immunoblots (as already described here).
Immunocytochemistry.
Rat kidneys were perfusion-fixed, and sections were prepared as described previously (7). A blocking agent for nonspecific binding sites (50 mL PBS, 0.5 g BSA, 0.188 g glycine; pH 7.2) was added to the samples for 20 minutes, followed by the primary antibody (10 μg/mL), for overnight incubation at 4°C. The sections were washed and incubated with species-specific fluorophor-conjugated secondary antibodies (1:100; Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA) for 2 hours at 4°C. Labeled sections were examined with a Zeiss LSM410 confocal microscope.
Results
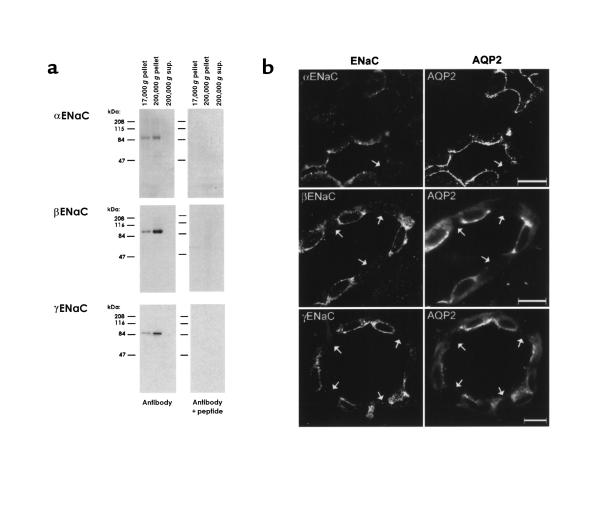
Figure 1 shows experiments done to characterize the antibodies to the 3 subunits of ENaC. Each antibody labeled a distinct, single band at approximately 83–90 kDa in membrane fractions from kidney cortex, but not in cytosol (Figure 1a). The bands were not present when identically loaded blots were probed with the peptide-preadsorbed antibodies. Figure 1b shows immunofluorescence labeling with the 3 ENaC subunit antibodies in rat renal cortex compared with labeling using an antibody to the collecting duct principal cell marker, aquaporin-2. As can be seen, the ENaC antibodies labeled only aquaporin-2–positive cells, i.e., principal cells. Intercalated cells (Figure 1, arrows) were unlabeled.
Figure 1.
Characterization of ENaC subunit antibodies. (a) Immunoblots of rat renal cortical proteins obtained by differential centrifugation. Blots were probed with the 3 ENaC subunit antibodies and with the antibodies preadsorbed with 1 mg of the respective immunizing peptides. Differential centrifugation was carried out as described (6), yielding membrane fractions (17,000 g and 200,000 g pellets) and a cytosolic fraction (200,000 g supernatant). (b) Immunofluorescence localization of ENaC subunits in rat renal cortical collecting duct. Three sections are shown, each double-labeled with 1 of 3 rabbit antibodies to ENaC subunits (left-hand panels) and a chicken anti–aquaporin-2 (right-hand panels). Arrows point to cells lacking aquaporin-2, i.e., intercalated cells. All tissue sections were from sodium-restricted rats. Scale bars: 10 μm.
To test the effect of aldosterone, we carried out experiments in rats using dietary NaCl restriction, which increases endogenous levels of aldosterone (Figure 2), and experiments using aldosterone infusion (Figure 3) as described next.
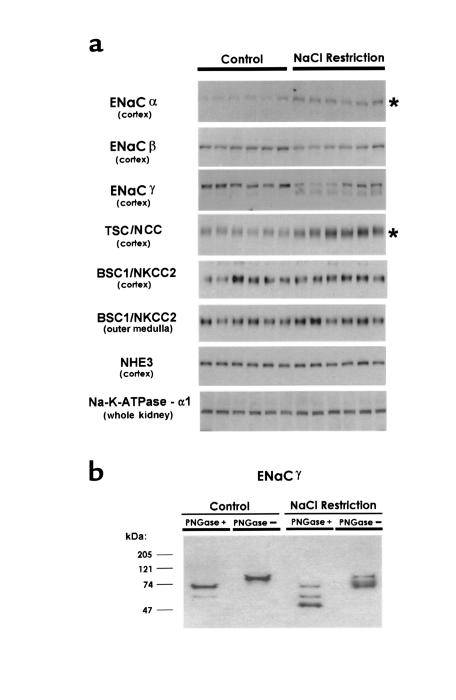
Figure 2.
Effect of dietary NaCl restriction for 10 days. (a) Semiquantitative immunoblots for ENaC subunits and other major renal sodium transporters. For each blot, each lane was loaded with a homogenate from a different rat (n = 6 for both control and sodium-restricted rats). Preliminary Coomassie-stained gels demonstrated equality of loading among lanes. Asterisks mark statistically significant increases. (b) Deglycosylation with PNGase. SDS-solubilized samples from control rats and NaCl-restricted rats were incubated with PNGase F (200 U/40 μg protein; New England Biolabs Inc., Beverly, Massachusetts, USA) or vehicle.
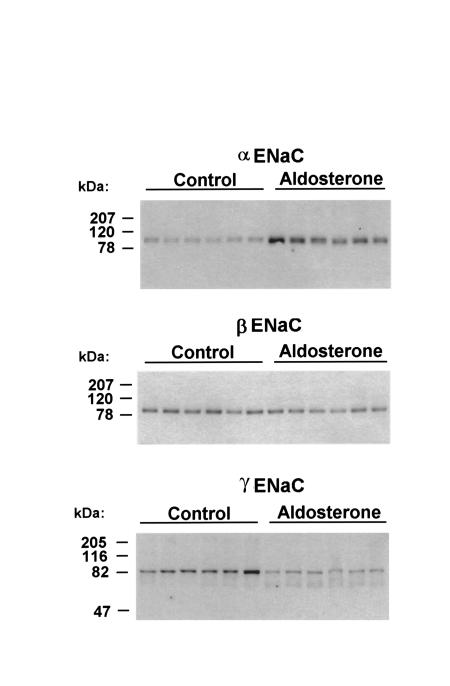
Figure 3.
Semiquantitative immunoblots showing effect of aldosterone infusion for 10 days on the abundance and molecular weight of each ENaC subunit in whole kidneys of rat. For each blot, each lane was loaded with 30 μg cortical homogenate from a different rat.
Dietary NaCl restriction.
Plasma aldosterone concentrations were significantly increased by NaCl restriction (3.5 ± 1.3 nM vs. 0.78 ± 0.32 nM in controls; P < 0.05; Figure 2). Figure 2a shows semiquantitative immunoblotting carried out for the 3 ENaC subunits and several other sodium transport proteins expressed along the nephron. The principal finding was a large increase in αENaC protein abundance in response to dietary NaCl restriction. Normalized band density increased to 285 ± 28% of control (P < 0.001). In contrast, no increase was seen in the abundance of the β and γ subunits. For the γ subunit, the NaCl restriction was associated with the appearance of a broad band centered around 70 kDa and with a reduction in the density of the 85-kDa band. To determine whether the total quantity of the γ subunit protein was altered with a low-salt diet, we carried out dot immunoblotting using the same samples. Dot densities did not differ significantly (normalized dot densities: NaCl restriction, 87 ± 21; control, 100 ± 15%; P = NS). Responses of the ENaC subunits in the outer medullas of the same rats were qualitatively the same as those seen in the cortex (results not shown). NaCl restriction also resulted in a large increase in the abundance of TSC/NCC (283 ± 35% of control; P < 0.005), as we have reported previously (7). There was little or no change in the abundance of BSC1/NKCC2 (thick ascending limb), NHE3 (proximal tubule), and the α1 subunit of the Na/K/ATPase (found in all renal tubule segments).
When samples from control and NaCl-restricted animals were deglycosylated with PNGase and immunoblotted using anti-γENaC (Figure 2b), the 85-kDa band shifted to 72 kDa, and the broad 70-kDa band was resolved into a distinct 52-kDa band and a weaker 60-kDa band.
Aldosterone infusion.
Plasma aldosterone concentrations were significantly increased in aldosterone-infused rats (80.4 ± 3.3 nM vs. 1.8 ± 0.5 nM in controls; P < 0.005; Figure 3). As with NaCl restriction, aldosterone infusion resulted in a large increase in αENaC protein abundance (normalized band density: 472 ± 84% of control; P < 0.01). Aldosterone infusion did not increase the abundance of the β and γ subunits. For the γ subunit, aldosterone infusion was associated with the appearance of a band at 70 kDa similar to the additional band seen with NaCl restriction. The responses of TSC/NCC, BSC1/NKCC2, NHE3, and the Na/K/ATPase were similar to those obtained with NaCl restriction (data not shown).
Cellular distribution of ENaC.
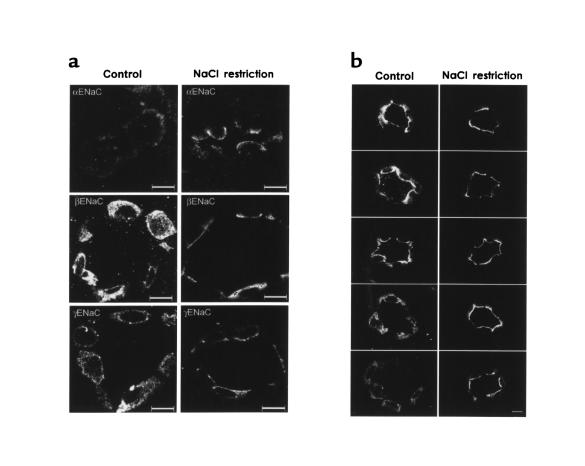
Figure 4 compares ENaC immunofluorescence labeling in collecting ducts from NaCl-restricted and control rats. These observations are typical of a large number of collecting ducts from 5 NaCl-restricted and 5 control rats. Figure 4a shows all 3 subunits of ENaC. Principal cells in control rats exhibited diffuse punctate labeling throughout the cell, consistent with labeling of intracellular vesicles. As can be seen, NaCl restriction was associated with a redistribution of the labeling to the apical regions of the principal cells. In addition, there was a marked increase in the strength of αENaC labeling in response to sodium restriction, confirming results from semiquantitative immunoblotting. Figure 4b shows several other examples of γENaC localization in control rats (left) and NaCl-restricted rats (right). Although there was variation in labeling among principal cells, there was a clear, consistent shift to the apical region in response to dietary NaCl restriction.
Figure 4.
Immunofluorescence localization of ENaC subunit proteins in control rats and rats undergoing dietary NaCl restriction. (a) Comparison of the 3 subunits. Left-hand panels are from rats on control diet; right-hand panels are from salt-restricted rats. Scale bars: 10 μm. (b) γENaC labeling in multiple rat renal cortical collecting ducts. Left-hand panels are from rats on control diet; right-hand panels are from salt-restricted rats. Scale bars: 10 μm.
Discussion
In this study, we have developed a novel set of polyclonal antibodies to the 3 individual subunits of ENaC, and have used these antibodies to identify 3 distinct effects of aldosterone on ENaC subunit proteins in the intact rat (as detailed later here).
Aldosterone produced a selective increase in the abundance of the α subunit.
Increases in circulating aldosterone induced by either NaCl restriction or aldosterone infusion were associated with a multifold increase in αENaC protein abundance. Thus, αENaC appears to be an aldosterone-induced protein. However, the increase in protein abundance does not necessarily imply increased transcription of the αENaC gene, but could be due to alterations in protein synthesis or degradation.
Whether activation of sodium transport by aldosterone is associated with an increase in ENaC gene expression in collecting duct cells has been controversial. Renard et al. (5) demonstrated no changes in renal mRNA levels for any of the 3 subunits in response to dietary sodium restriction. However, other investigators (11–14) found moderate increases in renal αENaC mRNA levels in response to increased circulating aldosterone concentrations, at least in some portions of the kidney. The present studies establish, both by immunoblotting and immunocytochemistry, that αENaC protein abundance in collecting duct principal cells is very low under control conditions and undergoes a multifold increase in response to aldosterone. In contrast, the abundance of the β and γ subunit proteins did not increase. Thus, the pattern of changes in ENaC subunit proteins matches reported changes for ENaC subunit mRNAs in some, but not all, studies.
Previously, May et al. (15) suggested that the synthesis of the α subunit is a limiting factor in the assembly of the ENaC complex. Based on this view, the regulation of the abundance of the α subunit would be expected to suffice in the regulation of the abundance of the mature ENaC complex. The increase in ENaC abundance presumably couples with the previously demonstrated (7) aldosterone-induced increase in the abundance of the thiazide-sensitive cotransporter (confirmed in this study), to account in large part for the sodium-retaining effect of aldosterone.
The abundance of αENaC protein could also be regulated through direct effects on protein synthesis. In cultured A6 renal epithelial cells, aldosterone increased the abundance of all 3 subunit mRNAs but had a selective effect to increase the rate of synthesis of α subunit protein as measured in pulse-chase experiments, suggesting regulation of translation (15).
Finally, the observed changes in αENaC protein abundance could also be due to an alteration of protein half-life. It has been proposed that ENaC half-life is regulated as a result of selective ubiquitination by a ubiquitin ligase, Nedd4, that binds to PY regions of the COOH-terminus of the individual ENaC subunit proteins (16). However, there is presently no evidence that this process is affected by aldosterone.
Aldosterone caused a redistribution of all 3 subunits to the apical region of the collecting duct principal cells.
In rats, patch-clamp studies of intact collecting ducts showed marked increases in sodium channel number in the apical plasma membrane in response to both dietary salt restriction and aldosterone administration (17). The present studies, which closely match the physiological conditions of the previous study, demonstrated marked redistribution of ENaC to the apical region of the collecting principal cells (Figure 4), suggesting that the increase in channel density was due to redistribution of ENaC to the plasma membrane. In Liddle’s syndrome, a clinical state that mimics hyperaldosteronism, hyperactivation of ENaC is associated with increased ENaC cell-surface expression (18) due to a decreased rate of endocytosis (19) and a loss of Nedd4 binding to truncated β and γ subunits (20). Taken together, these results suggest that redistribution of ENaC to the apical plasma membrane may play an important role in aldosterone-mediated sodium transport regulation in the mammalian collecting duct.
Aldosterone induces a shift in the molecular weight of the γ subunit from 85 kDa to 70 kDa.
Based on the magnitude of the molecular weight shift of the γ subunit seen in response to aldosterone, a likely explanation would be a physiological proteolytic cleavage. With deglycosylation, the apparent molecular weight of the unmodified protein was 72 kDa, very close to the expected molecular weight based on the open reading frame of the cloned γENaC cDNA (3). Thus, removal of a preexisting modifying group (e.g., de-ubiquitination) seems unlikely as the explanation for the aldosterone-induced molecular weight shift. Based on the size of the major fragment (52 kDa) recognized by our antibody to the COOH-terminus of γENaC, the putative proteolytic cleavage would have to occur in the early portion of the extracellular loop of the γ subunit, i.e., just beyond the first membrane-spanning region. Studies of ENaC expressed in Xenopus oocytes have demonstrated that serine proteases applied from the extracellular side activate the channel by increasing the open probability (21). Recently, Vallet et al. have identified a GPI-linked epithelial serine protease, CAP1, that activates ENaC and is induced by aldosterone (22), providing a possible explanation for the observed molecular weight shift of the γ subunit.
Conclusion.
The development of polyclonal antibodies to the 3 ENaC subunits has led to the identification of 3 effects of aldosterone in the renal collecting duct: selective increase in αENaC protein abundance, redistribution of ENaC to the apical region of collecting duct principal cells, and a reduction of the molecular weight of the γ subunit. The heterogeneity of the response at the level of ENaC protein subunits appears to be in accord with the complexity of the electrophysiological response to aldosterone in intact tissues (1). A major task for the future is to determine the detailed mechanisms involved in each of these identified actions of aldosterone and to learn how the demonstrated effects integrate to regulate sodium transport in the intact epithelium.
References
- 1.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 2.Canessa CM, Horisberger J-D, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- 3.Canessa CM, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 4.Duc C, Farman N, Canessa CM, Bonvalet J-P, Rossier BC. Cell-specific expression of epithelial sodium channel α, β, and γ subunits in aldosterone-responsive epithelia from the rat: localization by in situ hybridization and immunocytochemistry. J Cell Biol. 1994;127:1907–1921. doi: 10.1083/jcb.127.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renard S, Voilley N, Bassilana F, Lazdunski M, Barbry P. Localization and regulation by steroids of the α, β, and γ subunits of the amiloride-sensitive Na+ channel in colon, lung and kidney. Pflugers Arch. 1995;430:299–307. doi: 10.1007/BF00373903. [DOI] [PubMed] [Google Scholar]
- 6.Ecelbarger CA, et al. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol. 1995;269:F663–F672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- 7.Kim G-H, et al. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA. 1998;95:14552–14557. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim G-H, et al. Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle’s loop. Am J Physiol. 1999;276:F96–F103. doi: 10.1152/ajprenal.1999.276.1.F96. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Llama P, Andrews P, Ecelbarger CA, Nielsen S, Knepper MA. Concentrating defect in experimental nephrotic syndrome: altered expression of aquaporins and thick ascending limb Na+ transporters. Kidney Int. 1998;54:170–179. doi: 10.1046/j.1523-1755.1998.00984.x. [DOI] [PubMed] [Google Scholar]
- 10.Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rat. Am J Physiol. 1996;271:F414–F422. doi: 10.1152/ajprenal.1996.271.2.F414. [DOI] [PubMed] [Google Scholar]
- 11.Stokes JB, Sigmund RD. Regulation of rENaC mRNA by dietary NaCl and steroids: organ, tissue, and steroid heterogeneity. Am J Physiol. 1998;274:C1699–C1707. doi: 10.1152/ajpcell.1998.274.6.C1699. [DOI] [PubMed] [Google Scholar]
- 12.Ono S, Kusano E, Muto S, Ando Y, Asano Y. A low-Na+ diet enhances expression of mRNA for epithelial Na+ channel in rat renal inner medulla. Pflugers Arch. 1997;434:756–763. doi: 10.1007/s004240050462. [DOI] [PubMed] [Google Scholar]
- 13.Asher C, Wald H, Rossier BC, Garty H. Aldosterone-induced increase in the abundance of Na+ channel subunits. Am J Physiol. 1996;271:C605–C611. doi: 10.1152/ajpcell.1996.271.2.C605. [DOI] [PubMed] [Google Scholar]
- 14.Escoubet B, Coureau C, Bonvalet J-P, Farman N. Noncoordinate regulation of epithelial Na channel and Na pump subunit mRNAs in kidney and colon by aldosterone. Am J Physiol. 1997;272:C1482–C1491. doi: 10.1152/ajpcell.1997.272.5.C1482. [DOI] [PubMed] [Google Scholar]
- 15.May A, Puoti A, Gaeggeler HP, Horisberger JD, Rossier BC. Early effect of aldosterone on the rate of synthesis of the epithelial sodium channel alpha subunit in A6 renal cells. J Am Soc Nephrol. 1997;8:1813–1822. doi: 10.1681/ASN.V8121813. [DOI] [PubMed] [Google Scholar]
- 16.Staub O, et al. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J. 1997;16:6325–6336. doi: 10.1093/emboj/16.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol. 1994;104:693–710. doi: 10.1085/jgp.104.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firsov D, et al. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci USA. 1996;93:15370–15375. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimkets RA, Lifton RP, Canessa CM. The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem. 1997;272:25537–25541. doi: 10.1074/jbc.272.41.25537. [DOI] [PubMed] [Google Scholar]
- 20.Abriel H, et al. Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle’s syndrome. J Clin Invest. 1999;103:667–673. doi: 10.1172/JCI5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol. 1998;111:127–138. doi: 10.1085/jgp.111.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]