Abstract
Gd-EOB-DTPA is a hepatocyte-specific MRI contrast agent. Due to its hepatocyte-specific uptake and paramagnetic properties, functioning areas of the liver exhibit shortening of the T1 relaxation time. We report the potential use of T1 relaxometry of the liver with Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) for estimating the liver function as expressed by the MELD score. 3 T MRI relaxometry was performed before and 20 min after Gd-EOB-DTPA administration. A strong correlation between changes in the T1 relaxometry and the extent of liver disease, expressed by the MELD score, was documented. Reduced liver function correlates with decreased Gd-EOB-DTPA accumulation in the hepatocytes during the hepatobiliary phase. MRI-based T1 relaxometry with Gd-EOB-DTPA may be a useful method for assessing overall and segmental liver function.
The liver-specific magnetic resonance imaging (MRI) contrast agent gadolinium-ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA, Primovist®; Bayer Schering Pharma, Berlin, Germany) is preferentially used for detecting and characterizing focal hepatic lesions and for evaluating the biliary system1. After administration, Gd-EOB-DTPA is eliminated from the body in almost equal amounts via the following two pathways: organic anion transporting polypeptide (OATP)-mediated uptake in the hepatocytes with subsequent excretion into the biliary tract and glomerular filtration through the kidneys2,3. Hence, Gd-EOB-DTPA produces both dynamic perfusion and liver-specific hepatobiliary MR images4.
In animal models, there is an inverse correlation between experimentally induced hepatic dysfunction and Gd-EOB-DTPA enhancement of the liver parenchyma5,6. Moreover, the reduced signal intensity after Gd-EOB-DTPA administration has been linked to down-regulated membrane transporter expression in cirrhotic livers7.
Hepatic parenchymal enhancement in patients with impaired hepatocyte function decreases during the hepatobiliary phase (HP)8,9. Few studies have investigated the potential clinical factors that influence the liver parenchyma signal intensity on Gd-EOB-DTPA-enhanced MR images10,11,12,13. However, in addition to the measurement of relative signal intensities of the liver enhancement effects, MR relaxometry has recently received increased attention as a tool for quantitative analyses.
Because of the hepatocellular uptake of Gd-EOB-DTPA and its paramagnetic properties, functioning areas of the liver show T1 shortening in the longitudinal relaxation times14. Based on these T1 shortening effects, the quantitative evaluation of hepatocellular Gd-EOB-DTPA uptake allows for the direct measurement of liver function15,16. Therefore, we determined the T1 relaxation times before and 20 min after contrast medium administration in patients with and without diffuse liver disease.
The purpose of this study was to determine whether post-Gd-EOB-DTPA T1 relaxometry at 3 T is affected by the degree of liver disease, as expressed by the model for end-stage liver disease (MELD) score, a commonly applied scoring system for liver function.
Results
No significant difference in the age distribution was found between the patient group with normal liver function and the patient group with liver disease. The patient characteristics are summarized in Table 1.
Table 1. Patient characteristics.
| Group | All (n = 233) | MELD ≤ 10(n = 176) | MELD 11–18 (n = 46) | MELD > 18 (n = 11) | P-value |
|---|---|---|---|---|---|
| Age (years) | 59.5 ± 12.9 | 59.8 ± 13.0 | 60.3 ± 13.6 | 58.7 ± 8.2 | p = 0.93 |
| Gender | |||||
| men | 158 (68%) | 110 (63%) | 40 (87%) | 8 (73%) | p = 0.006 |
| women | 75 (32%) | 66 (38%) | 6 (13%) | 3 (27%) | |
| Height (m) | 1.72 ± 0.09 | 1.72 ± 0.09 | 1.75 ± 0.07 | 1.73 ± 0.09 | p = 0.044 |
| Weight (kg) | 82.7 ± 19.1 | 81.6 ± 19.1 | 87.6 ± 18.1 | 81.6 ± 21.0 | p = 0.16 |
Values indicate the mean ± standard deviation.
MELD (model for end-stage liver disease) score.
The mean T1 relaxation times of non-enhanced MRI (762.7 ms ± 149.0 ms) and Gd-EOB-DTPA-enhanced MRI (340.0 ms ± 118.8 ms) were significantly different (p < 0.001) (all patients). The pre-contrast mean T1 relaxation times were not significantly different between the groups with MELD ≤ 10 (760.7 ms ± 145.3 ms), MELD 11–18 (760.4 ms ± 152.6 ms), and MELD > 18 (803.2 ± ms ± 196.0 ms) (MELD ≤ 10 to MELD 11–18, p = 1.0, 95% CI [−58.01 to 58.66]; MELD ≤ 10 to MELD > 18, p = 0.632, 95% CI [−151.92 to 67.04], MELD 11–18 to MELD > 18, p = 0.670, 95% CI [−160.99 to 75.46]).
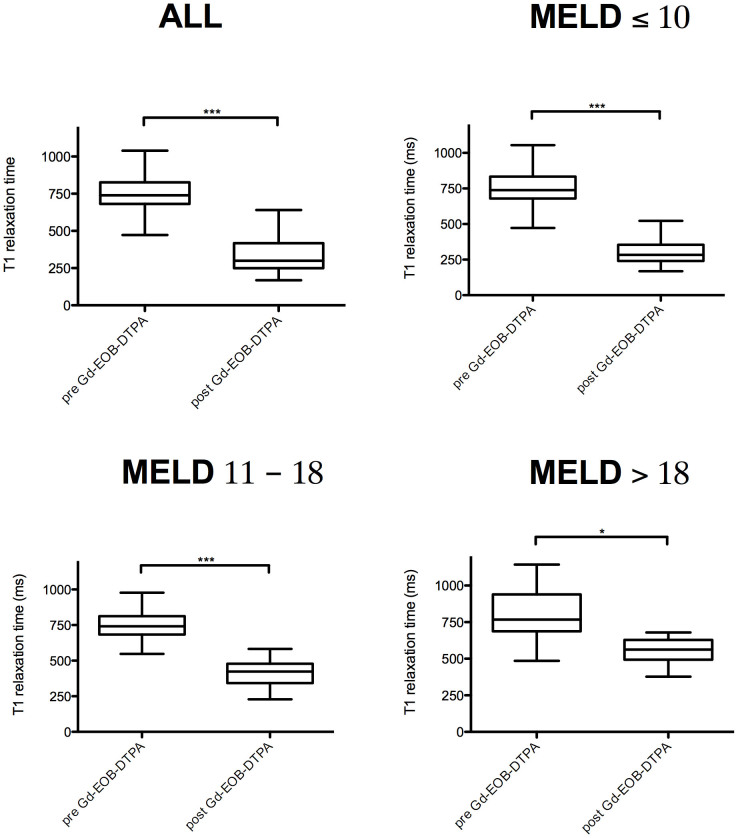
After Gd-EOB-DTPA administration, the T1 relaxation times decreased significantly in the respective groups (p < 0.001). Comparison of the patient group with MELD ≤ 10 and the patient groups with impaired liver function (MELD 11–18 and MELD > 18) showed significant increases in the mean T1 relaxation times with increasing liver damage (MELD ≤ 10 to MELD 11–18, p < 0.001, 95% CI [−156.65 to −78.91]; MELD ≤ 10 to MELD > 18, p < 0.001, 95% CI [−313.69 to −167.78], MELD 11–18 to MELD > 18, p = 0.001, 95% CI [−201.73 to −44.17]). The shortening of the T1 relaxation times was significantly lower for patients with higher MELD scores (Table 2, Figure 1).
Table 2. T1 relaxation times and reduction rates of the liver in non-enhanced MRI and Gd-EOB-DTPA-enhanced MRI.
| All (n = 233) | MELD ≤ 10 (n = 176) | MELD 11–18 (n = 46) | MELD > 18 (n = 11) | |
|---|---|---|---|---|
| T1pre (ms) | 762.7 ± 149.0 | 760.7 ± 145.3 | 760.4 ± 152.6 | 803.2 ± 196.0 |
| T1post (ms) | 340.0 ± 118.8 | 305.4 ± 92.4 | 423.1 ± 124.2 | 546.1 ± 95.4 |
| Reduction rate (%) | 55 ± 14 | 59 ± 11 | 44 ± 15 | 30 ± 11 |
Values indicate the mean ± standard deviation.
MELD (model for end-stage liver disease) score.
T1pre, T1 relaxation time before Gd-EOB-DTPA administration.
T1post, T1 relaxation time 20 min after Gd-EOB-DTPA administration.
Figure 1. Pre- and post-contrast T1 relaxation times.
Boxplots indicating the T1 relaxation times before (pre) and after (post) Gd-EOB-DTPA administration in patients with normal liver function (MELD score ≤ 10) and patients with impaired liver function (MELD score 11–18 and MELD score > 18). After Gd-EOB-DTPA administration, the reduction in the T1 relaxation times was highly significant (p ≤ 0.001) in patients with MELD scores of ≤10 and 11–18 and significant (p ≤ 0.05) for patients with severely impaired liver function (MELD > 18). MELD (model for end-stage liver disease) score. Data are given as the mean T1 relaxation times ± standard deviation. Tukey-adjusted post-hoc pairwise comparisons were used to compare the groups. ***p ≤ 0.001, *≤0.05.
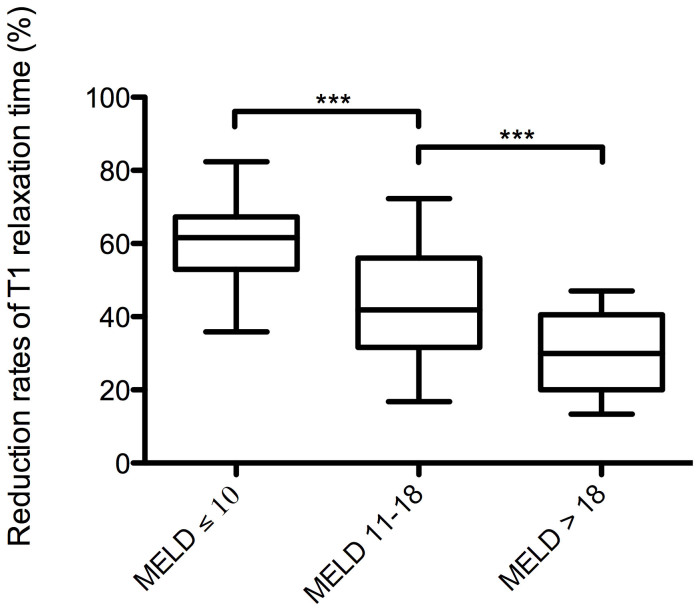
Twenty minutes after Gd-EOB-DTPA administration, we found a constant, significant decrease in the reduction rates of the T1 relaxation times. The reduction rates of the T1 relaxation times were significantly higher for patients with MELD ≤ 10 (59% ± 11%) than for patients with MELD 11–18 (44% ± 15%) and with MELD > 18 (30% ± 11%) (MELD ≤ 10 to MELD 11–18, p < 0.001, 95% CI [0.11 to 0.20]; MELD ≤ 10 to MELD > 18, p < 0.001, 95% CI [0.20 to 0.38]; MELD 11–18 to MELD > 18; p < 0.001, 95% CI [0.04 to 0.22]) (Table 2, Figure 2). Examples of the pre- and post-contrast T1 maps for all groups were color-coded and are shown in Figure 3.
Figure 2. Reduction rates for the T1 relaxation times.
Boxplots of the reduction rates for the T1 relaxation times of the liver in patients with normal liver function, MELD score ≤ 10, and patients with impaired liver function, MELD score 11–18 and MELD score > 18. Reduction rates were significantly reduced with an increasing degree of liver damage. The reduction rate (%) was calculated as follows: ([T1pre − T1post]/T1pre) × 100; here, T1pre is the T1 relaxation time before Gd-EOB-DTPA administration, and T1post is the T1 relaxation time after Gd-EOB-DTPA administration. MELD (model for end-stage liver disease) score. Data are given as the mean T1 reduction rate ± standard deviation. Tukey-adjusted post-hoc pairwise comparisons were used to compare the groups. ***p ≤ 0.001.
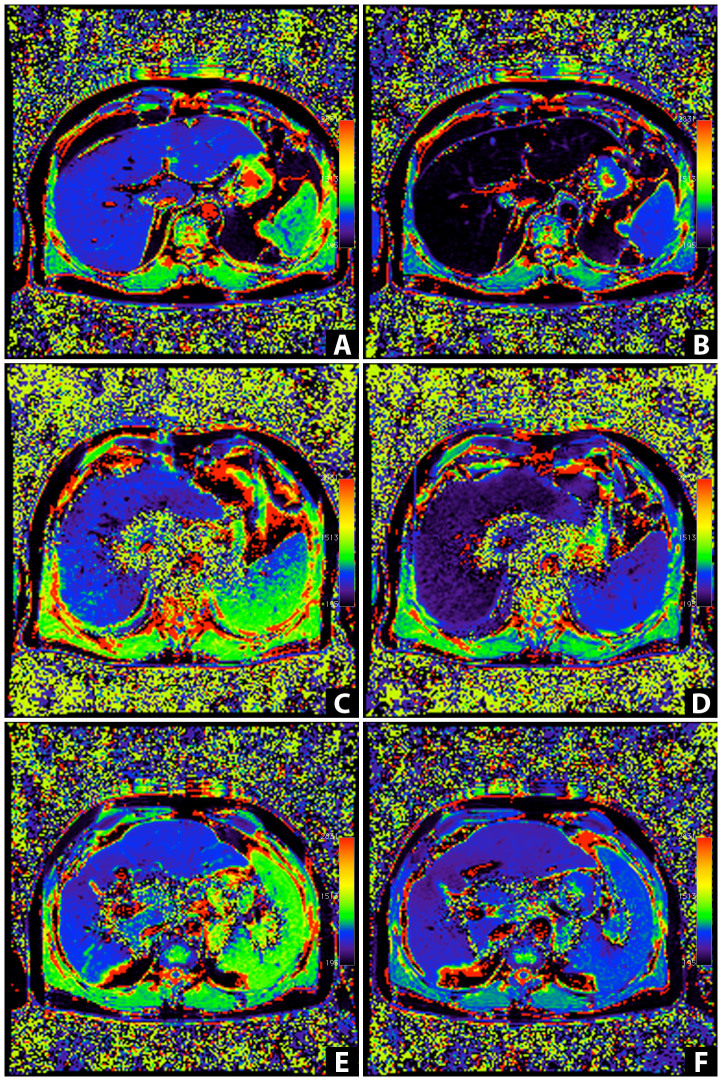
Figure 3. T1 relaxation time color-coded T1 maps.
(A–F) T1 maps calculated from two TurboFlash sequences (TI = 400 ms, 1000 ms) were obtained before (A, C, E) and 20 minutes after Gd-EOB-DTPA administration (B, D, F) in a patient with a MELD score of 6 (man, 44 years of age, height: 1.86 m, weight: 99 kg; A, B), a patient with a MELD score of 14 (man, 71 years of age, height: 1.75 m, weight: 91 kg; C, D), and a patient with a MELD score of 20 (man, 50 years of age, height: 1.80 m, weight: 92 kg; E, F). The mean T1 relaxation times of liver parenchyma were as follows: 751.4 ms (A), 216.0 ms (B), 718.7 ms (C), 321.8 ms (D), 785.4 ms (E), and 628.3.0 ms (F). The reduction rates of the T1 relaxation time were 71% (A, B), 41% (C, D), and 20% (E, F). In the control group (MELD = 6), the T1 relaxation time on the T1 mapping color-coded maps of the liver on post-contrast MRI (B) showed a markedly darker color distribution of the liver parenchyma than that on the pre-contrast mapping image (A), indicating strong Gd-EOB-DTPA-induced shortening of the T1 relaxation time 20 min after contrast medium administration. With an increasing degree of liver damage, the color distribution in liver parenchyma was visually confirmed to show a smaller change on the post-contrast T1 maps.
Using receiver-operating characteristics analysis, we determined the cut-off values and area under the curve (AUC) values for differentiating patients with MELD ≤ 10 from patients with impaired liver function and for differentiating between different grades of liver function based on the reduction rate. The cut-off values of the post-T1 relaxation times for distinguishing patients with MELD ≤ 10 from patients with MELD 11–18 and MELD > 18 were 341.0 ms and 419.5 ms, respectively; the corresponding cut-off value for distinguishing between patients with MELD 11–18 and those with MELD > 18 was 488.4 ms. The cut-off values for the reduction rate of the T1 relaxation times to differentiate patients with MELD ≤ 10 from the MELD 11–18 and MELD > 18 patient groups were 50.0% and 47.3%, respectively; the corresponding cut-off value for differentiating between the MELD 11–18 patient group and the MELD > 18 patient group was 35.6%. Table 3 summarizes the respective cut-off values for the T1 relaxation times and reduction rates as well as the corresponding AUC values, sensitivities, and specificities.
Table 3. ROC analysis indicating the various cut-off values and diagnostic performance for differentiating the control group (MELD ≤ 10) from patients with impaired liver function (MELD 11–18 and MELD > 18).
| Cut-offs T1post (ms) | AUC T1post (sensitivity, specificity) | Cut-off reduction rate (%) | AUC reduction rate (sensitivity, specificity) | |
|---|---|---|---|---|
| MELD ≤ 10 to MELD 11–18 | 341.0 | 0.80 (78%, 73%) | 50.0% | 0.79 (72%, 81%) |
| MELD ≤ 10 to MELD > 18 | 419.5 | 0.95 (91%, 89%) | 47.3% | 0.97 (100%, 85%) |
| MELD 11–18 to MELD > 18 | 488.4 | 0.81 (82%, 83%) | 35.6% | 0.75 (73%, 67%) |
Values indicate the mean ± standard deviation.
MELD (model for end-stage liver disease) score.
AUC (area under the receiver operating characteristic curve).
T1post, T1 relaxation time 20 min after Gd-EOB-DTPA administration.
Discussion
In this study, we compared the quantitative characterization of the T1 relaxation time changes in unenhanced and Gd-EOB-DTPA-enhanced 3 T MRI with the MELD score, which is a commonly applied score for liver function. The T1 relaxation time is a tissue parameter that depends on the tissue's physical, chemical, and biological characteristics. The results of our study show that the T1 relaxation time of the Gd-EOB-DTPA-enhanced liver parenchyma is a suitable method for distinguishing between healthy people and patients with liver disease.
Several scoring systems (Child-Pugh, MELD, or MELD-Na scores; ICG test) are widely accepted for assessing hepatic dysfunction17,18,19. The MELD score incorporates objective parameters, such as the creatinine and bilirubin levels, the pro-thrombin time, and international normalized ratio (INR). The MELD score is therefore not dependent on subjective clinical parameters such as hepatic encephalopathy. This condition — in addition to the albumin and bilirubin levels, INR, and ascites — defines the Child-Pugh score, creating problems due to its clinical parameter subjectivity and restricted discriminatory properties20,21,22. The MELD score has primarily been used for allocating organs for liver transplantation. Subsequently, this score has been validated as an accurate predictor of both the short-term and medium-term survival of patients with any type of liver disease, especially patients with fulminant hepatic failure, alcoholic hepatitis, hepatocellular carcinoma, liver infection, liver cirrhosis, or other chronic liver disease23,24.
Several studies have reported the evaluation of hepatic function based on the direct measurement of biliary enhancement25,26,27. The time and degree of Gd-EOB-DTPA biliary enhancement are related to hepatic function; the biliary enhancement is significantly weaker and delayed in patients with liver disease. Still, the most common approach for assessing hepatic function on Gd-EOB-DTPA-enhanced MRI is the direct or corrected measurement of hepatic parenchymal signal intensity, which is reduced in patients with hepatic dysfunction18,28,29,30. Based on a routine clinical imaging protocol, these approaches are simple, easy to implement in clinical practice and do not require additional MR sequences, mathematical modeling or sophisticated analysis of MR signal characteristics. However, the assessment of biliary enhancement is strongly influenced by biliary fluid dynamics, and signal intensity measurements reflect relative values depending on many technical parameters, such as the potency of the radiofrequency amplifiers, the receiver coil and the pulse sequence designed by different MRI system manufacturers19. Additionally, the signal intensity and gadolinium concentration do not have a linear pattern; therefore, the signal intensity measurements may not directly correlate with the gadolinium concentration31.
We used T1 relaxometry, indicating the absolute values of the T1 relaxation times, for the quantitative comparison of the pre-contrast and post-contrast enhancement of the hepatic parenchyma, which is not affected by those limitations.
In our study, the T1 relaxation times for non-enhanced MRI did not show any significant differences between the patients with MELD ≤ 10, indicating no liver disease, those with MELD 11–18, indicating moderate liver disease, and the group with MELD > 18, indicating severe liver disease32. However, the absolute T1 relaxation times of patients with severe liver disease tended to be higher than for other MELD score groups, although this effect was not significant (MELD ≤ 10: 760.7 ms, MELD 11–18: 760.4 ms; and MELD > 18: 803.2 ms). These results were in accordance with other published data on liver fibrosis and cirrhosis in non-enhanced MR imaging19,33,34. Increasing values for the T1 relaxation times have been found in patients with impaired liver function. These increases are caused by the pathophysiological processes of induced liver fibrogenesis, which is characterized by inflammation, edema, and synthesis of the extracellular matrix. This increase may be a result of the augmented hepatic water content that is caused by edema and the synthesis of collagen fibers, in which protons are less abundant and tightly bound. An additional reason may be the increase in the ratio of the free to bound water and the structure of intracellular water35,36. However, in the clinical situation, liver cirrhosis is a chronic condition that is not commonly combined with edema and acute inflammation. In contrast, other previous studies did not observe a correlation between liver disease and the T1 relaxation times, and some showed a decrease in the unenhanced T1 relaxation time with an increasing degree of cirrhosis37,38,39 These controversial findings indicate that non-enhanced T1 relaxometry is unlikely to be an appropriate method for detecting and staging liver disease.
Twenty minutes after Gd-EOB-DTPA administration, the mean T1 relaxation times of the patients in the control group were significantly lower than those of the patients with liver disease. Our results support the conclusion that the post-contrast T1 relaxation times may facilitate the differentiation between liver disease and normal liver as well as help with the evaluation of the degree of liver function. However, pre-contrast T1 relaxation times may modify the shortened T1 relaxation times after Gd-EOB-DTPA administration for patients with MELD ≤ 10 and the prolonged post-contrast T1 relaxation times for patients with liver disease. Therefore, the reduction rate in the T1 relaxation times was calculated to determine the uptake of Gd-EOB-DTPA in the liver parenchyma, and there was a significantly lower reduction rate in the T1 relaxation times for patients with liver disease (MELD 11–18, 44%; MELD > 18, 30%) than for patients with normal liver function (MELD ≤ 10, 59%).
We performed ROC analysis to estimate the cut-off values for differentiating healthy patients from patients with impaired liver function. The cut-off value for the reduction rate to distinguish patients with normal liver function from patients with moderately impaired liver function (MELD 11–18) was 50.0%. Therefore, the shortening (by half) of the T1 relaxation time after Gd-EOB-DTPA administration compared with the pre-contrast T1 relaxation time could serve as a possible point of reference for impaired liver function. The assessment of liver function plays a crucial role in many clinical settings. Most of the available liver tests provide an overall evaluation of global liver function. However, the assessment of regional or remnant hepatic function is important in both presurgical evaluations, before segmental liver resection, to avoid postoperative liver failure and in monitoring liver diseases that affect the liver in a non-homogeneous way40.
Our study has some limitations. For example, we compared the hepatic uptake of Gd-EOB-DTPA with only the MELD score. We did not use any other test, such as the ICG test, for evaluating liver function. ROI placement may also be an alternative method because of the potentially heterogeneous distribution of the parenchymal changes and the limited patient-to-patient reproducibility. However, averaging three repetitive measurements across a large area of the liver parenchyma should yield representative values. The lack of histopathology is another potential limitation because of the presence of early parenchymal changes, such as steatosis. Moreover, T1 relaxation has been shown to strongly depend on the strength of the magnetic field41; T1 relaxation times are reportedly longer and T1 shortening effects of contrast material are more prominent at 3 T than at 1.5 T. Compared with the direct hepatic signal intensity method, the MR relaxometry method requires additional scan sequences and dedicated software to calculate the relaxation times. However, the additional use of our liver MRI protocol to acquire pre- and post-contrast T1 relaxation times is limited to 32 s.
In conclusion, because T1 relaxometry for pre- and post-Gd-EOB-DTPA MRI is a useful parameter for indicating the degree of liver disease, it may have the potential to become a novel tool for monitoring liver function.
Methods
Patients
We obtained the approval of an institutional review board for this prospective study and written informed consent from all study participants before enrollment. Between June 2012 and August 2013, 302 consecutive patients underwent Gd-EOB-DTPA-enhanced MR imaging of the liver, including T1 mapping, for the presence of a suspicious liver lesion detected during previous computed tomographic or ultrasonographic examinations, for suspected chronic liver disease, or as part of a scheduled follow-up examination for known liver disease. Patients were excluded if they had previously received local treatment for liver disease (n = 29) or if they were unable to complete the entire MR imaging examination (n = 40). A total of 233 patients (158 men; 75 women; mean age, 59.9 ± 13.0 years) were included in this study. The severity of liver disease was classified according to the MELD score, an internationally accepted scoring system for assessing the severity of chronic liver disease. The MELD score was calculated with a standard formula that adds multiples of the natural logarithm (ln) to the values for the international ratio of coagulation (INR), creatinine, and bilirubin as follows: 11.2 × ln(INR) + 9.57 × ln(creatinine, in milligrams per deciliter) + 3.78 × ln(bilirubin, in milligrams per deciliter) + 6.43 (an intercept). The lower limit of all variables was set to 1, and creatinine was capped at 4 if patients received renal replacement therapy20.
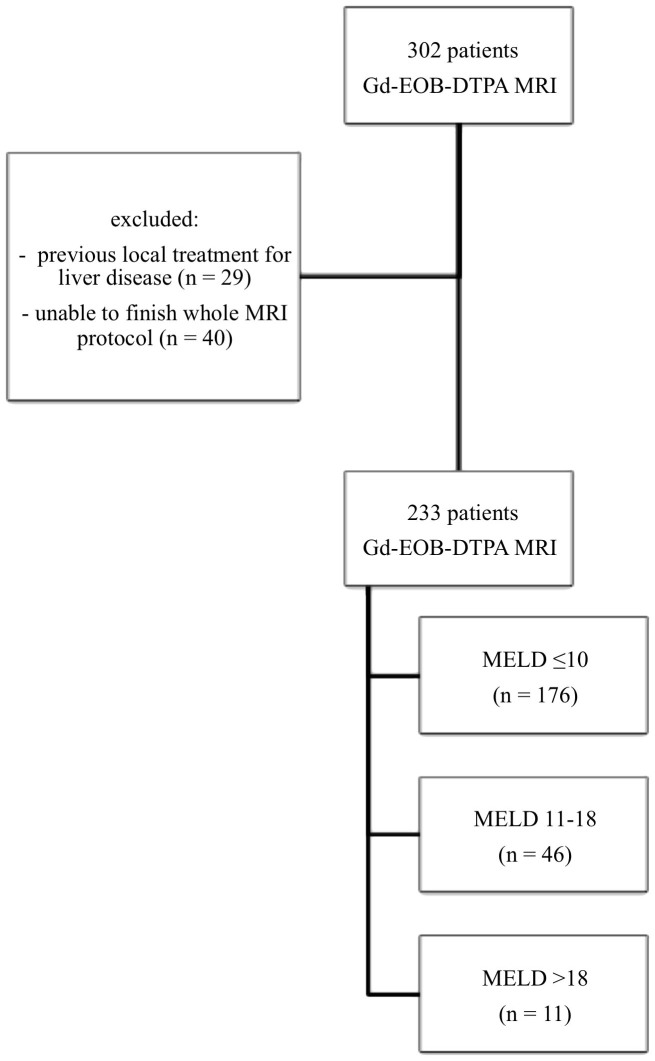
Thus, patients were classified as follows: patients with a MELD score ≤ 10 (MELD ≤ 10, n = 176), representing a healthy population; patients with a MELD score of 11 to 18 (MELD 11–18, n = 46), indicating moderate liver disease; and patients with a MELD score > 18 (MELD > 18, n = 11), indicating severe liver disease32. The parameters used to calculate the MELD score were acquired within 5 days before or after the MRI examination. The patient flowchart is shown in figure 4.
Figure 4. Flowchart of the included patients.
Three hundred two patients underwent Gd-EOB-DTPA-enhanced MRI. Twenty-nine patients were excluded because of previous local liver treatment, and 40 patients were unable to finish the entire MRI protocol. In total, 233 patients were included, with 176 having normal liver function and a MELD score ≤ 10 and 57 patients having impaired liver function (MELD 11–18, n = 46; MELD > 18, n = 11).
MR imaging protocol
All imaging procedures were conducted with a clinical, whole body 3 T system (Magnetom Skyra, Siemens Healthcare, Erlangen, Germany) using a combination of body and spine array coil elements (18-channel body matrix coil, 24-channel spine matrix coil) for signal reception. We obtained two TurboFLASH sequences (inversion time [TI], 400 ms and 1000 ms; repetition time [TR], 4000 ms; echo time [TE], 1.16 ms; flip angle 8°; slice thickness, 6 mm; FOV 400 mm × 400 mm; matrix size, 192 × 192) in a single breath hold. We acquired T1 maps of the porta hepatis before and 20 min after Gd-EOB-DTPA injection in addition to the routine Gd-EOB-DTPA MRI protocol; the peak of liver signal intensity has been shown to occur 20 min after Gd-EOB-DTPA administration2,42,43,44. The liver-specific contrast agent (Primovist; Bayer Schering Pharma AG, Berlin, Germany) was injected, body weight-adapted (0.025 mmol/kg body weight), via an intravenous bolus injection with a flow rate of 1 mL/s and flushed with 20 mL of NaCl.
Image analysis
Dedicated software was used to generate the parametric T1 maps (Siemens Healthcare, Erlangen, Germany) based on the acquired TurboFLASH sequences before and 20 min after Gd-EOB-DTPA administration.
To assess the T1 relaxation times of the liver, we used the region of interest (ROI) measurement in the T1 mapping images before and after Gd-EOB-DTPA administration. Two ROIs were placed in the right lobe, and one in the left lobe. Special care was taken to avoid focal hepatic lesions (e.g., cysts and hemangiomas) and major branches of the portal or hepatic veins and to avoid imaging artifacts. The mean T1 value for the 3 ROIs was considered the representative T1 value for the liver. ROIs of identical size and shape were placed at the same imaging sequence in T1 maps before and after Gd-EOB-DTPA administration.
We calculated the shortening of the T1 relaxation times for each group, indicating the absolute change in the T1 values between pre- and post-Gd-EOB-DTPA enhancement:
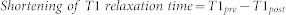 |
Additionally, the reduction rate, indicating the relative change in the T1 values between the pre- and post-Gd-EOB-DTPA enhancement, was calculated as follows:
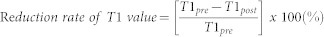 |
Here, T1pre is the T1 relaxation time before Gd-EOB-DTPA administration, and T1post is the T1 relaxation time after Gd-EOB-DTPA administration. We used the visualization tool of the open source OsiriX imaging software (OsiriX v.5.5) to generate color-coded maps.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). We used a one-way analysis of variance (ANOVA) to analyze the differences between patients with normal liver function and two patient groups with progressively impaired liver function. Post hoc pair-wise comparisons were made with the Tukey procedure. We used ROC analyses to differentiate between patient groups and estimated the optimal cut-off according to the Youden Index. Estimates for the area under the curve (AUC) and the true classification rates are reported. All tests were two-sided, and values of p < 0.05 indicate significant difference. All statistical analyses were performed with IBM SPSS Statistics (version 21, Chicago, IL) and R 3.0.1.
Author Contributions
M.H. performed the literature search, interpreted the data and drafted the manuscript. N.V. and A.G.S. participated in the study design, collected the data and edited the manuscript. C.F. and P.W. helped with the data acquisition, literature search and interpretation of the data. F.Z. performed the statistical analysis and interpreted the data. A.T. and S.F. revised the manuscript critically for important intellectual content and made substantial contributions to the data analysis. C.S. participated in the study design and coordination and helped draft the manuscript. All authors read and approved the final manuscript.
References
- Hammerstingl R. et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol 18, 457–467 (2008). [DOI] [PubMed] [Google Scholar]
- Hamm B. et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology 195, 785–792 (1995). [DOI] [PubMed] [Google Scholar]
- Leonhardt M. et al. Hepatic uptake of the magnetic resonance imaging contrast agent Gd-EOB-DTPA: role of human organic anion transporters. Drug Metab Dispos 38, 1024–1028 (2010). [DOI] [PubMed] [Google Scholar]
- Ahn S. S. et al. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology 255, 459–466 (2010). [DOI] [PubMed] [Google Scholar]
- Ryeom H. K. et al. Quantitative evaluation of liver function with MRI Using Gd-EOB-DTPA. Korean J Radiol 5, 231–239 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. et al. Experimental hepatic dysfunction: evaluation by MRI with Gd-EOB-DTPA. J Magn Reson Imaging 7, 683–688 (1997). [DOI] [PubMed] [Google Scholar]
- Tsuda N., Harada K. & Matsui O. Effect of change in transporter expression on gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging during hepatocarcinogenesis in rats. J Gastroenterol Hepatol 26, 568–576 (2011). [DOI] [PubMed] [Google Scholar]
- Tamada T. et al. Gd-EOB-DTPA-enhanced MR imaging: evaluation of hepatic enhancement effects in normal and cirrhotic livers. Eur J Radiol 80, e311–316 (2011). [DOI] [PubMed] [Google Scholar]
- Verloh N. et al. Impact of liver cirrhosis on liver enhancement at Gd-EOB-DTPA enhanced MRI at 3 Tesla. Eur J Radiol 82, 1710–1715 (2013). [DOI] [PubMed] [Google Scholar]
- Higaki A. et al. Potential clinical factors affecting hepatobiliary enhancement at Gd-EOB-DTPA-enhanced MR imaging. Magn Reson Imaging (2012). [DOI] [PubMed] [Google Scholar]
- Motosugi U. et al. Liver parenchymal enhancement of hepatocyte-phase images in Gd-EOB-DTPA-enhanced MR imaging: which biological markers of the liver function affect the enhancement? J Magn Reson Imaging 30, 1042–1046 (2009). [DOI] [PubMed] [Google Scholar]
- Kubota K. et al. Correlation of liver parenchymal gadolinium-ethoxybenzyl diethylenetriaminepentaacetic acid enhancement and liver function in humans with hepatocellular carcinoma. Oncol Lett 3, 990–994 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima T. et al. Relationship between liver function and liver signal intensity in hepatobiliary phase of gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. J Comput Assist Tomogr 34, 362–366 (2010). [DOI] [PubMed] [Google Scholar]
- Seale M. K., Catalano O. A., Saini S., Hahn P. F. & Sahani D. V. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics 29, 1725–1748 (2009). [DOI] [PubMed] [Google Scholar]
- Tsuda N., Okada M. & Murakami T. Potential of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) for differential diagnosis of nonalcoholic steatohepatitis and fatty liver in rats using magnetic resonance imaging. Invest Radiol 42, 242–247 (2007). [DOI] [PubMed] [Google Scholar]
- Shimizu J. et al. Evaluation of regional liver function by gadolinium-EOB-DTPA-enhanced MR imaging. Dig Dis Sci 44, 1330–1337 (1999). [DOI] [PubMed] [Google Scholar]
- Haimerl M. et al. Assessment of clinical signs of liver cirrhosis using T1 mapping on Gd-EOB-DTPA-enhanced 3T MRI. PLoS One 8, e85658 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A. et al. Quantitative evaluation of liver function with use of gadoxetate disodium-enhanced MR imaging. Radiology 260, 727–733 (2011). [DOI] [PubMed] [Google Scholar]
- Katsube T. et al. Estimation of liver function using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Invest Radiol 46, 277–283 (2011). [DOI] [PubMed] [Google Scholar]
- Kim W. R. et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 359, 1018–1026 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath P. S. & Kim W. R. The model for end-stage liver disease (MELD). Hepatology 45, 797–805 (2007). [DOI] [PubMed] [Google Scholar]
- Wiesner R., Lake J. R., Freeman R. B. & Gish R. G. Model for end-stage liver disease (MELD) exception guidelines. Liver Transpl 12, S85–87 (2006). [DOI] [PubMed] [Google Scholar]
- Kremers W. K. et al. MELD score as a predictor of pretransplant and posttransplant survival in OPTN/UNOS status 1 patients. Hepatology 39, 764–769 (2004). [DOI] [PubMed] [Google Scholar]
- Botta F. et al. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut 52, 134–139 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada T. et al. Gd-EOB-DTPA enhanced MR imaging: evaluation of biliary and renal excretion in normal and cirrhotic livers. Eur J Radiol 80, e207–211 (2011). [DOI] [PubMed] [Google Scholar]
- Tschirch F. T. et al. Contrast-enhanced MR cholangiography with Gd-EOB-DTPA in patients with liver cirrhosis: visualization of the biliary ducts in comparison with patients with normal liver parenchyma. Eur Radiol 18, 1577–1586 (2008). [DOI] [PubMed] [Google Scholar]
- Wibmer A. et al. Liver transplantation: impaired biliary excretion of gadoxate is associated with an inferior 1-year retransplantation-free survival. Invest Radiol 47, 353–358 (2012). [DOI] [PubMed] [Google Scholar]
- Watanabe H. et al. Staging hepatic fibrosis: comparison of gadoxetate disodium-enhanced and diffusion-weighted MR imaging--preliminary observations. Radiology 259, 142–150 (2011). [DOI] [PubMed] [Google Scholar]
- Motosugi U. et al. Staging liver fibrosis by using liver-enhancement ratio of gadoxetic acid-enhanced MR imaging: comparison with aspartate aminotransferase-to-platelet ratio index. Magn Reson Imaging 29, 1047–1052 (2011). [DOI] [PubMed] [Google Scholar]
- Verloh N. et al. Assessing liver function by liver enhancement during the hepatobiliary phase with Gd-EOB-DTPA-enhanced MRI at 3 Tesla. Eur Radiol (2014). [DOI] [PubMed] [Google Scholar]
- Materne R. et al. Assessment of hepatic perfusion parameters with dynamic MRI. Magn Reson Med 47, 135–142 (2002). [DOI] [PubMed] [Google Scholar]
- Wiesner R. et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 124, 91–96 (2003). [DOI] [PubMed] [Google Scholar]
- Thomsen C., Christoffersen P., Henriksen O. & Juhl E. Prolonged T1 in patients with liver cirrhosis: an in vivo MRI study. Magn Reson Imaging 8, 599–604 (1990). [DOI] [PubMed] [Google Scholar]
- Heye T. et al. MR relaxometry of the liver: significant elevation of T1 relaxation time in patients with liver cirrhosis. Eur Radiol 22, 1224–1232 (2012). [DOI] [PubMed] [Google Scholar]
- Mathur-De Vre R. Biomedical implications of the relaxation behaviour of water related to NMR imaging. Br J Radiol 57, 955–976 (1984). [DOI] [PubMed] [Google Scholar]
- Wynn T. A. Cellular and molecular mechanisms of fibrosis. J Pathol 214, 199–210 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg H. I. et al. Hepatic cirrhosis: magnetic resonance imaging. Radiology 153, 737–739 (1984). [DOI] [PubMed] [Google Scholar]
- Kreft B. et al. Evaluation of different models of experimentally induced liver cirrhosis for MRI research with correlation to histopathologic findings. Invest Radiol 34, 360–366 (1999). [DOI] [PubMed] [Google Scholar]
- Kim K. A. et al. Quantitative evaluation of liver cirrhosis using T1 relaxation time with 3 tesla MRI before and after oxygen inhalation. J Magn Reson Imaging 36, 405–410 (2012). [DOI] [PubMed] [Google Scholar]
- Wibmer A. et al. Liver failure after major liver resection: risk assessment by using preoperative Gadoxetic acid-enhanced 3-T MR imaging. Radiology 269, 777–786 (2013). [DOI] [PubMed] [Google Scholar]
- de Bazelaire C. M., Duhamel G. D., Rofsky N. M. & Alsop D. C. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 230, 652–659 (2004). [DOI] [PubMed] [Google Scholar]
- Vogl T. J. et al. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology 200, 59–67 (1996). [DOI] [PubMed] [Google Scholar]
- Frericks B. B. et al. Qualitative and quantitative evaluation of hepatocellular carcinoma and cirrhotic liver enhancement using Gd-EOB-DTPA. AJR Am J Roentgenol 193, 1053–1060 (2009). [DOI] [PubMed] [Google Scholar]
- Reimer P., Schneider G. & Schima W. Hepatobiliary contrast agents for contrast-enhanced MRI of the liver: properties, clinical development and applications. Eur Radiol 14, 559–578 (2004). [DOI] [PubMed] [Google Scholar]