The amiloride-sensitive epithelial Na+ channel (ENaC) is expressed in the apical membranes of many Na+-absorptive epithelia, such as renal tubules, distal colon, and lungs. The α subunit of ENaC (αENaC), and subsequently the β and γ subunits, were identified by expression cloning (1, 2). The latter subunits potentiate amiloride-sensitive Na+ currents more than 100-fold when coinjected with αENaC into Xenopus oocytes, but neither the β nor the γ subunit — alone or coexpressed — is sufficient to generate appreciable Na+ currents in this system in the absence of αENaC. Thus, this subunit appears to be required for the assembly or function of the ENaC complex. The 3 known ENaC subunits are homologous to each other, having about 35% amino acid identity and conserved membrane topology. Site-directed mutagenesis has defined a short segment (the P region depicted in Figure 1) that precedes the second transmembrane domain of all 3 ENaC subunits and is involved in ion permeation and sensitivity to amiloride. Several naturally occurring αENaC splice variants have been identified, but their physiological significance has not been characterized (Figure 1) (3).
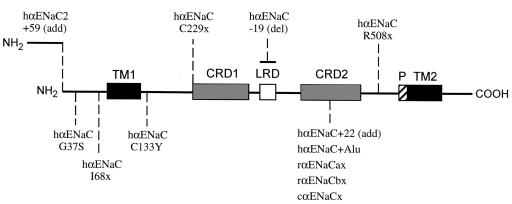
Figure 1.
αENaC-associated splice variants and mutations identified in human (h), rat (r), and chicken (c). The αENaC contains 2 transmembrane domains (TMs), 2 cysteine-rich domains (CRDs), a leucine-rich domain (LRD), and a pore-forming region (P) just before TM2. The human αENaC2+59 contains an additional 59 amino acids at the NH2-terminus (14). Three human αENaC splice variants (hαENaC C229x, hαENaC-19, and hαENaC+22) cause loss of function in Xenopus oocytes (3). The hαENaC+Alu contains an Alu fragment at the CRD2 (Y.S. Oh and D.G. Warnock, unpublished data). Two αENaC splice variants (αENaCax and αENaCbx) were identified in rat taste tissue (15), and αENaCx was identified in chicken cochlea (16); these splice variants, as well as hαENaC+22 and hαENaC+Alu, use the same splice site in the CRD2 exon (3). Four human αENaC mutations (G37S, I68x, C133Y, and R508x) have been identified in PHA-I individuals (6, 7). Here, “x” denotes a prematurely truncated protein, whether the new stop codon is generated by point mutation or frameshift.
Clinical disorders due to malfunction of the ENaC complex are well described (4). Liddle’s syndrome, an autosomal dominant form of volume-expanded low-renin hypertension, is caused by gain-of-function mutations in the COOH-termini of the β or γ ENaC subunits that constitutively activate ENaC activity (5). In contrast, loss-of-function mutations in the α and γ ENaC subunits have been found in autosomal recessive pseudohypoaldosteronism type I (PHA-I) with salt wasting, hyperkalemia, and metabolic acidosis (6, 7).
The critical role of αENaC in vivo has been demonstrated by αENaC gene knockouts in mice; these animals expire with severe respiratory insufficiency (8). In contrast, patients with PHA-I do not experience such respiratory disturbances but do exhibit abnormal renal Na+ handling. This discrepancy between the clinical phenotype and the knockout mouse models might be explained by physiological differences between the species, such as the existence of a protective mechanism that is either unique to the human lung or not expressed in the mouse lung. Alternatively, the αENaC knockout animal might prove to be an inadequate model for human disease if the human mutant alleles retain some biological function.
In support of the latter class of explanation, Bonny et al. (9) now provide evidence that an αENaC truncation mutant promotes the channel activity of ENaC complexes containing normal β and γ subunits. It appears that the previous failure to detect functional βγ ENaC channels was primarily due to delayed expression of these channels in oocytes and to the relatively low levels of the resulting amiloride-sensitive Na+ currents. Moreover, a truncated αENaC, equivalent to the R508X mutation that has been described in human PHA-I cases, can assemble with β and γ subunits to produce higher amiloride-sensitive Na+ currents than the βγ channels alone. Although these current levels are only about 10% of those found with wild-type αβγ ENaC, they could be sufficient, if expressed in vivo, to prevent development of the perinatal pulmonary phenotype in PHA-I patients. Bonny et al. have further proposed that the truncated αENaC, which retains the NH2-terminus and the extracellular cysteine-rich domain, may contain a sorting signal that promotes the assembly and proper intracellular trafficking of the βγ channels to the cell membrane.
Whether αENaC is necessary for expression of ENaC activity in cells remains a key question, but the formation of functional ENaC βγ complexes could account for the difficulty of detecting αENaC in some tissues (10). The special role of αENaC in channel expression is confirmed by the work of Masilamani et al., also in this issue of the JCI (11). For the first time, these authors have used subunit-specific antibodies to each of the ENaC subunits to investigate the in vivo regulation of ENaC expression in the renal collecting tubule, and, in particular, the physiologically relevant modulation of ENaC by aldosterone and dietary salt balance. Previous studies of ENaC expression have utilized heterologous expression systems, but the intrinsic limitations of this approach and the importance of examining protein expression and localization in vivo are now evident. Masilamani et al. demonstrate the apical localization of the assembled ENaC complex in response to aldosterone. They also show that αENaC is crucial for assembly and targeting of the channel complex to the apical membrane. There is also a provocative migration shift of the γ subunit on gel analysis, consistent with some sort of post-translational processing, perhaps by the CAP-1 serine protease? (12).
Beyond the evident importance of clarifying the pathophysiology of PHA-I, the significance of both of these papers (9, 11) lies in the insights they offer into normal cell physiology. The analysis of this inborn error lets us appreciate the special role of αENaC in “leading the charge” of ionic current flow across the apical membrane. Archibald Gerrod would be pleased but not surprised (13).
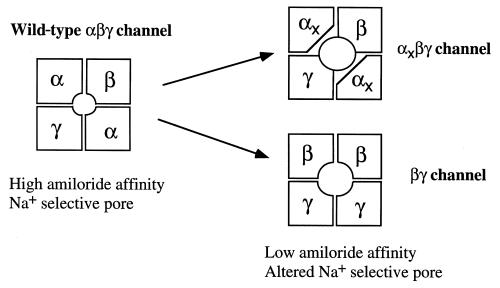
Figure 2.
Possible subunit arrangement of the ion-conducting pore in αxβγ channels and βγ channels. A possible ENaC structure consisting of 2 α, 1 β, and 1 γ subunit is shown as the wild-type channel. A truncated PHA-I mutation, R508x, was created in rat αENaC at the equivalent position (L535x), and αL535x, together with rat β and γ subunits, was expressed in Xenopus oocytes (9). Coexpression of rat β and γ ENaC subunits also generated amiloride-sensitive Na+ currents in oocytes. Interestingly, αL535xβγ and βγ channels displayed very similar macroscopic characteristics, such as reduced amiloride affinity and reduced Li+ selectivity, suggesting that the conductive pores of these channels are composed of P and TM2 domains of β and γ subunits, respectively.
References
- 1.Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. FEBS Lett. 1993;318:95–99. doi: 10.1016/0014-5793(93)81336-x. [DOI] [PubMed] [Google Scholar]
- 2.Canessa CM, Horisberger J-D, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- 3.Tucker JK, et al. Cloning and functional studies of splice variants of the α-subunit of the amiloride-sensitive Na+ channel. Am J Physiol. 1998;274:C1081–C1089. doi: 10.1152/ajpcell.1998.274.4.C1081. [DOI] [PubMed] [Google Scholar]
- 4.Scheinman SJ, Guay-Woodford LM, Thakker RV, Warnock DG. Genetic disorders of renal electrolyte transport. N Engl J Med. 1999;340:1177–1187. doi: 10.1056/NEJM199904153401507. [DOI] [PubMed] [Google Scholar]
- 5.Warnock DG. Polymorphism in the beta subunit and Na+ transport. J Am Soc Nephrol. 1996;7:2490–2494. doi: 10.1681/ASN.V7122490. [DOI] [PubMed] [Google Scholar]
- 6.Grunder S, et al. A mutation causing pseudohypoaldosteronism type 1 identifies a conserved glycine that is involved in the gating of the epithelial sodium channel. EMBO J. 1997;16:899–907. doi: 10.1093/emboj/16.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SS, et al. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet. 1996;12:248–253. doi: 10.1038/ng0396-248. [DOI] [PubMed] [Google Scholar]
- 8.Hummler E, et al. Early death due to defective neonatal lung liquid clearance in αENaC-deficient mice. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- 9.Bonny O, et al. Functional expression of a pseudohypoaldosteronism type I mutated epithelial Na+ channel lacking the pore-forming region of its α subunit. J Clin Invest. 1999;104:967–974. doi: 10.1172/JCI6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp UC, et al. Amiloride-sensitive Na+ channels in pelvic uroepithelium involved in renal sensory receptor activation. Am J Physiol. 1998;275:R1780–R1792. doi: 10.1152/ajpregu.1998.275.6.R1780. [DOI] [PubMed] [Google Scholar]
- 11.Masilamani S, Kim G-H, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β and γ subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–R23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coles JA, Orkand RK, Yamate CL, Tsacopoulos M. Free concentrations of Na, K, and Cl in the retina of the honeybee drone: Stimulus-induced redistribution and homeostasis. Ann NY Acad Sci. 1986;481:303–317. doi: 10.1111/j.1749-6632.1986.tb27160.x. [DOI] [PubMed] [Google Scholar]
- 13.Bearn, A.G. 1993. Archibald Gerrod and the individuality of man. Clarendon Press. Oxford, United Kingdom. 227 pp.
- 14.Thomas C, Auerbach S, Stokes JB, Volk KA. 5′ heterogeneity in epithelial sodium channel α-subunit mRNA leads to distinct NH2-terminal variant proteins. Am J Physiol. 1998;274:C1312–C1323. doi: 10.1152/ajpcell.1998.274.5.C1312. [DOI] [PubMed] [Google Scholar]
- 15.Li X-J, Xu R-H, Guggino WB, Snyder SH. Alternatively spliced forms of the α subunit of the epithelial sodium channel: distinct sites for amiloride binding and channel pore. Mol Pharmacol. 1995;47:1133–1140. [PubMed] [Google Scholar]
- 16.Killick R, Richardson G. Isolation of chicken alpha ENaC splice variants from a cochlear cDNA library. Biochim Biophys Acta. 1997;1350:3–37. doi: 10.1016/s0167-4781(96)00197-2. [DOI] [PubMed] [Google Scholar]