Abstract
Purpose of review
This review highlights recent developments in the management and monitoring of hypertension in adults with chronic kidney disease (CKD), not on dialysis.
Recent findings
Ambulatory blood pressure (BP) monitoring and self-measured BP monitoring can classify abnormal BP patterns better than clinic BP readings. Self-measured BP monitoring lowers BP and allows tailoring of antihypertensive treatment. Dosing of antihypertensive medication at night improves nocturnal hypertension. Recent guidelines recommend a BP target less than 140/90 mmHg for patients with CKD without proteinuria and less than 130/80 mmHg for those with proteinuria. Lower salt intake is associated with a greater effect of rennin–angiotensin–aldosterone system blockage in CKD. Lifestyle modification resulting in weight loss reduces BP in individuals with CKD. Of overweight or obese CKD patients, 8% report taking weight loss medication, which is a potential safety concern. Weight loss from intensive lifestyle modification in individuals with diabetes prevents CKD.
Summary
Although we have effective tools to monitor and lower BP, we still need clinical outcome studies to inform BP targets for specific age groups, types of CKD disease, and comorbidities. How to treat obesity to improve hypertension and other comorbidities in patients with CKD remains another important area of research.
Keywords: blood pressure target, chronic kidney disease, chronotherapy, evaluation, hypertension, obesity
INTRODUCTION
Nearly one-third of adults in the United States have hypertension [1]. The prevalence is even higher among those with chronic kidney disease (CKD) at 60% [2]. Treatment of elevated blood pressure (BP) is critical for preventing CKD, slowing its progression to kidney failure, and reducing the risk of adverse cardiovascular events in both diabetic and nondiabetic persons [3,4]. Over the last 2 years a number of organizations have issued new and updated guidelines pertinent to individuals with hypertension and CKD [5◎◎,6◎,7,8]. The focus of this narrative review is to highlight the recent developments in the field of hypertension and CKD with regard to evaluation of BP, BP targets, and use of certain antihypertensive agents. We conducted a systematic search of MEDLINE for publications indexed from 2011 to 8 June 2013 with the Medical Subject Heading (MeSH) terms of chronic renal failure and hypertension. We selected pertinent studies based on relevance to the above topics. We did not review literature in dialysis patients or children, nor did we cover topics reviewed in other papers in this issue such as renal sympathetic denervation.
DIAGNOSIS, CLASSIFICATION, AND EVALUATION OF HYPERTENSION
Evaluation of BP can be challenging. Elevated office BP is recognized as an important risk factor for future kidney and cardiovascular disease (CVD), and is the most widely used measure. Recent evidence, however, suggests that ambulatory BP monitoring (ABPM) may better classify a patient’s hypertensive status and risk for adverse events [9,10]. The 2011 guidelines from the National Institute for Clinical Excellence (NICE) recommend ABPM to confirm the diagnosis of hypertension in people with a clinic BP of 140/90 mmHg or higher [6◎]. Self-measured BP (SMBP) monitoring by a patient at home is recommended for persons unable to tolerate ABPM [6◎]. In a 2013 evidence update [11], the NICE guidelines cite the randomized controlled trial (RCT) by Myers et al. [12], which showed that in 522 hypertensive patients over the age of 45, both manual and automated office measurements of SBP were higher by 2.3 and 6.5 mmHg respectively than ABPM recordings. This study, however, excluded patients who had more than twice the normal value of serum creatinine, and the NICE guidelines did not specifically discuss the issue of BP measurement in persons with CKD. In 2004, the Kidney Disease: Outcomes Quality Initiative (KDOQI) guidelines recommended the use of ABPM in the setting of white-coat hypertension, resistant hypertension, hypotensive symptoms while taking antihypertensive medications, episodic hypertension, and autonomic dysfunction (Grade C recommendation) [13]. A recent consensus statement by a number of European and American societies has recommended that ABPM be used for the diagnosis of hypertension in persons at risk for CVD events such as those with CKD [14].
In the largest cross-sectional study of ABPM among CKD patients to date, Gorostidi et al. [15◎] evaluated differences between office and 24-h ABMP values in a cohort of 5693 patients with CKD stages 1–5 from the Spanish ABPM registry. Of this study population 68% had CKD 3, 7% had CKD 4, and only 2% had CKD 5. The investigators found control rates by 24-h BP (<130/80 mmHg) two times higher than office BP control rates (<140/90 mmHg) (43.5 versus 21.7%; P < 0.001). Compared to ABPM readings, the average SBP/DBP in the office was approximately 21/11 mmHg higher. The authors also noted that white-coat hypertension (i.e., office BP > 140/90 and 24-h ambulatory BP < 130/80 mmHg) was present in 36.8% of patients and masked hypertension (office BP < 140/90 and 24-h ambulatory BP > 130/80 mmHg) was present in 32.1% of patients. This study has also shown that in patients with CKD, 24-h control of BP is better than previously expected and this did not change with increasing stages of CKD. Nocturnal BP control, on the contrary, was worse as the severity of kidney disease increased. This nondipper pattern of BP appears to be associated with increased risk of adverse events. In a recent cross-sectional study of 540 Chinese patients with CKD who underwent ABPM, 42% had a nondipper pattern and 22% had a reversed dipper pattern [16]. Patients with a reversed dipper pattern had worse renal function and markers of cardiovascular damage when compared with patients with nondipper BP pattern after adjusting for other confounders.
These findings suggest that office BP recordings may not be sufficient for diagnosing and subsequent monitoring of hypertension during treatment. A systematic review of a large number of studies in individuals with hypertension has shown that SMBP improves BP control, especially when combined with additional support such as education, counseling or telemonitoring [17◎]. Although there is no reason this should not be the case in patients with hypertension and CKD, the data in this population are still limited. A small RCT of home BP telemonitoring in older patients with CKD and uncontrolled hypertension showed that this was feasible in this population [18]. A larger study showing effectiveness for BP control from telemonitoring and pharmacist BP management included almost a fifth of individuals with CKD [19].
BLOOD PRESSURE TARGETS
Prior guidelines recommended a BP target of less than 130/80 mmHg for high-risk individuals including patients with CKD, but recent guidelines have provided revised recommendations (Table 1) [5◎ ◎,6◎,8,20].
Table 1.
Recent guidelines on blood pressure targets
| Population | Target for pharmacological treatment | Comments | |
|---|---|---|---|
| NICE Guideline 2011 [6◎] | <80 years | <140/90 | If stage 1 hypertensiona and evidence of target organ damage or 10 y CVD risk equivalent ≥20%; or stage 2 hypertension a |
| >80 years | <150/90 | If newly diagnosed with stage 2 hypertension a | |
| KDIGO BP Guideline 2012 [5◎ ◎] | CKD, U alb <30 mg/day | <140/90 | 1B (strong recommendation, moderate quality evidence) |
| U alb 30–300 mg/day | <130/80 | 2D (discretionary recommendation, very low quality evidence) | |
| U alb >300 mg/day | <130/80 | 2C without DM, 2D with DM (discretionary recommendation, low or very low quality evidence, respectively) | |
| CKD and >65 years | <140/90 | ‘Prudent not to reduce BP much below the target <140/90’ | |
| ADA guideline [8] | DM | <140/80 | B level of evidence. ‘Based on patient characteristics and response to therapy, lower systolic blood pressure targets may be appropriate’ |
| ESH/ESC Guidelines 2013 [20] | CKD ≥3 | <140/90 | In patients with diabetes DBP < 85 mmHg is recommended |
| Overt proteinuria | <130 | This lower target ‘may be considered, provided that changes in eGFR are monitored’ | |
| <80 years | <140/90 | ‘In frail elderly, adapt goals to individual tolerability.’ | |
| >80 year | 140 to 150/<90 | If newly treated for stage 2 HTN (SBP ≥ 160 mmHg) |
ADA, American Diabetes Association; BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ESC, European Society of Cardiology; ESH, European Society of hypertension; KDIGO, Kidney Disease Improving Global Outcomes; NICE, National Institute for Clinical Excellence; U alb, urine albumin.
Definitions for hypertension stages: stage 1 is clinic BP ≥140/90 mmHg and subsequent ambulatory BP monitoring (ABPM) daytime average or home BP monitoring (HBPM) average BP is 135/85 mmHg or higher; stage 2 is clinic BP ≥160/100 mmHg and subsequent ABPM daytime average or HBPM average BP is ≥150/95 mmHg.
The BP target recommended for most patients with CKD is less than 140/90 mmHg. For those with proteinuria a lower target of less than 130/80 mmHg may be tried. The revised targets are based on a reevaluation of RCT evidence in CKD and non-CKD patients. Three main BP target trials in adults with predominantly nondiabetic CKD [21–23,24◎] failed to find benefit for progression of CKD, CVD, or death with SBP targets of less than 125–130 mmHg versus less than 140 mmHg [24◎]. An observational extension of one study found benefit for incidence of kidney failure in the lower BP group raising the question whether the 2–3 years follow-up in the trials was too short [25]. But the extension study of the other large trial did not find benefit [26]. In subgroups with proteinuria greater than 300 or 1000 mg/day, there may be benefit from the lower targets [24◎]. A recent meta-analysis confirmed lack of benefit from more intensive BP control in nonproteinuric CKD and a reduction in kidney failure in those with proteinuria [hazard ratio 0.73, 95% confidence interval (CI) 0.62–0.86] [27◎]. The analysis in proteinuric subgroups combined three large CKD studies in adults [21–23], one study in children [28], and one in older adults [29], thereby deriving more precision, but also introducing greater clinical heterogeneity regarding the populations and BP targets. For instance, the study in children compared a target of less than 50th percentile versus a target between 50th and 95th percentile for age and height reference BP by ABPM. The study in older adults compared an SBP target of less than 140 versus less than 160 mmHg. A large trial in diabetic patients, Action to Control Cardiovascular Risk in Diabetes (ACCORD), failed to show benefit from an SBP target less than 120 mmHg rather than less than 140 mmHg [30]. At baseline, 40% of the patients had albuminuria, mostly microalbuminuria. Two ongoing trials will provide more data in individuals with nondiabetic CKD [31,32]. There are no BP target trials in kidney transplant recipients; thus, recommendations for this group are extrapolated from evidence in nontransplant patients. Similarly, there are no BP target studies specifically in older adults with CKD. Guidelines recommend a less intensive BP target in older patients and to individualize BP targets based on benefit–harm assessment given the greater comorbidity and risk for falls (Table 1) [5◎ ◎].
CHRONOTHERAPY
Given the risk of nocturnal hypertension in CKD, recent trials have examined the role of daytime versus bedtime administration of antihypertensive agents. The first trial was conducted by Hermida et al. [33◎] in 661 hypertensive adults with CKD [estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2, urinary albumin excretion >30 mg/24-h urine or both]. The study evaluated the effect of changing one or more of the daily antihypertensive medications to bedtime as compared to giving all medications upon awakening. In this study in which hypertension was defined as an awake BP mean more than 135/85 mmHg or an asleep BP mean more than 120/70 mmHg, persons randomized to bedtime medication showed a better overall control of BP on ABPM (56 versus 45%; P < 0.03), greater reduction in mean asleep SBP (9.7 versus 4.4 mmHg; P < 0.01) and also a lower proportion of nondippers (41 versus 71%; P < 0.01). Persons randomized to bedtime therapy also had greater reduction in albuminuria compared to the control group. During the course of follow-up (median 5.4 years), eGFR did not change statistically significantly (−0.4 ml/min/1.73 m2; P = 0.6) in those assigned to the bedtime medication, but there was a decrease of −2.3 ml/min/1.73 m2 (P < 0.003) in those ingesting all medications upon waking (P between groups 0.043). The group receiving bedtime therapy had a significant reduction in the composite outcome of cardiovascular death, myocardial infaction, or stroke (adjusted hazard ratio 0.28; 95% CI 0.13–0.61; P < 0.001). This is an unexpectedly strong effect size for an important clinical outcome, for which CKD BP studies are often underpowered. In a subgroup of 448 patients with type 2 diabetes, the authors found similar improvements in BP control by ABPM and a significant risk reduction in CVD events (hazard ratio 0.25; CI 0.10–0.61; P = 0.003] [34]. The reduction of nocturnal BP with evening drug dosing was also seen in a smaller study [35]. In 27 patients with a mean eGFR of 58 ml/min/1.73 m2 who at baseline were taking three or more antihypertensive medications and demonstrating a nondipper or reversed dipper pattern of hypertension, changing all medications (except diuretics) to nocturnal dosing resulted in significant decrease in overall nocturnal SBP (−6.1 mmHg) and DBP (−4.2 mmHg) and overall 24 h SBP (−2.7 mmHg) and DBP (−2.0 mm Hg).
Another study in 147 former participants of the African American Study of Kidney Disease (AASK) with hypertensive kidney disease, however, failed to show nocturnal SBP reduction with nightly antihypertensive drug dosing [36]. Participants in this study were randomized to three groups: either all medications in the evening (p.m. dose); all medications in the morning with one additional evening dose (add-on dose); or all medications in the morning (a.m. dose). Compared to the a.m, group absolute reduction in nocturnal SBP in the p.m.-dose group (−1.7 mmHg; P = 0.16) or add-on dose group (−2.07 mmHg; P = 0.08) did not differ significantly. These findings did not change when analyzed in subgroups by dipping status, nocturnal BP, urinary sodium excretion, and number of antihypertensive medications at baseline. Some important population differences exist between this and the Hermida study [33◎], which may account for the differences in results. Participants in the Hermida study were younger compared to participants in AASK (approximately 60 versus 65 years), and had a shorter duration of hypertension (approximately 7 versus 30 years); higher eGFR (66 versus 45 ml/min/1.73 m2), and higher mean nocturnal SBP (129 versus 124 mmHg).
The low cost and simplicity of bedtime antihypertensive drug dosing make it an attractive therapeutic option in persons with nocturnal hypertension and relatively preserved eGFR (>45 ml/min/1.73 m2). However, the findings of the Hermida study need to be replicated across various clinical populations andstages of CKD, before one can recommend this practice as a treatment of proven efficacy for prevention of clinical outcomes.
DIURETIC THERAPY GUIDED BY BIOIMPEDENCE SPECTROSCOPY
Salt retention along with volume overload is a major cause of hypertension in patients with CKD. Bioimpedance spectroscopy (BIS) is a method for measuring body composition, which has been used to assess volume status in patients on dialysis [37]. Verdalles et al. [38] used BIS to assess fluid status and guide the use of diuretics to treat hypertension in CKD patients not on dialysis. They treated 30 patients with extracellular volume (ECV) expansion with a diuretic compared to 20 patients without ECV expansion who instead received another additional antihypertensive medication. At 6 months of follow-up, SBP decreased by 21 mmHg in patients with expansion of ECV compared with 9 mmHg in patients without expansion of ECV (P < 0.01). In addition nine of 30 patients with ECV expansion and two of 20 without ECV expansion achieved the target BP of less than 140/90 mmHg at 6 months. This novel approach to managing hypertensive CKD patients based on BIS assessment of volume status will need further study in larger cohorts before it can be considered for wider use.
TRIALS OF PARTICULAR ANTIHYPERTENSIVE AGENTS
Many effective antihypertensives are available, but drug therapy for the treatment of hypertension remains an active area of research. Recent studies examined newer agents such as aliskiren, a selective rennin inhibitor that has been shown to be associated with reduction in BP and sympathetic nerve activity [39,40], and sitaxsentan [41], a selective endothelial antagonist in the treatment of hypertension in persons with kidney disease. We however did not find new studies that reported clinical endpoints among patients with CKD. In a recent RCT of aldactone lasting 36 weeks, it was shown to be well tolerated in patients with early stage 3 CKD with fewer than 1% episodes of hyperkalemia (K+ ≥6 mmol/l) [42]. This is important as there has been an interest in its use for treating resistant hypertension.
DIETARY SALT INTAKE
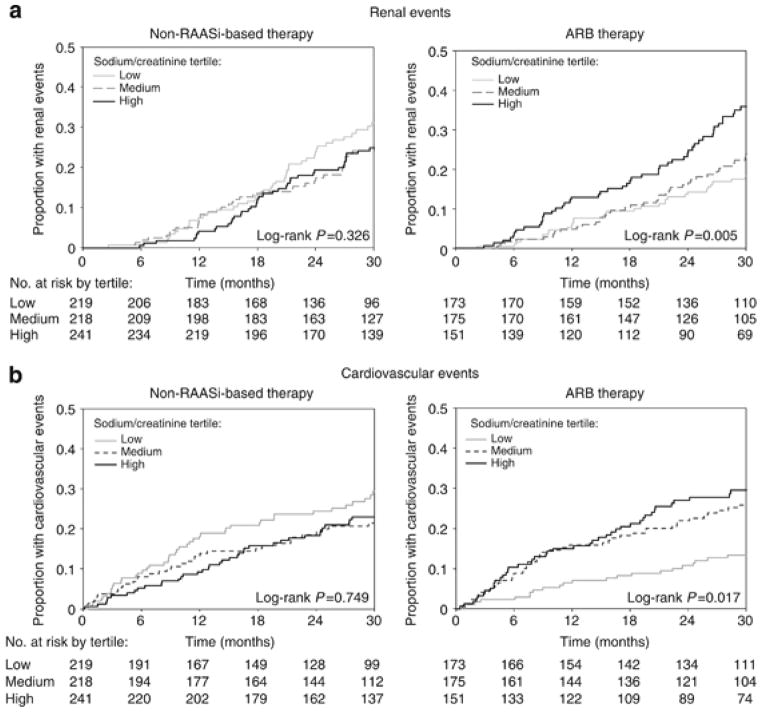
Recent studies have evaluated the interaction between dietary salt intake and rennin–angiotensin–aldosterone system (RAAS) blockade in CKD patients. A post hoc analysis included 1117 participants from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) and Irbesartan Diabetic Nephropathy Trial (IDNT) trials and evaluated how dietary sodium intake (assessed by 24-h urine sodium excretion) impacted the efficacy of angiotensin-receptor blockers (ARBs) in preventing CKD and CVD end points [43◎ ◎]. The authors reported that absolute reductions in 24-h urinary albumin-to-creatinine ratio and SBP were greatest in those with the lowest baseline urinary sodium-to-creatinine ratio. ARB use was not associated with a significant reduction in SBP, renal or CVD events among those in the highest tertile of sodium-to-creatinine ratio. Among those treated with irbesartan and losartan, individuals in the lowest tertile of sodium-to-creatinine ratio experienced significantly fewer CVD events compared to the highest tertile (Fig. 1).
FIGURE 1.
Kaplan–Meier curves according to tertiles of 24-h urinary sodium/creatinine ratio. From Lambers Heerspink et al. [43◎◎] (figure on page 332). Kaplan–Meier curves according to tertiles of 24-h urinary sodium/creatinine ratio. Kaplan–Meier curves for (a) renal and (b) cardiovascular events in participants who received angiotensin-receptor blocker (ARB) – and nonrenin–angiotensin–aldosterone system (non-RAASi)-based therapy stratified by tertiles of 24-h sodium/creatinine ratio: <121 mmol/g; 121–153 mmol/g; >153 mmol/g. gr1
Another study similarly shows increased risk for clinical endpoints in ACE-treated patients with greater salt intake. A post hoc analysis of 500 patients with CKD, but without diabetes treated with ramipril (5 mg/day) in the first and second Ramipril Efficacy in Nephropathy (REIN I & II) trials, were monitored with serial 24-h urinary sodium and creatinine measurements [44]. This study showed that the antiproteinuric effect of ramipril was blunted in persons with high sodium intake, independent of BP control. The risk of progression to end-stage renal disease (ESRD) increased linearly with increasing sodium intake with each 100 mEq/g increase in urinary sodium/creatinine excretion associated with a 1.61-fold (95% CI 1.15–2.24) higher risk for ESRD. These studies suggest that RAAS blockade is more effective in CKD patients with low sodium intake.
LIFESTYLE INTERVENTIONS AIMED AT WEIGHT LOSS
Data from nearly a decade ago showed that 13% of all patients initiating dialysis were obese (BMI > 35 mg/m2) [45] and that 60% of all patients receiving a transplant were overweight or obese [46]. Obesity is an independent risk factor for hypertension [47], CKD and ESRD [48,49]. However, data on weight loss interventions and their effect on BP and kidney function in CKD patients are limited. One uncontrolled study of 112 obese patients with CKD stages 1–5 and mean eGFR of 32 ml/min/1.73 m2 showed that sustained weight reductions can be achieved with a multidisciplinary approach and results in lower BP [50]. However, about half of the patients were dialysis patients and many may have been motivated to meet the BMI cutoff for transplant eligibility. The intervention consisted of nine individual sessions with a renal dietician and renal physiotherapist over 12 months, which included a program advising a low-fat, energy-reduced renal diet, increased physical activity, use of behavior therapy techniques, and the drug orlistat 120 mg three times daily [51]. Mean weight loss was 4% with over 22% of the participants achieving more than 10% weight loss, which was sustained at 24 months. In addition, there were statistically significant reductions in SBP at 24 months among participants who were partly compliant [−4 mmHg (95% CI −3 to −11)] or compliant [−8 mmHg (95% CI −2 to −14)] with the structured sessions. As orlistat may cause hyperoxaluria and kidney injury [52], it is noteworthy that the study did not report any patients who developed kidney stones or hyperoxalosis throughout the course of the therapy.
Using data from National Health and Nutrition Examination Survey Navaneethan et al. [53] showed that among individuals with CKD and BMI more than 25, 50% reported pursuing weight loss and 8% reported using medications to promote weight loss. This raises an important question about the safety of weight loss modalities in CKD because high protein diets and weight loss medications may need to be used with caution in persons with CKD. None of the FDA-approved weight loss medications can be safely used in patients with CKD, especially in patients with eGFR less than 60 ml/min/1.73 m2 [54]. It is also intriguing that the recent large Look AHEAD study in obese individuals with diabetes who did not yet have CKD reported benefit from intensive lifestyle modification for prevention of CKD [55◎].
CONCLUSION
ABPM and self-measured BP are tools to evaluate BP more comprehensively than clinic BP. They provide opportunity to tailor BP management to the BP pattern abnormality, for example, with chronotherapy. Recent guidelines recommend less aggressive BP targets for people with CKD without proteinuria than for those with. We still need more clinical outcome studies to inform BP targets for different age groups, types of CKD disease, and comorbidities. Lifestyle interventions play an important role in managing hypertension in CKD and management of obesity in CKD remains an important area for future research.
KEY POINTS.
Ambulatory BP and self-measured BP appear to better classify hypertension than office BP and allow tailoring of antihypertensive treatment.
BP targets have been reevaluated in guidelines and changed to less than 140/90 mmHg for patients with nonproteinuric CKD, and less than 130/80 mmHg for those with proteinuria.
Lower salt intake is associated with a greater effect of RAAS blockage in CKD. Lifestyle modifications resulting in weight loss lower BP in individuals with CKD.
A substantive proportion of overweight or obese CKD patients report use of weight loss medication, which is a potential safety concern.
Footnotes
Conflicts of interest
Dr. Pranav Garimella is supported by grant 5T32DK007777-13 from the National Institutes of Health and has received payment for educational content development from Elsevier Inc.
Dr. Katrin Uhlig has no conflicts of interest to declare.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
◎ of special interest
◎◎of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Ridao N, Luno J, Garcia de Vinuesa S, et al. Prevalence of hypertension in renal disease. Nephrol Dial Transplant. 2001;16(Suppl 1):70–73. doi: 10.1093/ndt/16.suppl_1.70. [DOI] [PubMed] [Google Scholar]
- 3.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden LG, He J, Lydick E, Whelton PK. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to the JNC VI risk stratification. Hypertension. 2000;35:539–543. doi: 10.1161/01.hyp.35.2.539. [DOI] [PubMed] [Google Scholar]
- 5◎◎.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337–414. The 2012 KDIGO guideline recommends that the target BP should be 140/90 mmHg or less in CKD patients without albuminuria. In patients with an albumin excretion rate of at least 30 mg/24 h, a lower target of 130/80 mmHg or less is suggested. These recommendations supersede those of the 2004 KDOQI guideline, which recommended a BP target of 130/80 mmHg or less in all CKD patients (see ref. [13]) [Google Scholar]
- 6.National Clinical Guideline Centre (UK) NICE Clinical Guidelines, No 127. London, UK: Royal College of Physicians; [Date of access June 26, 2013]. 2011. Hypertension: The Clinical Management of Primary Hypertension in Adults: Update of Clinical Guidelines 18 and 34 [Internet] http://www.ncbi.nlm.nih.gov/books/NBK83274/ [PubMed] [Google Scholar]
- 7.Hackam DG, Quinn RR, Ravani P, et al. The 2013 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2013;29:528–542. doi: 10.1016/j.cjca.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of medical care in diabetes 2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan HQ, Li Y, Thijs L, et al. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens. 2010;28:2036–2045. doi: 10.1097/HJH.0b013e32833b49fe. [DOI] [PubMed] [Google Scholar]
- 10.Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 11.Hypertension: Evidence Update March 2013: A summary of selected new evidence relevant to NICE clinical guideline 127 ‘Clinical management of primary hypertension in adults’. London, UK: Royal College of Physicians; 2011. [Date of access June 26, 2013]. 2013. [Google Scholar]
- 12.Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidney Disease Outcomes Quality Initiative (K/DOQI) K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2004;43(5 Suppl 1):S1–S290. [PubMed] [Google Scholar]
- 14.Hermida RC, Smolensky MH, Ayala DE, Portaluppi F. 2013 ambulatory blood pressure monitoring recommendations for the diagnosis of adult hypertension, assessment of cardiovascular and other hypertension-associated risk, and attainment of therapeutic goals. Chronobiol Int. 2013;30:355–410. doi: 10.3109/07420528.2013.750490. [DOI] [PubMed] [Google Scholar]
- 15◎.Gorostidi M, Sarafidis PA, de la Sierra A, et al. Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: a 5,693-patient cross-sectional analysis from Spain. Am J Kidney Dis. 2013;62:285–294. doi: 10.1053/j.ajkd.2013.03.025. This cross-sectional study compares ABPM and clinic BP in a large cohort with two thirds having CKD. It shows the prevalence for BP control, white-coat and masked hypertension. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Zhang J, Liu X, et al. Reversed dipper blood-pressure pattern is closely related to severe renal and cardiovascular damage in patients with chronic kidney disease. PloS one. 2013;8:e55419. doi: 10.1371/journal.pone.0055419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17◎.Uhlig K, Balk EM, Patel K, et al. Self-measured blood pressure monitoring: comparative effectiveness. Agency for Healthcare Research and Quality (US); Rockville, MD: 2012. This comprehensive systematic review summarizes the effectiveness of self blood pressure monitoring in the treatment of hypertension, with and without additional support. [PubMed] [Google Scholar]
- 18.Rifkin DE, Abdelmalek JA, Miracle CM, et al. Linking clinic and home: a randomized, controlled clinical effectiveness trial of real-time, wireless blood pressure monitoring for older patients with kidney disease and hypertension. Blood Press Monit. 2013;18:8–15. doi: 10.1097/MBP.0b013e32835d126c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310:46–56. doi: 10.1001/jama.2013.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 21.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 22.Wright JTJ, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 23.Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with nondiabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939–946. doi: 10.1016/S0140-6736(05)71082-5. [DOI] [PubMed] [Google Scholar]
- 24.Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Annals of internal medicine. 2011;154:541–548. doi: 10.7326/0003-4819-154-8-201104190-00335. [DOI] [PubMed] [Google Scholar]
- 25.Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142:342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 26.Appel LJ, Wright JTJ, Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27◎.Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949–957. doi: 10.1503/cmaj.121468. This meta-analysis confirmed lack of benefit from more intensive BP control in nonproteinuric CKD, but showed reduction of kidney failure with more intensive BP control in subgroups with proteinuria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wuhl E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi K, Saruta T, Goto Y, Ishii M. Impact of renal function on cardiovascular events in elderly hypertensive patients treated with efonidipine. Hypertens Res. 2010;33:1211–1220. doi: 10.1038/hr.2010.162. [DOI] [PubMed] [Google Scholar]
- 30.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ClinicalTrials.gov. SPRINT (Systolic Blood Pressure Intervention Trial) Clinical trial. database on the Internet. [cited 25July 1, 2013. www.clinicaltrial.gov/ct2/show/NCT01206062?term=SPRINT&rank=2on.
- 32.ClinicalTrials.gov. HALT Progression of Polycystic Kidney Disease (HALT PKD) Clinical trial. [database on the Internet]. [cited July 1, 2013]. www.clinicaltrial.gov/ct2/show/NCT00283686?term=HALT-PKD&rank=1.
- 33.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22:2313–2321. doi: 10.1681/ASN.2011040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270–1276. doi: 10.2337/dc11-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almirall J, Comas L, Martinez-Ocana JC, et al. Effects of chronotherapy on blood pressure control in nondipper patients with refractory hypertension. Nephrol Dial Transplant. 2012;27:1855–1859. doi: 10.1093/ndt/gfr557. [DOI] [PubMed] [Google Scholar]
- 36.Rahman M, Greene T, Phillips RA, et al. A trial of 2 strategies to reduce nocturnal blood pressure in blacks with chronic kidney disease. Hypertension. 2013;61:82–88. doi: 10.1161/HYPERTENSIONAHA.112.200477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crepaldi C, Soni S, Chionh CY, et al. Application of body composition monitoring to peritoneal dialysis patients. Contrib Nephrol. 2009;163:1–6. doi: 10.1159/000223772. [DOI] [PubMed] [Google Scholar]
- 38.Verdalles U, de Vinuesa SG, Goicoechea M, et al. Utility of bioimpedance spectroscopy (BIS) in the management of refractory hypertension in patients with chronic kidney disease (CKD) Nephrol Dial Transplant. 2012;27(Suppl 4):iv31–iv35. doi: 10.1093/ndt/gfs420. [DOI] [PubMed] [Google Scholar]
- 39.Sakai Y, Otsuka T, Ohno D, et al. Efficacy of aliskiren in Japanese chronic kidney disease patients with hypertension. Ren Fail. 2012;34:442–447. doi: 10.3109/0886022X.2011.649672. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqi L, Oey PL, Blankestijn PJ. Aliskiren reduces sympathetic nerve activity and blood pressure in chronic kidney disease patients. Nephrol Dial Transplant. 2011;26:2930–2934. doi: 10.1093/ndt/gfq857. [DOI] [PubMed] [Google Scholar]
- 41.Dhaun N, MacIntyre IM, Kerr D, et al. Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension. 2011;57:772–779. doi: 10.1161/HYPERTENSIONAHA.110.167486. [DOI] [PubMed] [Google Scholar]
- 42.Edwards NC, Steeds RP, Chue CD, et al. The safety and tolerability of spironolactone in patients with mild to moderate chronic kidney disease. Br J Clin Pharmacol. 2012;73:447–454. doi: 10.1111/j.1365-2125.2011.04102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43◎◎.Lambers Heerspink HJ, Holtkamp FA, Parving HH, et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82:330–337. doi: 10.1038/ki.2012.74. This study shows that the reduction of adverse kidney and CVD outcomes with RAAS blockade is present in patients with low sodium intake but blunted in those high sodium intake. [DOI] [PubMed] [Google Scholar]
- 44◎.Vegter S, Perna A, Postma MJ, et al. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol. 2012;23:165–173. doi: 10.1681/ASN.2011040430. The results of this observational trial demonstrate that the anti-proteinuric effect of angiotensin converting-enzyme inhibitors is significantly reduced among persons with high sodium intake independent of blood pressure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kramer HJ, Saranathan A, Luke A, et al. Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol. 2006;17:1453–1459. doi: 10.1681/ASN.2005111241. [DOI] [PubMed] [Google Scholar]
- 46.Friedman AN, Miskulin DC, Rosenberg IH, Levey AS. Demographics and trends in overweight and obesity in patients at time of kidney transplantation. Am J Kidney Dis. 2003;41:480–487. doi: 10.1053/ajkd.2003.50059. [DOI] [PubMed] [Google Scholar]
- 47.Wilson PW, D’Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 48.Gelber RP, Kurth T, Kausz AT, et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46:871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Iseki K, Ikemiya Y, Kinjo K, et al. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65:1870–1876. doi: 10.1111/j.1523-1755.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 50.MacLaughlin HL, Cook SA, Kariyawasam D, et al. Nonrandomized trial of weight loss with orlistat, nutrition education, diet, and exercise in obese patients with CKD: 2-year follow-up. Am J Kidney Dis. 2010;55:69–76. doi: 10.1053/j.ajkd.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 51.MacLaughlin HL, Sarafidis PA, Greenwood SA, et al. Compliance with a structured weight loss program is associated with reduced systolic blood pressure in obese patients with chronic kidney disease. Am J Hypertens. 2012;25:1024–1029. doi: 10.1038/ajh.2012.80. [DOI] [PubMed] [Google Scholar]
- 52.Courtney AE, O’Rourke DM, Maxwell AP. Rapidly progressive renal failure associated with successful pharmacotherapy for obesity. Nephrol Dial Transplant. 2007;22:621–623. doi: 10.1093/ndt/gfl684. [DOI] [PubMed] [Google Scholar]
- 53.Navaneethan SD, Kirwan JP, Arrigain S, et al. Overweight, obesity and intentional weight loss in chronic kidney disease: NHANES 1999–2006. Int J Obes (Lond) 2012;36:1585–1590. doi: 10.1038/ijo.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kramer H, Tuttle KR, Leehey D, et al. Obesity management in adults with CKD. Am J Kidney Dis. 2009;53:151–165. doi: 10.1053/j.ajkd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55◎.Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. In this recent large RCT of intensive weight loss in persons with diabetes, the authors showed a sustained reduction in weight among those assigned to the treatment. Although there was no reduction in CVD events, unpublished data from the trial reportedly showed that the intensive lifestyle intervention was associated with a 31% reduction in the risk of advanced kidney disease. [DOI] [PMC free article] [PubMed] [Google Scholar]