Abstract
Background
Amphiphilic block copolymers acting as biological response modifiers provide an attractive approach to improving the transfection efficiency of polycationic polymer/DNA complexes (polyplexes) by altering cellular processes crucial to efficient transgene expression.
Methods
The objective of this study was to investigate the effect of the poloxamine Tetronic T904, a 4-arm polyethylene oxide / polypropylene oxide block copolymer, on polyplex transfection and determine its mechanism of action by analyzing cellular uptake of polyplex, nuclear localization of plasmid, and RNA transcript production.
Results
T904 significantly increased the transfection efficiency of polyplexes based on 25 kDa branched polyethylenimine in a dose-dependent manner in the presence of serum in C6 glioma cells, as well as human fibroblasts and mesenchymal stem cells. The activity of T904 was not promoter-dependent, increasing expression of reporter genes under both CMV and SV40 promoters. While T904 did not affect internalization or nuclear uptake of plasmid, mRNA expression levels from both promoters showed dose-dependent increases that closely paralleled increases in gene expression.
Conclusions
This study demonstrates that T904 significantly increases polyplex transfection efficiency and suggests a mechanism of increased transcriptional activity. As a 4-arm, hydroxyl-terminated polymer, T904 is amenable to a variety of end group functionalization and covalent crosslinking strategies that have been developed for preparing hydrogels from multi-arm polyethylene glycol, making it particularly attractive for scaffold-mediated gene delivery.
Keywords: Gene delivery, poloxamine, polyplex, gene expression, promoter, amphiphilic block copolymer
1. Introduction
Gene delivery provides an attractive approach for the production of therapeutic proteins at sites of tissue disease or injury. Currently, most pre-clinical studies utilize viral vectors due to their high transfection efficiency. However, viral vectors have several safety-related drawbacks including immunogenicity and the risk of insertional mutagenesis that have hindered clinical translation (1). Nonviral vectors generally exhibit less adverse reactions than viral vectors, but the transfection efficiency of current nonviral vectors remains relatively low due to complex barriers that must be overcome to reach the nucleus of target cells. Local delivery of nonviral vectors through incorporation within implantable biomaterial scaffolds can overcome several extracellular barriers to systemic gene delivery such as serum aggregation, early elimination by the reticulo-endothelial system, and targeting to the intended site of action (2–4). After release from the scaffold, nonviral vectors are confronted with a further series of cellular barriers including internalization, endosomal escape, intracellular trafficking, and nuclear import (5). Each of these represents a potential rate-limiting step to which various vectors may be susceptible (6). Methods to enhance the transfection efficiency of nonviral vectors by addressing these barriers must be developed in order to increase the clinical relevancy of nonviral delivery systems.
One method that has recently gained increased attention for improving nonviral gene delivery is the addition of amphiphilic block copolymers to help stabilize vectors and overcome intracellular barriers to transfection. Pluronics® (poloxamers) are a family of triblock copolymers composed of polyethylene oxide (PEO) and polypropylene oxide (PPO) that have been widely applied as micellar carriers and thermoreversible gels to increase drug solubility and prolong release (7,8). Although originally viewed as biologically inert excipients, Pluronics have recently demonstrated potential as potent biological response modifiers with effects on ATPase activity, inhibition of drug efflux transporters such as Pgp (9), and the ability to enter difficult to penetrate cells such as neurons with pathogen-like intracellular trafficking to mitochondria and neuronal dendrites (10). Recently, addition of Pluronics to naked plasmid DNA has been shown to significantly increase gene expression after intramuscular injection (11–15). Pluronics have also been found to enhance polyplex gene delivery by providing protection from serum aggregation (16), polyplex stabilization (17), enhancing receptor mediated targeted gene delivery (18), increasing cellular internalization through endocytosis independent membrane fusion (19), increasing nuclear import through activation of NF-κB transcription factor in a promoter-dependent manner (20), and increasing transcriptional activity (21). Tetronics® (poloxamines) are a related family of 4-arm PEO/PPO block copolymers that are also capable of increasing gene expression from naked plasmid DNA following intramuscular or intradermal injection (14,15,22,23) However, unlike Pluronics, the potential efficacy and mechanisms of poloxamines for improving polyplex gene delivery are unknown.
Our long term goal is to develop hydrogels incorporating amphiphilic block copolymers that will be delivered in soluble form as a degradation product to surrounding cells and increase the activity of released polyplex vectors. The objective of this study was to investigate the efficacy of Tetronic T904 as a biological response modifier for improving transfection efficiency of polyplex vectors based on the widely studied 25 kDa branched polyethylenimine (PEI). We investigated the effect of T904 on polyplex-mediated transfection using plasmids containing both cytomegalovirus (CMV) and Simian virus 40 (SV40) promoters that have shown different efficacy in previous studies with Pluronics (11,24). We show that T904 significantly increases transfection efficiency and gene expression in a dose-dependent manner that is not promoter-dependent. In addition, we observe that T904 has little effect on polyplex internalization or nuclear uptake, while mRNA transcript levels closely parallel increases in gene expression, suggesting that T904 acts through a mechanism of increasing transcriptional activity.
2. Methods
2.1 Cell culture
Rat C6 glioma (C6, ATCC, Manassas, VA) were cultured in Dulbecco’s Modification of Eagle’s Medium/Ham’s F-12 50/50 mix with L-glutamine (DMEM/F12, Mediatech, Manassas, VA) with 5% bovine growth serum (BGS) (Hyclone, Logan, UT) and 1% penicillin and streptomycin (Mediatech). Normal human dermal fibroblasts (NHDF, Lonza, Walkersville, MD) were cultured in DMEM/F12 with 10% BGS and 1% penicillin and streptomycin. Human mesenchymal stem cells (hMSC, Lonza) were cultured in low glucose DMEM (Life Technologies, Carlsbad, CA) with 10% MSC-qualified fetal bovine serum (FBS, Life Technologies), 10 ng/mL fibroblast growth factor-2 (Peprotech, Rocky Hill, NJ), 1% GlutaMax (Life Technologies), and 1% penicillin and streptomycin. All cells were grown in a humidified incubator at 37°C in 5% CO2 atmosphere.
2.2 Polyplex formation and characterization
Plasmids encoding Monster Green Fluorescent Protein phMGFP Vector under a CMV promoter (pCMV-GFP, Promega, Madison, WI) and beta-galactosidase under SV-40 promoter (pSV40-β-gal, Promega) were transformed into Escherichia coli DH5α (Life Technologies), amplified in the presence of ampicillin, and purified using the Qiagen Mega Plus Prep kit (Qiagen, Valencia, CA). Plasmid concentrations were determined by UV spectrophotometry with Take 3 (Biotek, Winooski, VT). Polyplexes for transfection were prepared by mixing pCMV-GFP or pSV40-β-gal and 25 kDa branched polyethylenimine (PEI, Sigma-Aldrich, St. Louis, MO) in water at PEI nitrogen to pDNA phosphate ratios (N/P) of 7.5/1 and incubated for 30 minutes at 37°C. T904 (generously donated by BASF, Florham Park, NJ) was dissolved in water (1 mM) and added to polyplex solutions to yield final concentrations specified in each experiment and thoroughly mixed prior to characterization or transfection. Particle size and zeta potential of polyplexes mixed with varying amounts of T904 were measured by dynamic light scattering and electrophoresis using a ZetaPlus analyzer (Brookhaven, Worcestershire, UK) with sample size n=6.
2.3 Transfection efficiency and cytotoxicity
C6, NHDF, and hMSC cells were seeded in 12 well plates at densities yielding approximately 70% confluence after 24 hours incubation. Prior to transfection, growth medium was exchanged with fresh medium containing 5% serum. 100 μL polyplex solution (PEI/pDNA at N/P ratio 7.5/1, 2 μg pDNA) with T904 (0–10 μM final concentration in wells) was added drop wise into each well. Medium was exchanged with fresh medium containing 5% serum at 24 h post-transfection and incubated an additional 24 hrs. Transfection efficiency or cytotoxicity was assessed 48 h post-transfection. Flow cytometry was used to determine transfection efficiency in cells transfected with pCMV-GFP. Cells were washed with PBS, trypsinized, centrifuged at 1500 rpm for 3 minutes, fixed with 1% formaldehyde, and assayed using 5000 counts per sample in a Guava easyCyte flow cytometer (Millipore, Billerica, MA). Transfection efficiency for PEI/pCMV-GFP at each T904 concentration was expressed as % total cells transfected as well as transfection efficiency relative to PEI/pCMV-GFP with 0 μM T904 using mean fluorescence per cell (each experiment (n=3) independently replicated 3 times). Relative transfection was calculated as the ratio of mean fluorescence per cell for each individual T904 concentration to the mean fluorescence per cell at 0 μM T904. Gene expression in cells transfected with pSV40-β-gal was measured using the Pierce β-Galactosidase Assay Kit (Thermo Scientific). β-gal expression was normalized to total protein content determined by Pierce BCA Protein Assay Kit (Thermo Scientific). Transfection efficiency for PEI/ pSV40-β-gal at each T904 concentration was expressed as transfection efficiency relative to PEI/ pSV40-β-gal with 0 µM T904 using absorbance per mg protein with sample size n=6. Relative transfection was calculated as the ratio of absorbance per mg protein for each individual T904 concentration to the absorbance per mg protein at 0 μM T904. Cytotoxicity was evaluated by MTT assay (each experiment (n=3) independently replicated 3 times). For visualization of transfection efficiency, cells were fixed using 4% paraformaldehyde 48 h after transfection and imaged using an epifluorescent microscopy (Zeiss Axiovert 200).
2.4 Effect of delayed addition of T904
These studies evaluated the effect of T904 when added separately at various time points post-transfection. C6 cells were seeded, transfected with polyplexes (7.5/1 N/P ratio, 2 μg DNA / well) in the presence of 5% serum for 4 h, and then changed to fresh medium with 5% serum. After an additional 0, 4, 8, or 24 h incubation, soluble T904 was added drop-wise into each well to a final concentration of 5 or 10 μM with medium exchange 24 h following the addition of T904. Transfection efficiency was assessed by flow cytometry 48 h after the initial 4 h transfection period regardless of the time point of T904 addition with sample size n=3.
2.5 Plasmid and T904 labeling
All reagents for labeling were purchased from Sigma-Aldrich unless otherwise stated. For transfection using labeled plasmids, pDNA was incubated with the nucleic acid stain YOYO-1 iodide (YOYO-1, Life Technologies) at a ratio of 0.02 μL YOYO-1 per microgram pDNA for 10 minutes at room temperature prior to complexation with PEI. T904 was labeled with rhodamine B isothiocyanate (RITC) using an adapted protocol (20). The hydroxyl groups of T904 (200 mg) were activated using 213 mg 1,1′-carbonyldiimidazole in 10 mL of acetonitrile for 2 h at 37°C and then reacted with 313 mg ethylenediamine in 20 mL ethanol for 12 h at room temperature. The reaction mixture was first dialyzed in a 2 kDa MWCO membrane (Spectrum Laboratories, Rancho Dominguez, CA) against 15% ethanol for 24 h and then dialyzed against deionized water for 48 h and finally lyophilized to obtain amino-T904. Amino-T904 (5 mg) was dissolved in 2 mL acetonitrile, mixed with 2 mL 0.1 M sodium carbonate buffer, and reacted with 10 mg RITC in 1 mL dimethylformamide. After 2 h reaction at room temperature T904-RITC was dialyzed against 20% ethanol for 24 h and then deionized water for 48 h at 4 °C in the dark and lyophilized.
2.6 Cellular uptake and localization of polyplex and T904
Cells were transfected as described above using polyplexes containing YOYO-1 labeled pCMV-GFP or pSV40-β-gal with 0, 5, or 10 μM T904. At 0.5, 2, and 4 h post-transfection, cells were washed with cold PBS, fixed, and cellular uptake of polyplexes measured using flow cytometry and expressed as mean fluorescence per cell relative to PEI/pCMV-GFP at 30 minutes with sample size n=3 replicated 3 times. The same experiment was performed at 4 °C where endocytosis is inhibited as a negative control to account for the possibility of non-internalized vector associated with exterior of the plasma membrane. Localization of polyplex and T904 was examined using a Nikon Ti laser-scanning confocal microscope (Melville, NY). C6 cells were seeded (150,000 cells/mL) in chambered cell culture slides (Thermo Scientific, Rochester, NY) with 1 mL working volume and surface area similar to 12 well plate wells. After 24 h, media was exchanged with DMEM/F12 without phenol-red (Mediatech) containing 5% serum and polyplex solution (2 μg YOYO-1 labeled pCMV-GFP) with or without a 5 µM T904-RITC mixture (T904-RITC: T904 = 1:10) was added drop-wise to each well. In addition, 5 μL Hoechst 33342 trihydrochloride (2 mg/mL in water) was added to each chamber 10 minutes prior to imaging to visualize nuclei. Fluorescent images were obtained in a z-stack with 1 horizontal image at the approximate center of each stack used to visualize the distribution of internalized T904-RITC and YOYO-1-labeled pDNA and the nucleus.
2.7 Nuclear localization
C6 cells were transfected as previously described with YOYO-1 labeled pCMV-GFP or pSV40-β-gal and 0–10 μM T904. After 2, 4, or 8 h; cells were trypsinized, washed with cold PBS, and centrifuged at 1500 RPM for 3 minutes. To isolate nuclei, cell pellets with approximately 5 × 106 cells were gently resuspended in 500 μL hypotonic buffer containing 20 mM Tris-HCl (pH 7.4), 10 mM NaCl, and 3 mM MgCl2 and incubated on ice for 15 minutes. Nuclei were isolated with the addition of 25 μL 10% NP-40 (Life Technologies) and vortexing on highest setting for 8 seconds. The homogenate was then centrifuged for 10 minutes at 3,000 RPM and 4°C and washed twice with cold PBS. Recovered nuclei pellets were resuspended in PBS and assayed by flow cytometry. Nuclear localization for each group was expressed as mean fluorescence per nucleus relative to YOYO-1 labeled pCMV-GFP at 2 h (each experiment (n=3) independently replicated 3 times).
2.7 Quantitative RT-PCR
Total RNA was isolated from C6 cells transfected with PEI/pCMV-GFP or PEI/pSV40-β-gal and 0–10 µM T904 at 24 h post-transfection using the Qiagen RNeasy mini kit (Qiagen). Isolated RNA was treated with Turbo DNA-free DNase I Kit (Life Technologies) to remove trace amounts of genomic or plasmid DNA. cDNA was synthesized using 1 μg of total RNA from each sample as template using Retroscript Kit (Life Technologies). Real-time RT-PCR was performed with Quantitect SYBR green PCR Kit (Qiagen) using primers designed for the GFP (5′-ATCATGGCCGACAAGCAGAAGAAC-3′ and 5′-TCTTGAAGTCGCAGCGGTAG-3′) and β-galactosidase reporter genes (5′CGGACACGGACAGGATTGACAGAT-3′ and 5′-TCAATCTCGGGTGGCTGAACGC-3′) as well as endogenous genes Interleukin-6 (IL-6, 5′-GTATGAACAGCGATGATGCAC-3′ and 5′-GACCAGAAGACCTCAAGGCA-3′), IκB-α (5′-TGGAGCACTTGGTGACTTTG-3′ and 5′-CAGAGCGAGGACAACTTCAC-3′), and heat shock protein (hsp70, 5′-GCGAGGCTGACAAGAAGAAG-3′ and 5′-CACGTTGGGCTAGTAGTCG-3′) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) internal standard in a Rotor-Gene 3000 Real-Time Thermal Cycler (Qiagen). Relative gene expression levels were calculated using 2−ΔΔCt method with GAPDH as an internal standard (25) (each experiment (n=3) independently replicated 3 times).
2.8 Statistical Analysis
Results were analyzed using one-way ANOVA with Tukey’s test for mean differences (significance p<0.05).
3. Results
3.1 Particle size and zeta potential
We first examined the potential effect of T904 on the physical/chemical properties of 25 kDa branched PEI-based polyplexes (Table I). Particle size of PEI/pDNA complexes was about 137 nm and increased slightly in the presence of T904. Zeta potential of PEI/pDNA was about 41.16 mV and modestly decreased at higher T904 concentrations. Overall, all complexes retained particle size less than 200 nm and positive charge suitable for efficient cellular uptake.
Table 1.
Mean particle size (PS) and zeta potential (ZP) of PEI/pDNA (N/P = 7.5/1) polyplexes with various concentrations of T904
| T904 Concentration (μM) | PS (nm) | ZP (mV) |
|---|---|---|
| 0 | 137.03±0.62 | 41.16±0.48 |
| 1 | 146.37±1.11 | 41.70±0.13 |
| 2.5 | 142.37±1.18 | 40.26±1.56 |
| 5 | 152.23±1.62 | 43.18±2.58 |
| 7.5 | 136.67±2.07 | 36.88±0.67 |
| 10 | 156.10±2.26 | 34.98±0.40 |
3.2 Transfection efficiency and cytotoxicity
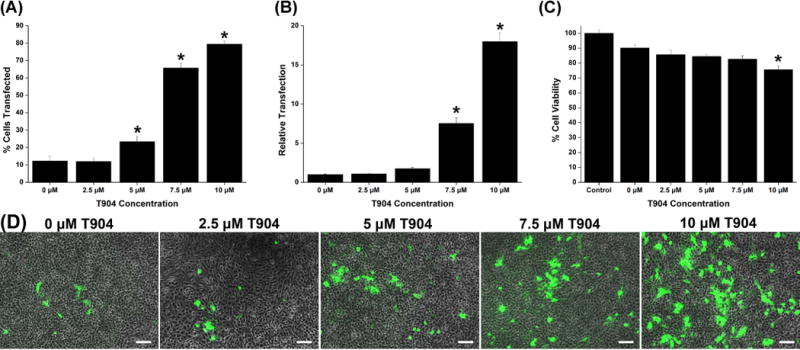
We next investigated the effect of T904 at varying concentrations on polyplex-mediated transfection and cell viability. Addition of T904 caused significant dose-dependent increases in transfection in C6 cells (Figure 1A). Transfection efficiency increased from 12.3% without T904 to 79.5% with 10 μM T904. Relative transfection measured by mean fluorescence increased approximately 2-, 8-, and 18-fold at 5, 7.5 and 10 μM T904, respectively (Figure 1B). Cell viability measured by MTT assay significantly decreased only at 10 μM relative to cells transfected with PEI/pCMV-GFP alone (Figure 1C). Cell viability in cultures transfected with PEI/pCMV-GFP alone was approximately 90% and greater than 75% for all groups transfected in the presence of T904. In a separate experiment, we also tested the cytotoxicity of T904 in non-transfected cells and found that T904 had no significant effect on viability up to 20 μM, the maximum concentration tested (data not shown). Figure 1D shows representative images of cells transfected with varying concentrations of T904.
Figure 1.
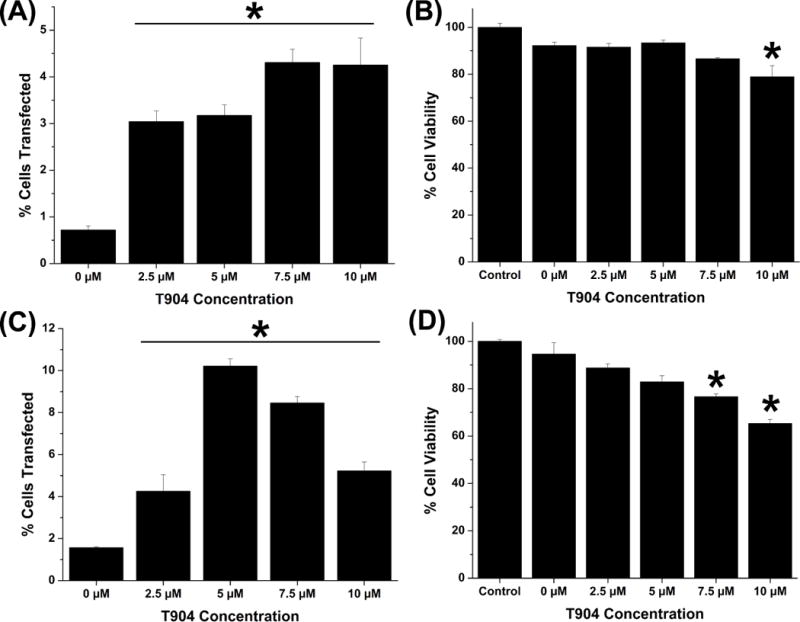
We also evaluated the effect of T904 on transfection and cell viability in primary human cell cultures. Transfection efficiency in NHDF increased with increasing T904 concentration and all groups transfected in the presence of T904 exhibited significantly higher transfection than the group transfected with PEI/pCMV-GFP alone (Figure 2A). Cell viability significantly decreased at 10 μM T904 relative to cells transfected with PEI/pCMV-GFP alone (Figure 2B). T904 also significantly increased transfection efficiency in hMSC at all concentrations, although the highest level was achieved at 5 μM T904 with 10.2% cells transfected compared to 1.6% cells transfected in the absence of T904 (Figure 2C). However, transfection efficiency subsequently decreased at 7.5 μM and 10 μM T904, likely caused by moderate cytotoxicity at higher T904 concentrations (Figure 2D).
Figure 2.
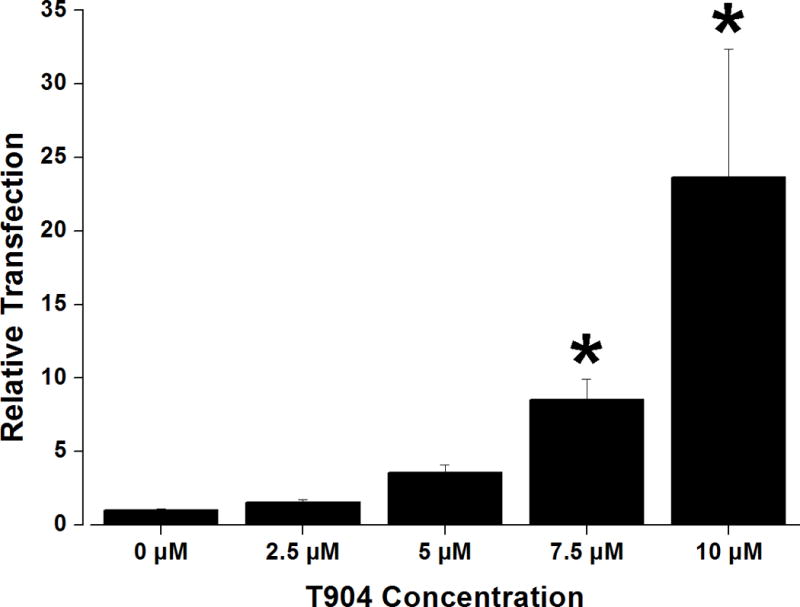
In order to investigate whether the ability of T904 to increase transfection was promoter-dependent, transfection studies were also performed using a plasmid encoding beta-galactosidase under the SV-40 promoter. Transfection increased in a dose-dependent manner with increasing T904 concentration, achieving an approximately 23-fold increase in the presence of 10 μM T904 relative to transfection with PEI/pSV40-β-gal alone (Figure 3).
Figure 3.
3.3 Effect of delayed T904 addition
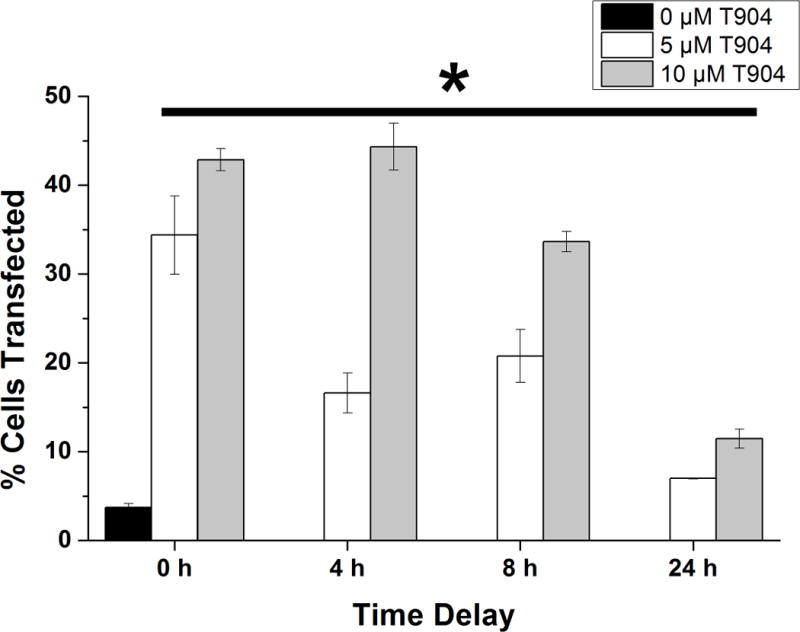
As a first step towards differentiating between possible effects of T904 on cellular internalization of polyplex versus later intracellular events, we performed a 4 hour transfection using PEI/pCMV-GFP polyplex alone followed by medium exchange and addition of soluble T904 either immediately (time 0) or at 4, 8, and 24 hours later during culture (Figure 4). Our expectation was that if cellular uptake of polyplex or to a lesser extent nuclear import of plasmid were the primary mechanisms by which T904 acted to increase transfection efficiency, delaying the delivery of T904 to cells after transfection would render T904 ineffective. While transfection efficiency decreased with increasing delay of T904 addition, all transfection conditions including a delay of 24 h between polyplex incubation and T904 addition resulted in significant increases in transfection efficiency compared to PEI/pCMV-GFP without T904. Overall transfection levels both with and without T904 were slightly lower than observed in the initial dose-response studies, likely due to the 4 h exposure to polyplex in these studies as opposed to the 24 h exposure time used earlier.
Figure 4.
3.4 Polyplex internalization and plasmid nuclear localization
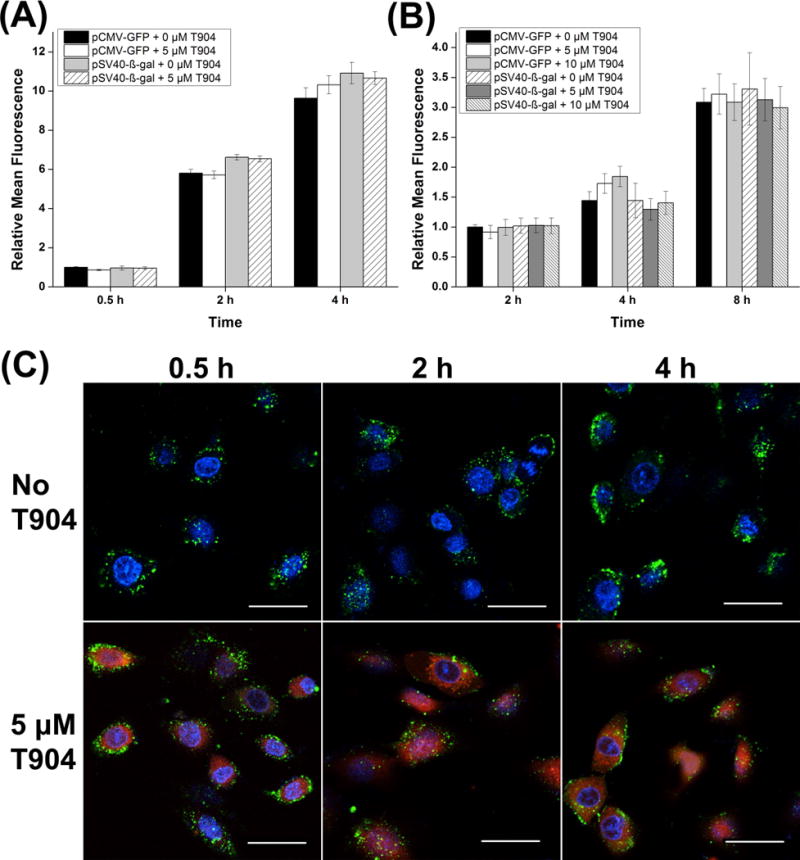
Fluorescently labeled pDNA and T904 were used to directly investigate the effects of T904 on polyplex internalization and nuclear uptake using both pCMV-GFP and pSV40-β-gal plasmids. Both cellular uptake and nuclear localization of plasmid measured by mean fluorescence increased as the duration of polyplex incubation increased (Figure 5 A and B). However, cell internalization of polyplex was not significantly affected by T904 for either plasmid at 0.5, 2, or 4 h. The impact of cell membrane bound polyplex on the mean fluorescence was minimal as transfection at 4 °C yielded <5% mean fluorescence relative to corresponding transfection conditions at 37 °C (data not shown). Likewise, T904 also did not increase the amount of plasmid in the nucleus at 2, 4, or 8 h. Figure 5C shows representative single horizontal slices from confocal microscopy demonstrating high cell uptake of polyplex with and without 5 μM T904 after transfection with YOYO-1 labeled pCMV-GFP. At every time point T904-RITC was detected in both the cytoplasm and nuclei with elevated perinuclear localization, leading to the formation of a distinct border around the nuclei. No qualitative difference in cell internalization or nuclear localization of YOYO-1 labeled plasmid between transfection with and without 5 μM T904 was conclusively evident by confocal microscopy.
Figure 5.
3.5 Transcriptional activity
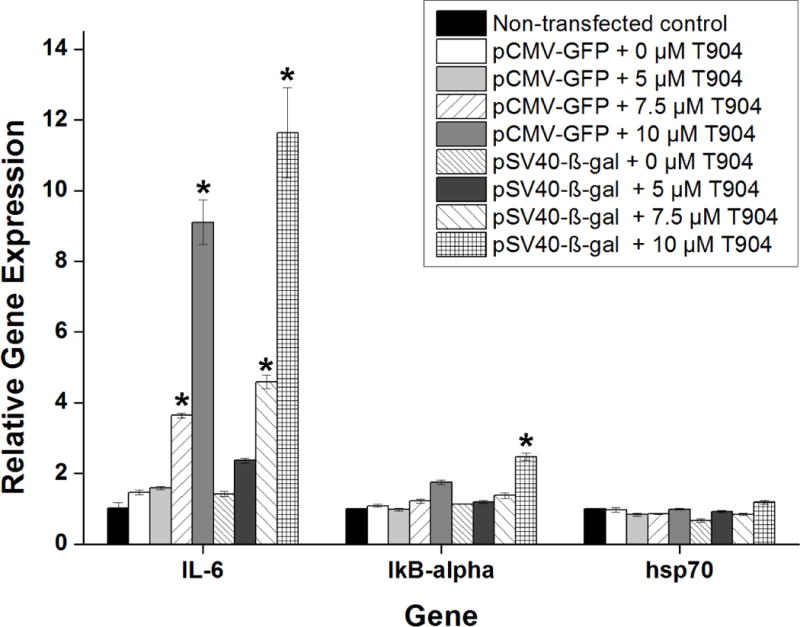
The effect of T904 on GFP and β-galactosidase mRNA expression levels was examined by qRT-PCR after transfection of both pCMV-GFP and pSV40-β-gal. Regardless of plasmid promoter type, the addition of T904 to PEI/pDNA polyplexes increased mRNA levels in a dose-dependent manner (Figure 6). Following 24 h incubation with polyplex and 10 μM T904, relative mRNA expression levels were significantly higher at 7.5 and 10 μM T904 relative to polyplex alone. Expression levels of β-gal were significantly higher than GFP at 7.5 μM T904, but not at 10 μM. The effect of T904 on mRNA expression levels of several endogenous genes associated with the NF-κB pathway was also examined. The addition of T904 significantly increased IL-6 expression in a dose dependent manner with both plasmid types at 7.5 and 10 μM T904 relative to polyplex alone (Figure 7). However, the expression levels of IκB-α and hsp70 remained relatively unchanged except for transfection with pSV40-β-gal and 10 μM T904 where a significant increase in IκB-α mRNA expression was detected.
Figure 6.
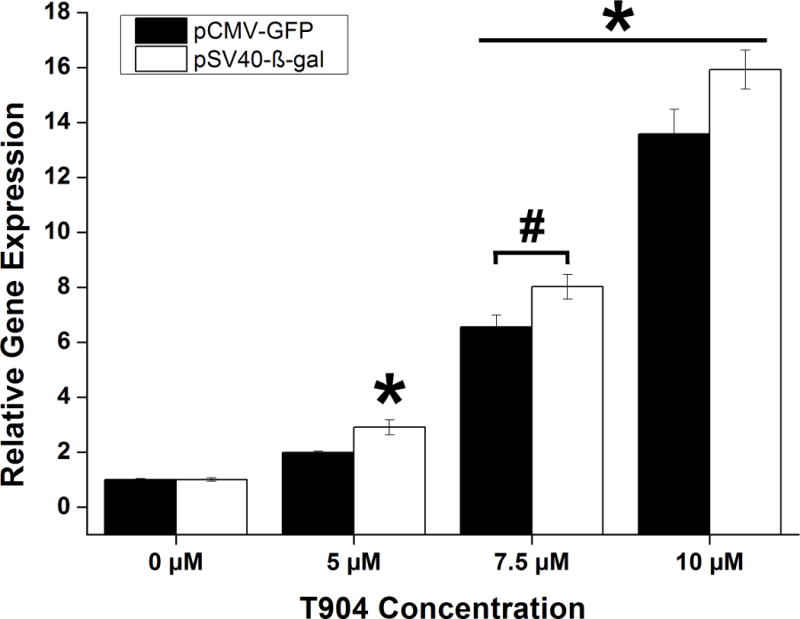
Figure 7.
4. Discussion
Due to the potential therapeutic benefits of safe and effective gene delivery systems, a significant literature exists pertaining to the development and optimization of nonviral vectors (26). A majority of studies have focused on modifications of vector chemical composition to address relatively early barriers to transfection such as serum aggregation/inhibition, endosomal entrapment, and cytotoxicity (27–30). However, even after reaching the cytoplasm, nonviral vectors face additional barriers such as intracellular trafficking, nuclear uptake, and efficient and stable transcription/translation (31,32). Amphiphilic block copolymers have recently emerged as a complimentary approach that offers the potential to assist vectors in navigating these later intracellular barriers by acting as biological response modifiers that activate intracellular biochemical processes that facilitate transgene expression. Several Pluronics have been shown to have broad effects on gene expression, enzymatic activity, and to potently increase the transfection efficiency of nonviral vectors (9). Despite similarities in chemical composition between Pluronics (poloxamers) and Tetronics (poloxamines), no investigations have been made on the efficacy of the latter for polyplex transfection.
In the present study, we examined the ability of the poloxamine Tetronic T904 to enhance polyplex transfection by delivering T904 in conjunction with polyplexes based on 25 kDa branched PEI. T904 was able to significantly increase polyplex-mediated transfection of C6, NHDF, and hMSC in a generally dose-dependent manner without substantially changing the physical/chemical characteristics of the polyplex itself. Overall gene expression levels as well as the impact of T904 on transfection efficiency were substantially higher in C6 cells compared to NHDF or hMSC, likely due to the inherently higher levels of gene expression observed in transformed and immortalized cell lines compared to primary cells (6). Cells were exposed to a maximum of 10 μM (0.0067% w/v) T904 for 24 h in all transfection experiments due to a reduction in cell viability at higher T904 concentrations. However, in the absence of polyplex, 10 μM T904 did not cause any toxicity in C6 cells, indicating that T904 alone does not have a significant impact on cell viability. Many previous studies have used considerably higher concentrations of Pluronics (0.03–1.0%), consistent with bolus delivery and subsequent diffusion in intramuscular gene transfer applications (20–22,24). Because our long-term goal is to create T904-based hydrogels for local nonviral gene delivery, we used relatively lower concentrations of T904 and longer exposure times to more closely approximate release of T904 during hydrogel degradation.
Many previous studies have observed that the ability of Pluronics to increase nonviral transfection and gene expression is dependent on the use of the CMV promoter that contains binding sites for the NF-κB transcription factor (11,24,33). Pluronic P85 has been shown to activate the NF-κB signaling pathway in cultured cells and inhibition of this pathway blocked Pluronic-mediated increases in transfection in intramuscular injection models (20,34). The possibility that T904 may share this promoter-dependent activity with Pluronics was assessed by transfecting C6 cells using PEI/pSV40-β-gal. Beta-galactosidase expression increased significantly with T904 dosage, achieving 23-fold higher expression. We have also qualitatively observed substantial increases in gene expression from another vector using the human EF-1α promoter (data not shown). These results suggest that unlike certain Pluronic formulations, T904 may act independent of promoter type and not rely on the NF-κB pathway to enhance transfection efficiency.
In order to gain more insight into T904’s mechanism of action, we investigated its effect on several key steps in transfection. One potential explanation for the activity of T904 is increased cell uptake of polyplex due to membrane destabilization previously observed with some PEO/PPO block copolymers such as Lutrol® (19). We first tested this possibility indirectly by adding T904 to transfected cultures at varying time points after removal of polyplex. If T904 acts by facilitating vector internalization, this approach would be expected to significantly reduce transfection. However, transgene expression was enhanced significantly following addition of T904 at every time point, consistent with a previous in vivo test of delayed administration (34). In addition, direct measurements of the internalization of fluorescently labeled plasmid by flow cytometry also revealed no increase in cellular uptake in the presence of T904. These results strongly suggest that the primary mechanism of T904 activity was modification of intracellular processes affecting transfection efficiency and not interactions with the cell membrane for increased uptake of polyplex. Recent work from Wasungu et al. also showed that Pluronic L64 increases gene expression without affecting membrane permeability or DNA transport in electroporation (35). The difference in polyplex internalization activity between Lutrol and Pluronic L64 and Tetronic T904 is likely a result of the difference in their composition, specifically hydrophilic / lipophilic balance [Lutrol: 24; L64/T904: 12–18] that influences their interactions will cellular membranes (36,37).
In order to achieve transgene expression, vectors must ultimately reach the cell nucleus. The nuclear membrane is widely considered a critical barrier to efficient nonviral transfection, particularly in non-dividing cells (38). Recently, Yang et al. demonstrated significant increases in nuclear localization of plasmid vectors containing a CMV promoter following polyplex transfection with Pluronic® P123 (20). The proposed mechanism was that Pluronic-activated NF-κB binds to sites within the CMV promoter providing a nuclear localization signal that can facilitate association with importins and active transport across the nuclear membrane. Gonçalves recently reported similar results where a plasmid containing a minimal promoter modified with three NF-κB binding sites achieved significantly increased transgene expression and nuclear uptake in cells stimulated with the NF-κB activator TNF-α, while nuclear uptake was significantly reduced in the presence of an NF-κB inhibitor (39). Similar DNA targeting sequences (DTS) capable of facilitating nuclear transport have been identified in other promoters as well (40). In particular, specific regions of the SV40 viral promoter often used in plasmid design facilitates active transport of cytoplasmic DNA through the nuclear pore complex (41). In our studies, addition of T904 did not affect the amount of plasmid detected in the nucleus for either plasmid containing CMV or SV-40 promoters. This may reflect the relatively rapid cell division rate of C6 cells since breakdown of the nuclear membrane during mitosis is a key mechanism of nuclear access in dividing cells (42). One confounding variable in the analysis of plasmid within the nucleus is potential interference from vectors bound to the exterior of the nuclear membrane (43). However, we did not observe any aggregation of nuclei indicative of excessive membrane bound polyplexes as has been previously described (44) and attempts at decomplexation of nuclear bound polyplexes with anionic sodium tripolyphosphate or non-labeled pDNA and digestion with restriction enzymes for both plasmid types did not significantly alter any flow cytometric observations (data not shown).
Since T904 did not affect the amount of plasmid within the nucleus, we considered potential changes in mRNA expression levels to be the most probable mechanism of action. qRT-PCR confirmed that mRNA transcript levels increased significantly with the addition of T904 in a dose-dependent manner with increasing T904 concentration that closely paralleled gene expression levels. This response was consistent for transfection with both pCMV-GFP and pSV40-β-gal plasmids. These results demonstrate that the increases in gene expression observed following polyplex transfection with T904 results primarily from the higher availability of mRNA transcripts. This outcome is consistent with an earlier study from Kabanov’s group in which it was shown that Pluronics P85 and L64 increased mRNA levels and protein expression in stably transfected cell lines where internalization / nuclear import were not variables (21).
In order to further investigate the potential role of transcription factor activity in T904-mediated increased transfection, we examined the expression levels of three endogenous genes associated with the NF-κB pathway: IL-6 known to be regulated primarily by AP-1 driven transcription and indirectly by NF-κB (45), IκB-α stimulated by the activation of NF-κB as part of a negative feedback mechanism (46), and hsp70 which is part of a family of stress inducible heat shock proteins previously examined by Sriadibhatla et al. for its activation by Pluronics in association with NF-κB activity (21). If NF-κB activity played a critical role in transcriptional activation, all three endogenous genes would be expected to experience increased mRNA expression levels with increasing amounts of T904 during transfection with pCMV-GFP. However, neither IκB-α or hsp70 mRNA levels were substantially affected by T904. In contrast, IL-6 expression was significantly increased by both pCMV-GFP and pSV40-β-gal. The contrasting transcriptional response of IL-6 and the other two endogenous genes suggests that T904 may act through transcription factors other than NF-kB, possibly including AP-1. Altogether, our findings suggest that enhanced transfection efficiency of branched PEI following the addition of T904 was primarily a result of increased transcription factor activity independent of the NF-κB pathway.
5. Conclusions
The present studies demonstrate that T904 shares the ability of some Pluronics to have a significant positive impact on the transfection efficiency of polyplex nonviral vectors and that the underlying mechanism is elevated mRNA expression levels, most likely attributable to T904-mediated activation of endogenous transcription factors. While the activity of Pluronics has been closely associated with the CMV promoter and activation of the NF-κB pathway, the similar results obtained in the present work with CMV and SV-40 promoters suggests that T904 may target a wider range of intracellular signaling pathways and have broader applicability. Although beyond the scope of the present studies, future work will further investigate these possibilities through microarray analysis, Western blotting to measure transcription factor levels in nuclear/cytoplasmic fractions, and transfection with minimal promoters activated by defined transcription factors. Finally, T904 is compatible with a variety of end group functionalization and covalent crosslinking methods that have been developed for preparing hydrogels from multi-arm polyethylene glycol. Hydrogels capable of releasing soluble T904 in combination with nonviral vectors during degradation may be particularly attractive for scaffold-mediated gene delivery applications.
Acknowledgments
This project was support by grants from the National Center for Research Resources (5P20RR021949-04) and the National Institute of General Medical Sciences (8 P 20 GM103444-04) from the National Institutes of Health. We gratefully acknowledge the assistance of Dr. Terri Bruce and the SC Biomat Histology and Imaging Core Facility for assistance with confocal imaging. We thank Dr. Alexey Vertegel for access to his particle size analyzer.
Footnotes
Conflicts of Interest Statement
There are no conflicts of interest to report.
Contributor Information
Jeremy Zhang, Email: jeremyz@clemson.edu.
Sooneon Bae, Email: sbae@clemson.edu.
Jeoung Soo Lee, Email: ljspia@clemson.edu.
Ken Webb, Email: kwebb@clemson.edu.
References
- 1.Tomanin R, Scarpa M. Why do we need new gene therapy viral vectors? Characteristics, limitations and future perspectives of viral vector transduction. Curr Gene Ther. 2004;4:357–372. doi: 10.2174/1566523043346011. [DOI] [PubMed] [Google Scholar]
- 2.Bonadio J. Tissue engineering via local gene delivery. J Mol Med. 2000;78:303–311. doi: 10.1007/s001090000118. [DOI] [PubMed] [Google Scholar]
- 3.Laporte L De, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliver Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Rorke S, Keeney M, Pandit A. Non-viral polyplexes: Scaffold mediated delivery for gene therapy. Prog Polym Sci. 2010;35:441–458. [Google Scholar]
- 5.Wang T, Upponi JR, Torchilin VP. Design of multifunctional non-viral gene vectors to overcome physiological barriers: dilemmas and strategies. Int J Pharm. 2012;427:3–20. doi: 10.1016/j.ijpharm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Van Gaal EVB, Van Eijk R, Oosting RS, Kok RJ, Hennink WE, Crommelin DJa, et al. How to screen non-viral gene delivery systems in vitro? J Control Release. 2011;154:218–232. doi: 10.1016/j.jconrel.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliver Rev. 2002;54:37–51. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 8.Kabanov AV, Alakhov V. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug. 2002;19:1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 9.Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130:98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahay G, Gautam V, Luxenhofer R, Kabanov AV. The utilization of pathogen-like cellular trafficking by single chain block copolymer. Biomaterials. 2010;31:1757–1764. doi: 10.1016/j.biomaterials.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alakhov V, Klinski E, Lemieux P, Pietrzynski G, Kabanov AV. Block copolypmerc biotransport carriers as versatile vehicles for drug delivery. Expert Opin Biol Th. 2001;1:583–602. doi: 10.1517/14712598.1.4.583. [DOI] [PubMed] [Google Scholar]
- 12.Guiraud S, Alimi-Guez D, Van Wittenberghe L, Scherman D, Kichler A. The reverse block copolymer Pluronic 25R2 promotes DNA transfection of skeletal muscle. Macromol Biosci. 2011;11:590–4. doi: 10.1002/mabi.201000463. [DOI] [PubMed] [Google Scholar]
- 13.Pitard B, Pollard H, Agbulut O, Lambert O, Vilquin J-T, Cherel Y, et al. A nonionic amphiphile agent promotes gene delivery in vivo to skeletal and cardiac muscles. Hum Gene Ther. 2002;13:1767–1775. doi: 10.1089/104303402760293592. [DOI] [PubMed] [Google Scholar]
- 14.Richard P, Bossard F, Desigaux L, Lanctin C, Bello-Roufai M, Pitard B. Amphiphilic block copolymers promote gene delivery in vivo to pathological skeletal muscles. Hum Gene Ther. 2005;16:1318–1324. doi: 10.1089/hum.2005.16.1318. [DOI] [PubMed] [Google Scholar]
- 15.Roques C, Fattal E, Fromes Y. Comparison of toxicity and transfection efficiency of amphiphilic block copolymers and polycationic polymers in striated muscles. J Gene Med. 2009;11:240–249. doi: 10.1002/jgm.1304. [DOI] [PubMed] [Google Scholar]
- 16.Kuo J-HS. Effect of Pluronic-block copolymers on the reduction of serum-mediated inhibition of gene transfer of polyethyleneimine-DNA complexes. Biotechnol Appl Bioc. 2003;37:267–271. doi: 10.1042/BA20020123. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal A, Vilensky R, Stockdale A, Talmon Y, Unfer RC, Mallapragada SK. Colloidally stable novel copolymeric system for gene delivery in complete growth media. J Control Release. 2007;121:28–37. doi: 10.1016/j.jconrel.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Cho CW, Cho YS, Lee HK, Yeom YI, Park SN, Yoon DY. Improvement of receptor-mediated gene delivery to HepG2 cells using an amphiphilic gelling agent. Biotechnol Appl Biochem. 2000;32:21–26. doi: 10.1042/ba20000022. [DOI] [PubMed] [Google Scholar]
- 19.Chèvre R, Le Bihan O, Beilvert F, Chatin B, Barteau B, Mével M, et al. Amphiphilic block copolymers enhance the cellular uptake of DNA molecules through a facilitated plasma membrane transport. Nucleic Acids Res. 2011;39:1610–1622. doi: 10.1093/nar/gkq922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z, Sahay G, Sriadibhatla S, Kabanov AV. Amphiphilic block copolymers enhance cellular uptake and nuclear entry of polyplex-delivered DNA. Bioconjug Chem. 2008;19:1987–1994. doi: 10.1021/bc800144a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sriadibhatla S, Yang Z, Gebhart C, Alakhov VY, Kabanov A. Transcriptional activation of gene expression by pluronic block copolymers in stably and transiently transfected cells. Mol Ther. 2006;13:804–813. doi: 10.1016/j.ymthe.2005.07.701. [DOI] [PubMed] [Google Scholar]
- 22.Prokop A, Kozlov E, Moore W, Davidson JM. Maximizing the in vivo efficiency of gene transfer by means of nonviral polymeric gene delivery vehicles. J Pharm Sci. 2002;91:67–76. doi: 10.1002/jps.1171. [DOI] [PubMed] [Google Scholar]
- 23.Pitard B, Bello-Roufaï M, Lambert O, Richard P, Desigaux L, Fernandes S, et al. Negatively charged self-assembling DNA/poloxamine nanospheres for in vivo gene transfer. Nucleic Acids Res. 2004;32:e159. doi: 10.1093/nar/gnh153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, Zhu J, Sriadibhatla S, Gebhart C, Alakhov V, Kabanov A. Promoter- and strain-selective enhancement of gene expression in a mouse skeletal muscle by a polymer excipient Pluronic P85. J Control Release. 2005;108:496–512. doi: 10.1016/j.jconrel.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Mintzer MA, Simanek EE. Nonviral Vectors for Gene Delivery. Chem Rev. 2009;11(2):259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 27.El-Sayed M, Hoffman AS, Strayton PS. Smart polymeric carriers for enhanced intracellular delivery of therapeutic macromolecules. Expert Opin Biol Th. 2005;5:23–32. doi: 10.1517/14712598.5.1.23. [DOI] [PubMed] [Google Scholar]
- 28.Xiong MP, Forrest ML, Karls AL, Kwon GS. Biotin-triggered release of poly(ethylene glycol)-avidin from biotinylated polyethylenimine enhances in vitro gene expression. Bioconjug Chem. 2007;18:746–753. doi: 10.1021/bc0602883. [DOI] [PubMed] [Google Scholar]
- 29.Gosselin MA, Guo W, Lee RJ. Efficient gene transfer using reversibly cross-linked low molecular weight polyethylenimine. Bioconjug Chem. 2001;12:989–994. doi: 10.1021/bc0100455. [DOI] [PubMed] [Google Scholar]
- 30.Needham CJ, Williams AK, Chew SA, Kasper FK, Mikos AG. Engineering a polymeric gene delivery vector based on poly(ethylenimine) and hyaluronic acid. Biomacromolecules. 2012;13:1429–1437. doi: 10.1021/bm300145q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lechardeur D, Lukacs GL. Intracellular barriers to non-viral gene transfer. Curr Gene Ther. 2002;2:183–194. doi: 10.2174/1566523024605609. [DOI] [PubMed] [Google Scholar]
- 32.Vaughan EE, DeGiulio JV, Dean DA. Intracellular trafficking of plasmids for gene therapy: mechanisms of cytoplasmic movement and nuclear import. Curr Gene Ther. 2006;6:671–681. doi: 10.2174/156652306779010688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavigne MD, Pohlschmidt M, Novo JF, Higgins B, Alakhov V, Lochmuller H, et al. Promoter dependence of plasmid-pluronics targeted alpha galactosidase A expression in skeletal muscle of Fabry mice. Mol Ther. 2005;12:985–990. doi: 10.1016/j.ymthe.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 34.Lavigne MD, Yates L, Coxhead P, Górecki DC. Nuclear-targeted chimeric vector enhancing nonviral gene transfer into skeletal muscle of Fabry mice in vivo. FASEB J Biology. 2008;22:2097–2107. doi: 10.1096/fj.07-093765. [DOI] [PubMed] [Google Scholar]
- 35.Wasungu L, Marty aL, Bureau MF, Kichler A, Bessodes M, Teissie J, et al. Pre-treatment of cells with pluronic L64 increases DNA transfection mediated by electrotransfer. J Control Release. 2011;149:117–125. doi: 10.1016/j.jconrel.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 36.Alexandridis P, Hatton TA. Poly(ethylene oxide)—poly(propylene oxide)—poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: thermodynamics, structure, dynamics, and modeling. Colloid Surface A. 1995;96:1–46. [Google Scholar]
- 37.Alvarez-Lorenzo C, Gonzalez-Lopez J, Fernandez-Tarrio M, Sandez-Macho I, Concheiro A. Tetronic micellization, gelation and drug solubilization: Influence of pH and ionic strength. Eur J Pharm Biopharm. 2007;66:244–252. doi: 10.1016/j.ejpb.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Peters R. Nuclear envelope permeability measured by fluorescence microphotolysis of single liver cell nuclei. J Biol Chem. 1983;258:11427–11429. [PubMed] [Google Scholar]
- 39.Goncalves C, Ardourel M-Y, Decoville M, Breuzard G, Midoux P, Hartmann B, et al. An optimized extended DNA kappa B site that enhances plasmid DNA nuclear import and gene expression. J Gene Med. 2009;11:401–411. doi: 10.1002/jgm.1312. [DOI] [PubMed] [Google Scholar]
- 40.Lam AP, Dean DA. Progress and prospects: nuclear import of nonviral vectors. Gene Ther. 2010;17:439–447. doi: 10.1038/gt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dean DA. Import of plasmid DNA into the nucleus is sequence specific. Exp Cell Res. 1997;230:293–302. doi: 10.1006/excr.1996.3427. [DOI] [PubMed] [Google Scholar]
- 42.Brunner S, Sauer T, Carotta S, Cotten M, Saltik M, Wagner E. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther. 2000;7:401–7. doi: 10.1038/sj.gt.3301102. [DOI] [PubMed] [Google Scholar]
- 43.Glover D, Leyton D. The efficiency of nuclear plasmid DNA delivery is a critical determinant of transgene expression at the single cell level. J Gene Med. 2010;12:77–85. doi: 10.1002/jgm.1406. [DOI] [PubMed] [Google Scholar]
- 44.Cohen RN, Van der Aa MAEM, Macaraeg N, Lee AP, Szoka FC. Quantification of plasmid DNA copies in the nucleus after lipoplex and polyplex transfection. J Control Release. 2009;135:166–74. doi: 10.1016/j.jconrel.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao W, Hodge DR, Wang L, Yang X, Shang Z, Farrar WL. NF-kB activates IL-6 expression through cooperation with c-jun and IL6-AP1 site, but is independent of its IL6-NF-kB regulatory site in autocrine human multiple myeloma cells. Cancer Biol Ther. 2004;3:1007–17. doi: 10.4161/cbt.3.10.1141. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman A, Baltimore D. Circuitry of nuclear factor kB signaling. Immunol Rev. 2006;210:171–86. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]


