Abstract
We studied AML patients over age 50 in CR1 after adult unrelated donor (URD; n = 441, 8/8 and n = 94 7/8 HLA-matched) or umbilical cord blood (UCB; n = 205) transplantations. UCB recipients less often achieved CR1 within 8 weeks, more often received reduced-intensity conditioning, and cyclosporin-based graft-versus-host disease (GVHD) prophylaxis. Neutrophil recovery was slower in UCB (69% by day 28) vs. 8/8 URD (97%); 7/8 (91%) (p<0.001). Three-year transplant-related mortality (TRM) was higher and leukemia-free survival (LFS) lower with UCB (35% and 28%, respectively) vs. 8/8 URD (27% and 39%). TRM was higher in 7/8 URD (41%, p=0.01), but LFS similar 34% (p=0.39). Three-year chronic GVHD was least in UCB (28%) vs. 53% and 59% in 8/8 and 7/8 URD recipients. Three-year survival was 8/8 URD 43% (95% CI 38-48), UCB 30% (95% CI 23-37) (p=0.002) and 7/8 URD 37% (95% CI 27-46). Allotransplantation for AML in CR1 with any of these grafts extends LFS for over a third of older patients. In the absence of an 8/8 HLA-matched URD or when transplantation is needed urgently, UCB can provide extended survival. Less frequent chronic GVHD with UCB transplantation may be of particular value for older patients.
Keywords: Allogeneic transplantation, AML, umbilical cord blood, unrelated donors
INTRODUCTION
Post-remission allogeneic transplantation is increasingly used for treatment of the high-risk acute myeloid leukemia (AML) affecting older adults [1-14]. While previous reports have validated the utility of several alternative donor sources, little data exists to carefully examine differences in toxicities, outcome, and potency of leukemia control amongst the available donor choices in these patients. Reports that have compared fully HLA-matched bone marrow and peripheral blood progenitor cell to mismatched umbilical cord blood (UCB) transplantations confirm comparable leukemia-free survival between the three graft sources [15,16]. Transplant-related mortality (TRM) rates are higher and hematopoietic recovery and chronic graft-versus-host disease (GVHD) lower after UCB compared to matched bone marrow and peripheral blood progenitor cell transplantation. A limitation of published reports is the inclusion of patients with both acute myeloid and lymphoblastic leukemia and inclusion of adults of all ages with the median ages of study cohorts ranging from 30 - 40 years. Data from the Center for International Blood and Marrow Transplant Research show 60% of allogeneic transplant recipients with hematologic malignancies in the United States are now over the age of 50. Because older adults (aged 50 years or older) less frequently have available healthy siblings to be donors, volunteer adult unrelated donors (URD) or umbilical cord blood (UCB) grafts can facilitate the curative potential of allotransplantation and to date, haploidentical transplants are rarely performed in this age group. While physicians may have little hesitation in recommending HLA-matched sibling donor transplantation, higher GVHD and mortality risks associated with unrelated donor (URD) transplantation might limit others from offering this treatment option for older adults [17-20]. As GVHD risks are high after mismatched URD transplantation there is further hesitation in proceeding to transplantation when an HLA-matched adult unrelated donor is not available. Therefore, in the current analyses we examined outcomes in 742 adults over the age of 50 years with AML in first complete remission (CR1) to compare the effectiveness of transplantation of grafts from HLA-matched and mismatched adult donors to that after HLA-mismatched UCB..
METHODS
Patients
Data were obtained from the Center for International Blood and Marrow Transplant Research or Eurocord. Included are allogeneic transplant recipients with AML in CR1, aged 50 years and older. Patient characteristics and treatment details are summarized in Table 1. Three treatment groups were created based on graft type and donor-recipient HLA-match. These include adult URD donor-recipient pairs matched at HLA-A, -B, -C, -DRB1 at the allele-level (8/8 matched), mismatched at a single locus (7/8 matched) and UCB donor-recipient pairs with the majority (96%) mismatched at one or two HLA-loci. HLA-matching between units and recipients considered lower resolution (antigen-level) match at HLA-A and –B and allele-level at DRB1. Matching at HLA-C or allele-level HLA-matching at HLA-A, -B were not considered as these data were not available. HLA-match between UCB units (N=125) was not considered as the sample size prohibits exploring any affect on transplantation outcomes. Among the 535 recipients of adult donor grafts, 458 (86%) received peripheral blood progenitor cells and the remaining 77 (14%), bone marrow. Of the 205 recipients of UCB grafts, 80 (39%) received one UCB unit and 125 (61%) received two units. UCB transplants with a single unit and two units were grouped together as reports have not shown significant differences in survival [21]. Transplants were performed between 2005 and 2010. The institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Table 1.
Patient, disease and transplantation characteristics
| Variables | 8/8 HLA-matched | 7/8 HLA-matched | Cord blood | p-value |
|---|---|---|---|---|
| Total number | 441 | 94 | 205 | |
| Age, years | 0.72 | |||
| Median (range) | 58 (50-75) | 58 (50-72) | 59 (50-71) | |
| 50 - 60 | 263 (60%) | 58 (62%) | 117 (57%) | |
| 61 - 75 | 178 (40%) | 36 (38%) | 88 (43%) | |
| Gender | 0.05 | |||
| Male | 262 (59%) | 53 (56%) | 99 (48%) | |
| Female | 179 (41%) | 41 (44%) | 105 (52%) | |
| CMV serostatus | 0.90 | |||
| Negative | 172 (39%) | 34 (36%) | 72 (35%) | |
| Positive | 260 (59%) | 58 (62%) | 128 (62%) | |
| Not reported | 9 ( 2%) | 2 ( 2%) | 2 ( 2%) | |
| WBC at diagnosis | <0.001 | |||
| < 25 x 109/L | 328 (74%) | 72 (77%) | 129 (63%) | |
| ≥ 25 x 109/L | 82 (19%) | 13 (14%) | 38 (19%) | |
| Not reported | 31 ( 7%) | 9 (10%) | 38 (19%) | |
| Time to achieve CR1 | <0.001 | |||
| < 8 weeks | 307 (70%) | 66 (70%) | 105 (51%) | |
| > 8 weeks | 127 (29%) | 24 (26%) | 68 (33%) | |
| Not reported | 7 ( 2%) | 4 ( 4%) | 32 (16%) | |
| Cytogenetic risk | <0.001 | |||
| Favorable | 14 ( 3%) | 1 ( 1%) | 5 ( 2%) | |
| Intermediate | 154 (35%) | 33 (35%) | 95 (46%) | |
| Unfavorable | 133 (30%) | 31 (33%) | 76 (37%) | |
| Conditioning regimen | ||||
| Myeloablative | <0.001 | |||
| TBI + other agents* | 49 (11%) | 7 ( 7%) | 18 ( 9%) | |
| Busulfan + cyclophosphamide | 78 (18%) | 15 (16%) | 10 ( 5%) | |
| Busulfan + fludarabine | 92 (21%) | 22 (23%) | 14 ( 7%) | |
| Reduced intensity | ||||
| TBI 200 cGy + cyclophosphamide + fuldarabine | 1 (<1%) | 137 (67%) | ||
| Fludarabine + alkylating agent | 188 (43%) | 40 (43%) | 23 (11%) | |
| TBI 200 cGy + fludarabine | 33 ( 7%) | 10 (11%) | 3 ( 1%) | |
| GVHD prophylaxis | <0.001 | |||
| Tacrolimus + mycophenolate | 94 (21%) | 24 (26%) | 30 (15%) | |
| Tacrolimus + methotrexate | 247 (56%) | 47 (50%) | 4 ( 2%) | |
| Tacrolimus alone | 36 ( 9%) | 4 ( 4%) | 12 ( 5%) | |
| Cyclosporine + mycophenolate | 35 ( 8%) | 14 (15%) | 149 (73%) | |
| Cyclosporine + methotrexate | 27 ( 6%) | 4 ( 4%) | 2 ( 1%) | |
| Cyclopsorine alone | 2 (<1%) | 1 ( 1%) | 1 (<1%) | |
| In vivo T-cell depletion | <0.001 | |||
| None | 267 (61%) | 47 (50%) | | 130 (63%) | |
| Yes | 174 (39%) | 47 (50%) | 65 (32%) | |
| Not reported | -- | -- | 10 ( 5%) | |
| Graft type | ||||
| Peripheral blood progenitor cells | 377 (85%) | 81 (86%) | -- | |
| Bone marrow | 64 (15%) | 13 (14%) | -- | |
| One cord blood unit | -- | 80 (39%) | ||
| Two cord blood units | -- | -- | 125 (61%) | |
| Transplant period | <0.001 | |||
| 2005 - 2007 | 249 (56%) | 57 (61%) | 42 (20%) | |
| 2008 - 2010 | 192 (44%) | 37 (39%) | 163 (80%) | |
| Follow-up, median (range), months | 50 (3 - 86) | 61 (3 - 91) | 37 (3 - 85) |
Abbreviations: TBI = total body irradiation;
Other agents include: 8/8 HLA-matched transplants: cyclophosphamide n=48; melphalan n=1 7/8 HLA-matched transplants: cyclophosphamide n=6; busulfan n=1 cord blood: cyclophosphamide n=14; fludarabine n=1; etoposide n=1; not reported n=2
Outcomes
Neutrophil recovery was defined as achieving an absolute neutrophil count ≥0.5 × 109/L for three consecutive days and platelet recovery to 20 x 109/L by day 90. Grade II - IV acute GVHD and chronic GVHD were assigned using standard criteria [22, 23] as data to determine NIH chronic GVHD classifications were not available for most of the patients. TRM was defined as death occurring in the absence of leukemia relapse. Leukemia relapse was defined as molecular, cytogenetic or morphologic evidence of recurrence. Treatment failure was defined as relapse or death from any cause; the inverse of leukemia-free survival. Overall mortality was defined as death from any cause.
Statistical analysis
We compared demographics, treatment characteristics and outcomes amongst the three treatment groups: 8/8 HLA-matched URD, 7/8 HLA-matched URD and mismatched UCB transplantations. Chi-square or the Wilcoxon statistic was used to compare patient, disease and transplantation characteristics between the three treatment groups for categorical or continuous variables, respectively. The probabilities of neutrophil recovery, acute and chronic GVHD, TRM and leukemia relapse were calculated using the cumulative incidence estimator [23], and leukemia-free and overall survival were calculated using the Kaplan and Meier estimator [24]. To study the association between treatment groups and outcomes, multivariate Cox regression models were built [25]. Results are expressed as hazard ratios (HR). All p-values are two-sided and values ≤0.05 were considered significant.
Multivariate models were built using the forward step-wise selection process considering variables shown in Table 1. Treatment group, 8/8 HLA-matched, 7/8 HLA-matched UCB transplants, as the main study question was included in all steps of model building regardless of level of significance. The other variables tested were retained in the final multivariate model if the variable attained the level of significance set for these analyses. All models met the assumptions of proportionality except for TRM. Therefore, a time-dependent model was created for TRM and the effects are shown as within 3 months after transplantation and beyond this period. There were no first order interactions. Analyses were done using SAS (version 9.3, Cary, NC).
RESULTS
Patients, disease and transplantation characteristics
Amongst the 441 8/8 HLA matched URD, 94 7/8 HLA matched and 205 UCB transplants, their ages at transplantation were similar. The median ages at transplantation were 58 years for URD recipients and 59 years for UCB. However, slightly more UCB recipients were female and a smaller fraction had a lower white blood count at diagnosis. Performance score at transplantation was not available for 89 UCB recipients (43%). However, among those for whom performance score was available, there were no differences across the three treatment groups; 274 of 441 (62%) recipients of 8/8 HLA-matched, 60 of 94 (64%) recipients of 7/8 HLA-matched and 87 of 116 recipients of UCB transplantations reported scores of 90 or 100 (p=0.15). UCB recipients were less likely to achieve CR1 within eight weeks from diagnosis. Recipients of 7/8 HLA-matched (22/94; 23%) transplants were more likely to have had myelodysplastic syndrome preceding AML compared to 8/8 HLA-matched (70/441; 16%) and UCB (31/205; 15%) transplants (p=0.05). In a subset of patients (N=352; transplantations between 2008 and 2010) data on chemotherapy delivered prior to transplantation was available. In these patients, 119/192 (61%) recipients of 8/8 HLA-matched, 25/37 (68%) recipients of 7/8 HLA-matched and 67/96 (70%) recipients of UCB transplantation proceeded to transplantation after induction therapy (p=0.12). Approximately 45% of recipients of 8/8 HLA-matched and UCB transplantations received a single cycle of induction therapy compared to 50% of recipients of 7/8 HLA-matched transplants (p=0.94). Recipients of 8/8 HLA-matched and UCB transplantations were more likely to proceed to transplantation within 3 months of achieving CR1; 175 of 441 (40%) recipients of 8/8 HLA-matched URD and 90 of 205 (44%) recipients of UCB transplants compared to 24 of 94 (26%) of recipients of 7/8 HLA-matched URD transplants (p<0.001). Data on cytogenetic risk was not available for a third of adult donor transplants and 14% of UCB transplants. Among those for whom data were available, unfavorable risk cytogenetics was equally likely across the three treatment groups: 37% among UCB recipients compared to 30% and 33% among matched and mismatched URD recipients. There were differences between UCB and URD transplants with respect to transplant conditioning and GVHD prophylaxis. UCB recipients were more likely to receive reduced intensity conditioning (RIC) whereas URD recipients were just as likely to receive myeloablative or RIC. The combination of low dose (200 cGy) total body irradiation (TBI) with cyclophosphamide and fludarabine was used exclusively with UCB grafts. On the other hand, fewer than 10% of URD recipients received TBI 200 cGy and fludarabine. Nearly half of all RIC URD transplants received an alkylating agent with fludarabine. Tacrolimus-based GVHD prophylaxis was used most often in the URD recipients and cyclosporine combinations for UCB. In vivo T depletion using antithymocyte globulin (ATG) or alemtuzumab was used for a smaller fraction of the UCB transplants (32%) compared to the URD groups (39% and 50%, after 8/8 and 7/8 HLA-matched transplants, respectively). Most URD transplant recipients received filgrastim mobilized peripheral blood progenitor cells (85% and 86% for 8/8 and 7/8 HLA-matched transplants, respectively). URD transplants were initiated in earlier study years for this older age population and included 56% of 8/8 URD and 61% of 7/8 transplants between 2005 and 2007 while 80% of UCB transplants were performed between 2008 and 2010.
Hematopoietic recovery, graft-versus-host disease and transplant-related mortality
After median follow-up of 50, 61 and 37 months after 8/8 HLA-matched, 7/8 HLA-matched URD and UCB transplants, respectively, univariate cumulative incidence analyses of hematopoietic recovery and acute and chronic GVHD are shown in Table 2. The probabilities of hematopoietic recovery were lower after UCB compared to 8/8 and 7/8 HLA-matched transplants (p<0.0001). The probability of day-100 grade II to IV acute GVHD, however, was similar in all three groups, but the 3-year probability of chronic GVHD was significantly lower after UCB transplants compared to 8/8 and 7/8 HLA-matched transplants (p<0.0001). Compared to 8/8 HLA-matched transplants, TRM was higher after 7/8 HLA-matched and UCB transplants (p=0.01 and p=0.05, respectively), but rates were similar after UCB and 7/8 HLA-matched transplants (p=0.42).
Table 2.
Univariate Analysis
| Probability (95% CI) | Probability (95% CI) | Probability (95% CI) | 8/8 HLA-matched vs. cord blood | 7/8 HLA-matched vs. cord blood | |
|---|---|---|---|---|---|
| 8/8 HLA- matched | 7/8 HLA- matched | Cord blood | p-value | p-value | |
| Outcomes | |||||
| Neutrophil recovery @ day-28 | 97% (96 - 99) | 91% (85 - 96) | 69% (63 - 75) | <0.0001 | <0.0001 |
| Platelet recovery to 20x 109/L @ day-90 | 91% (88 - 93) | 89% (83 - 94) | 69% (62 - 75) | <0.0001 | <0.0001 |
| Acute GVHD Grade II-IV @ day-100 | 36% (32 - 41) | 44% (34 - 54) | 35% (28 - 41) | 0.69 | 0.14 |
| Chronic GVHD @ 3-years | 53% (48 - 58) | 59% (49 - 69) | 28% (22 - 34) | <0.0001 | <0.0001 |
Results of multivariate analyses, adjusting for other significant factors showed that grade II to IV acute GVHD risks were similar after UCB compared to 8/8 HLA-matched transplants (HR 0.96, 95% CI 0.73 – 1.26, p=0.75). Acute GVHD risks were higher after 7/8 compared to 8/8 HLA-matched transplants (HR 1.46, 95% CI 1.06 – 2.03, p=0.01). In vivo T-cell depletion was associated with significantly lower risks of acute GVHD, independent of graft type (HR 0.56, 95% CI 0.43 – 0.72, p<0.0001). Compared to 8/8 HLA-matched transplants, chronic GVHD risks, analyzed with death as a competing hazard, were significantly lower after UCB transplants (HR 0.49, 95% CI 0.37 – 0.66, p<0.0001), but risks were higher after 7/8 HLA-matched transplants (HR 1.38, 95% CI 1.03 – 1.85, p=0.03). As with acute GVHD, in vivo T-cell depletion was associated with lower chronic GVHD risks (HR 0.52, 95% CI 0.42 – 0.66, p<0.0001).
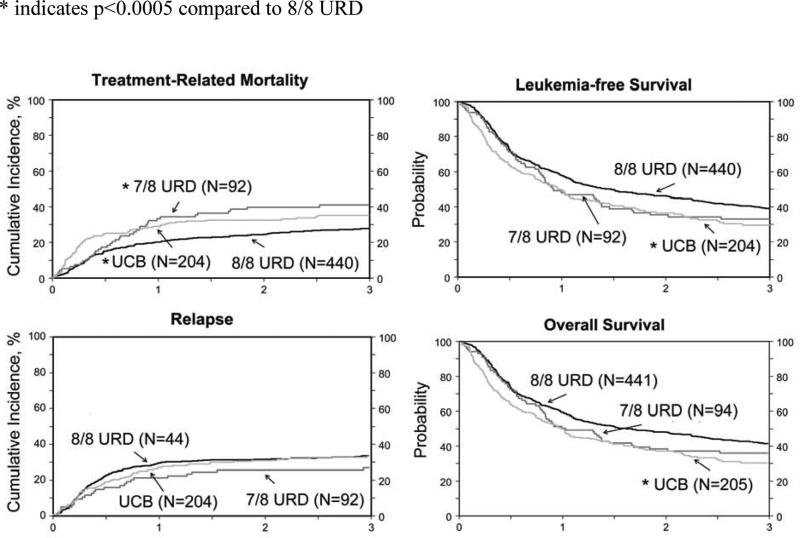
TRM was high after both UCB and 7/8 HLA-matched compared to 8/8 HLA-matched transplants, but the timing of mortality differed. As shown, compared to 8/8 HLA-matched transplants, TRM risks were significantly higher after UCB transplants within the first 3 months after transplantation (Table 3) and after 7/8 beyond 3 months after transplantation (Table 3). The 3-year probabilities of TRM after 8/8 HLA-matched and UCB transplants were 27% (95% CI 23 – 31) and 35% (95% CI 28 – 42), p=0.05 (Figure). The corresponding probability after 7/8 HLA-matched transplant was 41% (95% CI 31 – 51), p=0.01. There were no significant differences in TRM rates after 7/8 HLA-matched and UCB transplants (p=0.30). The time to CR1 and time from diagnosis or from CR1 to HCT were not significantly associated with TRM or other outcomes. To examine these influences more fully, we reassessed the demographic and transplant characteristics of those patients surviving leukemia-free at 3-months after transplantation. At that time point, the surviving UCB recipients were slightly younger (median age 58 vs. 60 years, p=0.01), but other clinical and demographic characteristics were similar between those who died of transplant-related complication within the first three months or those who survived into the latter follow-up period (data not shown). Similarly, there were no time period differences in clinical characteristics of 8/8 and 7/8 HLA-matched transplant recipients. Thus the excess toxicities of UCB grafting apparent in these first 3 post-transplant months were associated in part with slower engraftment and greater risks of graft failure. The higher TRM after 7/8 HLA-matched transplants is attributable to higher risks of GVHD and its associated morbidity and mortality. In addition, we tested for the effect of performance score on the subset of patients for whom these data were available; TRM risks were higher for patients who reported performance scores of 80 or lower (HR 1.45, 95% CI 1.08 – 1.94, p=0.01).
Table 3.
Multivariate analysis
| A. Transplant-related mortality | Hazard Ratio (95% confidence interval) | P-value |
|---|---|---|
| Transplant-related mortality | ||
| Early effect (0 - 3 months) | ||
| 8/8 HLA-matched BM or PBPC | 1.00 | |
| 7/8 HLA-matched BM or PBPC | 1.19 (0.52 – 2.72) | 0.68 |
| Umbilical cord blood | 2.83 (1.73 – 4.62) | <0.0001 |
| Effect beyond 3 months | ||
| 8/8 HLA-matched BM or PBPC | 1.00 | |
| 7/8 HLA-matched BM or PBPC | 1.73 (1.18 – 2.54) | 0.005 |
| Umbilical cord blood | 1.00(0.68 – 1.47) | 0.99 |
| Relapse * | ||
| 8/8 HLA-matched BM or PBPC | 1.00 | |
| 7/8 HLA-matched BM or PBPC | 0.86 (0.57 – 1.29) | 0.47 |
| Umbilical cord blood | 1.15 (0.85 – 1.54) | 0.36 |
| Treatment failure * | ||
| 8/8 HLA-matched BM or PBPC | 1.00 | |
| 7/8 HLA-matched BM or PBPC | 1.18 (0.91 – 1.53) | 0.22 |
| Umbilical cord blood | 1.35 (1.09 – 1.65) | 0.005 |
| Overall mortality ** | ||
| 8/8 HLA-matched BM or PBPC | 1.00 | |
| 7/8 HLA-matched BM or PBPC | 1.24 (0.95 – 1.62) | 0.12 |
| Umbilical cord blood | 1.43 (1.16 – 1.76) | 0.0008 |
Adjusted for cytogenetic risk
Adjusted for cytogenetic risk and patient age
FIGURE.
Treatment related mortality (*compared to 8/8 URD p<0.0001 within 3 months of HCT; compared to 7/8 URD p<0.001 beyond 3 months), LFS, Relapse incidence and Survival following allogeneic HCT. Adjusted p values reflect the multivariate analyses shown in Table 3.
* indicates p<0.0005 compared to 8/8 URD
Relapse, leukemia-free and overall survival
Relapse risks were not significantly different between the three treatment groups (Table 3). Cytogenetic risk group and intensity of transplant conditioning regimen were the only two factors that influenced relapse risks and these effects were independent of donor-graft type. Relapse risks were higher in patients with unfavorable cytogenetic risk compared to intermediate cytogenetic risk (HR 1.89, 95% CI 1.41 – 2.50, p<0.0001) and good cytogenetic risk (HR 3.85, 95% CI 1.25 – 12.5, p=0.02). Relapse risks were higher after RIC compared to myeloablative regimens (HR 1.36, 95% CI 1.04 – 1.77, p=0.02). White blood cell count at diagnosis was not associated with leukemia relapse. The 3-year probabilities of relapse, adjusted for cytogenetic risk and intensity of transplant conditioning regimens were 35% (95% CI 30 – 40) and 35% (95% CI 28 – 41) after 8/8 HLA-matched and UCB transplants (p=0.95), (Figure). The corresponding probability after 7/8 HLA-matched transplants was 26% (95% CI 18 – 35) and not different from that after 8/8 HLA-matched (p=0.09) and UCB transplants (p=0.13).
Treatment failure (relapse or death; inverse of leukemia-free survival) and overall mortality were higher after UCB transplants compared to 8/8 HLA-matched transplants (Table 3). Treatment failure and mortality risks after UCB transplants and 7/8 HLA-matched transplants were not significantly different. Cytogenetic risk group and patient age influenced treatment failure and mortality. Mortality risks were also associated with performance score. These effects were independent of donor-graft type. Treatment failure was higher in patients with unfavorable cytogenetic risk compared to intermediate cytogenetic risk (HR 1.49, 95% CI 1.06 – 1.85, p=0.0001) and good cytogenetic risk (HR 2.04, 95% CI 1.06 – 3.85, p=0.03); and was also higher in patients age 61-75, p=0.046) without a significant interaction with graft source. The 3- year probabilities of leukemia-free survival adjusted for cytogenetic risk and age were 39% (95% CI 34 – 43) and 28% (95% CI 22 – 35) after 8/8 HLA-matched and UCB transplants (p=0.01), (Figure). The corresponding probability after 7/8 HLA-matched transplants was 34% (95% CI 24 – 43) and not different from that after 8/8 HLA-matched (p=0.32) or UCB transplants (p=0.39). Similarly, overall mortality was higher in patients with unfavorable cytogenetic risk compared to intermediate cytogenetic risk (HR 1.47, 95% CI 1.19 – 1.82, p=0.0001) and good cytogenetic risk (HR 2.04, 95% CI 1.04 – 4.00, p=0.04). Mortality risks were also higher in patients aged 61 – 75 years compared to those age 50 – 60 years (HR 1.21, 95% CI 1.01 – 1.45, p=0.038) with no interaction between age and graft source. Poor performance score (≤ 80) was associated with higher overall mortality (HR 1.27, 95% CI 1.03 – 1.57, p=0.026) and was independent of graft source. The 3- year probabilities of overall survival adjusted for cytogenetic risk and age were 43% (95% CI 38 – 48) and 30% (95% CI 23 – 37) after 8/8 HLA-matched and UCB transplants (p=0.002), (Figure). The corresponding probability after 7/8 HLA-matched transplants was 37% (95% CI 27 – 46) and not different from that after 8/8 HLA-matched or UCB transplants (p=0.25).
As 60% of UCB transplants infused two UCB units and 40%, a single UCB unit we examined whether transplant outcomes were comparable between the two groups. With the exception of higher TRM at 3-years after transplantation of two UCB units (41%, 95% CI 32 – 50) compared to one UCB unit (27%, 95% CI 18 – 37), p=0.04, there were no significant differences in relapse or overall survival. The 3-year probabilities of relapse after transplantation of two UCB units compared to one unit were 35% (95% CI 26 – 44) and 48% (95% CI 35 – 62), p=0.09. The corresponding probabilities of overall survival were 28% (95% CI 20 – 37) and 25% (95% CI 14 – 38), p=0.70.
We also tested for other factors that may potentially influence survival after transplantation including patient cytomegalovirus seropositivity (HR 1.01, 95% CI 0.83 – 1.22, p=0.96), conditioning regimen intensity (HR 1.04, 95% CI 0.84 – 1.27, p=0.74) and transplantation of grafts from female donors to male recipients compared to other gender combinations (HR 0.99, 95% CI 0.77 – 1.28, p=0.97) and found none. Mortality risks were lower, but not significant for patients for whom the interval between achieving CR1 and transplantation was longer than 3 months compared to those transplanted within 3 months (HR 0.83, 95% CI 0.68 – 1.01, p=0.06). The interval between CR1 and transplantation was forced into the final model and the results were consistent with that reported above (data not shown).
DISCUSSION
These data examining a large group of alternative donor transplants in older patients reported to two large international observational registries demonstrate better outcomes after 8/8 HLA-matched transplants compared to 7/8 HLA-matched or UCB transplantations. These observations differ somewhat from those previously reported from our groups as well as others [15-20]. The current analysis includes a homogenous group with AML and all were in CR1 at transplantation whereas other reports included other leukemias and all disease stages at transplantation. Our observations confirm that for the older population with AML, allotransplantation in CR1 can provide extended leukemia-free survival for 30%-43% of patients using any of the donor-graft sources available to them. Although an 8/8 HLA-matched adult unrelated donor is preferred for patients who can promptly obtain such a donor there are known limitations in timely donor availability. For those ethnic or racial minorities, or mixed race populations where suitably HLA-matched adult unrelated donors are uncommonly identified, banked unrelated UCB or adult unrelated donors mismatched at a single HLA-locus provide a meaningful chance of extended leukemia-free survival [27, 28, 29]. The higher TRM after mismatched transplantations with UCB and adult unrelated donors must be considered when opting for a mismatched transplantation. Additionally, the rapid availability of UCB units provides effective therapy for older patients with expected short initial remissions; settings where ongoing consolidation therapy has not been effective in limiting risks of relapse [28-36]. Another option of recent interest is a haploidentical family donor, but as yet, it is uncommonly chosen for this age group. During the study period (2005-2010), only 29 haploidentical HCTs for patients over age 50 with AML in CR1 were reported to the CIBMTR, precluding meaningful analysis of this approach. Other factors including polymorphisms for immunologically or pharmacologically relevant genetic elements might also differentially influence outcomes, but such data were not available for our analysis [40-44].
Other factors may influence the choices of HCT using different donors and grafts, particularly with broader and extended experience in this older population; or affect the choice whether to proceed to HCT at all [37]. The generally disappointing outcomes for older patients with AML treated more conventionally cannot be compared directly in this observational HCT dataset, though other reports have suggested value for this allografting approach. [3,5,7,17,27-29,31-33]. Nonetheless, when considering HCT for older patients, the significantly lower risks of chronic GVHD, occurring without apparent compromise in anti-leukemic protection, might be associated with lesser morbidity and late mortality in recipients of UCB transplantation [9]. Avoiding the need for long-term immunosuppression and its associated morbidities may be of added importance for these older patients. An additional study, directed towards the functional recovery and quality of life of survivors after allotransplantation at one, two or more years beyond transplantation may provide needed insights in assessing these differential late morbidities of an otherwise successful allograft [1]. Studies addressing functional recovery and health-related quality of life are outside the scope of registry-based studies such as ours.
Limited other data have described outcomes of alternative donor transplantation for this older population. Investigators from the University of Minnesota in the US, Hôpital Saint-Louis, and Nantes in France recently described similar overall and leukemia-free survival in a modest-sized population of patients with AML in the same age group [37]. These somewhat better, or at least comparable outcomes in these three experienced centers when judged against this multicenter observational registry data may reflect center experience in donor selection and specific management practices for this older population and suggests that specialized techniques may further improve the reported outcomes for this older population. Earlier, McClune and others reported similar outcomes for a cohort of sibling and URD recipients overlapping this age group, emphasizing that allotransplantation is an appropriate and valuable alternative for patients with AML in early remission. [38]
The current analysis was conducted using data collected by two large international registries and subject to several limitations. There were differences in patient and disease characteristics as well as transplant strategies between the comparison groups which were in part overcome by performing carefully controlled regression analyses. Further improvements in donor and graft selection to increase the fraction with HLA- allele better matched grafts, improved HLA- matching and cell dose for UCB unit selection plus elements of supportive care using in vivo T-cell depletion and optimal GVHD prophylaxis could further improve survival for this older population. While older patients with AML are often presumed to be too old or too sick for transplantation and infrequently have available matched sibling donors, these encouraging results suggest that allotransplantation need not be withheld and for the right AML patients can produce extended and even curative long-term survival.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict – of –interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1.Deschler B, Binek K, Ihorst G, et al. Prognostic factor and quality of life analysis in 160 patients aged > or =60 years with hematologic neoplasias treated with allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:967–975. doi: 10.1016/j.bbmt.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Devine S, Owzar K, Blum W, et al. A Phase II study of allogeneic transplantation for older patients with AML in first complete remission using a reduced intensity conditioning regimen: Results from CALGB 100103/BMT CTN 0502. Blood. 2012 doi: 10.1200/JCO.2015.62.7273. Abstract 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25:1908–1915. doi: 10.1200/JCO.2006.10.2731. [DOI] [PubMed] [Google Scholar]
- 4.Wong R, Giralt SA, Martin T, et al. Reduced-intensity conditioning for unrelated donor hematopoietic stem cell transplantation as treatment for myeloid malignancies in patients older than 55 years. Blood. 2003;102:3052–3059. doi: 10.1182/blood-2003-03-0855. [DOI] [PubMed] [Google Scholar]
- 5.Farag SS, Maharry K, Zhang MJ, et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60-70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant. 2011;17:1796–1803. doi: 10.1016/j.bbmt.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koreth J, Aldridge J, Kim HT, et al. Reduced-intensity conditioning hematopoietic stem cell transplantation in patients over 60 years: hematologic malignancy outcomes are not impaired in advanced age. Biol Blood Marrow Transplant. 2010;16:792–800. doi: 10.1016/j.bbmt.2009.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurosawa S, Yamaguchi T, Uchida N, M, et al. Comparison of allogeneic hematopoietic cell transplantation and chemotherapy in elderly patients with non-M3 acute myelogenous leukemia in first complete remission. Biol Blood Marrow Transplant. 2011;17:401–411. doi: 10.1016/j.bbmt.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Majhail NS, Brunstein CG, Shanley R, et al. Reduced-intensity hematopoietic cell transplantation in older patients with AML/MDS: umbilical cord blood is a feasible option for patients without HLA-matched sibling donors. Bone Marrow Transplant. 2012;47:494–498. doi: 10.1038/bmt.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida N, Wake A, Takagi S, et al. Umbilical cord blood transplantation after reduced-intensity conditioning for elderly patients with hematologic diseases. Biol Blood Marrow Transplant. 2008;14:583–590. doi: 10.1016/j.bbmt.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Appelbaum FR. Dose intensity and the toxicity and efficacy of allogeneic hematopoietic cell transplantation. Leukemia. 2005;19:171–175. doi: 10.1038/sj.leu.2403609. [DOI] [PubMed] [Google Scholar]
- 11.Barrett AJ, Savani BN. Stem cell transplantation with reduced-intensity conditioning regimens: a review of ten years experience with new transplant concepts and new therapeutic agents. Leukemia. 2006;20:1661–1672. doi: 10.1038/sj.leu.2404334. [DOI] [PubMed] [Google Scholar]
- 12.Finke J, Nagler A. Viewpoint: What is the role of allogeneic haematopoietic cell transplantation in the era of reduced-intensity conditioning – is there still an upper age limit? A focus on myeloid neoplasia. Leukemia. 2007;21:1357–1362. doi: 10.1038/sj.leu.2404741. [DOI] [PubMed] [Google Scholar]
- 13.Lazarus HM, Rowe JM. Reduced-intensity conditioning for acute myeloid leukemia: is this strategy correct. Leukemia. 2006;20:1673–1682. doi: 10.1038/sj.leu.2404328. [DOI] [PubMed] [Google Scholar]
- 14.Aoudjhane M, Labopin M, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia. 2005;19:2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 15.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haematopoietic stem-cell transplantation in adults with acute leukemia: a retrospective analysis. Lancet Oncol. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunstein CG, Eapen M, Ahn KW, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119:5591–5598. doi: 10.1182/blood-2011-12-400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohty M, de Lavallade H, Padaique P, et al. The role of reduced intensity conditioning allogeneic stem cell transplantation in patients with acute myeloid leukemia: a donor vs. no donor comparison. Leukemia. 2005;19:916–920. doi: 10.1038/sj.leu.2403770. [DOI] [PubMed] [Google Scholar]
- 18.Shimoni A, Hardan I, Shem-Tov N, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20:322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 19.Shimoni A, Kroger N, Zabelina T, et al. Hematopoietic stem-cell transplantation from unrelated donors in elderly patients (age>55 years) with hematologic malignancies: older age is no longer a contraindication when using reduced intensity conditioning. Leukemia. 2005;19:7–12. doi: 10.1038/sj.leu.2403591. [DOI] [PubMed] [Google Scholar]
- 20.Alousi AM, Le-Rademacher J, Saliba RM. Who is the better donor for older hematopoietic transplant recipients: an older-aged sibling or a young, matched unrelated volunteer? Blood. 2013;121:2567–73. doi: 10.1182/blood-2012-08-453860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scaradavou A, Brunstein CG, Eapen M, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121:752–758. doi: 10.1182/blood-2012-08-449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, et al. Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1994;1995;15:825–828. [PubMed] [Google Scholar]
- 23.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13:1091–1112. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 24.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Klein JP, Moeschberger ML. Survival Analysis: Statistical Methods for Censored and Truncated Data. 2nd ed. Springer-Verlag; New York, NY: 2003. [Google Scholar]
- 26.Cox DR. Regression model and life tables. J R Stat Soc B. 1972;34:187–200. [Google Scholar]
- 27.Ustun C, Lazarus HM, Weisdorf D. To transplant or not: a dilemma for treatment of elderly AML patients in the twenty-first century. Bone Marrow Transplant. 2013;48:1497–1505. doi: 10.1038/bmt.2013.67. [DOI] [PubMed] [Google Scholar]
- 28.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97:1916–1924. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker H, Marcucci G, Maharry K, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:596–604. doi: 10.1200/JCO.2009.25.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frohling S, Schlenk RF, Kayser S, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood. 2006;108:3280–3288. doi: 10.1182/blood-2006-04-014324. [DOI] [PubMed] [Google Scholar]
- 32.Gardin C, Turlure P, Fagot T, et al. Postremission treatment of elderly patients with acute myeloid leukemia in first complete remission after intensive induction chemotherapy: results of the multicenter randomized Acute Leukemia French Association (ALFA) 9803 trial. Blood. 2007;109:5129–5135. doi: 10.1182/blood-2007-02-069666. [DOI] [PubMed] [Google Scholar]
- 33.Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624–627. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 34.Itzykson R, Gardin C, Pautas C, et al. Impact of post-remission therapy in patients aged 65-70 years with de novo acute myeloid leukemia: a comparison of two concomitant randomized ALFA trials with overlapping age inclusion criteria. Haematologica. 2011;96:837–844. doi: 10.3324/haematol.2010.036921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leith CP, Kopecky KJ, Godwin J, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89:3323–3329. [PubMed] [Google Scholar]
- 36.Kantarjian H, O'Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 37.Peffault de Latour R, Brunstein CG, et al. Similar Overall Survival Using Sibling, Unrelated Donor, and Cord Blood Grafts after Reduced-Intensity Conditioning for Older Patients with Acute Myelogenous Leukemia. Biol Blood Marrow Transplant. 2013;19:1355–1360. doi: 10.1016/j.bbmt.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 38.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eapen M, Klein JP, Sanz GF, et al. Eurocord-European Group for Blood and Marrow Transplantation; Netcord; Center for International Blood and Marrow Transplant Research Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. Lancet Oncol. 2011;12:1214–21. doi: 10.1016/S1470-2045(11)70260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Middleton PG, Taylor PRA, Jackson G, Protor SJ, Dickinson AM. Cytokine gene polymorphisms associated with severe acute-graft-versus-host disease in HLA-identical sibling transplants. Blood. 1998;92:3943–3948. [PubMed] [Google Scholar]
- 41.Lin M-T, Storer B, Martin PJ, et al. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349:2201–2210. doi: 10.1056/NEJMoa022060. [DOI] [PubMed] [Google Scholar]
- 42.Dickinson AM, Harrold JL, Cullup H. Haematopoietic stem cell transplantation: can our genes predict clinical outcome? Expert Rev Mol Med. 2007;9:1–19. doi: 10.1017/S1462399407000488. [DOI] [PubMed] [Google Scholar]
- 43.Newell LF, Gooley T, Hansen JA, Stirewalt DL, Petersdorf EW, Deeg HJ. Tumor necrosis factor polymorphism affects transplantation outcome in patients with myelodysplastic syndrome but not in those with chronic myelogenous leukemia, independent of the presence of HLA-DR15. Biol Blood Marrow Transplant. 2010;16:1700–1706. doi: 10.1016/j.bbmt.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haowen Xiao, Weijie Cao, Xiaoyu Lai, et al. Immunosuppressive cytokine gene polymorphisms and outcome after related and unrelated hematopoietic cell transplantation in a Chinese population. Biol Blood Marrow Transplant. 2011;17:542–549. doi: 10.1016/j.bbmt.2010.04.013. [DOI] [PubMed] [Google Scholar]