SUMMARY
Salient stimuli redirect attention and suppress ongoing motor activity. This attentional shift is thought to rely upon thalamic signals to the striatum to shift cortically driven action selection, but the network mechanisms underlying this interaction are unclear. Using a brain slice preparation that preserved cortico- and thalamostriatal connectivity, it was found that activation of thalamostriatal axons in a way that mimicked the response to salient stimuli induced a burst of spikes in striatal cholinergic interneurons that was followed by a pause lasting more than half a second. This patterned interneuron activity triggered a transient, presynaptic suppression of cortical input to both major classes of principal medium spiny neuron (MSN), that gave way to a prolonged enhancement of postsynaptic responsiveness in striatopallidal MSNs controlling motor suppression. This differential regulation of the corticostriatal circuitry provides a neural substrate for attentional shifts and cessation of ongoing motor activity with the appearance of salient environmental stimuli.
INTRODUCTION
Salient environmental stimuli induce shifts in attention that are typically accompanied by a halt in ongoing motor behavior (Desimone and Duncan, 1995). The neural mechanisms responsible for redirection of attention are incompletely understood, but the intralaminar nuclei of thalamus, long viewed as part of the reticular activating system (Smith et al., 2009), are essential components of this circuitry. Lesions of these nuclei produce contralateral sensory neglect and deficits in learning paradigms that demand appropriate responses to sensory stimuli (Matsumoto et al., 2001; Minamimoto and Kimura, 2002).
The intralaminar thalamic neurons implicated in sensory attention robustly innervate the striatum (Smith et al., 2004). There are two major targets of this projection. One target is the principal neuron of the striatum, the medium spiny neuron (MSN). Although this input is robust, MSNs are dominated by cortical signals conveying information about internal state, sensory events and ongoing motor activity (Stern et al., 1997). Two classes of MSN are thought to play complementary roles in determining the motor response to changing internal and environmental conditions, commonly referred to as action selection (Wichmann and DeLong, 1996). Based upon their connectivity with other basal ganglia nuclei, striatonigral MSNs are thought to be responsible for the initiation of appropriate movements, whereas striatopallidal MSNs are responsible for suppressing competing movement.
The other major striatal target of the intralaminar thalamus is the cholinergic interneuron (Lapper and Bolam, 1992; Matsumoto et al., 2001; Minamimoto and Kimura, 2002). The sole source of acetylcholine in the striatum, cholinergic interneurons have widespread and rich connections within the striatum (Bolam et al., 1984) and have long been known to play an important role in motor behavior and striatally-based associative learning (Aosaki et al., 1994). The receptors for acetylcholine are broadly distributed on both classes of MSN, as well as on the synaptic terminals of corticostriatal axons. The presynaptic muscarinic receptors on corticostriatal terminals transducing the response to acetylcholine are largely of the M2-class (M2/4 type), leading to presynaptic inhibition at corticostriatal terminals (Alcantara et al., 2001; Barral et al., 1999; Calabresi et al., 1998b; Higley et al., 2009). In contrast, postsynaptic muscarinic receptors are largely of the M1 class, leading to an enhancement in the response to depolarizing corticostriatal input, particularly in striatopallidal MSNs (Akins et al., 1990 ; Galarraga et al., 1999; Shen et al., 2005; Shen et al., 2007).
How these seemingly opposing cholinergic signaling events are coordinated has not been elucidated. One potential clue comes from response pattern of cholinergic interneurons to salient stimuli, which typically consists of a burst of spikes, followed by a prolonged pause in spiking. In associative learning paradigms, cholinergic interneurons begin to spike in a burst-pause pattern following presentation of a conditioned stimulus that has become salient because of its linkage to reward (Aosaki et al., 1994). Although this pattern of discharge is lost following lesioning of the dopaminergic neurons innervating the striatum, it is also lost following lesioning of the intralaminar nuclei of the thalamus, implicating them in its generation (Matsumoto et al., 2001).
To gain a better understanding of the thalamic mechanisms controlling the corticostriatal circuitry underlying action selection, horizontal brain slice that preserved both cortico- and thalamostriatal connectivity were obtained from transgenic mice in which one or the other major subclass of principal MSN were labeled with green fluorescent protein (GFP) (Ding et al., 2008). The goals of the studies described here were to first determine the response pattern of cholinergic interneurons to thalamic activation and second to determine how thalamic activation of interneurons influenced corticostriatal signaling through MSNs. These studies show that thalamic stimulation alone evoked a burst-pause pattern of spiking in cholinergic interneuron. Moreover, this pattern of activity led to a transient interruption of cortical signaling to MSNs followed by a period of enhanced responsiveness in striatopallidal MSNs, providing a potential neural mechanism underlying the redirection of attention and the interruption of ongoing motor activity with the presentation of salient stimuli.
RESULTS
Thalamic stimulation evoked a burst-pause pattern in cholinergic interneurons
To examine the interaction between cortical and thalamic inputs to the striatum, a parahorizontal brain slice was used (Fig. 1A) (Ding et al., 2008). The connectivity in these slices was assessed initially using the carbocynanine dye 1,1’-dioctadecyl -3,3,3’3’- tetramethylindocarbocyanine perchlorate (DiI) (Supplementary Fig. 1). Both cortical and thalamic axons were preserved in this slice. The striatal regions where both inputs overlapped were targeted for recording. In these regions, cholinergic interneurons spiked autonomously when the temperature was near physiological values (32-35°C) (~2.5-6.6 Hz, median spike rate=3.4 Hz; n= 6); this rate was similar to that described previously for cholinergic interneurons (Bennett et al., 2000) (Supplementary Fig 2). Surprisingly, a train of near threshold thalamic stimuli (50 Hz, 10 pulses) evoked not just a burst of spikes in cholinergic interneurons, but a burst followed by a pause in spiking (Fig. 1B). During the burst, the spiking rate was on average about 14.2 Hz (n=6, P<0.05 compared to baseline firing rate; Mann-Whitney), considerably slower than the 50 Hz stimulus. The pause that followed thalamic stimulation was variable in duration, ranging from 360 to 1300 ms (median=845 ms; n=6, Fig. 1D). When the same stimulus train was delivered to the cortex, spiking in interneurons increased much more modestly and was not accompanied by a pause (median firing rate within 400 ms after stimulation train: 4.34 Hz; P<0.05; Mann-Whitney; n=6; median firing rate 400 ms after stimulation: 3.42 Hz; P>0.05 compared to baseline before stimulation; Mann-Whitney) (Fig. 1C). Increasing the stimulus intensity three-fold had little effect on either response pattern, in spite of the fact that the cortical response grew (Fig. 1D).
Figure 1. Thalamic stimulation generated a ‘burst-pause’ firing pattern in cholinergic interneurons.
(A) Composite diagram of a sagittal slice showing cortex (Ctx), striatum (CPu), lateral globus pallidus (LGP), internal capsule (i.c.). Shaded area indicates the region where EPSCs can be reliably evoked by cortical stimulation in the striatum. Insert: Experimental configuration. (B, C) Sample traces of cell-attached recordings of 10 consecutive responses from a cholinergic interneuron in response to a train (50 Hz, 10 pulses) of thalamic stimulation and cortical stimulation (Top). PSTHs and rasters of an autonomously firing cholinergic interneuron in response to thalamic and cortical stimulation (Bottom). A train of thalamic stimulation produced a ‘burst-pause’ firing pattern in the cholinergic interneuron. A train of cortical stimulation (arrow) produced a slight increase in firing rate. (D) Averaged population responses of cholinergic interneurons showing that trains of thalamic stimulation generate a ‘pause’ (left). In the same set of neurons, averaged population responses of cholinergic interneurons showing increased firing rate following cortical stimulation (right), but no pause. Rasters and histograms aligned to onset of stimulation train. (E) Example of an autocorrelogram of autonomous interneuron spiking, showing periodic activity. The autocorrelogram was aligned with PSTH to compare the mean duration of the pause and the mean ISI during autonomous spiking. (F) The action potential number generated during the stimulation train was plotted against the ratio of the first ISI after stimulation divided by the average ISI before stimulation. Thalamic stimulation (blue dots) produced more action potential during the stimulation and a longer pause compared to cortical stimulation (red circles). (G) In the same set of neurons, the average number of action potential evoked was plotted against the ratio of the first ISI after stimulation divided by the average ISI before stimulation. Thalamic 50 Hz stimulation generated 3-4 action potentials on average (3.73±1.07 action potential counts) The ratio between the first ISI and the average ISI was greater than one (First ISI/Ave ISI =2.85±1.27). In contrast, cortical stimulation produced less than 2 action potentials during the stimulation (1.42±0.56 action potential counts; P<0.05 compared to thalamic stimulation; Mann-Whitney) and the ratio of first ISI and the average ISI of the same neuron was one (First ISI/Ave ISI =1.04±0.32, P<0.05 compared to thalamic stimulation; Mann-Whitney). Experiments were performed at 32-35°C.
To discriminate between a resetting of the pacemaking rhythm and an active pause, the average inter-spike interval (ISI) during autonomous activity (estimated from an autorcorrelogram) was compared to the stimulus-generated pause (estimated from the peristimulus time histogram (PSTH)). The autocorrelogram computed from interneuron pacemaking showed a period of only 300-600 ms during which spike probability was low (Fig. 1E). In contrast, PSTHs computed from thalamic stimulation of the same cell consistently had periods of low spike probability that were significantly longer; overlaying the thalamic PSTH and the autocorrelogram from the same cell made the distinction clear-cut (Fig. 1E).
The pause generated by thalamic stimulation appeared to be correlated with the number of spikes in the burst. To quantitatively assess the relationship the number of spikes generated by cortical or thalamic stimulation was plotted against the relative duration of the first inter-spike interval after the cessation of stimulation. When only a few spikes were evoked, the relative duration of the first interval was near one, meaning pacemaking simply resumed at the normal rate. However, as the number of spikes evoked increased, the first post-stimulus interval became several fold longer that the normal pacemaking interval, creating a pause (Fig. 1E, F, R2=0.41). These results argue that thalamic stimulation evokes a burst-pause pattern of spiking by virtue of its ability to effectively drive cholinergic interneurons to spike.
The thalamically-evoked pause was dependent upon dopamine
In vivo, the development of burst-pause response in cholinergic interneurons to salient stimuli is dependent upon dopamine and activation of D2 dopamine receptors (Aosaki et al., 1994). By modulating Nav1 and HCN channels, D2 receptors create the pause by transiently suppressing pacemaking (Deng et al., 2007; Maurice et al., 2004). Yet, in the brain slice preparation where dopamine fibers were not being ostensibly stimulated, burst spiking induced a pause in activity that was very reminiscent of that seen in vivo. To test for involvement of D2 dopamine receptors in the thalamic response, sulpiride (10 μM) was applied. Sulpiride didn't affect the burst of spikes evoked by thalamic stimulation (median inter-spike interval (ISI) control: 369.7 ms, in sulpiride: 370.3 ms, P>0.05; Mann-Whitney), however it dramatically attenuated the pause (n=4, median ISI control: 973.6 ms, in sulpiride: 426.7 ms, P<0.05; Mann-Whitney) (Fig. 2A). Another measure of the pause – the ratio between the first ISI and the average ISI – was greater than one in control conditions, but decreased significantly in the presence of sulpiride (Fig. 2C; FirstISI/AveISI control: 2.4±0.1; sulpiride: 1.2±0.1, P<0.05; Mann-Whitney). Additional evidence for the involvement of dopamine release and D2 receptors came from experiments examining the effects of the dopamine transporter antagonist cocaine (5 μM). Cocaine had no effect on the initial burst of spikes evoked by thalamic stimulation, however it significantly enhanced the duration of the pause following the burst (Fig. 2B,D; FirstISI/AveISI control: 1.9±0.11; in cocaine: 2.7±0.1; P<0.05; n=4; Mann-Whitney).
Figure 2. The thalamically stimulation evoked a dopamine-dependent pause.
(A) PSTHs and rasters from a cholinergic interneuron in response to a train (50 Hz, 10 pulses) of thalamic stimulation in the absence and presence of the D2 receptor antagonist sulpiride (10 μM). (B) PSTHs and rasters from a cholinergic interneuron in response to a train (50 Hz, 10 pulses) of thalamic stimulation in the absence and presence of dopamine transporter blocker, cocaine (5 μM). (C) The number of action potential evoked in a trial was plotted against the ratio of the first ISI after stimulation divided by the average ISI before stimulation. Blockade of D2 receptor by sulpiride (red dots) significantly shorten the pause without affecting the mean firing frequency (control = 2.4±0.1; sulpiride=1.2±0.1, P<0.05, Mann-Whitney). (D) The number of action potentials evoked was plotted against the ratio of the first ISI after stimulation divided by the average ISI before stimulation. Application of DA transporter blocker, cocaine (5 μM, green dots), significantly prolonged the pause without affecting the mean firing frequency (Mean firing frequency, control = 3.52±0.38; cocaine=3.48±0.12, P>0.05, Mann-Whitney; First ISI/Ave ISI control = 1.9±0.1; cocaine=2.7±0.1, P<0.001, Mann-Whitney). (E) The number of action potentials evoked was plotted against the ratio of the first ISI after stimulation divided by the average ISI before stimulation. Blockade of nAChR receptor by mecamylamine (yellow dots) also significantly shortened the pause (mean firing frequency, control = 2.32±0.11; mecamylamine=2.35±0.08, P>0.05, Mann-Whitney; First ISI/Ave ISI control = 1.9±0.1; mecamylamine=1.5±0.1, P<0.001, Mann-Whitney). (F) Summary of the effects of sulpiride, cocaine and mecamylamine in the thalamically evoked pause; for each spike count (2-5) the difference between the drug treatment median First ISI/Ave ISI value and the control value was calculated; this difference was then averaged across spike counts. If there was no change in this ratio, the average should be zero; if the first ISI is shortened by drug treatment, the average should be negative; if the first ISI is lengthened by drug treatment, the average should be positive. Experiments were performed at 32-35°C.
How did thalamic stimulation evoke dopamine release from terminals in the striatum? Dopamine terminals express nicotinic ACh (nACh) receptors capable of inducing dopamine release, raising the possibility that the thalamically-evoked burst in interneurons was responsible (Rice and Cragg, 2004; Threlfell et al., ; Zhang and Sulzer, 2004; Zhou et al., 2002). In agreement with this hypothesis, the nACh receptor antagonist, mecamylamine (10 μM) significantly diminished the thalamically evoked pause, while having no effect on the initial burst of spikes (Fig. 2E,F; FirstISI/AveISI control = 1.9±0.1; mecamylamine=1.5±0.1; P<0.05; n=5; Mann-Whitney).
Although these results suggest that the thalamically evoked pause depends upon nicotinic stimulation of dopamine terminals and activation of D2 receptors, pauses in activity can be created in other ways. For example, depolarizing current injection through the recording electrode can evoke a burst-pause pattern (Reynolds et al., 2004). However, these pauses were not sensitive to sulpiride (n=5, data not shown). It is possible that the key difference between this protocol and thalamic stimulation is the ability of thalamic stimulation to recruit neighboring interneurons that cooperate in producing dopamine release.
Thalamic stimulation evoked a facilitating response in interneurons
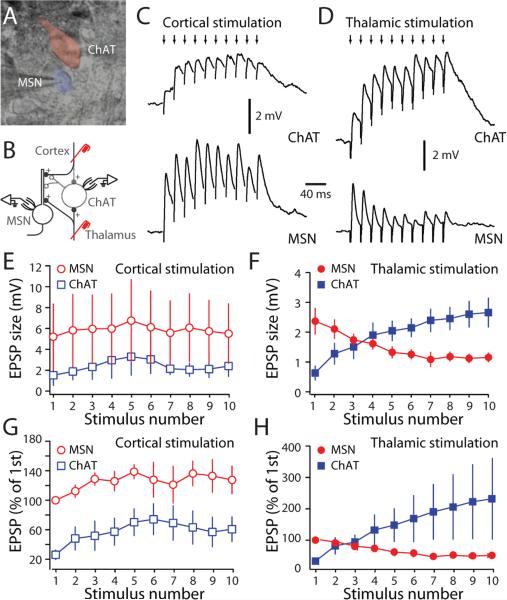
What makes the thalamic synapses on cholinergic interneurons more effective in generating a burst of spikes than cortical synapses? One factor might be that thalamic axons preferentially innervate cholinergic interneurons. To test this hypothesis, simultaneous recordings were made from neighboring MSNs and cholinergic interneurons and their responses to cortical and thalamic stimulation measured (Fig. 3A, B). In cell-attached recordings, thalamic stimulation generated a burst-pause firing pattern in interneurons, as shown above. In contrast, cortical stimulation was not capable of interrupting the tonic firing of cholinergic interneurons (Supplementary Figure 3). Next, the membrane patch was ruptured and whole cell recordings were made to measure EPSPs. To avoid interactions with pacemaking currents, interneurons were hyperpolarized to -65 mV. As previously reported (Ding et al., 2008), cortical stimulation in the horizontal slice evoked facilitating excitatory postsynaptic potentials (EPSPs) in MSNs (n=5 pairs, Fig. 3C,E,G). In neighboring cholinergic interneurons, the same stimulation evoked a much smaller initial EPSP, but the synaptic response was also facilitating (n=5 pairs, Fig. 3D,F,H). On average, the corticostriatal EPSP in MSNs was 3.8 times larger than that evoked in a cholinergic interneuron (P<0.05; Mann-Whitney; n=5 pairs).
Figure 3. Thalamic stimulation elicited distinctive postsynaptic responses in interneurons and MSNs.
(A) Infrared differential interference contrast image showing an MSN and a cholinergic interneuron. (B) Schematic of the experimental configurations. (C) Paired recording from a cholinergic interneuron (ChAT) and a neighboring MSN during cortical stimulation. Cortical stimulation (small arrows, 50 Hz) evoked EPSPs in both cell types. However, EPSPs recorded in MSNs had a bigger amplitude. (D) Paired recording from ChAT) and neighboring MSN in response to train of thalamic stimulation. Thalamic stimulation (small arrows, 50 Hz) evoked EPSPs that summed differently in cholinergic interneurons and MSNs. (E) The evoked cortical EPSPs amplitudes in MSNs and cholinergic interneurons were plotted against stimulus number. (F) The evoked thalamic EPSPs amplitude in MSNs and cholinergic interneurons were plotted against stimulus number. (G) Normalized cortical EPSPs amplitude was plotted against stimulus number. Cortical EPSPs in cholinergic interneurons are smaller than those in MSNs. (H) Normalized thalamic EPSPs amplitude was plotted against stimulus number. Thalamic EPSPs in cholinergic interneurons summed differently than those in MSNs. To unmask EPSPs, hyperpolarizing current was injected into cholinergic interneuron to prevent cells from firing action potentials. Experiments were performed at 32-35°C.
The thalamically-evoked EPSPs in MSNs and interneurons were strikingly different. First, the initial EPSP evoked by thalamic stimulation was consistently larger in MSNs than in cholinergic interneurons (Fig. 3E-H). On average, the peak thalamostriatal EPSP in MSNs was 3.0 times larger than that in interneurons (P<0.05; Mann-Whitney; n=4 pairs). Second, with repetitive stimulation, the thalamic synapses formed on MSNs consistently depressed, whereas the thalamic synapses on interneurons did not (Fig. 3E-H). The slow decay of the EPSPs in interneurons, reflecting their longer membrane time constant (Kawaguchi et al., 1989; Wilson et al., 1990), was clearly a factor in the EPSP summation with repetitive stimulation. With thalamic bursts of four or more stimuli, the synaptic response in interneurons was larger than that in neighboring MSNs (Fig. 3E-H, by the last stimulation pulse, EPSP amplitude in cholinergic interneurons was 4.5 times larger than that in MSNs; P<0.05; Mann-Whitney; n=4 pairs). These results suggest that the efficacy in interneurons of thalamic burst stimulation is not attributable to preferential innervation, but rather to the integrative properties of the thalamic synapse.
Thalamic interneuron bursts transiently inhibited MSN corticostriatal and thalamostriatal responses
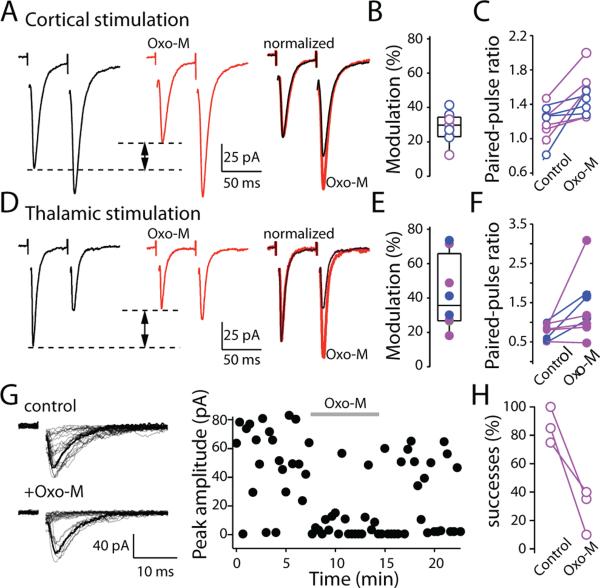
Previous studies have shown that many glutamatergic terminals forming synapses on MSNs possess M2-class receptors and that activation of these receptors leads to inhibition of glutamate release (Alcantara et al., 2001; Barral et al., 1999; Calabresi et al., 1998a; Higley et al., 2009). To determine whether both cortical and thalamic synapses were regulated in this way, paired pulses (50 ms interstimulus interval) were applied to the cortex or thalamus every 20-30s and evoked excitatory postsynaptic currents (EPSCs) measured in MSNs before and after application of a muscarinic receptor agonist (oxotremorine-M, Oxo-M, 10μM). Muscarinic receptor activation inhibited corticostriatal glutamatergic synapses (median inhibition at corticostriatal synapses=29.7%; n=9; P<0.05; Mann-Whitney; Fig. 4A,B). The modulation was the same in magnitude in D1R and D2R MSNs (median inhibition at D1 MSN corticostriatal synapses=29.9%; n=4; at D2 MSN synapses=29.7%, n=5). The paired-pulse ratio (PPR) also was increased by oxotremorine (corticostriatal PPR=1.2±0.1; after Oxo-M, PPR=1.5±0.1; P<0.05; n=9; Wilcoxon; Fig. 4A,C). The muscarinic modulation was very similar at thalamostriatal synapses. Oxo-M reduced the amplitude of the initial EPSC in both D1 and D2 MSNs (median inhibition at D1 MSN thalamostriatal synapses=41.1%; n=3; at D2 MSN synapses=27.1%, n=5, P>0.05; Mann-Whitney; Fig. 4D,E). As at corticostriatal synapses, Oxo-M increased the paired pulse ratio at thalamostriatal synapses (PPR=0.8±0.1; after Oxo-M=1.4±0.3; P<0.05; n=8; Wilcoxon; Fig. 4F). In support of a presynaptic locus of action, when stimulation intensity was decreased to produce release failures, Oxo-M significantly increased the failure rate (13% in control; 72% in Oxo-M; n=3; P<0.05; Wilcoxon; Fig. 4G,H).
Figure 4. Muscarinic receptor activation produced a presynaptic modulation of corticostriatal synapses in MSNs.
(A) Sample EPSCs evoked by a paired-pulse cortical stimulation with 50 ms interstimulus interval. Application of muscarinic receptor agonist, oxotremorine-M (Oxo-M) decreased first EPSC amplitude and increased paired-pulse ratios (PPRs). (B) Box-plot summary of reduction in first EPSC amplitude. (C) Box-plot summary of changes in PPRs. (D) Sample EPSCs evoked by paired-pulse stimulation delivered to thalamus with 50 ms interstimulus interval. Oxo-M also decreased first EPSC amplitude and increased PPRs (right panels) (E) Box-plot summary of reduction in first EPSC amplitude. (F) Box-plot summary of changes in PPRs. (G) Sample EPSCs evoked by thalamic stimulation. Stimulation threshold was reduced to allow transmission failures. (H) Oxo-M significantly decreased success rate. In all plots, data collected from D2 MSNs were shown as magenta dots, data from D1 MSNs were shown as blue dots. Experiments were performed at room temperature.
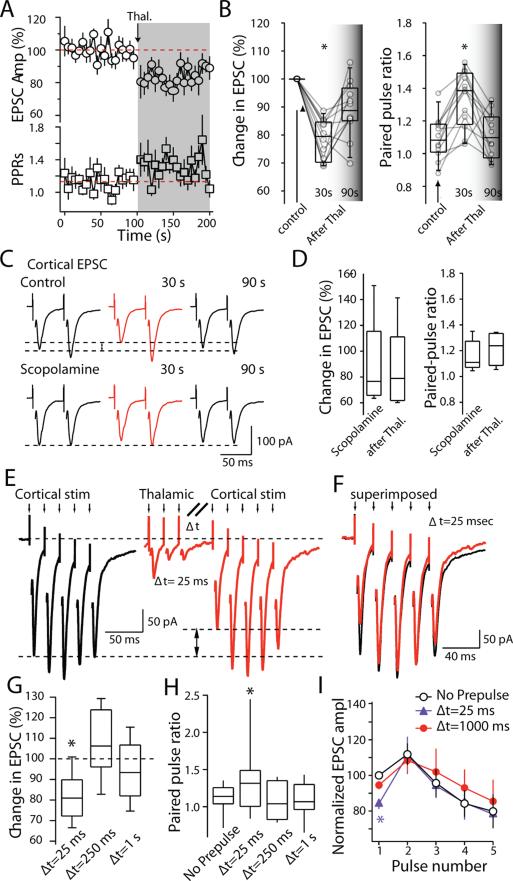
Next, the ability of cholinergic interneurons to produce the same presynaptic modulation of corticostriatal terminals was examined. Cholinergic interneurons were activated by thalamic stimulation. Corticostriatal EPSCs were evoked in MSNs before and after a thalamic conditioning burst stimulus (25 stimuli at 50Hz). Cells were voltage-clamped using a Cs+-based internal solution. As predicted from the bath application of muscarinic agonists, thalamic stimulation produced a reversible depression in the amplitude of corticostriatal EPSCs in MSNs (Fig. 5A). Corticostriatal EPSC amplitude was reduced 30 seconds after the conditioning volley (78±2% of control; P<0.05) and slowly returned to control levels (89±3% of control at 90 seconds after stimulation; P<0.05 compared to 30s; Wilcoxon; n=12; Fig. 5B). In parallel, corticostriatal PPRs increased soon after thalamic stimulation to 1.3 from 1.1 and then recovered to baseline (P<0.05; Wilcoxon; n=12; Fig. 5A,B). The change in EPSC amplitude and PPR induced by thalamic stimulation was blocked by the muscarinic receptor antagonist scopolamine (10μM) (Fig. 5C,D) In the presence of scopolamine, the EPSCs amplitude was 87.8±16% of baseline (P>0.05 compared to control; Mann-Whitney; n=5). In this situation, thalamic stimulation did not produce a significant change in either peak amplitude (84.9±14.7%; P>0.05 compared to before stimulation train; Wilcoxon; n=5) or PPRs (1.16±0.05 to 1.21±0.06; P>0.05; Wilcoxon; n=5). These results argue that effects of thalamic stimulation on corticostriatal signaling were mediated by cholinergic interneurons and not by direct thalamic input to MSNs or other striatal interneurons.
Figure 5. Corticostriatal synaptic transmission was depressed by thalamic stimulation.
(A) Time course of changes in cortical EPSC amplitude and PPRs in response to thalamic burst stimulation. Consistent with a presynaptic mechanism, there was a clear increase in PPRs accompanied by reduction in ESPC amplitude. (B) Box-plot summary of the change in first EPSC amplitude (left) and change in PPRs (right). (C) Sample EPSCs evoked by a paired-pulse cortical stimulation with 50 ms interstimulus interval. Traces illustrate the suppression of EPSC amplitude and increase in PPRs at cortical afferent synapses after burst stimulation of thalamic afferents. Scopolamine blocked the depression. (D) Box-plot summary of change in first EPSC amplitude (left) and changes in PPRs (right) in the presence of scopolamine. (E) Sample traces of cortical EPSCs recorded from a medium spiny neuron in the absence (left) and presence (right) of a thalamic pre-pulse with 25 ms interval (Δt=25 ms) at physiological temperature. (F) Cortical EPSCs were superimposed to illustrate the changes in EPSCs amplitudes (Δt=25 ms). (G) Box-plot summary of the reduction in first EPSC amplitude. Presynaptic modulation of cortical EPSCs by thalamic burst stimulation recovered quickly at higher temperature (Median amplitude=82% of control; P<0.05; median amplitude=108% of control at 250 ms, P>0.05, n=10;median amplitude=94% of control at 1s , P>0.05, ANOVA; n=10). (H) Box-plot summary of the paired pulse ratio (Mean control PPR=1.11; PPR(25ms)=1.30, P<0.05; PPR(250ms)=1.09, P>0.05, ;PPR(1s)=1.09, P>0.05, ANOVA; n=10). (I) Pooled data (n = 10) showing the effect of the thalamic pre-pulse on short term plasticity of EPSCs evoked by 50 Hz cortical afferent stimulation. Experiments illustrated in A-D were performed at room temperature; E-I were performed at 32-35°C.
The initial experiments characterizing the effects of thalamic stimulation on corticostriatal synaptic transmission were done at room temperature (20-22 °C). However, the clearance of ACh in the synaptic cleft by cholinesterase is highly dependent on temperature. To gain a better picture of the kinetics of the modulation at physiological temperatures, the experiments were repeated at (33-35 °C). Five cortical stimuli (50 Hz; 5 stimuli) were delivered before and after thalamic conditioning using the same stimulation protocol (10 stimuli at 50Hz) that evoked the burst-pause response in interneurons described above. With a short delay between the end of the thalamic stimulation and the beginning of the cortical stimulation (Δt=25 ms), thalamic conditioning produced a significant depression in the amplitude of the first corticostriatal EPSCs in MSNs (Fig. 5E; median amplitude=82% of control; P<0.05; ANOVA; n=10). As at room temperature, the inhibition was most prominent for the first EPSC in a train, reversing in subsequent EPSCs in a fashion typical of a presynaptic modulation (Fig. 5F-H). If the cortical stimulation was delayed 250 or 1000 ms, the inhibitory effect of thalamic stimulation was lost (Fig. 5G-I). The inhibition of the first cortical EPSCs with a 25 msec lag between conditioning and test volleys was observed in both D1 and D2 MSNs (median response in D1 MSN=87%; n=3; D2 MSN=79%, n=7; P>0.05; Mann-Whitney).
The response to paired corticostriatal pulses was consistent with a presynaptic mechanism. With a 25 ms delay, the amplitude of the second EPSC increased to 130% of the first EPSC, whereas it only increased to 115% in the absence of thalamic stimulation (Δt=25 ms, P<0.05; Wilcoxon; n=10). Paired pulse responses were back to normal with delays of 250 and 1000 ms (Δt=250 ms, 122%;Δt=1 s, 118%; P<0.05 compared to 25 ms after thalamic stimulation; P>0.05 compared to control; Wilcoxon; n=10). Interestingly, if the EPSC amplitude was normalized by the amplitude of the first control EPSC, the only effect of the thalamic conditioning shortly afterwards (Δt=25 ms) was on the initial response (Fig 5H, P<0.01 compared to control; Wilcoxon; n=10); the amplitude of the second, third, fourth and fifth EPSC was normal (Fig. 5H, P>0.05 compared to control; Wilcoxon; n=10).
Cholinergic interneurons enhanced corticostriatal responses of striatopallidal MSNs
Having isolated the presynaptic effects of thalamic activation of cholinergic interneurons, the postsynaptic effects were examined using a physiological K+ based internal solution that allowed dendritic mechanisms to interact with the currents evoked by synaptic stimulation. At near physiological temperatures (33-35°C), five cortical stimuli (50 Hz; 5 stimuli) produced a post-synaptic potential that initially increased in amplitude and then slighted declined (Fig. 6A). A thalamic conditioning train (50 Hz, 10 pulses) was given at three times (Δt=25 ms;Δt=250; andΔt=1 s; same as Fig. 5D) before the corticostriatal test train (50 Hz, 5 pulses).
Figure 6. Thalamic stimulation produced a biphasic modulation of corticostriatal EPSPs.
(A) Corticostriatal EPSPs recorded from a D2 MSN (inset: Experimental configuration). (B) When a thalamic stimulus train was given 25 ms before the cortical EPSPs, cortical EPSPs were depressed. (C) Cortical EPSPs before and after conditioning were superimposed to illustrate the changes in first EPSP amplitude and EPSP summation (Δt=25 ms). (D) Pooled data (n = 9) showing the effect of thalamic pre-pulse on EPSPs summation evoked by 50 Hz cortical afferent stimulation (Δt=25 ms). (E) The reduction in first EPSP amplitude was blocked by the muscarinic receptor antagonist, scopolamine. (F) When the interval between thalamic pre-pulse and cortical stimulation was prolonged to 1 s, the thalamic pre-pulse had no significant effect on first EPSP amplitude, whereas the amplitude of the 5th EPSP was significantly increased (Δt=1 s). (G) Pooled data (n = 9) showing that 1 s after thalamic pre-pulse EPSPs summation evoked by 50 Hz cortical afferent stimulation was significantly increased. (H) There was no reduction in first EPSP amplitude (Δt=1 s). Scopolamine did not significantly change the first EPSP amplitude. Experiments were performed at 32-35°C.
In D2 MSNs, the thalamic conditioning train at short delays (Δt=25 ms) diminished the cortical response, in agreement with the presynaptic inhibition seen in voltage-clamp recordings (Fig. 6B,C). When normalized by the first EPSP in the control records, only the first EPSP in the train was significantly reduced in amplitude by thalamic conditioning (Fig. 6D). On average, the first EPSP amplitude was reduced by 21±7% (P<0.05 compared to control; Wilcoxon; n=9). The inhibition of the first EPSP was still evident with a delay of 250 ms between the end of the conditioning train and the beginning of the test train (30±5% compared to control; P<0.05; Wilcoxon; n=9), but was gone when the delay was extended to a second (Δt=1 s) (9±8% compared to control; P>0.05; Wilcoxon; n=9), as seen in the voltage-clamp experiments. The muscarinic receptor antagonist scopolamine blocked the suppression of the first EPSP by thalamic conditioning (Fig. 6E).
In contrast, with a longer delay between the conditioning and test trains, a postsynaptic enhancement of EPSP summation was revealed (Fig. 6F). With a delay of 250 ms, the ratio of the fifth/first EPSP was increased to 188±23% (P<0.05 compared to control; Wilcoxon; n=9); with a delay of 1 second, the ratio was similar (187±41%; P<0.05 compared to control; Wilcoxon; n=9). Normalizing EPSP amplitude by that of the first EPSP in the control records showed that the elevation in amplitude was progressive (Fig. 6G). Muscarinic receptor antagonism had no effect on initial EPSP amplitude with the longer delay, in agreement with the voltage clamp results (Fig. 6H). To get a better understanding of the mechanism underlying the enhanced summation, summed EPSPs were fit with an alpha function that allowed the rise and decay time constants to be estimated. With short (Δt=25 ms) delay, neither the rise or decay time constants of the EPSPs were altered by thalamic conditioning, but with the 1 second delay the decay time constant of the EPSPs was increased by conditioning (Fig. 7; median control τrise =1.86 ms;Δt=25ms τrise=1.76 ms;Δt=1s τrise =1.91, P>0.05; Wilcoxon; median control τdecay =12.8 ms;Δt=25ms τdecay=11.8 ms;Δt=1s τdecay =15.3, P<0.05 compared to control andΔt=25ms; Wilcoxon; n=9). The slower EPSP decay provided an explanation for the change in EPSP summation, as the amplitudes of the fitted EPSPs were not different from those of the control (Fig. 7).
Figure 7. Summation of corticostriatal EPSPs was increased by slowing their decay rate.
(A) EPSPs were fitted with five double-exponential functions (thick line). (B) Each individual fit EPSP was generated and plotted based on fitted values. (C, D) The first and fifth fitted EPSP were superimposed (C) and normalized (D) to compare amplitude and kinetics of each individual EPSP. (E) Box-plot summary of fit EPSP rise time constant. There are no significant changes in rise time constant when thalamic pre-pulse was introduced. (F) Box-plot summary of fit EPSP decay time constant. Cortical EPSP decay time constant significantly increased when the thalamic pre-pulse was given one second before cortical stimulation, but not immediate before cortical stimulation. (G) Summary graph of normalized fit EPSP amplitude plot against stimulation pulse number. Reduction in the first EPSP amplitude was only seen inΔt=25 ms (median= 80% of control, P<0.05;Wilcoxon; n=9). The amplitudes of the fit EPSPs at the longer delay (Δt=1 s) were not different from those of the control (P>0.05;Wilcoxon; n=9; in all panels: blue,Δt=25 ms; red,Δt=1 s). Experiments were performed at 32-35°C.
The initial inhibition of EPSP amplitude following thalamic conditioning also was seen in D1 MSNs, but the slower enhancement in EPSP summation was not. Consistent with the voltage clamp data, the first EPSP in the test train delivered 25 ms after the end of the conditioning train was significantly reduced in amplitude in D1 MSNs (Fig. 8A, left).The magnitude of the reduction in the initial EPSP amplitude was similar to that in D2 MSNs (Δt=25 ms; 82±5% of control; P<0.05; Wilcoxon; n=8). Subsequent EPSPs were not different from control (Fig. 8A, blue traces). With a 1 second delay, the initial presynaptic modulation had recovered and the trailing EPSPs in the test response were unaffected in D1 MSNs, in stark contrast to the situation with D2 MSNs (Fig. 8A, red traces). Another obvious difference between D1 and D2 MSNs was the extent to which EPSPs summed, even in the absence of thalamic conditioning (c.f., Figs. 7D, 8A); this difference was accentuated by thalamic activation of cholinergic interneurons (c.f., grey line in Fig. 8A).
Figure 8. Presynaptic and postsynaptic modulation was dependent on muscarinic receptor activation.

(A) Left: Box-plot summary of changes in first EPSP amplitude recorded from D1 MSNs with thalamic pre-pulse (Δt=25 ms; median 82% of control; P<0.05; Wilcoxon; n=8;Δt=1 s; 93.4 of control; P>0.05; Wilcoxon; n=8). Right: Pooled data showing the effect of thalamic pre-pulse on EPSPs summation in D1 MSNs evoked by 50 Hz cortical stimulation. The normalized fifth EPSP was 78 ±9% in control;Δt=25 ms; 69±8%; P>0.05; Wilcoxon; n=8;Δt=1 s, 79±8 %; P>0.05; Wilcoxon; n=8. (B) Left: Box-plot summary of changes in first EPSP amplitude in D2R neurons with thalamic pre-pulse in the presence of scopolamine (Δt=25 ms; median 104.9% of control; P>0.05; Wilcoxon; n=7;Δt=1 s, 92.1% of control; P>0.05; Wilcoxon; n=7). Right: Pooled data showing the effect of thalamic pre-pulse on EPSPs summation in D2 MSNs evoked by 50 Hz cortical stimulation in the presence of scopolamine. The normalized fifth EPSP was 137±14% in control;Δt=25 ms; 145±23%; P>0.05; Wilcoxon; n=7;Δt=1 s, 143±31%;P>0.05; Wilcoxon; n=7). (C) Left: Box-plot summary of changes in first EPSP amplitude in D2 MSNs with thalamic pre-pulse in M1 muscarinic receptor knockout mice (Δt=25 ms; 88% of control; P<0.05; Wilcoxon; n=7;Δt=1 s, 108% of control; P>0.05; Wilcoxon; n=7). Right: Pooled data showing the effect of thalamic pre-pulse on EPSPs summation in M1 knockout D2 MSNs. (The normalized fifth EPSP was 94±9% in M1 knockout control;Δt=25 ms; 101±9%; P>0.05; Wilcoxon; n=7;Δt=1 s, 92±10%; P>0.05; Wilcoxon; n=7). Experiments were performed at 32-35°C.
In D2 MSNs, both rapid and slow effects of thalamic conditioning were dependent upon cholinergic interneurons as judged by the sensitivity of the modulation to disruption of muscarinic signaling. In the presence of the broad spectrum muscarinic receptor antagonist scopolamine (10 μM), both the initial and late effects of thalamic conditioning on corticostriatal EPSPs was abolished (Fig. 8B). There were no significant changes in first EPSP amplitude (Fig 8B) or PPRs (from 1.56±0.14 to 1.47±0.19;Δt=25 ms; to 1.40±0.16;Δt=250 ms; 1.46±0.18;Δt=1 s; P>0.05; Wilcoxon; n=7). The normalized fifth EPSP was 137±14% before thalamic stimulation, was 145±23% right after thalamic stimulation (Δt=25 ms; P>0.05 compared to control; Wilcoxon; n=7), and 143±31% after 1 s (P>0.05 compared to control; Wilcoxon; n=7).
Previous work has shown a similar asymmetry between D1 and D2 MSNs in muscarinic modulation of dendritic Kir2 channels controlling synaptic integration (Shen et al., 2007). This modulation was mediated by postsynaptic M1 muscarinic receptors. To test whether the asymmetry in the effects of thalamic conditioning stimuli was also dependent upon M1 receptors, D2 BAC GFP mice lacking functional M1 muscarinic receptors were studied. In brain slices from these mice, thalamic conditioning trains reduced the amplitude of the initial corticostriatal EPSP in D2 MSNs at short lag times (Δt=25 ms; median 88% of control; P<0.05; Wilcoxon; n=7), but not at longer lags (Δt=250 ms; 98% of control;Δt=1 s; 107% of control; P>0.05; Wilcoxon; n=7), suggesting that the presynaptic M2/4 muscarinic receptor modulation was intact. However, there was no enhancement of EPSP summation at longer lag times (Fig. 8C).
DISCUSSION
Our results show that thalamic input to the striatum engages cholinergic interneurons in a feed-forward circuit to differentially gate corticostriatal control of striatopallidal and striatonigral networks. By virtue of their distinctive facilitating short-term plasticity, repetitive activation of thalamic synapses generated a burst-pause pattern of spiking in cholinergic interneurons, mimicking the activity pattern seen in vivo following presentation of salient stimuli (Aosaki et al., 1994; Matsumoto et al., 2001). The initial interneuron burst transiently suppressed excitatory cortical (and thalamic) input to both major classes of MSN through presynaptic M2-class muscarinic receptors. It also served to initiate a slower, M1 muscarinic receptor mediated postsynaptic facilitation of dendritic responsiveness in striatopallidal MSNs that lasted about a second, roughly matching the duration of the interneuron pause. Because of the transience of the presynaptic inhibition, the pause created a window in which cortical networks could effectively drive activity in the striatopallidal network thought to control action suppression (Fig. 9). Thus, this sub-cortical, thalamostriatal circuit provides a means by which salient stimuli transiently halt behavior or action selection, providing a partial explanation of why lesion of these nuclei produce contralateral sensory neglect and deficits in learning paradigms that demand appropriate responses to sensory stimuli (Matsumoto et al., 2001; Minamimoto and Kimura, 2002).
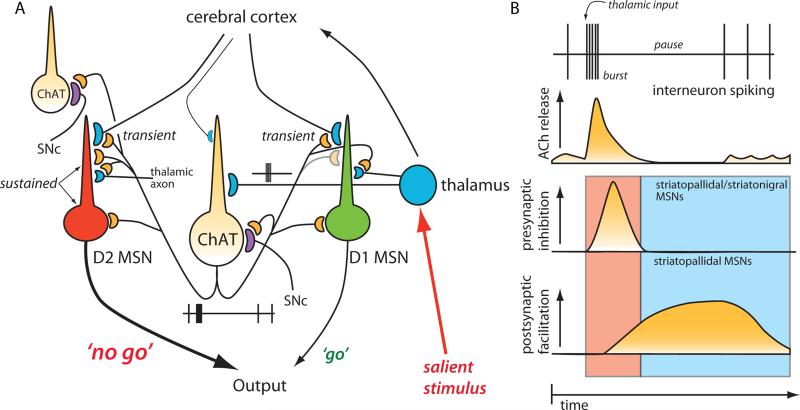
Figure 9. Schematic summary of thalamic gating of glutamatergic signaling in the striatum.
(A). Schematic illustration of cortico- and thalamostriatal glutamatergic projections. Both D1 and D2 MSNs receive glutamatergic afferents from the cortex and the thalamus. However, cholinergic interneuron receives glutamatergic inputs primarily from the thalamus. (B). Thalamic inputs efficiently drive cholinergic interneuron and generate a burst-pause firing pattern. By acting at presynaptic M2-class receptors, acetylcholine release transiently suppresses release probability at corticostriatal synapses formed on both D1 and D2 MSNs. By acting at postsynaptic M1 receptors, acetylcholine release primarily enhances the responsiveness of D2 MSNs to corticostriatal input for about a second. The pause in cholinergic interneuron activity ensures that there is not a concomitant presynaptic suppression in this window. Thalamic stimulation should activate neighboring cholinergic interneurons as well. The pause is generated in part by recurrent collateral or neighboring interneuron activation of nicotinic receptors on dopaminergic terminals. In this way, the burst of thalamic spikes engages cholinergic interneurons to transiently suppress cortical drive of striatal circuits and then create a second long period in which the striatal network is strongly biased toward cortical activation of D2 MSNs.
Thalamic stimulation produced a burst-pause pattern of activity in cholinergic interneurons
Thalamic axons of neurons in the intralaminar nuclei of the thalamus richly innervate both principal MSNs and cholinergic interneurons (Smith et al., 2004). The initial synaptic response in neighboring MSNs and interneurons evoked by stimulation of thalamic axons was similar in amplitude, suggesting a similar innervation density. In MSNs, the thalamic synaptic responses were balanced by a strong cortical input, unlike the situation with cholinergic interneurons where cortical responses were typically small (Fig 3C). The relative weakness of the cortical innervation of cholinergic interneurons seen in our in vitro experiments is consistent with previous electrophysiological work in vivo (Wilson et al., 1990) and anatomical studies (Lapper and Bolam, 1992; Meredith and Wouterlood, 1990).
What distinguished the thalamic responses of MSNs and interneurons was how it was shaped by repetitive activation. In contrast to the rapidly depressing synaptic response in MSNs (Ding et al., 2008), repetitive stimulation of thalamic synapses on interneurons produced a facilitating synaptic response. In part, the relatively slow membrane time constant of interneurons was a factor promoting summation. But taking this factor into account by fitting the current clamp response with a sum of slower EPSP-like waveforms suggested that glutamate release also increased with repetitive stimulation. The basis for this difference in short-term plasticity was not entirely clear. It is possible that the innervation of interneurons and MSNs is derived from different thalamic neurons with distinctive terminal release probabilites (Smith et al., 2009). However, it is also possible that the synaptic properties are determined by the postsynaptic neuron (Koester and Johnston, 2005; Pouille and Scanziani, 2004). Approaches like those that rely upon optogenetics will be required to distinguish these possibilities.
Regardless of its origins, the facilitating, short-term plasticity enabled burst stimulation of thalamic axons to evoke a burst of spikes in cholinergic interneurons. This burst was typically followed by a pause in the ongoing autonomous spiking of cholinergic interneurons, creating a burst-pause pattern that strongly resembled that seen in vivo following presentation of a salient stimulus (Aosaki et al., 1994; Blazquez et al., 2002; Graybiel et al., 1994; Matsumoto et al., 2001; Minamimoto and Kimura, 2002). As in vivo (Aosaki et al., 1994), the thalamically-evoked pause in the slice was dependent upon dopamine release and activation of D2 dopamine receptors that slow on-going autonomous pacemaking (Deng et al., 2007; Maurice et al., 2004). The ability of mecamylamine to attenuate the pause is consistent with the hypothesis that acetylcholine released in response to the thalamic stimulation activated nicotinic receptors on intrastriatal dopaminergic terminals, inducing dopamine release. Presynaptic nicotinic receptors are well known to enhance intrastriatal dopamine release (Rice and Cragg, 2004; Threlfell et al., ; Zhang and Sulzer, 2004; Zhou et al., 2002). Nicotinic receptors also are capable of driving intrastriatal GABAergic interneurons that synapse on cholinergic interneurons, providing another means by which thalamic stimulation could induce a pause (Koos and Tepper, 2002; Sullivan et al., 2008). Because our experiments were performed in the presence of GABAA receptor antagonists, this aspect of the thalamic response was not seen. A slowing in interneuron spiking also can be generated directly by injecting depolarizing current through the recording electrode (Reynolds et al., 2004). This slowing was readily reproducible in our hands, but it was not sensitive to D2 receptor antagonism, suggesting its origins were intrinsic, as suggested previously. Although distinctive, this mechanism should work in concert with those described here to promote a synchronous pause within the interneuron population in response to thalamic stimulation.
A subcortical network for cessation of ongoing motor behavior?
The thalamically generated burst-pause pattern of interneuronal activity had a profound effect on cortical regulation of principal MSNs. Although only a few percent of all striatal neurons, the ‘giant’ cholinergic interneurons have large, dense terminal axonal fields, leading to striatal markers of cholinergic signaling being among the highest in the brain (Zhou et al., 2003). In agreement with previous studies of exogenous muscarinic receptor agonists (Alcantara et al., 2001; Barral et al., 1999; Calabresi et al., 1998a; Higley et al., 2009; Shen et al., 2005; Shen et al., 2007), thalamically evoked interneuron activity had both pre- and postsynaptic effects on corticostriatal synaptic signaling. The initial effect was mediated by presynaptic M2/4 muscarinic receptors. This presumed membrane delimited signaling event produced a rapidly developing and transient (<100 msec) reduction in glutamate release at corticostriatal (and thalamostriatal) terminals on both D1 and D2 MSNs. This spatially diffuse modulation should effectively reduce the ability of spatial summed excitatory input to generate up-states and spiking in MSNs (Stern et al., 1997).
This transient presynaptic inhibition was followed by a period of enhanced postsynaptic excitability triggered by activation of M1 muscarinic receptors in striatopallidal D2 MSNs. These receptors are coupled to intracellular signaling enzymes that turn on more slowly but have a more lasting impact than the presynaptic, M2/4 receptors (Shen et al., 2005). In this case, M1 receptor stimulation of phospholipase C depletes the membrane of phosphatidylinositol 4,5-bisphosphate (PIP2), leading to closure of Kir2 K+ channels governing the resting excitability of striatopallidal dendrites (Shen et al., 2007). M1 receptor signaling also down-regulates Kv4 K+ channels in striatopallidal MSNs, as in other neurons, contributing to an enhancement in EPSP summation (Chen et al., 2006; Day et al., 2008). The ineffectiveness of M1 receptors in striatonigral MSNs to produce this modulation is incompletely understood, but is attributable in part to differences in the subunit composition of the Kir2 channels and their sensitivity to PIP2 depletion (Shen et al., 2007). In this way, the burst of thalamic activity creates a temporal window in which the striatal network is strongly biased toward cortical activation of striatopallidal MSN ensembles. The pause in interneuron spiking ensures that in this window, the release probability of corticostriatal synapses is not depressed. The so-called indirect pathway anchored by D2 MSNs is widely thought to be responsible for action suppression, creating a ‘no-go’ signal to the motor thalamus (Frank and Claus, 2006; Wichmann and DeLong, 1996).
This sub-cortical, thalamostriatal network could be a key component of the circuitry controlling the response to salient environmental events. When a salient stimulus is presented to a subject, ongoing motor behavior typically ceases and the subject orients to the stimulus. How does this happen? Salient stimuli produce a burst of spikes in intralaminar thalamic neurons that project to the striatum (Smith et al., 2009). This component of the reticular activating system is critical to sensory orientation as lesions of this network lead to a failure to orient to stimuli in the contralateral hemifield (Matsumoto et al., 2001; Minamimoto and Kimura, 2002). Our work shows that this burst of spikes engages cholinergic interneurons to transiently suppress cortical drive of striatal circuits controlling action selection and then create a second long period in which the activity of corticostriatal networks responsible for action suppression are enhanced. This latter modulation was mediated by M1 muscarinic receptors. Indeed, selective M1 but not M2-class muscarinic receptor blockade impairs reversal learning (McCool et al., 2008), suggesting this network is important for shifting attention and re-directing behavior.
In associative learning paradigms, cholinergic interneurons respond in a similar burst-pause pattern to presentation of stimuli that have become salient by virtue of having been paired with reward or aversive events (Aosaki et al., 1994; Graybiel et al., 1994). In the initial stages of training, the interneuron pause is tightly correlated in time with a burst in the activity of midbrain dopaminergic neurons thought to be signaling reward prediction errors (Morris et al., 2004). As a consequence, the pause has been attributed to an active suppression of pacemaking by dopamine acting at D2 dopamine receptors (Aosaki et al., 1994; Deng et al., 2007; Maurice et al., 2004). Although plausible in the early stages of learning, the stereotyped pattern of interneuron activity is also disrupted by thalamic lesions that should have no impact on dopaminergic signaling (Graybiel, 2000; Graybiel et al., 1994). Moreover, the interneuron response continues with over-training, at which point dopaminergic (but not thalamic) neurons have stopped responding to the conditioned stimulus (because of a diminished reward prediction error). Our data reconciles these results, showing that thalamically induced burst spiking is capable of engaging a local network mechanism involving dopaminergic terminals.
What is less clear is whether the thalamically induced burst-pause pattern of interneuron activity plays a direct role in the striatal plasticity accompanying learning. What distinguishes this situation is the rise in striatal dopamine levels during the pause in interneuron spiking when the presynaptic effects have waned but the postsynaptic effects on excitability are still manifest. In D1 MSNs, the enhancement of somatic excitability mediated by M1 receptors (Perez-Rosello et al., 2005; Shen et al., 2008) should promote the induction of long-term potentiation (LTP) of corticostriatal synapses thought to underlie selection of actions leading to reward (Shen et al., 2008). Although M1 receptors have been implicated in MSN LTP induction (Calabresi et al., 1999), the mechanisms underlying this effect remain to be determined, particularly in light of recent work that failed to find clear M1 receptor effects on dendritic excitability in striatonigral MSNs (Day et al., 2008). In striatopallidal MSNs, it is more likely that the lingering effects of M1 receptor activation and the rise in D2 receptor activity during the pause would lead to activity dependent induction of long-term depression (LTD) needed to ‘de-select’ actions for suppression (Day et al., 2008; Shen et al., 2008).
EXPERIMENTAL PROCEDURES
Slice preparation and solutions
Horizontal slices (275-300 μm) were obtained from 21-31 day old FVB mice. Three populations of mice were used: one in which GFP expression was driven by the D1 receptor promoter, one in which GFP expression was driven by the D2 receptor promoter (both of these lines were obtained from GENSAT), and one in which these D2 GFP mice were crossed with mice lacking the M1 muscarinic receptor (Hamilton et al., 1997) Mice were sacrificed and their brains removed using standard techniques that were approved by the Northwestern University ACUC committee. Briefly, the mice were anesthetized deeply with ketamine (50 mg/kg)/xylazine (4.5 mg/kg), transcardially perfused with oxygenated, ice-cold, artificial cerebral spinal fluid (ACSF) and decapitated. Brains were rapidly removed. The horizontal slice preparation was obtained using the same method as previously described (Ding et al., 2008). The brain was laid on a chilled cutting surface ventral side down. After the cerebellum was removed the brain was blocked along the midline. Both hemispheres were laid medial side down, then an approximately 20° oblique horizontal cut was made on dorsal side because single axon tracing work suggests that thalamostriatal afferents run toward striatum in a lateral, anterior and dorsal direction (Deschenes et al., 1996). Blocked brains were sectioned in oxygenated, ice-cold, ACSF using a Leica VT1000S vibratome (Leica Microsystems). The ACSF contained the following (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 25 NaHCO3, 1.25 NaH2PO4, and 12.5 glucose. The slices were transferred to a holding chamber where they were completely submerged in ACSF bubbled with 95% O2 and 5% CO2 and incubated for 30 minutes at 34 °C. The slices were then maintained at room temperature (22-23°C) before use.
DiI Labeling
28 days old D1 and D2 GFP mice were anesthetized with a ketamine (50 mg/kg)/xylazine (4.5 mg/kg) mixture and perfused transcardially with phosphate buffered saline (PBS) followed by 60 ml 4% paraformaldehyde (PFA) (in 0.2 M phosphate buffer, pH 7.4). Brains were then removed, postfixed for 2 days. Horizontal brain slices (275 μm) were cut using a Leica VT1000S vibratome (Leica Microsystems). 1,1’-dioctadecyl-3,3,3’,3’-Tetramethylindo-carbocyanine perchlorate (DiI) crystalline was glued on the surface of the brain slice either in the cortex or the thalamus. The slice was allowed to air-dry for 2-3 min to promote adhesion and prevent DiI from spreading along the surface of the brain slice. If the DiI comes in contact with external or internal capsule, it will diffuse along those fibers and may obscure the labeling results. During application of the dye, we made sure the dye is precisely placed in the cortex or thalamus without contacting external or internal capsule. Brains slices were then kept in 4% PFA at 35 °C for 5-7 days. DiI fluorensence images were captured on a Zeiss LSM 510 confocal microscope.
Electrophysiology
Individual slices were transferred to a submersion-style recording chamber and continuously superfused with ACSF at a rate of 2-3 ml/min at room temperature (20-22ºC) or elevated temperature (32-35°C) as stated in the text. Cell-attached, whole-cell voltage-clamp or current-clamp recordings were performed using standard techniques. Recordings were performed on striatal medium spiny neurons or cholinergic interneurons visually identified in the slice with the help of infrared-differential interference contrast (IR-DIC) video microscopy with an Olympus OLY-150 camera/controller system (Olympus, Japan). For all experiments, 10 μM (-) SR95531 (gabazine) or 50 μM picrotoxin was added to the superfusion medium to block GABAA receptor-mediated synaptic responses; 10 μM CGP55845 was used to block GABAB receptors in some experiments. For voltage-clamp experiments, pipettes (3-5 MΩ) were filled with Cs+ internal solution containing the following (in mM): 120 CsMeSO3, 15 CsCl, 8 NaCl, 10 TEA-Cl, 10 HEPES, 2-5 QX-314, 0.2 EGTA, 2 Mg-ATP, 0.3 Na-GTP, pH 7.3 adjusted with CsOH. Voltage-clamp Experiments were performed at room temperature or 32-35 °C as stated. For cell-attached and current-clamp experiment, the K+ internal solution consisted of (in mM) 135 KMeSO4, 5 KCl, 0.5 CaCl2, 5 HEPES, 5 EGTA, 2 Mg-ATP, 0.3 Na-GTP, pH=7.3 with KOH. Experiments were done close to physiological temperature (32-35 °C). Data were recorded with Multiclamp 700A (filtered at 2-5 kHz and digitized at 10-20 kHz). Voltage measurements were not corrected for the experimentally determined junction potential (~8-9mV). Stimulation (50-200 μs) was performed using steel concentric electrodes (Frederick Haer & Co, ME). The cortical afferents were stimulated by placing stimulation electrode between layer V and VI in the cortex; thalamic afferents were stimulated by placing stimulation electrode in the thalamus close to the border of thalamic reticular nucleus.
To determine EPSC amplitudes in paired pulse paradigms with short inter-stimulus intervals, the first EPSC was fitted with a bi-exponential, EPSC-like function (A1*(1-exp(-(t-t0)/taurise)) * exp(-(t-t0)/taudecay)) and the amplitude of the second EPSC was determined after subtracting the first EPSC. To determine EPSC amplitudes in 50 Hz high frequency stimulation train, the EPSCs (5 stimuli, 50 Hz) were fitted with a sum of five bi-exponential alpha functions of the same form with appropriate time delays. The kinetics of the EPSCs (taurise, taudecay) were assumed to be constant, with only the amplitude (Ai) varying as a function of pulse number. EPSCs early in the trains were well-fit with this constraint, but the decay phase of the last EPSC often had a slower component. Estimates of EPSC amplitude from the fits were measured and plotted against stimulus number.
Data analysis and statistical methods
Data analysis was done with Clampfit 9.2 (Axon Instruments) and Igor Pro 5.0 (WaveMetrics, Lake Oswego, OR). Statistical analysis was performed using Sigmastat 3.0 (SPSS Inc.). Summary data are reported as mean±SEM when sample sizes exceeded 10 and as medians for smaller samples where sampling distributions were less well defined. Box plots were used for graphic illustration of data. Non-matched samples were analyzed with the nonparametric Mann-Whitney Rank Sum test. Matched samples were analyzed with Wilcoxon signed ranks test and paired t-test.
Reagents and chemicals
All reagents were obtained from Sigma except DiI (Invitrogen Molecular Probes), KMeSO4 (ICN Biochemicals Aurora, OH), Na2GTP (Boehringer Mannheim, Indianapolis, IN), SR95531, D-APV, CGP55845 (Tocris).
Supplementary Material
Acknowledgements
The authors would like to thank Dilyan Dryanovski, Qing Ruan, Karen Saporito and Sasha Ulrich for excellent technical assistance. Supported by NS 34696 (D.J.S.) and the Picower Foundation (D.J.S.). Jun B. Ding's present address: Department of Neurobiology, Harvard Medical School, Boston, MA 02115, jun_ding@hms.harvard.edu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akins PT, Surmeier DJ, Kitai ST. M1 muscarinic acetylcholine receptor in cultured rat neostriatum regulates phosphoinositide hydrolysis. J Neurochem. 1990;54:266–273. doi: 10.1111/j.1471-4159.1990.tb13310.x. [DOI] [PubMed] [Google Scholar]
- Alcantara AA, Mrzljak L, Jakab RL, Levey AI, Hersch SM, Goldman-Rakic PS. Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J Comp Neurol. 2001;434:445–460. doi: 10.1002/cne.1186. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994;265:412–415. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- Barral J, Galarraga E, Bargas J. Muscarinic presynaptic inhibition of neostriatal glutamatergic afferents is mediated by Q-type Ca2+ channels. Brain Res Bull. 1999;49:285–289. doi: 10.1016/s0361-9230(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000;20:8493–8503. doi: 10.1523/JNEUROSCI.20-22-08493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez PM, Fujii N, Kojima J, Graybiel AM. A network representation of response probability in the striatum. Neuron. 2002;33:973–982. doi: 10.1016/s0896-6273(02)00627-x. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Bernardi G. Activation of M1-like muscarinic receptors is required for the induction of corticostriatal LTP. Neuropharmacology. 1999;38:323–326. doi: 10.1016/s0028-3908(98)00199-3. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Blockade of M2-like muscarinic receptors enhances long-term potentiation at corticostriatal synapses. Eur J Neurosci. 1998a;10:3020–3023. doi: 10.1111/j.1460-9568.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Pisani A, Sancesario G, North RA, Bernardi G. Muscarinic IPSPs in rat striatal cholinergic interneurones. J Physiol. 1998b;510(Pt 2):421–427. doi: 10.1111/j.1469-7793.1998.421bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P, Zhang Y, Xu ZC. Involvement of I(h) in dopamine modulation of tonic firing in striatal cholinergic interneurons. J Neurosci. 2007;27:3148–3156. doi: 10.1523/JNEUROSCI.5535-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Doan VD, Parent A. A single-cell study of the axonal projections arising from the posterior intralaminar thalamic nuclei in the rat. Eur J Neurosci. 1996;8:329–343. doi: 10.1111/j.1460-9568.1996.tb01217.x. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Galarraga E, Hernandez-Lopez S, Reyes A, Miranda I, Bermudez-Rattoni F, Vilchis C, Bargas J. Cholinergic modulation of neostriatal output: a functional antagonism between different types of muscarinic receptors. J Neurosci. 1999;19:3629–3638. doi: 10.1523/JNEUROSCI.19-09-03629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Hamilton SE, Loose MD, Qi M, Levey AI, Hille B, McKnight GS, Idzerda RL, Nathanson NM. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc Natl Acad Sci U S A. 1997;94:13311–13316. doi: 10.1073/pnas.94.24.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Soler-Llavina GJ, Sabatini BL. Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat Neurosci. 2009;12:1121–1128. doi: 10.1038/nn.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol. 1989;62:1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science. 2005;308:863–866. doi: 10.1126/science.1100815. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51:533–545. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Minamimoto T, Graybiel AM, Kimura M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J Neurophysiol. 2001;85:960–976. doi: 10.1152/jn.2001.85.2.960. [DOI] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci. 2004;24:10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool MF, Patel S, Talati R, Ragozzino ME. Differential involvement of M1-type and M4-type muscarinic cholinergic receptors in the dorsomedial striatum in task switching. Neurobiol Learn Mem. 2008;89:114–124. doi: 10.1016/j.nlm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Wouterlood FG. Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: a light and electron microscopic study. J Comp Neurol. 1990;296:204–221. doi: 10.1002/cne.902960203. [DOI] [PubMed] [Google Scholar]
- Minamimoto T, Kimura M. Participation of the thalamic CM-Pf complex in attentional orienting. J Neurophysiol. 2002;87:3090–3101. doi: 10.1152/jn.2002.87.6.3090. [DOI] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Perez-Rosello T, Figueroa A, Salgado H, Vilchis C, Tecuapetla F, Guzman JN, Galarraga E, Bargas J. Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J Neurophysiol. 2005;93:2507–2519. doi: 10.1152/jn.00853.2004. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J Neurosci. 2004;24:9870–9877. doi: 10.1523/JNEUROSCI.3225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci. 2005;25:7449–7458. doi: 10.1523/JNEUROSCI.1381-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, Surmeier DJ. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci. 2007;10:1458–1466. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009;78:60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Stern EA, Kincaid AE, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol. 1997;77:1697–1715. doi: 10.1152/jn.1997.77.4.1697. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Chen H, Morikawa H. Recurrent inhibitory network among striatal cholinergic interneurons. J Neurosci. 2008;28:8682–8690. doi: 10.1523/JNEUROSCI.2411-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson C, Dani JA. Muscarinic and nicotinic cholinergic mechanisms in the mesostriatal dopamine systems. Neuroscientist. 2003;9:23–36. doi: 10.1177/1073858402239588. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.