Abstract
Aim:
To examine whether danshensu could protect vascular endothelia in a rat model of hyperhomocysteinemia.
Methods:
The model was established by feeding rats with a methionine-rich diet (1 g·kg−1·d−1) for 3 months. Immediately following the discontinuation of methionine-rich diet, rats were treated with danshensu (67.5 mg·kg−1·d−1, po) or saline for 3 additional months. One group of rats receiving vitamin mixture (folic acid, vitamin B12 and vitamin B6) was included as a positive control. One group of rats not exposed to methionine-rich diet was also included as a blank control. The expression of tumor necrosis factor-alpha (TNF-alpha) and intercellular adhesion molecule-1 (ICAM-1) protein in the descending aorta was examined using immunohistochemistry and Western blot. Homocysteine and blood concentration of endothelin and nitric oxide (NO) was also examined.
Results:
Methionine-rich diet resulted in accumulation of “foam cells”, up-regulated expression of TNF-alpha and ICAM-1 in the descending aorta, and significantly increased serum homocysteine. Plasma endothelin concentration was significantly increased; NO was decreased. Danshensu treatment, either simultaneous to methionine-rich diet or afterwards, attenuated the above mentioned changes.
Conclusion:
Chronic treatment with danshensu could prevent/attenuate the formation of atherosclerosis. Potential mechanisms include inhibited expression of representative proinflammatory cytokines and adhesion molecules in arterial endothelia. Changes in homocysteine and circulating molecules that control vascular contraction/relaxation via endothelial cells (eg, endothelin and NO) were also implicated.
Keywords: hyperhomocysteinemia, danshensu, endothelin, nitric oxide, tumor necrosis factor-alpha, intercellular adhesion molecule-1
Introduction
Hyperhomocysteinemia (elevated circulating level of homocysteine) often occurs in patients with one or more defective genes (eg, methylenetetrahydrofolate reductase) that control the breakdown of homocysteine1, and is an independent risk factor for atherosclerosis2. Deficiency of folate, vitamin B6 and B12 could also result in hyperhomocysteinemia3. High level of homocysteine damages endothelial cells in vasculature via increased formation of reactive oxygen species (ROS) and inflammation, and therefore increases the likelihood to develop heart attack and stroke4, 5, 6, 7. Hyperhomocysteinemia also promotes LDL oxidation and internalization by macrophages, which in turn is the initial step of atherosclerosis8.
Supplementation of folate, vitamin B6 and B12 could reduce the level of homocysteine in patients with hyperhomocysteinemia9, but do not affect the process of atherosclerosis directly10. As a result, the benefit is limited11, 12, 13.
Radix salviae miltiorrhizae (commonly known as “Danshen”) has been used by practitioners of the Traditional Chinese Medicine for decades in the treatment of a variety of cardiovascular diseases. Danshensu [3-(3,4-dihydroxy-phenyl)-2-hydroxy-propionic acid], a major active component of Danshen, could improve microcirculation, suppress the formation of ROS, inhibit platelet adhesion and aggregation, and protect myocardium against ischemia14, 15, 16. Danshensu could also protect endothelial cells against injury induced by hyperhomocysteinemia17 or inflammation18.
Effects of danshensu on homocysteine, TNF-alpha, ICAM-1, endothelin and NO have been reported by many labs. For example, Cao and colleagues19 reported decreased homocysteine level in rats in response to danshensu treatment. Ding et al20 showed that danshensu inhibits the expression of ICAM-1 induced by TNF-alpha in endothelial cells. In addition, danshensu also inhibits proliferation of vascular smooth muscle cells induced by elevated NO level and decreased ET-121. In the current study, we examined potential effects of danshensu on the formation of atherosclerosis in a rat model of hyperhomocysteinemia. Signaling molecules that control vascular contraction/relaxation, and representative proinflammatory cytokines and ICAM-1 were also investigated.
Materials and methods
Animal treatment
Animal experiments were conducted in compliance with the Animal Management Rules of the Ministry of Health of the People's Republic of China (Document #55, 2001) and were approved by the Animal Care and Use Committee of Shandong University. Wistar rats were purchased from Shandong Academy of Medical Sciences, and housed individually in stainless cages at 24 °C with a 12-h light–dark cycle. Rats (200 g) received a methionine-rich diet (provided by Dr Wei-dong ZHANG, Shandong Academy of Medical Sciences) for 3 months. Intake of methionine-rich diet was monitored daily and adjusted to a level of 1 g methionine per day per kg body weight. Access to water was unrestricted. At the end of the 3-month methionine-rich diet, rats received daily treatment of danshensu (67.5 mg·kg−1·d−1, po; (Ang Sheng Shaanxi Biomedical Technology, Xi'an, China), a vitamin mixture (folic acid 0.45 mg·kg−1·d−1, vitamin B12 6.75 μg·kg−1·d−1, vitamin B6 2.7 mg·kg−1·d−1; po), or saline for another 3 months before sacrifice. Each group consisted of 5 male and 5 female rats. An additional “prevention” group received danshensu (67.5 mg·kg−1·d−1, po) during the 3-month period of methionine-rich diet. Data collected from this group were compared to a “model” group that was sacrificed immediately after the 3-month methionine-rich diet. A group of rats not exposed to methionine-rich diet was also included as a blank control.
At the end of the experiments, rats were sacrificed with chloral hydrate (0.36 g/kg). Trunk blood was collected; blood samples were stored at −80 °C. Segments of descending aorta were collected for Western blot analysis, or fixed with 4% paraformaldehyde and processed for immunohistochemical analysis or routine H&E staining.
ICAM-1 and TNF-alpha expression
The expression of ICAM-1 and TNF-alpha was examined with a 3-step streptavidin-biotin immunoperoxidase method22. Tissue sections (5 μm) were deparaffinized and rehydrated, and then heated in a microwave oven for 10 min to enhance antigen retrieval. Slides were incubated with 3% H2O2 for 10 min to quench endogenous peroxidase, and then blocked with 5% normal goat serum for 20 min. Tissue sections were incubated with a rabbit anti-rat ICAM-1 or rabbit anti-rat TNF-alpha antibody (1:200 dilution; Bosider, Wuhan, China) for 2 h in a moisture chamber at 37 °C. After extensive washing, sections were incubated with biotinylated goat anti-rabbit IgG (1:200 dilution) and avidin for 30 min at 37 °C, and developed using a diaminobenzidine method. Sections were counterstained with hematoxylin for 15 s. For blank controls, primary antibody was omitted. Sections were examined under a microscope for positively stained cells (brown or yellow). Data were analyzed with CMIAS series of true color multi-functional pathological image analysis system (Beijing University of Aeronautics and Astronautics, Beijing, China). The mean optical density of positively stained cells from 10 randomly selected fields (×400) was calculated, and used to estimate the expression of ICAM-1 and TNF-alpha.
Western blot analysis
The descending aorta was homogenized and then digested with a lysis buffer, and centrifuged at 13 000×g for 15 min at 4 °C. Protein concentration in the supernatant was measured with a BCA method (Pierce Corporation, Tempe, AZ, US). Samples (20 μg protein) were separated with 10% SDS-PAGE, and transferred to a polyvinylidene difluoride membrane. The membrane was incubated with a rabbit anti-rat TNF-alpha or rabbit anti-rat ICAM-1 (both 1:500) for 1 h. After extensive washing, the membrane was incubated with a horseradish peroxidase (HRP)-conjugated goat-anti-rabbit IgG (1:1000; Bosider, Wuhan, China) prior to ECL visualization (Pierce Corporation). Optical density of the band at appropriate molecular weight was used to estimate the amount of protein. Experiments were repeated 3 times independently.
Blood biochemical analysis
Serum homocysteine concentration was measured using a HPLC method as described previously23. Blood endothelin concentration was measured using a radioimmunoassay (RIA Institute of People's Liberation Army General Hospital Science and Technology Development Center)24. Serum NO concentration was measured using a kit from Nanjing Jiancheng Bioengineering Research Institute (Nanjing, China)24.
Statistical analysis
Data are expressed as mean±SD. Statistical analysis was performed with one-way ANOVA followed by least-significant difference t-test for post hoc comparison. Statistical significance was set at P<0.05.
Results
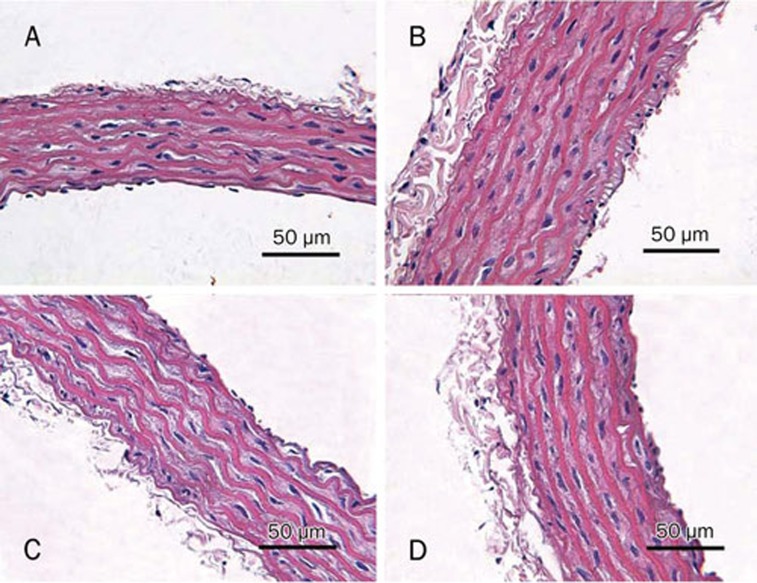
In contrast to the normal appearances in the blank control rats, the intima of descending aorta in rats receiving methionine-rich diet was thickened, and contained scattered foam cells (Figure 1). Danshensu treatment, either simultaneous with methionine-rich diet or afterwards, significantly decreased the number of foam cells.
Figure 1.
Morphology of aortic wall was observed by light microscopy. (A) blank controls; (B) model group; (C) “preventive” group; (D) danshensu group.
Effect of danshensu on homocysteine level
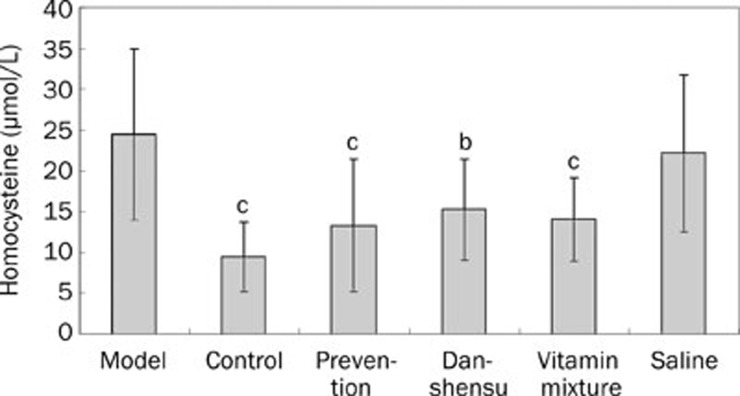
Methionine-rich diet significantly increased serum homocysteine concentration (P<0.01 vs blank controls; Figure 2). Such an increased homocysteine was significantly attenuated by treatment with folate-VitB6-VitB12 vitamin and danshensu after the 3-month methionine-rich diet (P<0.01 and <0.05 respectively). Preventive treatment (daily danshensu during the 3-month methionine-rich diet) also significantly decreased serum homocysteine level (P<0.01 vs the “model” group).
Figure 2.
Serum homocysteine concentration in different groups. bP<0.05, cP<0.01 vs model group.
Effect of danshensu on blood endothelin and NO
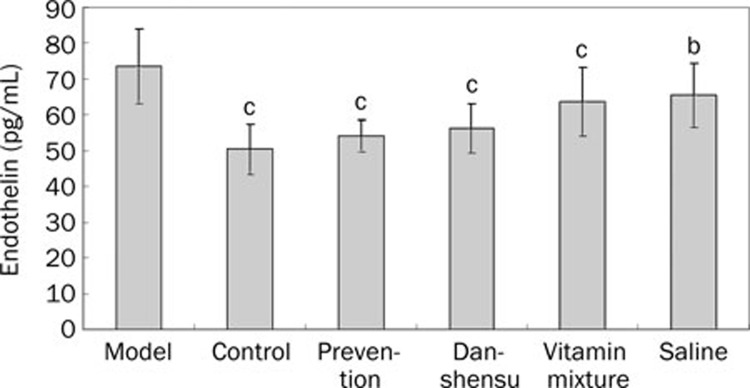
Methionine-rich diet significantly increased plasma endothelin concentration (P<0.01 vs blank controls; Figure 3). Such a response was significantly attenuated by treatment with vitamin mixture and danshensu (P<0.01 for both). Preventive danshensu treatment also significantly decreased endothelin concentration. Saline treatment also produced a statistically significant reduction in endothelin concentration in rats exposed to methionine-rich diet (P<0.05).
Figure 3.
Plasma endothelin levels in different groups. bP<0.05, cP<0.01 vs model group.
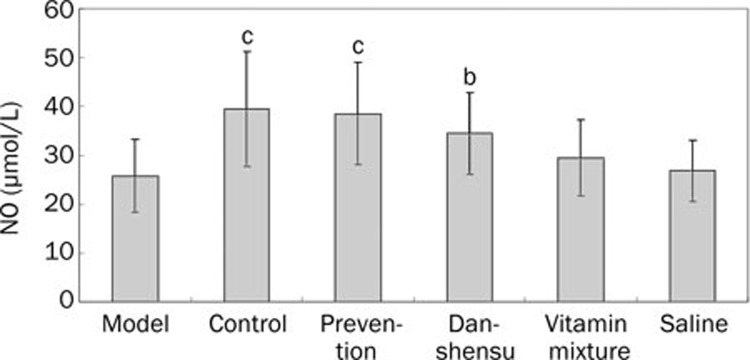
Serum NO level was significantly decreased by methionine-rich diet (P<0.01 vs blank controls; Figure 4). Treatment with danshensu, either after or simultaneously with methionine-rich diet, significantly increased serum NO level (P<0.05 and <0.01, respectively). Treatment with vitamin mixture produced a trend of increasing serum NO, but the difference was not statistically significant.
Figure 4.
Serum NO concentration in different groups. bP<0.05, cP<0.01 vs model group.
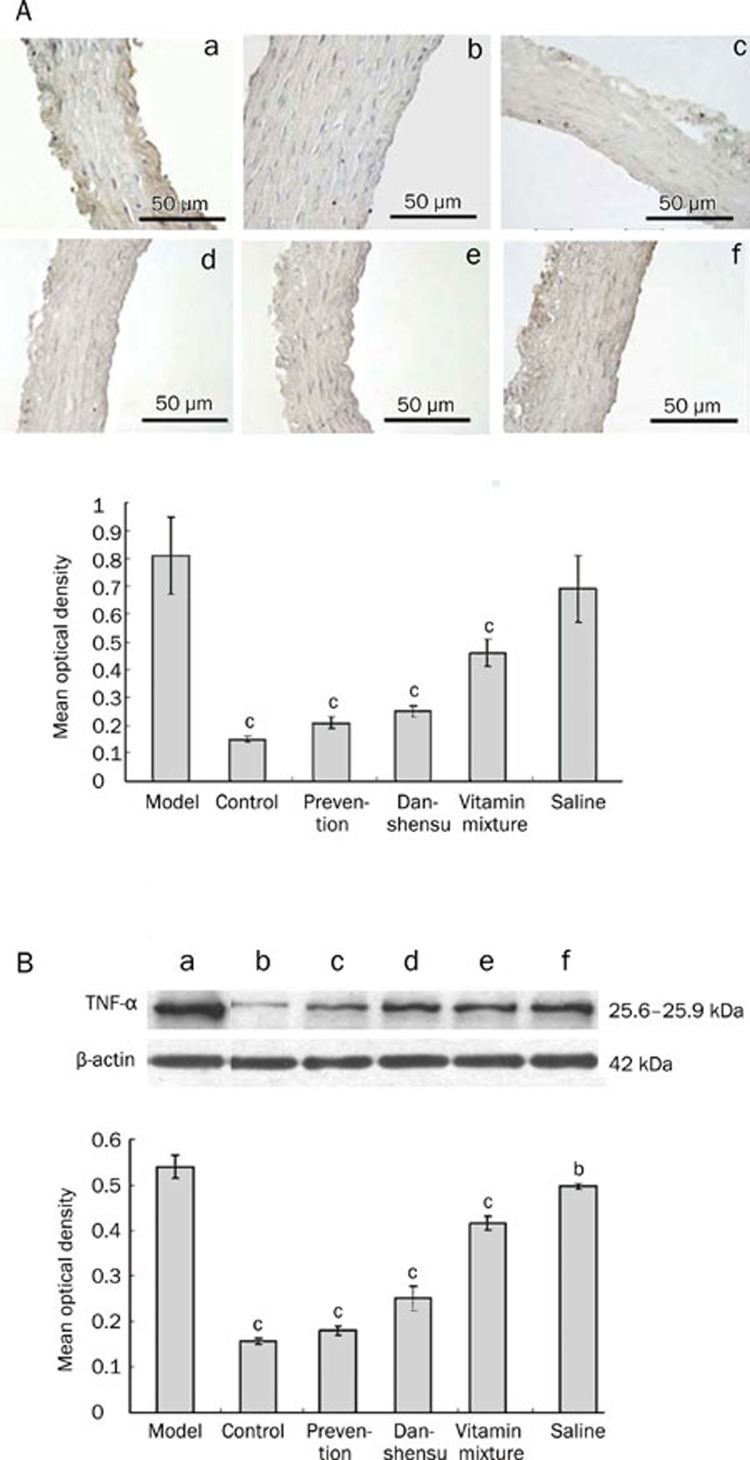
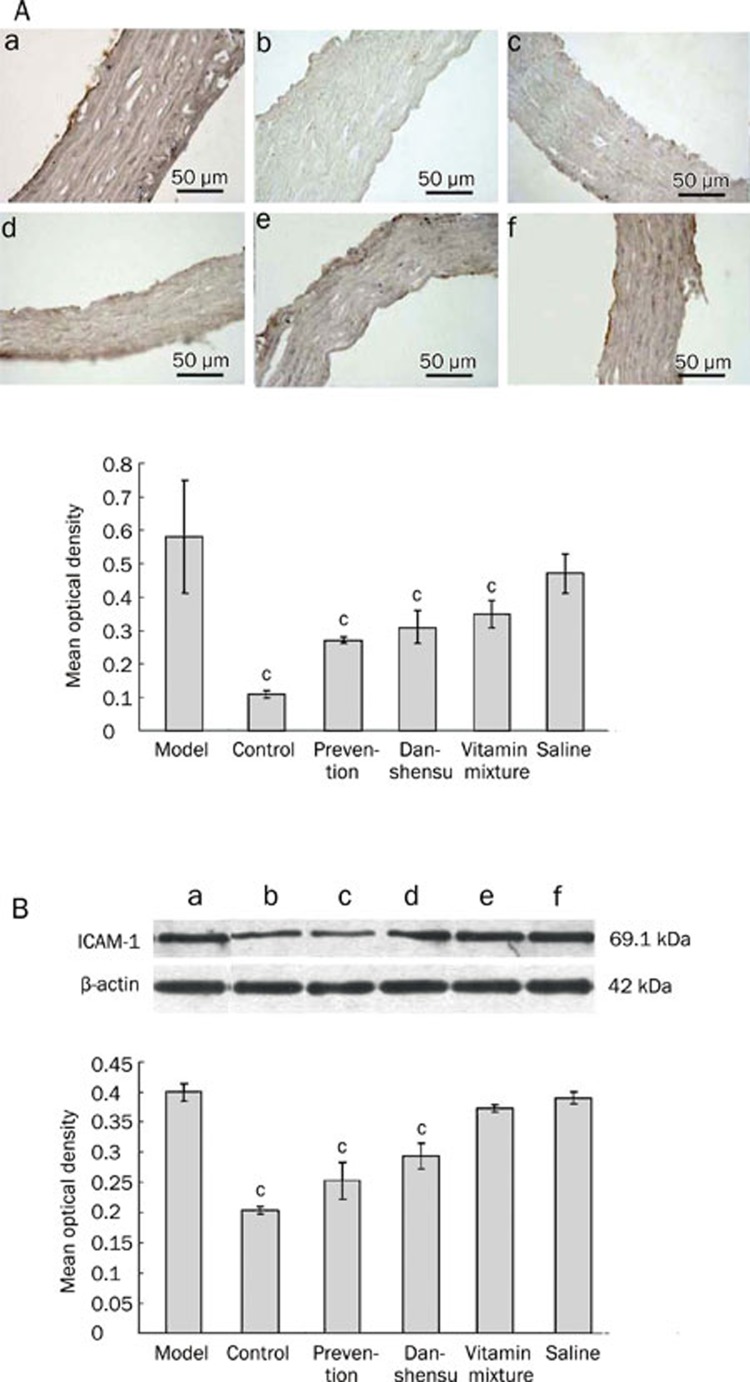
Effect of danshensu on TNF-alpha and ICAM-1
Immunohistochemical analysis revealed dramatically increased expression of TNF-alpha (Figure 5A) and ICAM-1 (Figure 6A) in endothelial cells in response to methionine-rich diet. Increased expression of TNF-alpha and ICAM-1 was significantly attenuated by vitamin mixture and danshensu treatment, regardless of the treatment timing (P<0.01 for all). Results obtained from Western blot analysis (Figure 5B and 6B) were largely consistent with the immunohistochemical findings.
Figure 5.
The effect of Danshensu on TNF-α expression in endothelium in a rat model of hyperhomocysteinemia. (A) TNF-α expression determined by immunohistochemistry. Upper: Representative photographs. Bottom: a statistical analysis of immunohistochemical results. cP<0.01 vs model group. (B) TNF-α expression determined by Western blot. Upper: Representative photographs. Bottom: a statistical analysis of Western blot results. (a) model group; (b) blank controls; (c) prevention group; (d) danshensu group; (e) vitamin mixture group; (f) saline group. bP<0.05, cP<0.01 vs model group.
Figure 6.
The effect of Danshensu on ICAM-1 expression in endothelium in a rat model of hyperhomocysteinemia. (A) ICAM-1 expression determined by immunohistochemistry. Upper: Representative photographs. Bottom: a statistical analysis of immunohistochemical results. cP<0.01 vs model group. (B) ICAM-1 expression determined by Western blot. Upper: Representative photographs. Bottom: a statistical analysis of Western blot results. (a) model group; (b) blank controls; (c) prevention group; (d) danshensu group; (e) vitamin mixture group; (f) saline group. cP<0.01 vs model group.
Discussion
Folate, vitamin B6 and vitamin B12 are key cofactors to the enzymes that metabolize homocysteine, and have been used to manage hyperhomocysteinemia25. Hyperhomocysteinemia is associated with increased risk of thrombotic and atherosclerotic vascular diseases25. Elevated level of homocysteine causes lipid peroxidation, inflammatory reactions, and vascular endothelial injury26. In the current study, danshensu treatment significantly lowered the serum homocysteine concentration in rats receiving a methionine-rich diet. The magnitude of such an action was similar to that of a vitamin mixture containing folate, VitB6 and VitB12. Effects of danshensu were more robust when given simultaneous to methionine-rich diet, indicating the superiority of preventive treatment. We speculate that contributing factors to this phenomenon is the ability of danshensu to promote the catabolism19 and excretion of homocysteine during the period of methionine intake.
A major function of NO in the cardiovascular system is to relax both large arteries and small resistance vessels26. In contrast, endothelin induces vasoconstriction, participates in inflammation, cellular injury, and vascular events27. Consistent with previous studies8, 28, 29, 30, 31, 32, our results indicated that NO is decreased upon hyperhomocysteinemia. In contrast, endothelin was significantly increased. These changes would result in increased vascular contraction, and might have contributed to the eventual development of atherosclerosis. Treatment with danshensu clearly attenuated the changes of NO and endothelin in response to methionine-rich diet, suggesting that the preventive/therapeutic effects of danshensu on atherosclerosis are at least partially mediated by vasodilation secondary to decreased level of serum homocysteine. In addition to indirect effect via decreased homocysteine level, danshensu has direct action on vascular endothelium. For example, a previous report33 indicated that danshensu could result in significant vasodilation through promoting the opening of non-selective potassium channels and small-conductance calcium-sensitive potassium channels in vascular smooth muscle cells. In our experiments, the level of endothelin but not NO was significantly lower in rats receiving 3-month saline treatment after methionine-rich diet (saline group) in comparison to immediately after the methionine-rich diet (“model” group), indicating differential spontaneous recovery of various pathological changes after discontinuation of the methionine-rich diet.
A previous study demonstrated that hyperhomocysteinemia could increase inflammatory responses mediated by NF-kappaB and TNF-alpha via activating the ERK(1/2)/p38MAPK pathway10. TNF-alpha is mainly secreted by monocytes and macrophages34. But under certain circumstances, TNF-alpha can also be secreted by endothelial cells, vascular smooth muscle cells, and cardiac fibroblast cells35. TNF-alpha could damage endothelial cells and vessel wall, and thus contribute to the formation of plaques36, possibly through stimulating the synthesis of matrix metalloproteinases-9 (MMP-9) by macrophage37, 38.
ICAM-1 is expressed constitutively at low levels by vascular endothelial cells under normal conditions, but expressed at large quantity upon stimulation by inflammatory cytokines such as TNF-alpha39. Increased ICAM-1 allows the attachment of leukocytes to the endothelium and subsequent migration into peripheral tissue39. Antioxidants can decrease TNF-alpha synthesis/release by a variety of cell types and ICAM-1 expression by vascular endothelial cells39. Danshensu is a known antioxidant. Therefore, decreased TNF-alpha and ICAM-1 expression upon danshensu treatment could partially be attributed to its antioxidant property.
In our experiment, high level of TNF-alpha and ICAM-1 expression was observed in rats exposed to methionine-rich diet. Danshensu treatment, either simultaneous to methionine-rich diet or afterwards, significantly decreased TNF-alpha and ICAM-1 expression. It remains to be investigated whether such effects are mediated by the ERK1/2/p38MAPK pathway as suggested by Bai et al10.
In summary, our findings demonstrated that danshensu could lower homocysteine level and attenuate atherosclerosis in rats receiving a methionine-rich diet. Signaling molecules that favor vasoconstriction, such as endothelin, were decreased by danshensu. Molecules that relax arteries, such as NO, were increased. Danshensu treatment also attenuated the overexpression of TNF-alpha and ICAM-l. Considering its excellent safety profile, we propose that danshensu could be used in people at high risk to develop atherosclerotic diseases.
Author contribution
Yu-xia ZHAO and Yun-fang LIU designed this research project; Rui-xue YANG carried out the experiments, analyzed the data, and wrote the manuscript; Shan-ying HUANG, Fang-fang YAN, Xiao-ting LU, Yi-fan XING, and Yan LIU contributed analytical tools and reagents; Yu-xia ZHAO revised the manuscript.
Acknowledgments
This study was supported by the National Basic Research Program of China (national “973” Project, 2005CB523301) and grants from the National Natural Science Foundation of China (No. 30801503, 30772810, 30873325). Assistance was provided by the key laboratory of cardiovascular remodeling and function research and the Chinese Ministry of Education.
References
- Guerzoni AR, Biselli PM, Godoy MF, Souza DR, Haddad R, Eberlin MN, et al. Homocysteine and MTHFR and VEGF gene polymorphisms: impact on coronary artery disease. Arq Bras Cardiol. 2009;92:263–8. doi: 10.1590/s0066-782x2009000400003. [DOI] [PubMed] [Google Scholar]
- Patrick A, O'Callaghan Fitzgerald A, Fogarty J, Gaffney P, Hanbidge M, et al. New and old cardiovascular risk factors: C-reactive protein, homocysteine, cysteine and von Willebrand factor increase risk, especially in smokers. Eur J Cardiovasc Prev Rehabil. 2005;12:542–7. doi: 10.1097/00149831-200512000-00005. [DOI] [PubMed] [Google Scholar]
- Vannucchi H, Melo SS. Hyperhomocysteinemia and cardiometabolic risk. Arq Bras Endocrinol Metabol. 2009;53:540–9. doi: 10.1590/s0004-27302009000500007. [DOI] [PubMed] [Google Scholar]
- Perez-de-Arce K, Foncea R, Leighton F. Reactive oxygen species mediates homocysteine-induced mitochondrial biogenesis in human endothelial cells: modulation by antioxidants. Biochem Biophys Res Commun. 2005;338:1103–9. doi: 10.1016/j.bbrc.2005.10.053. [DOI] [PubMed] [Google Scholar]
- Qureshi I, Chen H, Brown AT, Fitzgerald R, Zhang X, Breckenridge J, et al. Homocysteine-induced vascular dysregulation is mediated by the NMDA receptor. Vasc Med. 2005;10:215–23. doi: 10.1191/1358863x05vm626oa. [DOI] [PubMed] [Google Scholar]
- Chaava M, Bukia T, Shaburishvili T. Homocysteine as risk marker of cardiovascular disease. Georgian Med News. 2005;127:65–70. [PubMed] [Google Scholar]
- Fowler B. Homocystein — an independent risk factor for cardiovascular and thrombotic diseases. Ther Umsch. 2005;62:641–6. doi: 10.1024/0040-5930.62.9.641. [DOI] [PubMed] [Google Scholar]
- Murawska-clalowicz E, Januszewska L, Zuwala-jagiello J, Millczarska J, Zawadzki M, Paprocka-borowicz M, et al. Melatonin decreases homocysteine level in blood of rats. J Physiol Pharmacol. 2008;59:717–29. [PubMed] [Google Scholar]
- Postea O, Krotz F, Henger A, Keller C, Weiss N. Stereospecific and redox-sensitive increase in monocyte adhesion to endothelial cells by homocysteine. Arterioscler Thromb Vasc Biol. 2006;26:508–13. doi: 10.1161/01.ATV.0000201039.21705.dc. [DOI] [PubMed] [Google Scholar]
- Bai YP, Liu YH, Chen J, Song T, You Y, Tang ZY, et al. Rosiglitazone attenuates NF-kappaB-dependent ICAM-1 and TNF-alpha production caused by homocysteine via inhibiting ERK1/2/p38MAPK activation. Biochem Biophys Res Commun. 2007;360:20–6. doi: 10.1016/j.bbrc.2007.05.222. [DOI] [PubMed] [Google Scholar]
- Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–75. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- Cheng TO. Cardiovascular effects of danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Wang F, Liu YY, Liu LY, Zeng QJ, Wang CS, Sun K, et al. The attenuation effect of 3,4-dihydroxy-phenyl lactic acid and salvianolic acid B on venular thrombosis induced in rat mesentery by photochemical reaction. Clin Hemorheol Microcirc. 2009;42:7–18. doi: 10.3233/CH-2009-1180. [DOI] [PubMed] [Google Scholar]
- Han JY, Horie Y, Fan JY, Sun K, Guo J, Miura S, et al. Potential of 3,4-dihydroxy-phenyl lactic acid for ameliorating ischemia-reperfusion-induced microvascular disturbance in rat mesentery. Am J Physiol Gastrointest Liver Physiol. 2009;296:36–44. doi: 10.1152/ajpgi.90284.2008. [DOI] [PubMed] [Google Scholar]
- Chan K, Chui SH, Wong DY, Ha WY, Chan CL, Wong RN. Protective effects of danshensu from the aqueous extract of Salvia miltiorrhiza (danshen) against homocysteine-induced endothelial dysfunction. Life Sci. 2004;75:3157–71. doi: 10.1016/j.lfs.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Yang GD, Zhang H, Lin R, Wang WR, Shi XL, Liu Y, et al. Down-regulation of CD40 gene expression and inhibition of apoptosis with Danshensu in endothelial cells. Basic Clin Pharmacol Toxicol. 2009;104:87–92. doi: 10.1111/j.1742-7843.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- Cao YG, Chai JG, Chen YC, Zhao J, Zhou J, Shao JP, et al. Beneficial effects of danshensu, an active component of Salvia miltiorrhiza, on homocysteine metabolism via the trans-sulphuration pathway in rats. Br J Pharmacol. 2009;157:482–90. doi: 10.1111/j.1476-5381.2009.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Zhao GR, Yuan YJ, Guo ZX. Aqueous extract of salvia miltiorrhoza regulates adhesion molecule expression of tumor necrosis factor α-induced endothelial cells by blocking activation of nuclear factor kB. J Cardiovasc Pharmacol. 2005;45:516–24. doi: 10.1097/01.fjc.0000159643.82641.e9. [DOI] [PubMed] [Google Scholar]
- Wu L, Li X, Li Y, Wang L, Tang Y, Xue M. Proliferative inhibition of danxiongfang and its active ingredients on rat vascular smooth muscle cell and protective effect on the VSMC damage induced by hydrogen peroxide. J Ethnopharmacol. 2009;126:197–206. doi: 10.1016/j.jep.2009.08.045. [DOI] [PubMed] [Google Scholar]
- Costa FA, Prianti MG, Silva TC, Silva SM, Guerra JL, Goto H. T cells, adhesion molecules and modulation of apoptosis in visceral leishmaniasis glomerulonephritis. BMC Infect Dis. 2010;10:112. doi: 10.1186/1471-2334-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DE, Lehotay DC, Evrovski J. Simplified simultaneous assay of total plasma homocysteine and methionine by HPLC and pulsed integrated amperometry. Clin Chem. 1998;44:188–90. [PubMed] [Google Scholar]
- Chen Z, Li CS, Zhang J, Pang BS, Xia CQ, Liu XF. Relationship between endothelial dysfunction and serum homocysteine in patients with coronary lesions. Chin Med Sci J. 2005;20:63–6. [PubMed] [Google Scholar]
- Miyaki K. Genetic polymorphisms in homocysteine metabolism and response to folate intake: a comprehensive strategy to elucidate useful genetic information. J Epidemiol. 2010;20:266–70. doi: 10.2188/jea.JE20100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin KA, Abd EI-Twab TM. Oxidative markers, nitric oxide and homocysteine alteration in hypercholesterolimic rats: role of atorvastatine and cinnamon. Int J Clin Exp Med. 2009;2:254–65. [PMC free article] [PubMed] [Google Scholar]
- Guan H, Wang P, Hui R, Edin ML, Zeldin DC, Wang DW. Adeno-associated virus-mediated human C-reactive protein gene delivery causes endothelial dysfunction and hypertension in rats. Clin Chem. 2009;55:274–84. doi: 10.1373/clinchem.2008.115857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet S, Furukawa K, Maghazechi A, Hamid Q, Giaid A. Chemokine- and cytokine-induced expression of endothelin 1 and endothelin-converting enzyme 1 in endothelial cells. J Allergy Clin Immunol. 2000;105:333–8. doi: 10.1016/s0091-6749(00)90084-8. [DOI] [PubMed] [Google Scholar]
- Er H, Evereklioglu C, Cumurcu T, Turkoz Y, Ozerol E, Sahin K, et al. Serum homocysteine level is increased and correlated with endothelin-1 and nitric oxide in Behçet's disease. Br J Ophthalmol. 2002;86:653–7. doi: 10.1136/bjo.86.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Dai S, Fang CX, Sun R, Shavali S, Sharma SK, et al. Phytoestrogen alpha-zearalanol antagonizes homocysteine-induced imbalance of nitric oxide/endothelin-1 and apoptosis in human umbilical vein endothelial cells. Cell Biochem Biophys. 2006;45:137–45. doi: 10.1385/CBB:45:2:137. [DOI] [PubMed] [Google Scholar]
- de Valk-de Roo GW, Stehouwer CD, Lambert J, Schalkwijk CG, van der Mooren MJ, Kluft C, et al. Plasma Homocysteine is weakly correlated with plasma endothelin and von Willebrand factor but not with endothelium-dependent vasodilatation in healthy postmenopausal women. Clin Chem. 1999;45:1200–5. [PubMed] [Google Scholar]
- Sen U, Tyagi N, Kumar M, Moshal KS, Rodriguez WE, Tyagi SC. Cystathionine-β-synthase gene transfer and 3-deazaadenosine ameliorate inflammatory response in endothelial cells. Am J Physiol Cell Physiol. 2007;293:1779–87. doi: 10.1152/ajpcell.00207.2007. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zou H, Jin L, Wang J, Zhong MF, Huang P, et al. Biphasic effects of sodium danshensu on vessel function in isolated rat aorta. Acta Pharmacol Sin. 2010;31:421–8. doi: 10.1038/aps.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P, Pritchard MT, Nagy LE. Anti-inflammatory pathways and alcoholic liver disease: role of an adiponectin/interleukin-10/heme oxygenase-1 pathway. World J Gastroenterol. 2010;16:1330–6. doi: 10.3748/wjg.v16.i11.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AA. Monoclonal antibodies and other biologic agents in the treatment of asthma. MAbs. 2009;1:237–46. doi: 10.4161/mabs.1.3.8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriquez C, Alcudia JF, Martinez-Gonzalez J, Raposo B, Navarro MA, Badimon L. Lysyl oxidase (LOX) down-regulation by TNFalpha: a new mechanism underlying TNFalpha-induced endothelial dysfunction. Atherosclerosis. 2008;196:558–64. doi: 10.1016/j.atherosclerosis.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Rowsell S, Hawtin P, Minshull CA, Jepson H, Brockbank SM, Barratt DG, et al. Crystal structure of human MMP9 in complex with a reverse hydroxamate inhibitor. J Mol Biol. 2002;319:173–8. doi: 10.1016/S0022-2836(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50–9. [PubMed] [Google Scholar]
- Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–86. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]