Abstract
Anemia and red blood cell (RBC) transfusion occur frequently in hospitalized patients with cardiac disease. In this narrative review, we report the epidemiology of anemia and RBC transfusion in hospitalized adults and children (excluding premature neonates) with cardiac disease, and on the outcome of anemic and transfused cardiac patients. Both anemia and RBC transfusion are common in cardiac patients, and both are associated with mortality. RBC transfusion is the only way to rapidly treat severe anemia, but is not completely safe. In addition to hemoglobin (Hb) concentration, the determinant(s) that should drive a practitioner to prescribe a RBC transfusion to cardiac patients are currently unclear. In stable acyanotic cardiac patients, Hb level above 70 g/L in children and above 70 to 80 g/L in adults appears safe. In cyanotic children, Hb level above 90 g/L appears safe. The appropriate threshold Hb level for unstable cardiac patients and for children younger than 28 days is unknown. The optimal transfusion strategy in cardiac patients is not well characterized. The threshold at which the risk of anemia outweighs the risk of transfusion is not known. More studies are needed to determine when RBC transfusion is indicated in hospitalized patients with cardiac disease.
Keywords: Blood, Cardiac, Critical care, Erythrocyte, Hemoglobin, Intensive care, Practice, Risk factors, Surgery, Transfusion
Review
Introduction
Red blood cell (RBC) transfusion is common in critically ill adults and children [1-3]. Patients with cardiac disease are transfused at higher hemoglobin (Hb) thresholds than those with non-cardiac illness [1-3]. Both anemia and RBC transfusion are associated with increased mortality in cardiac patients. The risk/benefit balance of RBC transfusion in critically ill cardiac patients is currently a matter of debate.
This narrative review critically appraises the available data on the relationship between anemia, RBC transfusion and outcome of critically ill adults and children with cardiac disease. We discuss the prevalence and risks of anemia in the ICU, the rationale for RBC transfusion, the evidence supporting restrictive transfusion strategies, and the growing interest in goal-directed transfusion therapy. Premature infants and intraoperative transfusions will not be addressed in this paper, nor will a detailed discussion of the relationship between length of storage of RBC units and outcomes, since the latter is discussed in other papers [4-7].
Anemia
The prevalence of anemia in adults with heart failure ranges from 18 to 38% [8]; it was 19.1% in 27 observational studies indexed before July 2011 that enrolled 233,144 patients with acute coronary syndrome [9]. Anemia may result in insufficient oxygen delivery (DO2) to vital organs and tissues if DO2 drops below a critical DO2 (Figure 1). DO2 is determined by cardiac output (Q’) and arterial content in:
Figure 1.
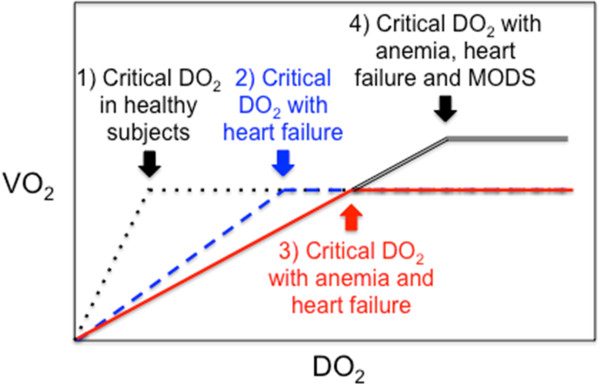
Critical oxygen delivery (DO2), cardiac failure, anemia and multiple organ dysfunction syndrome (MODS). X axis:systemic DO2; Y axis: global oxygen consumption (VO2). Critical DO2: level below which DO2 does not meet oxygen demand (O2 supply dependency). (1) In healthy subjects (dotted black and white line), as DO2 decreases, VO2 remains constant by compensatory mechanisms (increased cardiac output and cellular O2 extraction). (2) Cardiac failure limits compensatory increase in cardiac output (hatched blueline). (3) This limitation is worst in patients with anemia and heart failure (plain red line) because low hemoglobin decreases arterial oxygen content (CaO2). (4) Severe cardiac dysfunction may be associated with systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS), both of which increase systemic VO2 and therefore critical DO2 (double black line).
DO2 = Q’ × CaO2
and CaO2 = {(Hb × SaO2 × 1.39) + (PaO2 × 0.0031)} [10]
Any drop of the Hb level decreases CaO2 and DO2 if the compensatory increase of Q’ is not high enough. Anemia may be poorly tolerated by patients with cardiac failure and/or coronary disease because their ability to increase cardiac output to compensate for anemia is limited [11,12].
Carson et al. [13] studied the association between anemia and surgical mortality in 1,958 Jehovah’s Witness patients with cardiovascular disease who refused transfusion; the risk of mortality was inversely related to Hb level (Figure 2). Table 1 summarizes the results of two meta-analyses [9,14] and six observational studies [15-20] on the relationship in cardiac adults between anemia and adverse outcomes; a statistically significant association is reported in almost each instance. However, it must be underlined that the relationship between anemia and outcome is fundamentally confounded in all these studies by co-morbidities that might have caused the anemia (renal failure, gastro-intestinal bleeding, iron deficiency, and so on).
Figure 2.
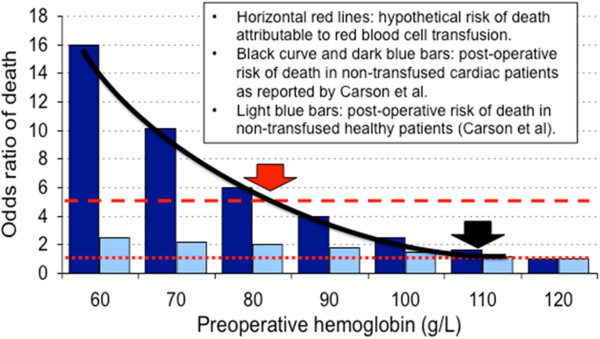
Cost-benefit analysis of red blood cell (RBC) transfusion. The background histogram is drawn from data published by Carson et al. [13] (with permission) illustrating the relationship between pre-operative anemia and surgical mortality in 1,958 Jehovah’s Witness patients. Dark blue bars: preoperative hemoglobin (Hb) versus odds ratio (OR) of death in cardiac patients (risk of death increases as Hb level falls). Light blue bars: pre-Hb versus OR of death in patients who were healthy before surgery. We draw on this histogram: (1) dotted horizontal red line: no risk of mortality attributable to RBC transfusion (OR = 1); (2) hatched horizontal red line: hypothetical higher risk of mortality attributable to RBC transfusion (OR = 5); (3) plain curved black line: pre-operative Hb versus odds ratio (OR) of death in cardiac patients. A RBC transfusion is probably more useful than harmful when Hb level is below the intersection between the black curve describing the risk of mortality associated with anemia and the red line describing the risk of mortality attributable to RBC transfusion. If RBC transfusions are perfectly safe (OR = 1), the dotted horizontal red line and the black curve cross together at a Hb level of about 110 g/L (black arrow). If the risk of mortality attributable to RBC transfusion is high (for example, OR = 5), the hatched horizontal red line and the black curve cross together at a Hb level of about 80 g/L (red arrow). Where the true curve lies for cardiac patients is unknown. The curved line would probably move to the right in severely ill cardiac patients, and to the left if cardiac dysfunction is milder. The actual risk of death attributable to RBC transfusions in different cardiac populations remains to be determined.
Table 1.
Anemia and outcomes in cardiac adults: observations studies
| Health problem | Patients | Studies | Risk | |
|---|---|---|---|---|
| Outcome |
|
(n) |
(n) |
(95% CI) |
| Meta-analyses |
|
|
|
|
| Mortalitya[14] |
ACS |
51,449 |
10 |
aOR: 1.49 (1.24 to 1.79) |
| Mortalityb[9] |
ACS |
171,915 |
27 |
aHR: 1.49 (1.23 to 1.81) |
| Studies not included in the meta-analyses |
|
|
|
|
| Mortality [15] |
New heart failure |
12,065 |
1 |
aHR: 1.34 (1.24 to 1.46) |
| Mortality [16] |
PCI |
6,116 |
1 |
aHR: 1.8 (1.3 to 2.3) |
| Mortality [17] |
PCI |
48,851 |
1 |
aOR: 2.29 (1.79 to 2.92) |
| Mortality [18] |
Cardiac surgery |
13,843 |
1 |
12.5% versus 7.5%, P = 0.014 |
| Mortality [19] |
CABG |
2,102 |
1 |
aOR: 0.99 (0.98 to 1.0) |
| Mortality [20] |
ACS |
7,922 |
1 |
aOR: 1.71 (1.34 to 2.17) |
| Meta-analyses on other outcomes |
|
|
|
|
| Heart failurea[14] |
ACS |
152,849 |
5 |
OR: 1.96 (1.47 to 2.62) |
| Cardiogenic shocka[14] |
ACS |
129,136 |
4 |
OR: 1.95 (1.04 to 2.64) |
| Reinfarctionb[9] | ACS | 22,115 | 6 | RR: 1.25 (1.02 to 1.53) |
ACS: acute coronary syndrome; aHR: adjusted HR; aOR: adjusted odds ratio; CABG: coronary artery bypass graft; CI: confidence interval; HR: hazard ratio; OR: odds ratio; PCI: percutaneous coronary intervention; RR: relative risk.
This table reports data from two meta-analyses [9,14] and six observational studies [15-20] that were not included in these meta-analyses.
aOverall, the meta analysis conducted by Liu et al. [14] included 241,293 patients enrolled in 19 studies; the analysis on mortality (10 studies) disclosed a very important heterogeneity (I 2 = 84%).
bOverall, the meta analysis conducted by Lawler et al. [9] included 233,144 patients. The analyses on mortality and reinfarction included 27 and 10 studies respectively; an important heterogeneity was found in both instances (I 2 = 98.2% and 48.4% respectively).
A number of host characteristics specific to children, like growth, fetal Hb, different cardiac diseases and physiology, may impair their adaptive mechanisms to anemia. There are few data on the relationship between anemia and outcomes in cardiac children. Kammache et al. [21] reported some anemia (Hb < 100 g/L) in 24% of 218 children with idiopathic dilated cardiomyopathy; mortality was more frequent in anemic children.
Transfusion
In the United States, 7.8% to 92.8% of adults undergoing cardiac surgery are transfused [22-24]. A transfusion is given after cardiac surgery in 38% to 74% of children [3,25-28].
RBC transfusions are sometimes given to cardiac patients to rapidly replace blood volume in cases of postoperative blood losses [29]. In the absence of active bleeding, some practitioners declare that low Hb concentration and/or signs of inadequate DO2 (low central venous O2 saturation (ScvO2), high lactate level, low SaO2/FiO2 ratio) would prompt them to prescribe RBC transfusions in this population [29]. There is no doubt that Hb level is a very important determinant of RBC transfusions in critically ill patients [30-32]. However, there are data suggesting that Hb level is not the only determinant of RBC transfusions in cardiac patients. In a retrospective study on transfusions in pediatric cardiac ICU [24], admission Hb ranged from 141 to 150 g/L, nadir Hb level was 121 g/L in patients who were not transfused, 119 g/L in a low transfusion group, and 115 g/L in a high transfusion group, suggesting that transfusions may have been given for reasons other than anemia. Age below one year, low weight, high severity of illness as measured by the acute physiology and chronic health evaluation (APACHE) or pediatric risk of mortality (PRISM) score, cardiopulmonary bypass, cyanotic heart condition and lower admission Hb level are also reported to be independently associated with increased administration of RBC transfusions [24,33].
Risks and benefits of RBC transfusions
Non-cardiac patients
RBC transfusion should be given only when the risk/benefit ratio is favorable (ratio < 1). The threshold at which the risks of anemia outweigh the risks of transfusion is currently unknown.
There are little available hard data on the clinical benefits of RBC transfusion. In a prospective study that enrolled 303 Kenyan children with Hb below 50 g/L at hospitalization, 116 (38%) did not receive a transfusion, mostly because of blood unavailability, while 187 did; the mortality was significantly higher in non-transfused children (41.4% versus 21.4%, P < 0.001) [34]. This study suggests that RBC transfusion may improve the survival of anemic hospitalized patients with Hb level below 50 g/L, but what threshold above 50 g/L should be used to prescribe a RBC transfusion in cardiac patients remains a matter of debate.
Adult cardiac patients
The available evidence suggests that RBC transfusion in patients with cardiac disease is an independent risk factor of mortality (Table 2).
Table 2.
Red blood cell (RBC) transfusions and outcomes in cardiac patients: observational studies a
| |
Health problem |
Patients |
Risk
c
|
|
|---|---|---|---|---|
| Outcome, first author, year b | (n) | (95% CI) | P- value | |
| Outcome: mortality in adults |
|
|
|
|
| Mortality, Alexander, 2008 [35] |
ACS |
44,242 |
OR: 3.2 (2.9 to 3.6) |
|
| |
ACS and Hct ≤ 24% |
|
aOR: 0.68 (0.45 to 1.02) |
|
| |
ACS and Hct = 24 to 27% |
|
aOR: 1.01 (0.79 to 1.30) |
|
| |
ACS and Hct = 27 to 30% |
|
aOR: 1.18 (0.92 to 1.50) |
|
| |
ACS and Hct > 30% |
|
aOR: 3.47 (2.30 to 5.23) |
|
| Mortality, Aronson, 2008 [36] |
AMI |
2,358 |
aHR: 0.13 (0.03 to 0.65)d,e |
0.013 |
| |
|
|
aHR: 2.2 (1.5 to 3.3)d,f |
< 0.0001 |
| Mortality, Jani, 2007 [37] |
AMI |
4,623 |
aOR: 2.02 (1.47 to 2.79) |
< 0.0001 |
| |
|
|
RR: 4.83 (3.81 to 6.12)d |
|
| Mortality, Jolicœur, 2009 [38] |
AMI |
5,188 |
RR: 6.38 (4.88 to 8.34) |
|
| |
|
5,532 |
aHR: 2.16 (1.20 to 3.88) |
< 0.0001 |
| |
|
5,188 |
RR: 6.38 (4.88 to 8.34)d |
|
| Mortality, Koch, 2006 [39] |
CABG |
5,814 |
OR: 1.77 (1.67 to 1.87) |
< 0.0001 |
| Mortality, Murphy, 2007 [40] |
Cardiac surgery (UK) |
8,518 |
HR: 6.69 (3.66 to 15.1) |
< 0.05 |
| Mortality, Nikolsky, 2009 [41] |
AMI |
2,060 |
HR: 4.71 (1.97 to 11.36) |
|
| |
|
|
RR: 2.92 (1.62 to 5.24)d |
|
| Mortality, Pattakos, 2012 [42] |
Cardiac surgery |
644 |
95% versus 89% |
0.007 |
| Mortality, Rao, 2004 [43] |
ACS |
24,112 |
aHR: 3.94 (3.26 to 4.75) |
< 0.05 |
| |
|
|
RR: 2.60 (2.22 to 3.03)d |
< 0.05 |
| Mortality, Shehata, 2012 [19] |
CABG |
2,102 |
OR: 0.44 (0.32 to 14.1) |
NS |
| Mortality, Shishehbor, 2009 [44] |
AMI |
3,575 |
aHR: 3.89 (2.66 to 5.68) |
< 0.001 |
| |
|
|
RR: 1.30 (0.90 to 1.88)d |
< 0.001 |
| Mortality, Singla, 2007 [45] |
AMI |
370 |
RR: 2.36 (1.49 to 3.76)d |
|
| Mortality, Wu, 2001, [46] |
AMI |
78,974 |
RR: 2.51 (2.42 to 2.61)d,g |
< 0.05 |
| Mortality, Yang, 2005 [47] |
AMI |
85,111 |
aOR: 1.67 (1.48 to 1.88) |
< 0.05 |
| |
|
|
RR: 3.03 (2.85 to 3.21)d |
< 0.05 |
| Outcomes: myocardial infarction or ischemic eventsh in adults |
|
|
|
|
| AMI, Jani, 2007 [37] |
AMI |
4,623 |
RR: 1.19 (0.82 to 1.74)d |
|
| AMI, Jolicœur, 2009 [38] |
AMI |
5,188 |
RR: 3.05 (1.85 to 5.04)d |
|
| IEh, Murphy, 2007 [40] |
Cardiac surgery (UK) |
8,518 |
aOR: 3.35 (2.68 to 4.35)c |
< 0.05 |
| AMI, Nikolsky, 2009 [41] |
AMI |
2,060 |
RR: 3.28 (1.44 to 7.49)d |
|
| AMI, Pattakos, 2012 [42] |
Cardiac surgery |
644 |
2.8% versus 0.31% |
< 0.01 |
| AMI + death, Rao, 2004 [43] |
ACS |
2,401 |
HR: 3.08 (2.84 to 3.35)d |
< 0.05 |
| AMI, Shishehbor, 2009 [44] |
ACS |
3,575 |
aHR : 3.44 |
< 0.001 |
| AMI + death, Singla, 2007 [45] |
AMI |
370 |
aOR: 2.57 (1.41 to 4.69) |
< 0.001 |
| |
|
|
RR: 2.10 (0.83 to 5.30)d |
|
| AMI, Yang, 2005 [47] |
AMI |
85,111 |
aOR: 0.95 (0.83 to 1.09)d |
< 0.05 |
|
Outcomes in children |
Health problem |
Children |
Risk
c
|
P
-value |
| LMV (days), 2011 [48] |
Cardiac surgery |
270 |
HR: 0.71 (0.54 to 0.92) |
0.009 |
| LMV (days), 2013 [49] |
Cardiac surgery |
335 |
HR: 2.6 (2.0 to 3.4) |
< 0.001 |
| PICU LOS (days), 2013 [49] |
Cardiac surgery |
335 |
LOS: 8 ± 0.9 versus 3.5 ± 2 |
< 0.001 |
| Hospital LOS, 2011 [24] |
Cardiac surgery |
802 |
aHR: 0.65 (0.49 to 0.87) |
< 0.001 |
| Wound infection, 2010 [50] | Cardiac surgery | 216 | aOR: 7.87 (1.63 to 37.92) | < 0.001 |
ACS: acute coronary syndrome; aHR: adjusted HR; AMI: acute myocardial infarction; aOR: adjusted OR; CABG: coronary artery bypass graft; card surg: cardiac surgery; CI: confidence interval; Hct: hematocrit; HR: hazard ratio; IE: ischemic events; LMV: length of MV; LOS: length of stay; MI: myocardial infarction; MV: mechanical ventilation; OR: odds ratio; PICU: pediatric intensive care unit; RBC: red blood cell; NS: not significant; RCT: randomized controlled trial; UK: United Kingdom.
aStudies on the relationship between the length of storage of RBC units and outcomes of transfused cardiac patients are excluded from this table.
bYear of publication.
cTransfused patients versus no RBC transfusion or restricted RBC transfusion strategy.
dChatterjee et al. [51] completed a systematic review that included the studies marked byd in this table, plus a small RCT conducted by Cooper et al. [52]. Overall, the risk ratio of death in transfused patients versus controls was 2.91 (95% confidence interval (CI): 2.46 to 3.44), but there was a very significant heterogeneity (I 2 = 92%).
ePre-transfusion hemoglobin concentration ≤ 80 g/L.
fPre-transfusion hemoglobin concentration > 80 g/L.
gThe study by Wu et al. [46] enrolled patients ≥ 65 years of age with AMI. RBC transfusion was associated with a reduction in 30-day mortality if hematocrit < 24% (aOR = 0.22; 95% CI: 0.11 to 0.45) or between 30% and 33% (aOR = 0.69; 95% CI: 0.53 to 0.89), but the risk of mortality was not increased in patients with a hematocrit > 33% who received RBC transfusion.
hIschemic events: myocardial infarction, stroke, renal impairment, or failure.
Three studies reported some transfusion benefits if the Hb level is low (<80 g/L), but they also reported harm with high Hb level [35,36,46]. A retrospective descriptive epidemiological study of 78,974 patients older than 65 years with acute myocardial infarction showed that RBC transfusion was associated with a lower risk of 30-day mortality if hematocrit was below 24% (odds ratio (OR): 0.22; 95% CI: 0.11 to 0.45) or between 30% and 33% (OR: 0.69; 95% CI: 0.53 to 0.89), but not in cardiac patients with hematocrit above 33% [46]. Alexander et al. [35] reported that RBC transfusion tended to have a beneficial impact on mortality if the nadir hematocrit was ≤ 24% (adjusted odds ratio (aOR): 0.68; 95% CI: 0.45 to 1.02), but the opposite was found if it was > 30% (aOR: 3.47; 95% CI: 2.30 to 5.23). Aronson et al. [36] reported that RBC transfusion in patients with myocardial infarction decreased the risk of mortality if the pre-transfusion Hb level was ≤ 80 g/L (adjusted hazard ratio (aHR): 0.13: 95% CI: 0.03 to 0.65), but the risk was increased if the Hb level was > 80 g/L (aHR: 2.2; 95% CI: 1.5 to 3.3). Shehata et al. [19] reported a lower risk of mortality in 2,102 adults who were transfused after coronary artery bypass graft (CABG), but the association was not statistically significant (adjusted OR (aOR) = 0.44; 95% CI: 0.32 to 14.1).
On the other hand, six studies of adults undergoing cardiac surgery, CABG or with myocardial infarction reported increased mortality in transfused cardiac patients [39,40,42-44,47]. Rao et al. [43] published a descriptive epidemiological study on 24,112 patients with acute coronary syndrome who were enrolled in three large randomized controlled trials (RCTs). They compared the outcomes of those who received at least one RBC transfusion (n = 21,711) and those who did not (n = 2,401); RBC transfusion was associated with an increased HR for 30-day mortality (HR = 3.94; 95% CI: 3.26 to 4.75). Probability of 30-day mortality was higher in transfused patients with nadir hematocrit values above 25%. In the systematic review of Chatterjee et al. [51], the risk ratio of death in transfused patients versus controls was 2.91 (95% CI: 2.46 to 3.44) and the risk of secondary myocardial infarction was 2.04 (95% CI: 1.06 to 3.93), but there was a very significant heterogeneity in both instances (I 2 : 92% and 98% respectively). Garfinkle et al. [53] also published a systematic review on 11 observational studies that enrolled 290,847 patients with acute coronary syndrome: the unadjusted OR of mortality in transfused patients ranged from 1.9 to 11.2; a meta-analysis was not performed because there was too much heterogeneity, but the data suggested a protective effect of RBC transfusion if nadir Hb drops below 80 g/L and neutral or harmful effects above 110 g/L. In summary, there is evidence in adults with cardiac disease that RBC transfusion is associated with mortality and ischemic events.
Pediatric cardiac patients
There is less evidence in children. Three studies reported prolonged length of mechanical ventilation in children who received RBC transfusion after cardiac surgery [48,49,54], three reported prolonged length of PICU and/or hospital stay [24,49,54] and three reported an increased incidence of infections [33,50,54]. In 657 consecutive children undergoing open heart surgery, Székely et al. [33] found an association between total volume of blood transfusion and the rate of infections (aOR: 1.01; 95% CI: 1.002 to 1.02, P < 0.01), but no association with mortality (aOR: 1.00; 95% CI: 0.99 to 1.02, P = 0.65).
Risks/benefits of RBC transfusions: summary
Is it justified to prescribe more RBC transfusions to cardiac than to non-cardiac ICU patients? The available data on this question are inconclusive: while some descriptive studies suggest a benefit to transfusion, most studies associate RBC transfusion to worse outcomes. Descriptive studies cannot prove that there is a cause-effect relationship between a risk factor and a given outcome: adjustment for confounders like volume of RBC transfusion and administration of other blood products can be done, but a possibility always remains that unknown confounders play a role. Moreover, severity of illness is associated with both transfusion and mortality [8,24]. A multivariate analysis cannot deconstruct such confounding by indication [55]; only RCTs can [56].
Transfusion related adverse events
The safety of blood products with respect to transfusion-transmitted infectious diseases has improved greatly in recent decades. Presently, the greatest concern is non-infectious serious hazards of transfusion (NISHOT) [57-59].
NISHOT may be non-immune or immune-mediated. Short-term non-immune NISHOT include transfusion-related circulatory overload (TACO) and overtransfusion. Overtransfusion is of concern because of the risk of TACO and/or hyperviscosity. Viscous blood flow may impair DO2 in small vessels and decrease coronary blood flow. Barr et al. [60] reviewed the data of 1,474 transfused patients: 19% of them were overtransfused according to the British RBC transfusion guidelines. Sabatine et al. [61] reported an increased mortality in patients with ST-elevation myocardial infarction when Hb value was > 170 g/L (OR = 1.79; 95% CI: 1.18 to 2.71, P = 0.007) and > 160 g/L (OR = 1.31; 95% CI: 1.03 to 1.66, P = 0.027) in patients with non-ST-elevation myocardial infarction.
Immune-mediated NISHOT include hemolytic and allergic reactions, transfusion-related immunomodulation (TRIM), transfusion-related acute lung injury (TRALI), nosocomial infections, transfusion-associated graft versus host disease, and alloimmunization to RBC and HLA antigens [57,62,63].
In critically ill cardiac patients, TRIM may represent a significant ‘second-hit’ when added to pre-existing organ dysfunction and/or a systemic inflammatory response syndrome (SIRS), which may result in TRALI and multiple organ dysfunction syndrome. Vlaar et al. [64] reported that in cardiac surgery patients, RBC transfusion was associated with an increase pulmonary leak index, an early marker of acute lung injury. Inflammatory markers in bronchoalveolar lavage were increased in transfused cardiac surgery patients when compared to controls [65]. TRALI may be an important underdiagnosed cause of lung injury in cardiac and non-cardiac ICU patients [66,67].
Although NISHOT are currently the most important causes of transfusion-related fatalities, their incidence rate in cardiac patients is not well characterized [58].
Restrictive or liberal RBC transfusion strategy
As illustrated in Figure 1, the relationship between DO2 and O2 consumption (VO2) remains horizontal as long as compensatory mechanisms (increasing cardiac output and O2 extraction) are still effective [10,68]. However, VO2 drops with DO2 below a critical threshold. Anemia decreases DO2 and it is unmistakably deleterious below a certain threshold. The critical Hb level threshold below which DO2 becomes significantly impaired and transfusion becomes less harmful than persisting anemia is unknown and likely varies depending upon the underlying condition and clinical status of each patient. Anemia is well endured by healthy subjects: acute isovolemic reduction of Hb level down to 50 g/L was hemodynamically well tolerated by 11 resting healthy humans [69], but delayed memory was observed in 31 healthy young volunteers after a similar reduction of Hb [70]. However, cardiac patients may have higher thresholds of critical DO2 than healthy adults and target Hb for transfusion might be different (Figures 1 and 2). Moreover, patients with cardiac disease may also be more vulnerable than the general population to adverse effects of transfusion (disturbed rheology [71] and coagulation [65], NISHOT [57], dysfunctional vasoregulation [72], circulatory overload, and so on).
Confronted with the risks of transfusion and a growing body of literature associating RBC transfusion with adverse outcomes, a number of RCTs have now compared the safety of adopting a restrictive versus liberal RBC transfusion strategy (low versus higher threshold Hb) in critically ill patients with cardiac disease (Table 3).
Table 3.
Restrictive versus liberal red blood cell (RBC) transfusion strategy in cardiac patients: randomized clinical trials
| |
Health problem |
Patients |
Mortality
b
|
|
|---|---|---|---|---|
| First author, year a | (n) | (95% CI) | P -value | |
| Adults |
|
|
|
|
| Bracey, 1999 [73] |
CABG |
428 |
RR: 0.52 (0.13 to 2.04) |
NS |
| Carson, 2011 [74] |
Hip surgeryc |
2,016 |
ARR: 0.9 (−1.5 to +3.4) |
NS |
| Cooper, 2001 [52] |
Myocardial infarction |
46 |
8% versus 5% |
1.0 |
| Hajjar, 2012 [23] |
Cardiac surgery |
502 |
6% versus 5% |
0.93 |
| Hébert, 2001 [75] |
ICU cardiac patients |
357 |
22.5% versus 22.7% |
1.00 |
| Johnson, 1992 [76] |
CABG |
38 |
No differenced |
NS |
| Shehata, 2012 [19] |
Cardiac surgery |
50 |
16% versus 4% |
NS |
| Pediatric cardiac surgery |
|
|
|
|
| Cholette, 2011 [77] |
Cyanotic |
60 |
1 death |
NS |
| de Gast-Bakker, 2013 [78] |
Non-cyanotic |
107 |
No death |
NS |
| Willems, 2010 [79] | Non-cyanotic | 125 | 12.7% versus 6.5% | 0.36 |
ARR: absolute risk reduction; CABG: coronary artery bypass graft; CI: confidence interval; RBC: red blood cell; NS: not statistically significant.
aYear of publication.
bRestrictive versus liberal transfusion strategy.
cHip surgery in patients older than 50 years with atherosclerosis.
dNo significant difference in duration or degree of exercise was demonstrated between the two groups.
RBC transfusion strategy in cardiac adults
We found seven RCTs conducted in adults with cardiac illness [19,23,52,73-76]; all enrolled only hemodynamically stable patients. All but one [23] reported that a restrictive strategy was as safe or safer than a liberal strategy. Three RCTs included more than 50 patients.
In 1999, Hébert et al. [80] published the ‘Transfusion Requirements in Critical Care’ (TRICC) study. Euvolemic patients were randomized to receive a RBC transfusion if Hb level was below 100 g/L (liberal strategy) or below 70 g/L (restrictive strategy). Patients with acute myocardial infarction and unstable angina were excluded. A subgroup analysis of 357 patients with cardiovascular disease was published in 2001 [75]; the 30-day all-cause mortality was similar (22.5% versus 22.7%, P = 1.00).
Hajjar et al. [23] published a single center non-inferiority RCT of 502 adults undergoing cardiac surgery who were allocated to receive a transfusion if hematocrit was below 24% or 30%. The pre-transfusion Hb level was 91 in the restrictive and 105 g/L in the liberal group. They found a similar incidence of 30-day all-cause mortality (6% in the restrictive and 5% in the liberal group, P = 0.93), cardiogenic shock (5% versus 9%, P = 0.42), acute respiratory distress syndrome (1% versus 2%, P = 0.99) and acute renal failure (5% versus 4%, P = 0.99). The number of RBC units transfused was independently associated with 30-day all-cause mortality (HR = 1.2/unit; 95% CI: 1.1 to 1.4, P = 0.002).
Carson et al. [74] published the ‘Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair’ (FOCUS) study, a large multicenter RCT of 2,016 adults over 50 years of age with known atherosclerotic disease undergoing a hip surgery and who had a Hb level below 100 g/L within 3 days post-surgery. The restrictive group was transfused if Hb level fell below 80 g/L, the liberal group if below 100 g/L. Primary outcome was ability to walk 10 feet unassisted or death 60 days post-randomization. No difference was found in any of the outcomes, including survival, postoperative complications, activities of daily living and disposition.
In summary, the available RCTs suggest that a restrictive transfusion strategy (threshold Hb level for transfusion: 70 or 80 g/L) appears as safe as a liberal strategy in stable adult ICU patients with cardiac disease.
RBC transfusion strategy in cardiac children
Three RCTs compared a restrictive and a liberal transfusion strategy after pediatric cardiac surgery.
In the ‘Transfusion Requirements In PICU’ (TRIPICU) study [81], 637 stabilized critically ill children were randomized to receive a RBC transfusion if their Hb dropped below 70 or 95 g/L [81]. Patients were considered stable if mean systemic arterial pressure was not less than two standard deviations below the normal mean for age and if cardiovascular treatment (fluids and/or medication) had not been increased for at least two hours before enrollment. Children with cyanotic cardiac disease and neonates under 28 days were excluded. A sub-group analysis of 125 cardiac children enrolled in TRIPICU demonstrated no significant difference in new or progressive multiple organ dysfunction syndrome (restrictive versus liberal group: 12.7% versus 6.5%, P = 0.36), PICU length of stay (7.0 ± 5.0 versus 7.4 ± 6.4 days) or 28-day mortality (3.2% versus 3.2%) [79].
An RCT compared the outcome of cardiac children older than 6 weeks randomized to receive a RBC transfusion if Hb level dropped below 80 g/L or 108 g/L [78]. Patients with cyanotic cardiac disease were excluded. Randomization occurred before surgery; the research protocol with respect to RBC transfusion was initiated in the operating room and maintained up to PICU discharge. One hundred patients were enrolled and retained for analysis. Duration of mechanical ventilation, length of PICU stay and incidence of adverse events were similar in both groups, but length of hospital stay was shorter in the restrictive group (median: 8 {interquartile range: 7 to 11} versus 9 {7 to 14} days, P = 0.063).
Physicians target higher Hb values for critically ill children with cyanotic heart disease [28,29]. In an RCT completed by Cholette et al. [77], 60 children were randomized after Glenn or Fontan palliation to a restrictive (transfusion if Hb < 90 g/L) or liberal group (<130 g/L). One death was observed (liberal group). There was no difference in mean and peak arterial lactate, arterio-venous and arterio-cerebral oxygen content. Although not powered to demonstrate statistical significance, mortality, PICU and hospital length of stay, duration of mechanical ventilation, dose and duration of inotropic support were similar.
Goal-directed transfusion therapy
Goal-directed RBC transfusion therapy targeting a physiological goal may be a more appropriate target than specific Hb levels. The concept of goal-directed therapy is well illustrated by the RCT conducted by Rivers et al. [82]: 266 adults with severe sepsis or septic shock were randomized to be monitored or not with ScvO2. Patients allocated to ScvO2 monitoring were also assigned to a bundle of treatment to maintain their ScvO2 over 70%; this bundle included, in sequential order, mechanical ventilation, fluid bolus, vasoactive drugs and RBC transfusion if the ScvO2 remained under 70% after all other interventions. Mortality was 30.5% with goal-directed therapy versus 46.5% in controls.
There are currently no hard data on goal-directed transfusion therapy in cardiac patients. Goals that can be considered include physiologic parameters like DO2 measured by a Swan-Ganz catheter or locally with near infrared spectroscopy (NIRS), global oxygen consumption (VO2), blood lactate, mixed venous O2 saturation (Sv’O2), ScvO2, tissue O2 saturation and O2 extraction rate [10]. Well-conducted research is required to demonstrate the reliability and clinical applicability of a test before we start to use it at the bedside. There are examples of tests that were too rapidly adopted by the medical community. For example, regional (splanchnic and/or renal) NIRS is frequently used to detect low cardiac output in ICU patients; a recent study showed that its positive predictive value is so low that it cannot be considered a reliable test [83]. Repeated (‘dynamic’) measurements of NIRS might be more informative than ‘static’ measures [84], but this remains to be proven. Transfusion therapy guided by ScvO2 is another candidate. The results of the trial by Rivers et al. [82] suggested that RBC transfusion might be useful in septic patients. Another RCT conducted in 102 children with severe sepsis or fluid refractory shock reported similar results (mortality: 11.8% versus 39.2% in controls) [85]. However, in the ProCESS three arms RCT [86], 1,341 adults were allocated to a protocol-based goal-directed therapy that required the placement of a central venous catheter, a protocol-based standard therapy without such catheter, or usual care; no difference was observed in any outcomes, which questions the results of the RCTs by Rivers and de Oliveira [82,85]. Moreover, the specific contribution of RBC transfusions was unclear in these RCTs and none enrolled cardiac patients.
Currently, we do not know what physiologic parameters we must use to guide a goal-directed transfusion therapy in cardiac patients. Further clinical studies are required before a given goal that would help practitioners to precisely decide when to transfuse cardiac patients can be recommended in this population.
Conclusion
Anemia is common in patients with cardiac disease and is associated with mortality and morbidity. RBC transfusion is the best way to rapidly increase the Hb level, but it is not risk-free: storage lesion and NISHOT, including prothrombotic and proinflammatory effects, may cause transfusion-related adverse events.
The Hb level at which the risk of anemia outweighs the risk of transfusion is not well known. Three observational studies suggest that there might be some benefit to give a RBC transfusion to adults with acute coronary syndrome if their Hb level is < 80 g/L, but many more studies suggest that RBC transfusion might be harmful in this population if their Hb level is higher than 80 to 100 g/L. The question of RBC transfusion to adults with acute coronary syndrome is not addressed in the guidelines of the American College of Cardiology, the American Heart Association and the Canadian Cardiovascular Society [87]. However, a number of RCTs in hemodynamically stable cardiac patients suggest a level of Hb (about 80 g/L) above which it appears safe not to transfuse, thereby avoiding the risks related to transfusions.
Data in children are scarce. In stable acyanotic cardiac children, a Hb level above 70 or 80 g/L appears to be well tolerated without RBC transfusion. In children with cyanotic heart lesions, Hb level over 90 g/L appears safe. The threshold Hb level for unstable cardiac children and for neonates is unknown. Goal-directed transfusion therapy is a promising avenue for future research.
Severity of illness, anemia and transfusions are interconnected with outcome in cardiac patients, and their individual contribution to outcome remains unresolved. Further RCTs are necessary to disentangle this relationship.
Abbreviations
ACS: acute coronary syndrome; aHR: adjusted HR; AMI: acute myocardial infarction; aOR: adjusted OR; APACHE: acute physiology and chronic health; CABG: coronary artery bypass graft; CI: confidence interval; DO2: O2 delivery; Hb: hemoglobin; Hct: hematocrit; ICU: intensive care unit; IE: ischemic events; HR: hazard ratio; MI: myocardial infarction; MODS: multiple organ dysfunction syndrome; MV: mechanical ventilation; NIRS: near infrared spectroscopy; NISHOT: non-infectious serious hazard of transfusion; OR: odds ratio; PCI: percutaneous coronary intervention; PICU: pediatric ICU; PRISM: pediatric risk of mortality; RBC: red blood cell; RCT: randomized controlled trial; RR: relative risk; ScvO2: central venous O2 saturation; Sv’O2: mixed venous O2 saturation; TACO: transfusion-associated circulatory overload; TRALI: transfusion-related acute lung injury; TRIM: transfusion-related immunomodulation; VO2: O2 consumption.
Competing interest
The authors declare that they have no competing interests.
Authors’ contribution
GD, KH and JL wrote, revised and approved the manuscript in its final form.
Contributor Information
Geneviève Du Pont-Thibodeau, Email: genevievedpt@hotmail.com.
Karen Harrington, Email: harrington.karen.p@gmail.com.
Jacques Lacroix, Email: j_lacroix@videotron.ca.
Source of funding
Supported by the Fonds de la Recherche en Santé du Québec (grant #24460) and by the Groupe de Recherche en Transfusion Sanguine (http://www.chu-sainte-justine.org/recherche).
References
- Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G. For the ABC Investigators. Anemia and blood transfusion in critically ill patients. JAMA. 2002;4:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ. The CRIT Study: anemia and blood transfusion in the critically ill - current clinical practice in the United States. Crit Care Med. 2004;4:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- Bateman ST, Lacroix J, Boven K, Forbes P, Barton R, Thomas N, Jacobs B, Markovitz B, Goldstein B, Hanson J, Randolph AG. for the Pediatric Acute Lung Injury and Sepsis Investigator’s (PALISI) Network. Anemia, blood loss and blood transfusion in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008;4:26–33. doi: 10.1164/rccm.200711-1637OC. [DOI] [PubMed] [Google Scholar]
- Tinmouth A, Fergusson D, Chin-Yee I, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;4:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- Lacroix J, Hébert PC, Fergusson D, Tinmouth A, Blajchman MA, Callum J, Cook D, Marshall J, McIntyre L, Turgeon A. for the ABLE study group, and the Canadian Critical Care Trials Group. The age of blood evaluation (ABLE) randomized controlled trial: study design. Transfus Med Rev. 2011;4:197–205. doi: 10.1016/j.tmrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Lacroix J, Tucci M. Impact clinique de la durée d’entreposage des globules rouges avant transfusion. Transfus Clin Biol. 2011;4:97–105. doi: 10.1016/j.tracli.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Aubrun C, Nichol A, Cooper DJ, Bellomo R. Age of red blood cells and transfusion in critically ill patients. Ann Intensive Care. 2013;4:2. doi: 10.1186/2110-5820-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SD, Li Y, Ellis SJ, Isitt JJ, Cheng S, Schulman KA, Whellan DJ. Associations between hemoglobin level, resource use, and medical costs in patients with heart failure: findings from HF-ACTION. J Card Fail. 2012;4:784–791. doi: 10.1016/j.cardfail.2012.08.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler PR, Filion KB, Dourian T, Atallah R, Garfinkle M, Eisenberg MJ. Anemia and mortality in acute coronary syndromes: a systematic review and meta-analysis. Am Heart J. 2013;4:143–153. doi: 10.1016/j.ahj.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Vallet B, Robin E, Lebuffe G. Venous oxygen saturation as a physiologic transfusion trigger. Crit Care. 2010;4:213. doi: 10.1186/cc8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn DR, Smith LR, Schell RM, Hoffman RD, Gillespie R, Leone BJ. Importance of severity of coronary artery disease for the tolerance to normovolemic hemodilution. Comparison of single-vessel versus multivessel stenoses in a canine model. J Thorac Cardiovasc Surg. 1994;4:231–239. [PubMed] [Google Scholar]
- Spahn DR, Smith LR, Veronee CD, McRae RL, Hu WC, Menius AJ, Lowe JE, Leone BJ. Acute isovolemic hemodilution and blood transfusion. Effects on regional function and metabolism in myocardium with compromised coronary blood flow. J Thorac Cardiovasc Surg. 1993;4:694–704. [PubMed] [Google Scholar]
- Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R, Noveck H, Strom BL. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;4:1055–1060. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang YM, Zhu J, Tan HQ, Liang Y, Li JD. Anaemia and prognosis in acute coronary syndrome: a systematic review and meta-analysis. J Int Med Res. 2012;4:43–55. doi: 10.1177/147323001204000105. [DOI] [PubMed] [Google Scholar]
- Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12,065 patients with new-onset heart failure. Circulation. 2003;4:223–225. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- Lee PC, Kini AS, Ahsan C, Fisher E, Sharma SK. Anemia is an independent predictor of mortality after percutaneous coronary intervention. J Am Coll Cardiol. 2004;4:541–546. doi: 10.1016/j.jacc.2004.04.047. [DOI] [PubMed] [Google Scholar]
- McKechnie RS, Smith D, Montoye C, Kline-Rogers E, O’Donnell MJ, DeFranco AC, Meengs WL, McNamara R, McGinnity JG, Patel K, Share D, Riba A, Khanal S, Moscucci M. Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) Prognostic implication of anemia on in-hospital outcomes after percutaneous coronary intervention. Circulation. 2004;4:271–277. doi: 10.1161/01.CIR.0000134964.01697.C7. [DOI] [PubMed] [Google Scholar]
- Ranucci M, Di Dedda U, Castelvecchio S, Menicanti L, Frigiola A, Pelissero G. Surgical and Clinical Outcome Research (SCORE) Group. Impact of preoperative anemia on outcome in adult cardiac surgery: a propensity-matched analysis. Ann Thorac Surg. 2012;4:1134–1141. doi: 10.1016/j.athoracsur.2012.04.042. [DOI] [PubMed] [Google Scholar]
- Shehata N, Burns LA, Nathan H, Hebert P, Hare GM, Fergusson D, Mazer CD. A randomized controlled pilot study of adherence to transfusion strategies in cardiac surgery. Transfusion. 2012;4:91–99. doi: 10.1111/j.1537-2995.2011.03236.x. [DOI] [PubMed] [Google Scholar]
- Sulaiman K, Prashanth P, Al-Zakwani I, Al-Mahmeed W, Al-Motarreb A, Al Suwaidi J, Amin H, Asaad N, Hersi A, Al Faleh H, Al Saif S, Alsheik-Ali AA, Al LJ, Al-Habib K. Impact of anemia on in-hospital, one-month and one-year mortality in patients with acute coronary syndrome from the Middle East. Clin Med Res. 2012;4:65–71. doi: 10.3121/cmr.2011.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammache I, Parrinello G, Marini D, Bonnet D, Agnoletti G. Anaemia is a predictor of early death or cardiac transplantation in children with idiopathic dilated cardiomyopathy. Cardiol Young. 2012;4:293–300. doi: 10.1017/S1047951111001442. [DOI] [PubMed] [Google Scholar]
- Bennett-Guerrero E, Zhao Y, O’Brien SM, Ferguson TB, Peterson ED, Gammie JS, Song HK. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;4:1568–1575. doi: 10.1001/jama.2010.1406. [DOI] [PubMed] [Google Scholar]
- Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, Fukushima J, Kalil Filho R, Sierra DB, Lopes NH, Mauad T, Roquim AC, Sundin MR, Leão WC, Almeida JP, Pomerantzeff PM, Dallan LO, Jatene FB, Stolf NA, Auler JO Jr. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;4:1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- Salvin JW, Scheurer MA, Laussen PC, Wypij D, Polito A, Bacha EA, Pigula FA, McGowan FX, Costello JM, Thiagarajan RR. Blood transfusion after pediatric cardiac surgery is associated with prolonged hospital stay. Ann Thorac Surg. 2011;4:204–210. doi: 10.1016/j.athoracsur.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Chambers LA, Cohen DM, Davis JT. Transfusion patterns in pediatric open heart surgery. Transfusion. 1996;4:150–156. doi: 10.1046/j.1537-2995.1996.36296181928.x. [DOI] [PubMed] [Google Scholar]
- Armano R, Gauvin F, Ducruet T, Hume H, Lacroix J. Determinants of red blood cell transfusions in a pediatric critical care unit: a prospective descriptive epidemiological study. Crit Care Med. 2005;4:2637–2644. doi: 10.1097/01.ccm.0000185645.84802.73. [DOI] [PubMed] [Google Scholar]
- Keung CY, Smith KR, Savoia HF, Davidson AJ. An audit of transfusion of red blood cell units in pediatric anesthesia. Pediatr Anesth. 2009;4:320–328. doi: 10.1111/j.1460-9592.2009.02939.x. [DOI] [PubMed] [Google Scholar]
- Demaret P, Tucci T, Ducruet T, Trottier H, Lacroix J. Red blood cell transfusion in critically ill children. Transfusion. 2014;4:365–375. doi: 10.1111/trf.12261. [DOI] [PubMed] [Google Scholar]
- Harrington K, Farrell C, Poirier N, Ducruet T, Lacroix J. Survey on red-cell transfusion practices after paediatric cardiac surgery. Pediatr Crit Care Med. 2011;4:A82. [Google Scholar]
- Hébert PC, Wells G, Martin C, Tweeddale M, Marshall J, Blajchman M, Pagliarello G, Schweitzer I, Calder L. For the Transfusion Requirements in Critical Care Investigators. A Canadian survey of transfusion practices in critically ill patients. Crit Care Med. 1998;4:482–487. doi: 10.1097/00003246-199803000-00019. [DOI] [PubMed] [Google Scholar]
- Laverdière C, Gauvin F, Hébert PC, Infante-Rivard C, Hume H, Toledano BJ, Lacroix J. Survey of transfusion practices in pediatric intensive care units. Pediatr Crit Care Med. 2002;4:335–340. doi: 10.1097/00130478-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Nahum E, Ben-Ari J, Schonfeld T. Blood transfusion policy among European pediatric intensive care physicians. J Intensive Care Med. 2004;4:38–43. doi: 10.1177/0885066603257966. [DOI] [PubMed] [Google Scholar]
- Székely A, Cserép Z, Sápi E, Breuer T, Nagy CA, Vargha P, Hartyánszky I, Szatmári A, Treszl A. Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg. 2009;4:187–197. doi: 10.1016/j.athoracsur.2008.09.079. [DOI] [PubMed] [Google Scholar]
- Lackritz EM, Hightower AW, Zucker JR, Ruebush TK, Onudi CO, Steketee RW, Were JB, Patrick E, Campbell CC. Longitudinal evaluation of severely anemic children in Kenya: The effect of transfusion on mortality and hematologic recovery. AIDS. 1997;4:1487–1494. doi: 10.1097/00002030-199712000-00013. [DOI] [PubMed] [Google Scholar]
- Alexander KP, Chen AY, Wang TY, Rao SV, Newby LK, LaPointe NM, Ohman EM, Roe MT, Boden WE, Harrington RA, Peterson ED. CRUSADE Investigators. Transfusion practice and outcomes in non-ST-segment elevation acute coronary syndromes. Am Heart J. 2008;4:1047–1053. doi: 10.1016/j.ahj.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Aronson D, Dann EJ, Bonstein L, Blich M, Kapeliovich M, Beyar R, Markiewicz W, Hammerman H. Impact of red blood cell transfusion on clinical outcomes in patients with acute myocardial infarction. Am J Cardiol. 2008;4:115–119. doi: 10.1016/j.amjcard.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Jani SM, Smith DE, Share D, Kline-Rogers E, Khanal S, O’Donnell MJ, Gardin J, Moscucci M. Blood transfusion and in-hospital outcomes in anemic patients with myocardial infarction undergoing percutaneous coronary intervention. Clin Cardiol. 2007;4(Suppl 2):II49–II56. doi: 10.1002/clc.20236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur EM, O’Neill WW, Hellkamp A, Hamm CW, Holmes DR, Al-Khalidi HR, Patel MR, Van de Werf FJ, Pieper K, Armstrong PW, Armstrong PW, Granger CB. APEX-AMI Investigators. Transfusion and mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur Heart J. 2009;4:2575–2583. doi: 10.1093/eurheartj/ehp279. [DOI] [PubMed] [Google Scholar]
- Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, Starr NJ, Blackstone EH. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;4:1608–1616. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;4:2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- Nikolsky E, Mehran R, Sadeghi HM, Grines CL, Cox DA, Garcia E, Tcheng JE, Griffin JJ, Guagliumi G, Stuckey T, Turco M, Fahy M, Lansky AJ, Stone GW. Prognostic impact of blood transfusion after primary angioplasty for acute myocardial infarction: analysis from the CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) Trial. JACC Cardiovasc Interv. 2009;4:624–632. doi: 10.1016/j.jcin.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Pattakos G, Koch CG, Brizzio ME, Batizy LH, Sabik JF, Blackstone EH, Lauer MS. Outcome of patients who refuse transfusion after cardiac surgery: A natural experiment with severe blood conservation. Arch Intern Med. 2012;4:1154–1160. doi: 10.1001/archinternmed.2012.2449. [DOI] [PubMed] [Google Scholar]
- Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, Moliterno DJ, Lindblad L, Pieper K, Topol EJ, Stamler JS, Califf RM. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;4:1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- Shishehbor MH, Madhwal S, Rajagopal V, Hsu A, Kelly P, Gurm HS, Kapadia SR, Lauer MS, Topol EJ. Impact of blood transfusion on short- and long-term mortality in patients with ST-segment elevation myocardial 639 infarction. J Am Coll Cardiol Intv. 2009;4:46–53. doi: 10.1016/j.jcin.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Singla I, Zahid M, Good CB, Macioce A, Sonel AF. Impact of blood transfusions in patients presenting with anemia and suspected acute coronary syndrome. Am J Cardiol. 2007;4:1119–1121. doi: 10.1016/j.amjcard.2006.11.056. [DOI] [PubMed] [Google Scholar]
- Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;4:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- Yang X, Alexander KP, Chen AY, Roe MT, Brindis RG, Rao SV, Gibler WB, Ohman EM, Peterson ED. CRUSADE Investigators. The implications of blood transfusions for patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol. 2005;4:1490–1495. doi: 10.1016/j.jacc.2005.06.072. [DOI] [PubMed] [Google Scholar]
- Kipps AK, Wypij D, Thiagarajan RR, Bacha EA, Newburger JW. Blood transfusion is associated with prolonged duration of mechanical ventilation in infants undergoing reparative cardiac surgery. Pediatr Crit Care Med. 2011;4:52–56. doi: 10.1097/PCC.0b013e3181e30d43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneyber MCJ, Grotenhuis F, Berger RFM, Ebels TW, Burgerhof JGM, Albers MJJJ. Transfusion of leukocyte-depleted red blood cells is independently associated with increased morbidity after pediatric cardiac surgery. Pediatr Crit Care Med. 2013;4:298–305. doi: 10.1097/PCC.0b013e3182745472. [DOI] [PubMed] [Google Scholar]
- Costello JM, Graham DA, Morrow DF, Morrow J, Potter-Bynoe G, Sandora TJ, Pigula FA, Laussen PC. Risk factors for surgical site infection after cardiac surgery in children. Ann Thorac Surg. 2010;4:1833–1834. doi: 10.1016/j.athoracsur.2009.08.081. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Wetterslev J, Sharma A, Lichstein E, Mukherjee D. Association of blood transfusion with increased mortality in myocardial infarction. Arch Intern Med. 2013;4:132–139. doi: 10.1001/2013.jamainternmed.1001. [DOI] [PubMed] [Google Scholar]
- Cooper HA, Rao SV, Greenberg MD, Rumsey MP, McKenzie M, Alcorn KW, Panza JA. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study) Am J Cardiol. 2011;4:1108–1111. doi: 10.1016/j.amjcard.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Garfinkle M, Lawler PR, Filion KB, Eisenberg MJ. Red blood cell transfusion and mortality among patients hospitalized for acute coronary syndromes: a systematic review. Int J Cardiol. 2013;4:151–157. doi: 10.1016/j.ijcard.2011.12.118. [DOI] [PubMed] [Google Scholar]
- Willems A, Van Lerberghe C, Gonsette K, De Villé A, Melot C, Hardy JF, Van der Linden P. The indication for perioperative red blood cell transfusions is a predictive risk factor for severe postoperative morbidity and mortality in children undergoing cardiac surgery. Eur J Cardiothorac Surg. 2014. In press. [DOI] [PubMed]
- Middelburg RA, van de Watering LMG, van der Bom JG. Blood transfusions: good or bad? Confounding by indication, an underestimated problem in clinical transfusion research. Transfusion. 2010;4:1881–1883. doi: 10.1111/j.1537-2995.2010.02675.x. [DOI] [PubMed] [Google Scholar]
- Lacroix J. Red cell transfusion: risk marker or risk factor in cardiac patients? Pediatr Crit Care Med. 2013;4:330–331. doi: 10.1097/PCC.0b013e31827d165b. [DOI] [PubMed] [Google Scholar]
- Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;4:759–769. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- Taylor C, Cohen H, Mold D, Jones H, Davies T, Asher D, Cawley C, Chaffe B, Chapman C, Gray A, Knowles S, Milkins C, New H, Norfolk D, Still E, Tinegate H, on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2008 Annual SHOT Report. 2009. p. 153.
- Tucci M, Lacroix J, Gauvin F, Toledano B, Robitaille N. In: Pediatric critical care medicine: Basic science and clinical evidence. 2. Wheeler DS, Wong HR, Shanley TP, editor. London: Springer-Verlag; 2014. Transfusion medicine. In press. [Google Scholar]
- Barr PJ, Donnelly M, Cardwell CR, Parker M, Morris K, Bailie KE. The appropriateness of red blood cell use and the extent of overtransfusion: right decision? Right amount? Transfusion. 2011;4:1684–1694. doi: 10.1111/j.1537-2995.2011.03130.x. [DOI] [PubMed] [Google Scholar]
- Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, Gibson CM, Braunwald E. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;4:2042–2049. doi: 10.1161/01.CIR.0000162477.70955.5F. [DOI] [PubMed] [Google Scholar]
- Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;4:327–348. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Gajic O, Gropper MA, Hubmayr RD. Pulmonary edema after transfusion: how to differentiate transfusion-associated circulatory overload from transfusion-related acute lung injury. Crit Care Med. 2006;4(5 Suppl):S109–S113. doi: 10.1097/01.CCM.0000214311.56231.23. [DOI] [PubMed] [Google Scholar]
- Vlaar AP, Cornet AD, Hofstra JJ, Porcelijn L, Beishuizen A, Kulik W, Vroom MB, Schultz MJ, Groeneveld AB, Juffermans NP. The effect of blood transfusion on pulmonary permeability in cardiac surgery patients: a prospective multicenter cohort study. Transfusion. 2012;4:82–90. doi: 10.1111/j.1537-2995.2011.03231.x. [DOI] [PubMed] [Google Scholar]
- Tuinman PR, Vlaar AP, Cornet AD, Hofstra JJ, Levi M, Meijers JC, Beishuizen A, Schultz MJ, Groeneveld AJ, Juffermans NP. Blood transfusion during cardiac surgery is associated with inflammation and coagulation in the lung: a case control study. Crit Care. 2011;4:R59. doi: 10.1186/cc10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaar AP, Binnekade JM, Prins D, van Stein D, Hofstra JJ, Schultz MJ, Juffermans N. Risk factors and outcome of transfusion-related acute lung injury in the critically ill: a nested case–control study. Crit Care Med. 2010;4:771–778. doi: 10.1097/CCM.0b013e3181cc4d4b. [DOI] [PubMed] [Google Scholar]
- Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O’Byrne MM, Evenson LK, Malinchoc M, DeGoey SR, Afessa B, Hubmayr RD, Moore SB. Transfusion-related acute lung injury in the critically ill: prospective nested case–control study. Am J Respir Crit Care Med. 2007;4:886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalavatti GS, DeBacker D, Vincent JL. Assessment of cardiac index in anemic patients. Chest. 2000;4:782–787. doi: 10.1378/chest.118.3.782. [DOI] [PubMed] [Google Scholar]
- Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, Leung JM, Fisher DM, Murray WR, Toy P, Moore MA. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;4:217–221. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- Weiskopf RB, Feiner J, Hopf HW, Viele MK, Watson JJ, Kramer JH, Ho R, Toy P. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology. 2002;4:871–877. doi: 10.1097/00000542-200204000-00014. [DOI] [PubMed] [Google Scholar]
- Frank SM, Abazya B, Ono M, Hogue CW, Cohen DB, Berkowitz DE, Ness PM, Barodka VM. Decreased erythrocyte deformability after transfusion and the effects of erythrocyte storage duration. Anesth Analg. 2013;4:975–981. doi: 10.1213/ANE.0b013e31828843e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S, Doctor A. In: Current Concepts in Pediatric Critical Care. Spinella PC, Nakagawa TA, editor. Des Plaines: Society of Critical Care Medicine; 2011. Vasoregulation by red blood cells; pp. 21–39. [Google Scholar]
- Bracey AW, Radovancevic R, Riggs SA, Houston S, Cozart H, Vaughn WK, Radovancevic B, McAllister HA, Cooley DA. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion. 1999;4:1070–1077. doi: 10.1046/j.1537-2995.1999.39101070.x. [DOI] [PubMed] [Google Scholar]
- Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J. for the FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;4:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert PC, Yetisir E, Martin C, Blajchman MA, Wells G, Marshall J, Tweeddale M, Pagliarello G, Schweitzer I. Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med. 2001;4:227–234. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Johnson RG, Thurer RL, Kruskall MS, Sirois C, Gervino EV, Critchlow J, Weintraub RM. Comparison of two transfusion strategies after elective operations for myocardial revascularization. J Thorac Cardiovasc Surg. 1992;4:307–314. [PubMed] [Google Scholar]
- Cholette JM, Rubenstein JS, Alfieris GM, Powers KS, Eaton M, Lerner NB. Children with single ventricle physiology do not benefit from higher hemoglobin levels following cavopulmonary connection: Results of a prospective, randomized controlled trial of a restrictive v. liberal red cell transfusion strategy. Pediatr Crit Care Med. 2011;4:39–45. doi: 10.1097/PCC.0b013e3181e329db. [DOI] [PubMed] [Google Scholar]
- de Gast-Bakker DH, de Wilde RBP, Hazekamp MG, Sojak V, Zwaginga JJ, Wolterbeek R, de Jonge E, der Veer BJG-v. Safety and effects of two red blood cell transfusion strategies in pediatric cardiac surgery patients; a randomized controlled trial. Intensive Care Med. 2013;4:2011–2019. doi: 10.1007/s00134-013-3085-7. [DOI] [PubMed] [Google Scholar]
- Willems A, Harrington K, Lacroix J, Biarent D, Joffe A, Wensley D, Hébert P, Tucci M. For the Canadian Critical Care Trials Group and the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery. Crit Care Med. 2010;4:649–656. doi: 10.1097/CCM.0b013e3181bc816c. [DOI] [PubMed] [Google Scholar]
- Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yertsir E. The Transfusion Requirements in Critical Care Investigators. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;4:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- Lacroix J, Hébert PC, Hutchison JH, Hume H, Tucci M, Ducruet T, Gauvin F, Collet JP, Toledano BJ, Robillard P, Joffe A, Biarent D, Meert K, Peters MJ. on behalf of the TRIPICU investigators, for the Canadian Critical Care Trials Group and the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;4:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;4:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Bhalala US, Nishisaki A, McQueen D, Bird GL, Morrison WE, Nadkarni VM, Nathan M, Starr JP. Change in regional (somatic) near-infrared spectroscopy is not a useful indicator of clinically detectable low cardiac output in children after surgery for congenital heart defects. Pediatr Crit Care Med. 2012;4:529–534. doi: 10.1097/PCC.0b013e3182389531. [DOI] [PubMed] [Google Scholar]
- Creteur J, Neves AP, Vincent JL. Near-infrared spectroscopy technique to evaluate the effects of red blood cell transfusion on tissue oxygenation. Crit Care. 2009;4(Suppl 5):S11. doi: 10.1186/cc8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira CF, de Oliveira DS, Gottschald AF, Moura JD, Costa GA, Ventura AC, Fernandes JC, Vaz FA, Carcillo JA, Rivers EP, Troster EJ. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med. 2008;4:1065–1075. doi: 10.1007/s00134-008-1085-9. [DOI] [PubMed] [Google Scholar]
- The ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;4:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Jr Smith SC. American College of Cardiology; American Heart Association; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction - executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;4:671–719. doi: 10.1016/j.jacc.2004.07.002. [DOI] [PubMed] [Google Scholar]


