Abstract
The past quarter century has seen a rapid increase in our knowledge about the natural history of auto-immune type 1 diabetes. However, we stand unable to achieve our ultimate goal of preventing or reversing this disease. This Viewpoint discusses controversies in current management of type 1 diabetes, the challenges in translating promising studies from mouse models of the disease to humans, hurdles faced in designing optimal prevention and intervention studies, and potential strategies to overcome these obstacles.
Keywords: Clinical trials, Intervention, Prevention, Type 1 diabetes
“…Two roads diverged in a wood, and I-I took the one less traveled by, And that has made all the difference.”
-The Road Not Taken
Robert Frost, 1920
Controversies in clinical care
With the discovery of insulin, type 1 diabetes (T1D) was transformed from a uniformly fatal diagnosis to a chronic disease; one characterized by the need for multiple daily insulin injections and sadly, complications driven by long-term hyperglycemia [1]. Undoubtedly, the development of recombinant human insulin, insulin analogues, meters for performing self monitoring of blood glucose, insulin pumps, point of care hemoglobin A1c testing and more recently, continuous glucose monitoring have dramatically improved the quality of life of T1D patients. Still, patients of all ages, and especially children, continue to struggle with the challenges of attempting to maintain normoglycemia [2, 3]. Added to this, the worldwide epidemic of diabetes (i.e. both type 1 and type 2), the increased burden of diabetes both to the individual and society, no reduction in acute complications, and the fact that benefits of improved glycemic control are reaching only a minority of patients mandates that increased efforts be made to hasten progress toward a biological cure.
Animal to human translation
As discussed in von Herrath’s accompanying Viewpoint [4], the non-obese diabetic mouse has provided an animal model for the study of autoimmune T1D for over 20 years. Countless ways to prevent, and a growing number of means to reverse, T1D have been documented using non-obese diabetic mice, yet in 2009, we stand unable to achieve the ultimate goal of preventing or reversing human T1D. The discrepancy between cure in animal models and humans exists to some degree because of the inherent differences between mouse and man. Specifically, when compared with animal models, the far greater heterogeneity of diabetes in humans and the potential differences in the pathological processes driving β-cell destruction at the time of immune intervention may explain many of the difficulties in translating observations from mouse to man. Although great strides have been made in our understanding of the natural history of human pre-T1D and the subsequent development of the disease itself, to date we have no reliable immunoregulatory, immunomodulatory, or immunoeffector markers of the disease process. Additionally, grounds for failure to translate across species also resides with the unpopular message that certain agents, while effective in laboratory settings, have little chance of being translated into practical clinical trials, and even less likelihood of ever being considered as adjunctive standard therapy for those afflicted or at high-risk for developing the disease. Among these challenges are route of administration, dose, as well as potential short- and long-term toxicities. Indeed as technological advances in management continue, the relative risk-benefit ratio involved in developing immunological-based preventions or cures will continue to move such that patients and physicians will tolerate less risk and will mandate increasingly safer approaches.
Given the vast number of potential agents initially considered, relatively few immune-based interventions have made their way from the laboratory to the patient bedside. That said, key lessons have already been learned from our experience with non-antigen specific immunosuppressive agents like azothiaprine [5], cyclosporine [6], antithymocyte globulin [7], and anti-CD3 [8, 9], antigen-specific immunotherapies such as insulin [10] and GAD65 [11], and β-cell replacement strategies such as islet transplantation [12].
Early efforts with non-antigen specific agents showed promise in preserving c-peptide, but were limited by intolerable side effect profiles and relatively short-term and perhaps clinically insignificant benefits. More recent efforts, specifically those involving anti-CD3 have gained momentum as two different monoclonal anti-CD3 products, hOKT3gl (Ala-Ala) and ChAglyCD3 (TRX4), have demonstrated initial efficacy in slowing the expected rate of c-peptide decay over a period of 18–36 months [8, 9]. While leading to short-term T-cell depletion following administration, anti-CD3 antibodies may selectively induce more long-term tolerance possibly via enhanced production of CD4+CD25+ Treg. Still, logistical, efficacy, and safety issues associated with anti-CD3 therapy must be considered. Current dosing regimens require a 1–2 wk intravenous infusion – a requirement that limits both patient enrollment and possible wide scale use of this agent. Among the safety issues are the commonly occurring cytokine release syndrome after initial dosing and reactivation of Epstein-Barr virus. As just one example of a potential non-antigen specific therapy, anti-CD3 therapy is representative of the ongoing equipoise debate as it relates to developing therapies that offer both acceptable risk profile and clinically meaningful efficacy endpoints.
Perhaps laying on the opposite side of the equipoise issue, recent efforts to develop antigen-specific immunotherapy (Insulin [10], DiaPep227 [13], GAD65 [11]) have generally been associated with more appealing risk profiles. Although neither nasal insulin in the Finnish Diabetes Prediction and Prevention Project nor oral insulin therapy in the Diabetes Prevention Trial were effective in preventing diabetes in at risk individuals, post-hoc analysis of the oral insulin arm demonstrated delay of diabetes onset in subjects with high insulin auto-antibodies resulting in a formal retesting of that observation in the ongoing TrialNet oral insulin trial. In addition, recent phase 1 and phase 2 experiences with GAD65 suggest that this easily administered subcutaneous therapy is safe in both children and adults with T1D and may preserve residual β-cell function for at least 15–30 months [11].
To date, the concept of performing pancreas and islet transplants in children with T1D has been untenable due to the considerable risks associated with long-term immunosuppression and the observation that even the most effective steroid-free protocols rarely result in sustained insulin independence [11]. Despite these concerns, potential surgical risks, and the paucity of tissue, further research is clearly indicated. Most importantly, even the most successful immunomodulatory therapies will require not only the replacement of a functional β-cell mass to provide meaningful benefit to long standing T1D patients but also the prevention of both allo and recurrence of autoimmunity. The potential development of β-cell replacements from autologous sources, including the recently described induced pluoripotent stem cells [14], suggests that issues related to alloimmunity may well be overcome before auto-immunity is successfully controlled.
Observations related to antigen specific, non-antigen specific, and β-cell replacement therapies are encouraging and mandate additional efforts. However, we must accept that the clinical impacts of these approaches are inadequate to achieve our ultimate goals of preventing and reversing diabetes.
Prevention versus intervention
Among the challenges arising in attempts to design therapies to interdict the natural history of T1D, one of the greatest has been deciding whether to focus on prevention- or intervention-based strategies [15]. This may largely be considered a semantic argument as to what denotes prevention versus intervention, but the fact that primary prevention (i.e. prevention of autoimmunity), secondary prevention (i.e. prevention of diabetes in subjects with established autoimmunity), and intervention studies (i.e. reversal of disease or preservation of c-peptide) are all currently being performed as part of large multi-center trials, demonstrates that the diabetes research community continues to struggle with choosing a dominant paradigm.
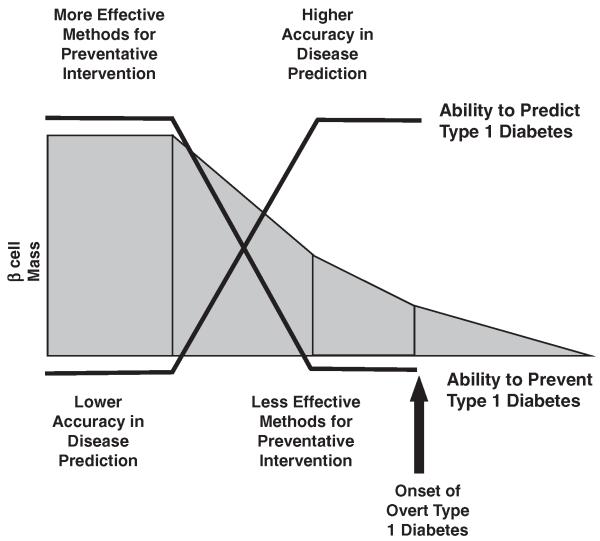
While consensus exists that intervening early (i.e. primary or secondary prevention) in the development of the disease process is more likely to succeed in ameliorating the autoimmune process [16], we are faced with the dilemma that the ability to accurately predict T1D improves only as the presumed ability to intervene declines (Fig. 1) [17]. Thus, the risk-benefit ratio of potential therapies is paramount. Low-risk therapies can ethically be tested in primary prevention studies but are logistically challenging. With low disease predictability, large numbers of subjects need to be followed over many years to document effect-cost and subject retention present difficult challenges for sponsors, investigators and subjects themselves. Conversely, high-risk therapies are more rightly reserved for intervention studies with smaller numbers of subjects who have T1D and 1–2 year studies have been used to document the potential efficacy. Thus, to ensure a state of equipoise between risk and benefit, intervention studies in recent onset T1D patients have emerged as the rationale format for studying potential novel therapies. Not only might preservation of c-peptide protect individuals themselves from acute complications (e.g. hypoglycemia, hyperglycemia and ketosis) and potentially long-term complications, but also, afford an opportunity to garner efficacy and safety data. Unfortunately, several agents tested to date have potentially significant side effect profiles that may limit their application; both for prevention studies as well as widespread use in new-onset T1D patients.
Figure 1.
The “treatment dilemma” for T1D. Many studies from animal models of T1D, in combination with a much more limited series of investigations in human beings, suggest that early intervention not only is more effective in terms of disease prevention, but also often requires more benign forms of therapy. In contrast, the ability to identify an individual who will truly develop T1D increases as the individual approaches onset of overt disease. Adapted from [10], with permission.
Statistically significant but clinically irrelevant
While prevention of diabetes onset is a relatively well defined and agreed upon endpoint, the determination of remaining β-cell mass or function in patients with established T1D remains somewhat enigmatic. Mixed meal stimulated c-peptide has become the accepted primary surrogate in intervention studies. Yet c-peptide as a marker of success does have its limitations [18]. Thus there is an ethical dilemma of exposing people without T1D to potentially risky interventions with the additional burden of differentiating between short-term statistically significant results and clinically relevant benefits. Absolute c-peptide (both stimulated and unstimulated) and the rate of c-peptide decay have been used in multiple trials (e.g. anti-CD3, GAD65, etc.) and both have their limitations [8]. For example, there would be debate as to which patient might be better off: the patient with a stimulated c-peptide of 1.5 pg/mL at diagnosis and 0.75 pg/mL at 1 year or the one with a stimulated c-peptide of 0.5 pg/mL at diagnosis and 0.4 pg/mL at one year. The first patient has a greater percentile loss in c-peptide but still maintains a more robust absolute c-peptide than the second. Translating this into clinically relevant outcomes including improved hemoglobin A1c, reduced insulin requirement (while maintaining control), less frequent hypoglycemia, ketosis, and even micro- and macro-vascular complications are vital.
Heterogeneity of disease
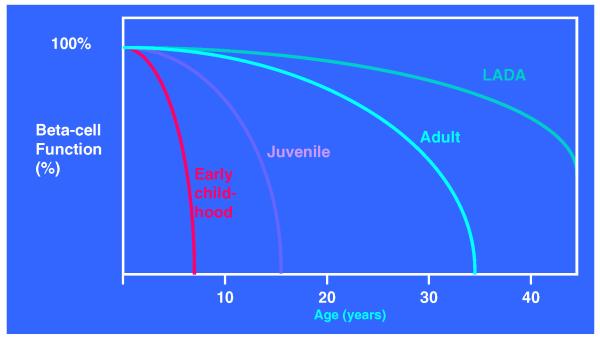
T1D affects young children, teenagers, young adults, and even the elderly [19]. The heterogeneity of the natural history of T1D adds yet another layer of complexity when attempting to define benefit for patients participating in intervention trials. The rate of decay of c-peptide is clearly age dependent and response to therapy appears dependent on multiple factors (e.g. how early the diagnosis is made, time to initiation of therapy, presence of diabetic ketoacidosis, glycemic management, etc.) (Fig. 2). Thus, the challenge of establishing both statistically significant and clinically relevant differences between study cohorts requires additional attention to detail when designing intervention trials and when interpreting study data.
Figure 2.
The heterogeneity of T1D. The onset of T1D can occur at any age and documents the marked heterogeneity of the natural history of the disease. The course of β-cell destruction after diagnosis may be equally heterogeneous, making assessment of interventions a difficult task. Adapted from [19], with permission.
Efficacy versus safety
Only one highly risky approach (non-myeloablative immunotherapy including cyclophosphamide, anti-thymocyte globulin and granulocyte colony stimulating factor followed by autologous cell infusion/rescue) has, to date, resulted in what patients, clinicians, and researchers alike would consider a definite (at least over the short-term) clinically relevant benefit (i.e. discontinued need for insulin injections and increased stimulated c-peptide) [20], [21] In this controversial approach, 14 of 15 very recent onset young adult patients achieved insulin free periods of at least 1 month with the majority achieving “remission” for at least 6 months. Even so, since showing initial promise, many have gone back to requiring insulin or other adjunct therapies to ensure that they maintain adequate glycemic control. While fortunately no mortality was observed (estimated mortality risk for such an approach is between 0.3 and 3%), considerable morbidity and prolonged hospital stays were required. Adding further to the equipoise debate, this study forces us to accept that “effective” approaches may not be achievable without accommodating considerable risk. The difficulty lies in defining the level of acceptable risk.
While T1D is undoubtedly a difficult chronic disease, management tools continue to improve the quality of life and reduce complication risks for our patients. Clinical trials involving low-risk therapies such as autologous umbilical cord blood infusion [22], docosahexaenoic acid [23] and Vitamin D3 supplementation [24], and avoidance of cow milk proteins (e.g. TRIGR trial) [25] are currently underway. However, other non-antigen-specific therapies with tolerable side effect profiles such as nicotinamide [26], ketotifen [27], and Bacille Calmette-Guerin [28] have been previously studied and failed to demonstrate benefit. Our own research group, admittedly one that cares primarily for children with T1D, continues to be a proponent of “safe therapies” accepting that these approaches may only incrementally improve our abilities to preserve c-peptide. However, with time, we hope that such an approach will allow development of statistically significant, clinically relevant, and acceptably low-risk therapeutic regimens.
Combination therapy
Novel, “out of the box”, and patient centered thinking is needed to effectively apply the lessons learned from the relative “successes” and failures of past clinical trials. It is noteworthy that previous attempts to reverse T1D have largely been focused on single agents designed to intervene in a singular aspect of the disorder’s pathogenesis. Indeed, some individual agents have shown promise in animal models of T1D, only to fall short of producing a similar response in humans. As a result, we and others have questioned whether the delivery of agents in combination might produce a synergistic response that would allow for the successful reversal of T1D [29]. The success of combination or “cocktail therapy” for advancing the treatment of patients with cancer, HIV, as well as transplantation (among others) demonstrates a model for multi-agent therapy in diseases involving multiple pathways. Combination therapy is not in reality a new concept; yet our desire to fully understand the mechanisms of potential single agent therapies has in part delayed the initiation of combination therapies in human T1D. Safe combination therapy with multiple agents aimed at providing islet protection (e.g. immunosuppression, immunomodulation), islet regeneration, and β-cell replacement are now being developed and must be actively pursued to achieve our ultimate goal.
Concluding remarks
The inability to cure T1D underscores the complex pathophysiology of the disease and our poor knowledge about the precise etiological triggers and immunological mechanisms that culminate in the disease. As such, we stand at the proverbial fork in the road and must choose how best to move forward in our search for a cure. We can either continue along our current path with the knowledge that it has failed to lead us to our destination, or we can shift paradigms and take the road less traveled.
Acknowledgements
We thank the Juvenile Diabetes Research Foundation and the National Institutes of Health for research support.
Abbreviation
- T1D
type 1 diabetes
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Bliss M. 25th Anniversary Edition Edn. The University of Chicago Press; Chicago: 2007. The Discovery of Insulin. [Google Scholar]
- 2.Gerstl EM, et al. Eur. J. Pediatr. 2008;167:447–453. doi: 10.1007/s00431-007-0586-9. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial Research Group J. Pediatr. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 4.von Herrath M, Nepom GT. Eur. J. Immunol. 2009;39:2049–2054. doi: 10.1002/eji.200939429. [DOI] [PubMed] [Google Scholar]
- 5.Silverstein J, et al. N. Engl. J. Med. 1988;319:599–604. doi: 10.1056/NEJM198809083191002. [DOI] [PubMed] [Google Scholar]
- 6.The canadian-european randomized control trial group cyclosporin-induced remission of IDDM after early intervention. Diabetes. 1988;37:1574–1582. [PubMed] [Google Scholar]
- 7.Eisenbarth GS, et al. Diabetes Res. 1985;2:271–276. [PubMed] [Google Scholar]
- 8.Keymeulen B, et al. N. Engl. J. Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 9.Herold KC, et al. N. Engl. J. Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 10.Group D-S. Diabetes. 1994;43:159A. [Google Scholar]
- 11.Ludvigsson J. Diabetes Metab. Res. Rev. 2009;25:307–315. doi: 10.1002/dmrr.941. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro AM, et al. N. Engl. J. Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 13.Raz I, et al. Lancet. 2001;358:1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 14.Huangfu D, et al. Nat. Biotech. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 15.Staeva-Vieira T, et al. Clin. Exp. Immunol. 148:17, 31. doi: 10.1111/j.1365-2249.2007.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller MJ, et al. Pediatr. Clin. North Am. 2005;52:1553–1578. doi: 10.1016/j.pcl.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Atkinson MA, Eisenbarth GS. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 18.Palmer JP, et al. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 19.Redondo MJ, et al. N. Engl. J. Med. 2008;359:2849–2850. doi: 10.1056/NEJMc0805398. [DOI] [PubMed] [Google Scholar]
- 20.Voltarelli JC, et al. JAMA. 2007;297:1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 21.Couri CEB, et al. JAMA. 2009;301:1573–1579. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- 22.Haller MJ, et al. Exp. Hematol. 2008;36:710–715. doi: 10.1016/j.exphem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chase HP. Infant Child Adolesc. Nutr. 2009;1:98–107. [Google Scholar]
- 24.The EURODIAB Substudy 2 Study Group Diabetologia. 1999;42:51–54. doi: 10.1007/s001250051112. [DOI] [PubMed] [Google Scholar]
- 25.Study design of the Trial to Reduce IDDM in the Genetically at Risk (TRIGR) Pediatr. Diabetes. 2007;8:117–137. doi: 10.1111/j.1399-5448.2007.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gale EA, et al. Lancet. 2004;363:925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 27.Bohmer KP, et al. Diabetes Care. 1994;17:138–141. doi: 10.2337/diacare.17.2.138. [DOI] [PubMed] [Google Scholar]
- 28.Huppmann M, et al. Diabetes Care. 2005;28:1204–1206. doi: 10.2337/diacare.28.5.1204. [DOI] [PubMed] [Google Scholar]
- 29.Schatz D, et al. Diabetes Care. 2003;26:3326–3328. doi: 10.2337/diacare.26.12.3326. [DOI] [PubMed] [Google Scholar]