Abstract
Congenital toxoplasmosis and toxoplasmic encephalitis can be associated with severe neuropsychiatric symptoms. However, which host cell processes are regulated and how Toxoplasma gondii affects these changes remain unclear. MicroRNAs (miRNAs) are small noncoding RNA sequences critical to neurodevelopment and adult neuronal processes by coordinating the activity of multiple genes within biological networks. We examined the expression of over 1000 miRNAs in human neuroepithelioma cells in response to infection with Toxoplasma. MiR-132, a cyclic AMP-responsive element binding (CREB)-regulated miRNA, was the only miRNA that was substantially upregulated by all three prototype Toxoplasma strains. The increased expression of miR-132 was also documented in mice following infection with Toxoplasma. To identify cellular pathways regulated by miR-132, we performed target prediction followed by pathway enrichment analysis in the transcriptome of Toxoplasma-infected mice. This led us to identify 20 genes and dopamine receptor signaling was their strongest associated pathway. We then examined myriad aspects of the dopamine pathway in the striatum of Toxoplasma infected mice 5 days after infection. Here we report decreased expression of D1-like dopamine receptors (DRD1, DRD5), metabolizing enzyme (MAOA) and intracellular proteins associated with the transduction of dopamine-mediated signaling (DARPP-32 phosphorylation at Thr34 and Ser97). Increased concentrations of dopamine and its metabolites, serotonin and 5-hydroxyindoleacetic acid were documented by HPLC analysis; however, the metabolism of dopamine was decreased and serotonin metabolism was unchanged. Our data show that miR-132 is upregulated following infection with Toxoplasma and is associated with changes in dopamine receptor signaling. Our findings provide a possible mechanism for how the parasite contributes to the neuropathology of infection.
Keywords: Toxoplasma gondii, miR-132, dopamine receptor pathway, alteration in expression, mouse striatum
Introduction
Toxoplasma gondii is an obligate intracellular pathogen within the phylum Apicomplexa. Toxoplasma is capable of infecting and replicating within virtually any nucleated mammalian or avian cell. Moreover, Toxoplasma is one of the few pathogens that regularly cross the placenta. Brain and eye lesions are the most common consequences of in utero infection. While infection of healthy adults is usually relatively mild, the tropism of Toxoplasma for brain tissue has been linked with specific behavioral changes in humans and in animals (Vyas and Sapolsky, 2010; Webster et al., 2013). In immunocompromised patients, severe neurological disease such as toxoplasmic encephalitis can occur due to either acute infection or reactivation of chronic infection. Taken together, these lines of evidence document that Toxoplasma infection has specific effects on the brain. However, which host cell processes are regulated and how the parasite effects these changes remain unclear.
Previous studies have indicated that Toxoplasma infection affects the levels of certain neurotransmitters (e.g. monoamines) and their metabolites in both the acute and chronic phases of infection (Stibbs, 1985; Gatkowska et al., 2013). Moreover, a study on rats has demonstrated that treatment with the dopamine antagonist haloperidol during the tachyzoite replicative stage diminishes the behavioral effects of Toxoplasma infection (Webster et al., 2006). In infected mice, dopamine uptake inhibitor GBR12909 modifies behavioral responses associated with latent toxoplasmosis (Skallová et al., 2006). It thus has been speculated that the dopaminergic system may be involved in the neurological effects of infection. Indeed, Toxoplasma harbors two genes encoding tyrosine hydroxylase catalyzing the rate-limiting step in dopamine biosynthesis (Gaskell et al., 2009), and an increase in dopamine level during infection of neural cells in vitro has been observed (Prandovszky et al., 2011).
Dopamine is a catecholamine neurotransmitter that controls a diverse range of physiological processes. Dopamine exerts its effects by acting on two primary receptor subtypes: D1-like (DRD1 and DRD5) and D2-like (DRD2, DRD3, and DRD4) receptors. Activation of D1-like receptors leads to the activation of adenylyl cyclase and increase in cyclic adenosine monophosphate (cAMP) and Ca2+ levels, whereas activation of D2-like receptors leads to a decrease in adenylyl cyclase and cAMP levels. DARPP-32 (dopamine and cyclic AMP-regulated 32-kDa phosphoprotein) was identified as a major target for dopamine-activated adenylyl cyclase in striatum. Two phosphorylation sites, threonine-34 (Thr34) and threonine-75 (Thr75), make DARPP-32 a bifunctional signal transduction molecule that controls the activities of protein phosphatase 1 (PP1) and protein kinase A (PKA), and thereby controls the phosphorylation state and activity of many downstream physiological effectors (Nairn et al., 2004; Svenningsson et al., 2004). Disturbances of dopaminergic signaling have been implicated in many pathological conditions including Parkinson's disease, schizophrenia, attention-deficit/hyperactivity disorder and addiction. Not surprisingly, dopaminergic signaling in the central nervous system (CNS) is highly regulated and subject to precise temporal control (Kotowski et al., 2011).
MicroRNAs (miRNAs) comprise a class of small noncoding RNAs (∼20-23 nt) that regulate gene expression. Dysregulation of a single miRNA can be sufficient to alter the gene-expression profile and developmental trajectory of cells (Lim et al., 2005; Friedman et al., 2009).
Approximately 70% of known miRNAs are expressed in the nervous system, often with a high degree of spatial and temporal specificity (Krichevsky et al., 2003). MiR-132 is a cyclic AMP-responsive element binding (CREB)-regulated miRNA and is enriched in neuronal cells (Cheng et al., 2007). MiR-132 function has been suggested within both the nervous and the immune systems with the majority of function in a neuronal context. Dysregulation of miR-132 is associated with several neurological disorders, such as schizophrenia, Alzheimer, Parkinson's disease and tauopathies (Miller et al., 2012; Wanet et al., 2012), suggesting a broader impact of this miRNA on diseases of brain development. A role for miRNAs in Toxoplasma infections was first documented in human fibroblasts, where the transcription of the miR-17/92 loci was specifically increased by two- to three-fold (Zeiner et al., 2010).
We first performed a comprehensive genomewide miRNA expression profiling of neural cells infected by Toxoplasma, with the objective of understanding the pathogenetic role of miRNA dysregulation during infection. This led us to identify the increased expression of miR-132 as a common effect of infection with three canonical Toxoplasma strains. In our further investigation on cellular pathways regulated by miR-132, we were surprised to find the dopamine receptor pathway had the strongest association. Therefore, we examined myriad aspects of the dopamine system in mice with acute Toxoplasma infection to evaluate the balance of dopaminergic neurotransmission. In addition to examining changes in the dopamine metabolism in the striatal regions of the mouse brain, we have evaluated the expression of genes that regulate dopaminergic pathways, such as dopamine receptors and some of the intracellular proteins associated with the transduction of dopamine-mediated signaling. Our findings support the hypothesis that abnormal dopamine signaling may account for numerous data suggesting some neuropsychiatric symptoms (e.g. mental impairment such as learning, motor disabilities and personality changes) observed in congenital toxoplasmosis and toxoplasmic encephalitis.
Experimental Procedures
Infection of human neuroepithelioma cells
MiRNA expression profiles were measured in RNA samples obtained from SK-N-MC cells (ATCC, HTB-10) infected or mock infected with three major clonal Toxoplasma strains: (RH-2F (type I), PRU (type II) or CTG (type III)). SK-N-MC is a human neuroepithelioma cell line expressing neuronal characteristics (Barnes et al., 1981). Cell culture of parasite strains and infection of SK-N-MC cells were conducted as previously described (Xiao et al., 2011) except that cells were infected at multiplicity of infection 5. Infections and mock-infected controls (no tachyzoites) were performed for each strain on three separate occasions in order to have biological replicates. RNA was extracted 20 hours following infection using the miRNeasy kit (QIAGEN, Valencia, CA).
MiRNA profiling and analysis
MiRNA expression was profiled using Affymetrix miRNA 2.0 arrays containing 1, 105 human mature miRNA and carried out at The Johns Hopkins Deep Sequencing & Microarray Core Facility. Briefly, RNA samples were prepared following the Affymetrix FlashTagTM Biotin HSR RNA Labeling kit protocol (Affymetrix Inc., Santa Clara, CA) and hybridized onto Affymetrix miRNA microarrays. After 16 hours, the arrays were stained and washed using the Affymetrix GeneChip Fluidics Station 450 and FS450_003 fluidics script. All arrays were scanned in the Affymetrix GeneChip Scanner 3000 and raw analysis performed with their miRNA QC Tool version 1.1.1.0. For data extraction, quality control (QC) and further analyses their raw Affymetrix CEL files were then imported into the Partek Genomics Suite (Partek Incorporated, St. Louis MO, USA). The CEL files' data were extracted, normalized using RMA quantile normalization and converted into log2 notation. Only those interrogating probesets that represent human sequence were imported so as to avoid any interference in the normalization process from non-human values. For QC, pre- and post-normalization fluorescence signal distributions were compared to previous miRNA 2.0 analyses, and across all samples to ensure there were no technical outliers. To determine miRNAs that were differentially expressed by infected cells for each of the three Toxoplasma strains, paired-sample t-tests were performed between infected and un-infected samples.
CD-1 mouse tissue samples
Animal protocols were approved by the Animal Care and Use Committee of Johns Hopkins University and were in accordance with the National Institutes of Health Guidelines. In this study, three replicate experiments (replicate 1, n = 5 per group; replicate 2, n = 20 per group; replicate 3, n = 7 or 8 per group) were conducted and samples from each experiment were used for subsequent studies. Six- to eight-week-old female outbred CD-1 mice (ICR-Harlan Sprague) were infected intraperitoneally with 500 tachyzoites of Toxoplasma GT1 strain (type I, virulent) diluted in 200 μL of PBS. For negative controls, un-infected mice groups received 200 μL of only the vehicle (PBS) intraperitoneally. The type I stain was chosen for study in light of the ability to largely affect genes related to central nervous system in our previous studies using neural cells (Xiao et al., 2011; Xiao et al., 2013). GT1 strain was maintained by passage in human glia cell line (A172). Mice were sacrificed at 5 days postinfection (dpi) by cervical dislocation and then decapitation. This time point was chosen based on our research focus on tachyzoite and the transcriptome of Toxoplasma-infected mice (Hill et al., 2012) we applied for target prediction and pathway enrichment analysis. Brains were placed on ice, and striata were dissected within 90 seconds. For neurochemical analysis, striatum was snap frozen in liquid nitrogen, and stored at −80 °C. For gene and protein analysis, striatum was suspended in 1 ml of RNAlater solution (Ambion, Austin, TX), and then stored at −70°C. To collect peritoneal cells, 5 ml of ice-cold PBS was injected into the peritoneal cavity, and then peritoneal lavage fluid was collected. Peritoneal cells were collected by centrifugation at 1,000 × g for 10 min, resuspended in 1 ml of RNAlater solution, and then stored at −70°C before RNA extraction. Infection was confirmed in the mouse spleen tissues by measuring the amplification of Toxoplasma 5S rRNA as previously described (Xiao et al., 2011).
Reverse transcription and quantitative PCR
Reverse transcription was performed using either Multiscribe reverse transcriptase and random primers (Applied Biosystems) to generate cDNA, or the Multiscribe miRNA Reverse Transcription kit (Applied Biosystems) using miRNA-specific primers to produce miRNA. Quantitative real-time PCR (qPCR) was performed using inventoried TaqMan miRNA and mRNA assays (Applied Biosystems) with standard ABI protocols and reagents. For human neuroepithelioma cells, RNA samples for microarray analysis were used to quantitate either miRNA level or mRNA transcript levels of genes. For mouse samples, miRNA qPCR reactions were performed using both the peritoneal cells and striatum samples, but mRNA qPCR reactions were performed only on striatum samples because of the limited amount/gene abundance of peritoneal cells. All samples were run in triplicate using RNU48, snoRNA135 and snoRNA202 as endogenous miRNA controls and β-actin as an endogenous mRNA control, Relative abundance was determined using the comparative ΔCt method.
MiR-132 target gene and regulated pathway analysis using IPA
To investigate dysregulated miR-132 target gene induced by Toxoplasma, previous gene-expression data were evaluated for overlap with miR-132 predicted targets using Ingenuity Pathway Analysis (IPA summer Release-content version number 9.0.162830). For human neuroepithelioma cells, our previous transcriptomes induced by 3 Toxoplasma strains (Xiao et al., 2011) were employed. For mouse samples, a recently published transcriptome of mouse peritoneal cells with an infection course identical to our mouse model was used for analysis (Hill et al., 2012). We also performed pathway enrichment analysis to identify the miR-132-regulated cellular pathways using those dysregulated target genes from Toxoplasma-infected mice.
Gene expression
For human neuroepithelioma cells, we examined mRNA expression of four genes: apoptotic peptidase activating factor 1 (APAF1), v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS), mitogen-activated protein kinase 3 (MAPK3) and protein phosphatase 2, regulatory subunit B′, epsilon isoform (PPP2R5E). For mouse striatum, we investigated mRNA expression of multiple genes that involved in dopamine receptor signaling, including five dopamine receptors: Drd1, Drd2, Drd3, Drd4 and Drd5; three metabolizing enzymes: monoamine oxidase A (Maoa) and B (Maob), catechol-O-methyltransferase (Comt); Calcyon, a gene which encodes the protein that interacts with Drd1; and Darpp-32.
Western blotting
Protein measurements were conducted for DRD1, DRD5, MAOA, DARPP32 and DARPP32 phosphorylation at multiple sites. The striatum of mouse brain was homogenized in RIPA buffer (Sigma) containing protease and phosphatase inhibitors, sonicated at 4 °C for 5 min, and centrifuged at 10,000 g for 5 min. Proteins were probed with primary antibodies for DRD1 (#sc-14001, 1:500), DRD5 (#sc-25650, 1:500) from Santa Cruz, MAOA (#B02P, 1:500, Abnova), DARPP-32 (#2302, 1:2000), Phospho-DARPP-32 at Ser97 (#3401, 1:2000), at Thr34 (#5393, 1:2000), at Thr75 (#2301, 1:2000) from Cell Signaling Technology. Bands were visualized using an enhanced chemiluminescence (ECL Prime Western Blotting Detection Reagent, GE Healthcare Life Sciences). All protein values were normalized for the corresponding values of β-actin. Relative optical density was assessed using Scanalytics image analysis software (BioRad, Hercule, CA).
Measurement of dopaminergic and serotoninergic amines by HPLC
Biogenic amine concentrations were measured by High-performance liquid chromatography with electrochemical detection (HPLC-ECD). Striatal tissue were sonicated in 0.2 ml ice cold 0.01 mM perchloric acid containing 0.01% EDTA and 60 ng 3,4-dihydroxybenzylamine (DHBA) as an internal standard. After centrifugation (15,000 × g, 30 min, 4°C), the supernatant was passed through a 0.2 μm filter. Twenty microliters of the supernatant were analyzed in the HPLC column (3 mm ×150mm C-18 RP-column, Acclaim Polar advantage II, Thermo Scientific) with detection by a dual channel Coulchem III electrochemical detector (Model 5300, ESA, Inc Chelmsford, MA, USA). The protein concentrations of tissue homogenates were measured using the BCA protein assay kit (Pierce, Rockford, IL, USA). Data were normalized to protein concentrations (ng neurotransmitters/μg protein).
Statistics
Initial analysis showed that results were normally distributed. Therefore, parametric statistical procedures were employed. Student's t test was used to analyze the differences between Toxoplasma infection and control group. A p value of less than 0.05 was considered statistically significant. Experiments were repeated at least three times with similar results. All experiments included at least triplicate samples for each group. Representative results from single experiments are presented. Statistical analyses were performed with STATA version 12 (STATA Corp LP, College Station, TX, USA).
Results
MiR-132 is upregulated in human neuroepithelioma cells infected by all three prototype Toxoplasma strains
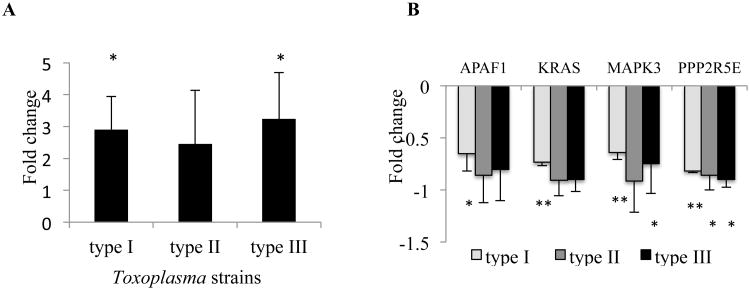
Toxoplasma isolates that have been identified in Europe and North America have been grouped as types I, II and III (Howe and Sibley, 1995). We infected human neuroepithelioma cells with a representative strain of each of the three types and used microarray analysis to investigate differences in host microRNA expression 20 h later (n = 3 per strain). Among the over 1000 miRNAs investigated, miR-132 was the only miRNA that substantially upregulated (> 2 fold change) by all three different Toxoplasma strains. To validate the miR-132 expression, the same RNA samples used for microarray were analyzed by real-time quantitative PCR. QPCR analysis confirmed an increase in the expression of miR-132 by the three main strains (2.9 + 1.0, 2.5 + 1.5, and 3.2 + 1.4 fold change for type I, II, and III, respectively, Fig. 1A), although the increase induced by type II did not achieve statistical significance.
Figure 1. Effects of Toxoplasma infection on the expression of miR-132 and its target genes determined by qPCR.
(A) MiR-132 is highly expressed in human neuroepithelioma cells 20 hours following infection with 3 canonical Toxoplasma strains. (B) Four putative miR-132 targets were downregulated in infected cells compared to controls, regardless of the parasite genotype. The fold expression of miR-132 was normalized by an endogenous control (RNU48), while the putative targets were normalized by β-actin. Each strain n = 3; error bars = 1 SD. *P < 0.05, **P < 0.01.
MiR-132 target genes were downregulated in Toxoplasma infected human cells
To identify those predicted targets of miR-132 with altered expression in cells infected by Toxoplasma, we compared the list of miR-132 targets with our previous transcriptomes of human neuroepithelioma cells (Xiao et al., 2011) using IPA. Because miR-132 is up-regulated in the infected cells, only putative miR-132 targets down-regulated in the previous dataset were considered. Four genes (APAF1, KRAS, MAPK3, and PPP2R5E) were downregulated following cellular infection with each of the three Toxoplasma lineages using these criteria. QPCR evaluation confirmed that cells infected with Toxoplasma displayed significant downregulation or a trend toward downregulation for each of these four genes, regardless of the parasite genotype (n = 3 per strain, Fig. 1B).
MiR-132 is upregulated in mice infected with Toxoplasma
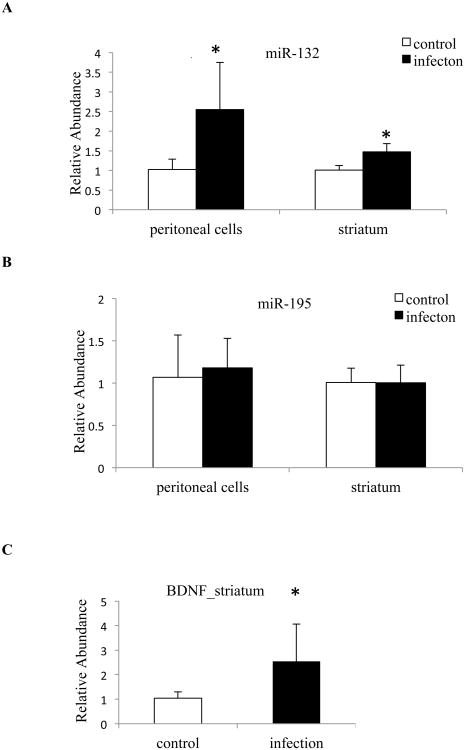
Toxoplasma infection was confirmed by DNA amplification of Toxoplasma 5S rRNA in the spleen tissues in all experimentally infected mice at 5 dpi (data not shown). QPCR results revealed that miR-132 was significantly upregulated in both peritoneal cells and striatum, at fold changes of 2.5 + 1.2 and 1.5 + 0.2, respectively (control n = 4, infection n = 5, Fig. 2A), similar to the effect seen in the human neuroepithelioma cells. This effect was specific to miR-132; miR-195 was not significantly affected by Toxoplasma infection (control n = 4, infection n = 5, Fig. 2B). In neuronal cells, brain derived neurotrophic factor (BDNF) is known to induce the transcription of miR-132 (Remenyi et al., 2010). We observed a corresponding increase of BDNF expression in the striatum of infected samples (2.5 + 1.3, control n = 4, infection n = 5, Fig. 2C).
Figure 2. Expression of miR-132, miR-195 and BDNF in mice on day 5 postinfection.
Quantitative real-time PCR analysis of (A) miR-132 and (B) miR-195 in peritoneal cells and in the striatum of mice. (C) Quantification of BDNF in the striatum. The expression of microRNA was normalized by two endogenous controls (snoRNA135 and snoRNA202), while BDNF was normalized by β-actin. Control mice: n = 4; Infected mice: n = 5; error bars = 1 SD; * < 0.05.
Downstream pathways affected by upregulated miR-132 in mice
To assess the relevance of the upregulated miR-132 in mice, we correlated its predicted target genes with transcriptomes of mouse peritoneal cells induced by Toxoplasma GT1 strain (Hill et al., 2012). This led us to identify a total of 20 target genes the expressions of which was downregulated more than 2-fold by infection (Table 1). We performed pathway analysis to identify cellular pathways enriched by the 20 selected genes. IPA identified 11 significantly enriched pathways. Dopamine receptor signaling, regulation of eIF4 and p70S6K signaling, EIF2 signaling, mTOR signaling, and ERK/MAPK signaling are the top 5 enriched pathways (Table 2).
Table 1. 20 predicted targets of upregulated miR-132 in mice at day 5 postinfection with Toxoplasma GT1 strain.
| Gene | FC | Gene | FC | Gene | FC | Gene | FC |
|---|---|---|---|---|---|---|---|
| ARID4B | -2.0 | CCDC117 | -2.2 | CENPQ | -4.1 | CRK | -2.3 |
| EDNRA | -2.4 | EIF4A2 | -2.7 | FAM76B | -2.4 | FOXN3 | -2.2 |
| GMFB | -2.0 | LEMD3 | -2.2 | MANEA | -2.0 | MAOA | -2.6 |
| PPP2R5C | -2.6 | RPL13A | -2.8 | SAP30L | -2.0 | SDF2 | -2.0 |
| SLC12A6 | -3.1 | SMAD5 | -2.6 | TIMM9 | -2.3 | ZNF644 | -2.1 |
FC: fold change
Table 2. Top five canonical pathways from ingenuity Pathway Analysis calculated using 20 downregulated targets of miR-132.
| Canonical pathways | p-value a | Ratiob | Genesc | Function |
|---|---|---|---|---|
| Dopamine Receptor Signaling | 2.97E-03 | 0.021 | PPP2R5C, MAOA | Important to vital brain functions like motor control and short term memory |
| Regulation of eIF4 and p70S6K Signaling | 9.54E-03 | 0.012 | PPP2R5C, EIF4A2 | Play critical roles in translational regulation |
| EIF2 Signaling | 1.22E-02 | 0.010 | EIF4A2, RPL13A | Important in the initiation phase of protein synthesis |
| mTOR Signaling | 1.51E-02 | 0.010 | PPP2R5C, EIF4A2 | Involved in cell survival and proliferation |
| ERK/MAPK Signaling | 1.65E-02 | 0.010 | PPP2R5C, CRK | Transducing cellular information on meiosis/mitosis, growth, differentiation and carcinogenesis within a cell |
the p-value denotes the significance of the enrichment of a function within the 20 downregulated targets of miR-132;
ratio is the number of genes from the 20 targets of miR-132 that map to the pathway divided by the total number of genes in a given pathway;
genes is the name of altered targets in the pathway.
mRNA expression of genes involved in dopamine receptor signaling
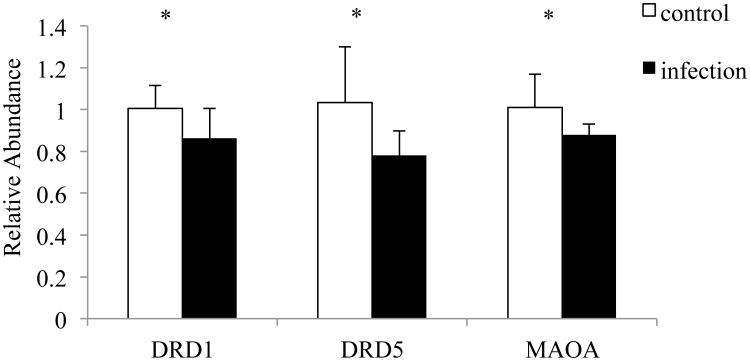
Since dopamine receptor signaling is the strongest finding within all the enriched pathways, we measured this pathway in the striatum of mouse brain since striatum has the highest density of dopamine projections in the brain. Figure 3 depicts mRNA expression levels for 3 genes involved in the dopamine receptor pathway: Drd1, Drd5 and Maoa. These genes showed a general pattern of decrease in Toxoplasma-infected mice (control n = 7, infection n = 8). There were no significant differences in gene expression for Drd2, Drd3, Drd4, Calcyon, Darpp-32, Maob and Comt (data not shown).
Figure 3. Three genes involved in dopamine pathway are downregulated in mice at day 5 postinfection.
The gene expression was normalized by β-actin. Control mice: n = 7; Infected mice: n = 8; error bars = 1 SD; * < 0.05.
Protein expression
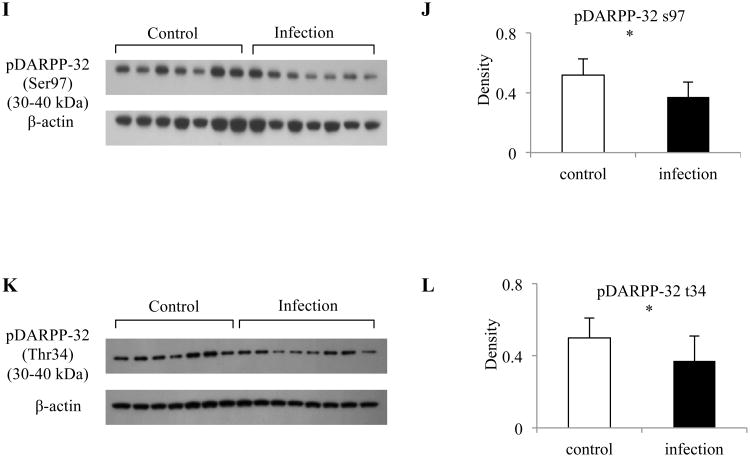
To test whether gene expression changes were accompanied by changes of the corresponding proteins, we determined protein levels for DRD1, DRD5 and MAOA. Western blot analyses revealed that the expression of DRD1, DRD5 and MAOA corresponded to the results obtained by mRNA qPCR. We found that Toxoplasma infection led to an approximately 44% reduction in DRD1, 37% reduction in DRD5, and 55% reduction in MAOA expression in the striatum of mouse brain compared to mock infected controls (control n = 6 - 7, infection n = 6 - 7, Fig. 4A-F). DARPP-32 function depends on its relative state of phosphorylation and activation by dopamine D1 receptors stimulates the phosphorylation of DARPP-32 at Thr34. We therefore measured the phosphorylation levels of DARPP-32 at 3 sites. Although the total amount of DARPP32 did not change (control n = 7, infection n = 8, Fig. 4G, H), we found a significantly reduced phosphorylation of DARPP-32 at both Ser 97 (28% lower, Fig.4I, J) and Thr34 (25% lower, Fig. 4K, L) in infected mice (control n = 7, infection n = 7). There was no significant difference for DARPP-32 phosphorylation at Thr75 in infected mice (data not shown).
Figure 4. Reduced expression of several proteins involved in dopamine pathway in mouse striatum at day 5 postinfection.
Protein expression of (A) DRD1; (C) DRD5; (E) MAOA; (I) DARPP-32 Phosphorylation at Ser97; and (K) DARPP-32 Phosphorylation at Thr34 between control and infected mice. Quantification of normalized level of (B) DRD1; (D) DRD5; (F) MAOA; (J) DARPP-32 Phosphorylation at Ser97; and (L) DARPP-32 Phosphorylation at Thr34 between control and infected mice. (G) Total DARPP-32 expression is comparable between control and infected mice. (H) Quantification of normalized level of DARPP-32 shown in (G). Protein levels were compared to β-actin. Control mice: n = 6 - 7; Infected mice: n = 6 - 8; Error bars = 1 SD. * < 0.05, ** < 0.01.
Altered dopamine metabolism in Toxoplasma-infected mice
HPLC data revealed that Toxoplasma infection produced a 38% increase in striatal dopamine levels. The dopamine metabolites: DOPAC was elevated by 24%, 3-MT was elevated by 36% and HVA was 19% in the infected group compared to controls. The HVA/dopamine and HVA/3-MT ratios were reduced by 14% and 15%, respectively, in the infected mice compared to controls (control n = 19, infection n = 20, Table 3).
Table 3. Profile of dopamine and serotonin metabolites in the mouse striatum at day 5 postinfection with Toxoplasma GT1 strain.
| DA | DOPAC | HVA | 3-MT | 5-HT | 5-HIAA | HVA/DA | HVA/3-MT | |
|---|---|---|---|---|---|---|---|---|
| Control | 1110+461 | 543+50 | 190+8.7 | 211+11 | 305+23 | 80+3.5 | 0.173+0.008 | 0.924+0.05 |
| Infection | 1539+88 | 672+48 | 226+11 | 287+14 | 368+21 | 100+6.8 | 0.150+0.006 | 0.792+0.02 |
| P value2 | <0.001 | 0.036 | 0.008 | <0.001 | 0.029 | 0.006 | 0.009 | 0.007 |
| % Change3 | 38% | 24% | 19% | 36% | 20% | 25% | -14% | -15% |
Expressed as nanograms per microgram protein, mean + S.E.M.;
Derived from a one-tailed Student's t-test;
Percentage change from controls. Control: n = 19; Infection: n = 20
We found 5-HT levels were 20% higher and 5-HIAA was 25% higher in the striatum of infected mice compared to uninfected controls but the ratio of 5-HIAA to 5-HT remained constant (control n = 19, infection n = 20, Table 3).
Discussion
Although the neurological consequences of congenital toxoplasmosis and toxoplasmic encephalitis are well documented, their exact mechanisms remain incompletely understood. In this study, we reported that acute infection with Toxoplasma induces the level of host miR-132 and this induction is associated with altered dopamine pathway in infected mice by repressing the expression of relevant proteins. Our findings provide a possible mechanism for how these parasites contribute to the neuropathology of infection and may shed light on how they manipulate their hosts. Furthermore, miR-132 may itself be a potential therapeutic target that can be exploited in the future.
MiR-132 can be upregulated by exposure to several toll like receptor (TLR) ligands such as lipopolysaccharides (LPS) and some viruses (Taganov et al., 2006; Lagos et al., 2010). KSHV (Kaposi's sarcoma-associated herpesvirus) infections of endothelial cells, as well as HSV-1 (herpes simplex virus-1) or HCMV (human cytomegalovirus) infection of monocytes, have been observed to induce this upregulation (Lagos et al., 2010). Such responses are likely to represent innate reactions to the virus, rather than being responses to virus gene expression. In our studies, we noted that infection with three major types of Toxoplasma resulted in a similar upregulation of miR-132 in human neuroepithelioma cells, suggesting this induction is not restricted to parasite genotype. An explanation for this observation is that several TLRs including TLR2, TLR4, and TLR11 are known to bind Toxoplasma-derived factors (Blader and Saeij, 2009). Our data, along with the published reports of others, indicate that miR-132 is a common target of a broad range of pathogens. It is also intriguing to speculate whether the implication of miR-132 upregulation on dopamine pathways is a general response of inflammatory stimulus-infected host cells or a specific response to Toxoplasma infection. In future studies it will therefore be important to address this by employing other inflammatory stimuli (e.g. LPS and other pathogens) in this mouse model.
Our finding that downregulation of four genes (APAF1, KRAS, MAPK3, and PPP2R5E) associated with mir-132 in human neuroepithelioma cells is of interest in light of the functions of these genes, although the extent of downregulation differed among the three Toxoplasma strains. These discrepancies might be related to differences in cellular tropism and virulence, viability of tachyzoites, and differential growth rates among the three strains. APAF1 plays a central role in the apoptotic process (Zou et al., 1997). KRAS performs an essential function in normal tissue signaling, and the mutation of a KRAS gene is an essential step in the development of many cancers (Kranenburg, 2005). MAPK3 and PPP2R5E are involved in phosphorylation and hence may have important roles in control of wide range of cellular processes in the infected cell (McCright and Virshup, 1995; Saxena et al., 1999). Additional experiment should be directed at defining the implications of altering these genes by Toxoplasma infection.
MiR-132 was recently classified as a “neurimmiR”, a class of miRNAs regulating both neuronal and immune functions and was suggested to function in the “cross-talk” between both systems (Soreq and Wolf, 2011). Indeed, we observed the increased expression of miR-132 not only in the striatum but also in the peritoneal cells of Toxoplasma-infected mice. Since the peritoneal cell population of Toxoplasma-infected mice contains macrophages, neutrophils and dendritic cells (Hill et al., 2012), miR-132 has a potential role in the immune response to this parasite. This possibility merits further investigation. Similarly, although the miR-132-mediated dopamine receptor pathway was first defined in terms of the transcriptome of mouse peritoneal cells, changes in this pathway have also been demonstrated in the striatum of mouse brain. Our results support the possible association of miR-132 with both neuronal and inflammatory processes.
Apart from the effects of miR-132 on infection and inflammation, the majority of miR-132 functions were described in a neuronal context. MiR-132 has been linked to neuronal differentiation, neurogenesis, neuronal outgrowth and sprouting and synaptic plasticity, as well as spine density (Hansen et al., 2010; Impey et al., 2010; Magill et al., 2010). Regarding the involvement of miR-132 in neuronal processes, its dysregulation is associated with several brain-related disorders (Miller et al., 2012; Wanet et al., 2012). It is notable that miR-132 was recently shown to regulate the differentiation of dopamine neurons from mouse embryonic stem cells by targeting the dopaminergic transcription factor Nurr1 (Yang et al., 2012). In the current study, we propose that Toxoplasma-induced upregulation of miR-132 is associated with changes in dopamine receptor pathway. This finding may have important implications for understanding some neuropsychiatric symptoms (e.g. mental impairment such as learning, motor disabilities and personality changes) that have been observed to occur in congenital toxoplasmosis and toxoplasmic encephalitis.
Dopamine exerts its effects on neurons through dopamine receptors. In Toxoplasma-infected mice, we demonstrated by real-time PCR that the D1-like receptor subtypes (D1 and D5) have decreased expression, and that these differences are maintained at the protein level. These results are in agreement with our previous finding showing that Toxoplasma type I infection decreased the expression of DRD1 in human neural cells (Xiao et al., 2013). We did not find changes in D2-like receptors. Since D1 receptors are involved in the negative feedback regulation of dopamine release in the brain (Saklayen et al., 2004), an increased level of dopamine might be expected. In support of this, we found that dopamine levels were elevated by 38% in the infected mice. This finding is consistent with previous in vitro study using neural cells (Prandovszky et al., 2011). This increased dopamine level may suggest an increase in the rate of dopamine biosynthesis, since a previous study has found that Toxoplasma can potentially supply a rate-limiting enzyme, tyrosine hydroxylase, in dopamine synthesis (Gaskell et al., 2009). It would be interesting to measure levels of tyrosine hydroxylase and L-DOPA in Toxoplasma infected samples in future studies.
DARPP-32 was identified as a major target for dopamine-activated adenylyl cyclase in the striatum and DARPP-32 function depends on its relative state of phosphorylation. We found that Toxoplasma infection induced a decrease in the phosphorylation of DARPP-32 at Thr34 and Ser97 within the striatum of mouse brain, although the total amount of DARPP-32 did not change. There was also no effect of Toxoplasma infection on DARPP-32 phosphorylation at Thr75. In striatonigral neurons, activation by dopamine D1 receptors stimulates the phosphorylation of DARPP-32 at Thr34. Therefore, the observed decreased in D1 receptors may be responsible for the decreased phosphorylation of DARPP-32 at Thr34. Moreover, it is known that Ser97 phosphorylation can increase the efficiency of phosphorylation of Thr34 by PKA (Svenningsson et al., 2004). We observed decreased phosphorylation at Ser97 in the infected mice, which suggests Toxoplasma is affecting DARPP-32 phosphorylation at Thr34 through a general strategy to control Ser97. When DARPP-32 is phosphorylated at Thr34, it is converted into a potent inhibitor of PP1 and thereby controls the phosphorylation state and activity of many downstream physiological effectors. This reduction in DARPP-32 phosphorylation at Thr34 would be likely to remove the inhibition of PP1 (Nairn et al., 2004; Svenningsson et al., 2004).
A significant elevation in the concentrations of dopamine and its metabolites, serotonin and 5-hydroxyindoleacetic acid was seen in the striatum of the infected mice. Similarly, previous observations made by Stibbs (1985) suggested a 40% rise in HVA levels during acute infection. However, when measuring the dopamine-metabolism (expressed as the ratio between HVA/dopamine), we found a significant decrease (14%), a finding consistent with the report of Gatkowska et al. (2013) using acutely infected female mice. The less robust increase in levels of HVA (19%) is responsible for the decreased ratio, as dopamine was found to be increased by 38%. HVA is a product of intra- and extra-neuronal metabolism of released dopamine. DOPAC primarily reflects intra-neuronal metabolism of dopamine, while 3-MT is the unique extracellular metabolite of dopamine. We observed an equivalent increases between 3-MT (36%) and dopamine (38%) suggesting that dopamine release mediated by COMT is normal, as supported by no change being seen at mRNA level of COMT. Thus, it is tempting to speculate that the less robust increase in HVA (19%) was caused by inefficient intraneuronal metabolism mediated through MAO. In support of this, we found that MAOA was decreased at both protein and mRNA levels in infected mice, a finding consistent with our previous reports using human neural cells (Xiao et al., 2013). In contrast to the decreased dopamine metabolism, the 5-HT metabolism (measured as 5-HIAA/5-HT ratio) remains constant, although MAOA preferentially oxidizes serotonin (Fowler et al., 2002). The selective effect of MAOA in the HVA pathway in Toxoplasma-infected mice is not known but should be the subject of additional investigations.
Although the molecular mechanisms underlying this finding are not known, there are several possibilities by which this may occur: (i) the cells in the striatum are unlikely infected by parasites at 5 dpi, so the effects in mice may be due to inflammatory responses. These could induce miR-132 and alter downstream dopamine as dopamine signaling is now known to occur in lymphocytes (Ilani et al., 2001; Taganov et al., 2006). Since both miR-132 and dopamine are “shared” by neuronal and immune processes, the changes observed in the striatum could due to reciprocal interplay between the body and brain; (ii) dopamine was recently suggested to be a beneficial factor in Toxoplasma multiplication in primary neonatal rat astrocyte cells (Strobl et al., 2012), which may explain the specific influence of infection on dopamine signaling. Certainly, further and more extensive studies are required and will be performed to strengthen and specify the data presented in this study. Moreover, the current finding underlines the need for a thorough study for the role of dopamine in the chronic stage of infection, because the majority of people infected with the parasite are in the chronic stage in which the parasite is largely present in the form of tissue cyst. Future studies with different intermediate host species in both sexes, including humans, should further elucidate the role of dopamine system on behavioral changes induced by Toxoplasma.
Acknowledgments
This work was supported by the Stanley Medical Research Institute and by a National Institute of Mental Health (NIMH) P50 Silvio O. Conte Center at Johns Hopkins (grant# MH094268). The authors thank Ms. Claudia Bordón for technical assistance for the in vitro experiments.
Abbreviations
- miRNA
MicroRNA
- BDNF
brain-derived neurotrophic factor
- DOPAC
3,4-dihydroxyphenylacetic acid
- 3-MT
3-Methoxytyramine
- HVA
homovanilic acid
- 5-HT
serotonin
- 5-HIAA
5-hydroxyindoleacetic acid
- MAOA
monoamine oxidase A
- MAOB
monoamine oxidase B
- COMT
catechol-O-methyltransferase
- DRD1
dopamine receptor D1
- DRD5
dopamine receptor D5
- DARPP-32
dopamine- and cAMP-regulated neuronal phosphoprotein of molecular mass of 32 kDa
- qPCR
quantitative real-time PCR
- HPLC
High-performance liquid chromatography
- IPA
Ingenuity Pathway Analysis
- dpi
days post infection
- PP1
protein phosphatase 1
References
- Barnes EN, Biedler JL, Spengler BA, Lyser KM. The fine structure of continuous human neuroblastoma lines SK-N-SH, SK-N-BE(2), and SK-N-MC. In Vitro. 1981;17:619–631. doi: 10.1007/BF02618461. [DOI] [PubMed] [Google Scholar]
- Blader IJ, Saeij JP. Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and virulence. APMIS. 2009;117:458–476. doi: 10.1111/j.1600-0463.2009.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, Obrietan K. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–829. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Volkow ND, Wang GJ, MacGregor RR, Ding YS. Monoamine oxidase: radiotracer development and human studies. Methods. 2002;27:263–277. doi: 10.1016/s1046-2023(02)00083-x. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One. 2009;4:e4801. doi: 10.1371/journal.pone.0004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatkowska J, Wieczorek M, Dziadek B, Dzitko K, Dlugonska H. Sex-dependent neurotransmitter level changes in brains of Toxoplasma gondii infected mice. Exp Parasitol. 2013;133:1–7. doi: 10.1016/j.exppara.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Hansen KF, Sakamoto K, Wayman GA, Impey S, Obrietan K. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS One. 2010;5:e15497. doi: 10.1371/journal.pone.0015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RD, Gouffon JS, Saxton AM, Su C. Differential gene expression in mice infected with distinct Toxoplasma strains. Infect Immun. 2012;80:968–974. doi: 10.1128/IAI.05421-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- Ilani T, Ben-Shachar D, Strous RD, Mazor M, Sheinkman A, Kotler M, Fuchs S. A peripheral marker for schizophrenia: Increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proc Natl Acad Sci U S A. 2001;98:625–628. doi: 10.1073/pnas.021535398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Davare M, Lesiak A, Fortin D, Ando H, Varlamova O, Obrietan K, Soderling TR, Goodman RH, Wayman GA. An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol Cell Neurosci. 2010;43:146–156. doi: 10.1016/j.mcn.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotowski SJ, Hopf FW, Seif T, Bonci A, von Zastrow M. Endocytosis promotes rapid dopaminergic signaling. Neuron. 2011;71:278–290. doi: 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenburg O. The KRAS oncogene: past, present, and future. Biochim Biophys Acta. 2005;1756:81–82. doi: 10.1016/j.bbcan.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9:1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, Milne RS, Gotch F, Boshoff C. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat Cell Biol. 2010;12:513–519. doi: 10.1038/ncb2054. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci U S A. 2010;107:20382–20387. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCright B, Virshup DM. Identification of a new family of protein phosphatase 2A regulatory subunits. J Biol Chem. 1995;270:26123–26128. doi: 10.1074/jbc.270.44.26123. [DOI] [PubMed] [Google Scholar]
- Miller BH, Zeier Z, Xi L, Lanz TA, Deng S, Strathmann J, Willoughby D, Kenny PJ, Elsworth JD, Lawrence MS, Roth RH, Edbauer D, Kleiman RJ, Wahlestedt C. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc Natl Acad Sci U S A. 2012;109:3125–3130. doi: 10.1073/pnas.1113793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault JA, Greengard P. The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology. 2004;47:14–23. doi: 10.1016/j.neuropharm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One. 2011;6:e23866. doi: 10.1371/journal.pone.0023866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenyi J, Hunter CJ, Cole C, Ando H, Impey S, Monk CE, Martin KJ, Barton GJ, Hutvagner G, Arthur JS. Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem J. 2010;428:281–291. doi: 10.1042/BJ20100024. [DOI] [PubMed] [Google Scholar]
- Saklayen SS, Mabrouk OS, Pehek EA. Negative feedback regulation of nigrostriatal dopamine release: mediation by striatal D1 receptors. J Pharmacol Exp Ther. 2004;311:342–348. doi: 10.1124/jpet.104.067991. [DOI] [PubMed] [Google Scholar]
- Saxena M, Williams S, Taskén K, Mustelin T. Crosstalk between cAMP-dependent kinase and MAP kinase through a protein tyrosine phosphatase. Nat Cell Biol. 1999;1:305–311. doi: 10.1038/13024. [DOI] [PubMed] [Google Scholar]
- Skallová A, Kodym P, Frynta D, Flegr J. The role of dopamine in Toxoplasma-induced behavioural alterations in mice: an ethological and ethopharmacological study. Parasitology. 2006;133:525–535. doi: 10.1017/S0031182006000886. [DOI] [PubMed] [Google Scholar]
- Soreq H, Wolf Y. NeurimmiRs: microRNAs in the neuroimmune interface. Trends Mol Med. 2011;17:548–555. doi: 10.1016/j.molmed.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Stibbs HH. Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Ann Trop Med Parasitol. 1985;79:153–157. doi: 10.1080/00034983.1985.11811902. [DOI] [PubMed] [Google Scholar]
- Strobl JS, Goodwin DG, Rzigalinski BA, Lindsay DS. Dopamine stimulates propagation of Toxoplasma gondii tachyzoites in human fibroblast and primary neonatal rat astrocyte cell cultures. J Parasitol. 2012;98:1296–1299. doi: 10.1645/GE-2760.1. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Sapolsky R. Manipulation of host behaviour by Toxoplasma gondii: what is the minimum a proposed proximate mechanism should explain? Folia Parasitol (Praha) 2010;57:88–94. doi: 10.14411/fp.2010.011. [DOI] [PubMed] [Google Scholar]
- Wanet A, Tacheny A, Arnould T, Renard P. miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res. 2012;40:4742–4753. doi: 10.1093/nar/gks151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JP, Kaushik M, Bristow GC, McConkey GA. Toxoplasma gondii infection, from predation to schizophrenia: can animal behaviour help us understand human behaviour? J Exp Biol. 2013;216:99–112. doi: 10.1242/jeb.074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JP, Lamberton PH, Donnelly CA, Torrey EF. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii's ability to alter host behaviour. Proc Biol Sci. 2006;273:1023–1030. doi: 10.1098/rspb.2005.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao JC, Jones-Brando L, Talbot CC, Jr, Yolken R. Differential Effects of Three Canonical Toxoplasma Strains on Gene Expression in Human Neuroepithelial Cells. Infect Immun. 2011;79:1363–1373. doi: 10.1128/IAI.00947-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Li Y, Jones-Brando L, Yolken RH. Abnormalities of neurotransmitter and neuropeptide systems in human neuroepithelioma cells infected by three Toxoplasma strains. J Neural Transm. 2013;120:1631–1639. doi: 10.1007/s00702-013-1064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Li T, Wang Y, Tang Y, Cui H, Tang Y, Zhang X, Chen D, Shen N, Le W. miR-132 regulates the differentiation of dopamine neurons by directly targeting Nurr1 expression. J Cell Sci. 2012;125:1673–1682. doi: 10.1242/jcs.086421. [DOI] [PubMed] [Google Scholar]
- Zeiner GM, Norman KL, Thomson JM, Hammond SM, Boothroyd JC. Toxoplasma gondii infection specifically increases the levels of key host microRNAs. PLoS One. 2010;5:e8742. doi: 10.1371/journal.pone.0008742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]