Mitochondrial Dysfunction in Aging-Associated Neurologic Disorders
Mitochondrial bioenergetic dysfunction is a key factor in the pathophysiology of neurologic disorders and diseases associated with aging, including stroke, Alzheimer’s and Parkinson’s diseases, and amyotrophic lateral sclerosis (ALS) (Soane et al., 2007; Starkov et al., 2004; Fiskum et al., 2008; Nicholls, 2009). The consequences of this dysfunction include oxidative stress, loss of cellular calcium homeostasis, promotion of apoptosis and metabolic failure (Fiskum et al., 2008; Robertson et al., 2009). Considering the multiple mechanisms by which mitochondrial impairment can lead to the death of brain cells, many experimental neuroprotective interventions have targeted mitochondria. A much better understanding of the effects of aging on the mitochondrial contribution to neuropathology is needed, however, for translation of preclinical findings to clinical implementation. One mechanism of cell death that has been implicated in many aging-related neurologic disorders is the mitochondrial inner membrane permeability transition (MPT). Thousands of articles about the MPT have been published but very few studies have investigated the effects of aging on this activity.
Mitochondrial Inner Membrane Permeability Transition
The MPT is defined as the sudden increase of inner mitochondrial membrane permeability to solutes of molecular mass less than 1,500 Daltons that is elicited in response to exposure to abnormally high levels of Ca2+ (Sullivan et al., 2005; Mazzeo et al., 2009; Soane et al., 2007). This transition is due to the opening of a non-selective mega-channel called the mitochondrial permeability transition pore (PTP) (Sullivan et al., 2005; Soane et al., 2007).
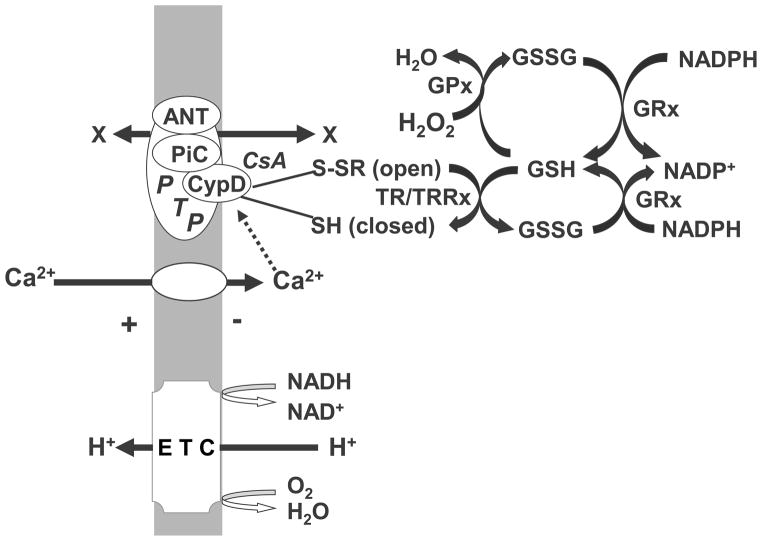
The actual protein that constitutes the pore has not been identified. Pore opening, or activation, appears to result from interactions between several different mitochondrial proteins, possibly including the adenine nucleotide translocase (ANT), cyclophilin D (CypD), the mitochondrial phosphate carrier (PiC), and other polypeptides (Figure 1) (Mazzeo et al., 2009; Bazil et al., 2010). CypD, a cis-proline isomerase, plays a crucial role in protein folding and is the only protein that definitely plays an important role in regulating PTP opening, as demonstrated with CypD knockout animals (Nakagawa et al., 2005). CypD is activated by elevated intramitochondrial Ca2+ and may also act as a redox sensor, promoting PTP opening when conditions favor oxidation of protein sulfhydryl groups (Tsujimoto and Shimizu, 2007; Linard et al., 2009). CypD is located in the mitochondrial matrix when the PTP is closed. Conditions that promote PTP opening result in the association of CypD with the mitochondrial inner membrane with conformational changes of the ANT (Mazzeo et al., 2009). CypD is also the target for specific cyclophilin drugs, e.g., cyclosporin A, which are effective at inhibiting PTP opening under many but not all conditions. While the prevailing dogma is that an altered conformation form of the ANT actually constitutes the PTP, recent evidence suggests that the PiC may actually constitute the pore or is at least as important as the ANT in regulating PTP opening (Halestrap, 2010).
Figure 1. Proteins associated with mitochondrial permeability transition pore and its regulation by mitochondrial redox state.
Electrogenic efflux of proteins driven by redox energy released from the mitochondrial electron transport chain (ETC) generate a positive outside membrane potential which drives the electrophoretic influx of extramitochondrial Ca2+ into the mitochondrial matrix. Excessive influx activates the permeability transition pore (PTP), mediated by binding of Ca2+ to cyclophilin D (CypD), which is also the site of PTP inhibition by the drug cyclosporin A (CsA). Other proteins that either mediate or regulate pore formation include the adenine nucleotide translocase (ANT) and the phosphate carrier (PiC). Pore opening is sensitive to mitochondrial redox state possibly via the sulfhydryl redox state of cysteines present on either CyD or ANT. Mitochondrial protein sulfhydryls are maintained in a reduced state through both the thioredoxin/ thioredoxin reductase system (TR/TRRx) or by the glutaredoxin/glutaredoxin reductase system. Either system is dependent on reduced glutathione (GSH), which is kept in a reduced redox state by the NADPH-dependent glutathione reductase (GRx). Oxidative stress can promote PTP opening by shifting mitochondrial glutathione redox state to a more oxidized level via the metabolism of peroxides by glutathione peroxidase (GPx).
Induction of the MPT results in increased conductance of the inner mitochondrial membrane and subsequent collapse of the electrochemical gradient of protons, causing uncoupling of oxidative phosphorylation and release of accumulated intramitochondrial Ca2+ (Rasola et al., 2010). Pore opening can also lead to osmotic swelling, due to the net influx of ions like K+, which is attracted by the exceptionally high concentration of net negatively charged protein present in the mitochondrial matrix. While the mitochondrial matrix space can increase dramatically due to expansion of the convoluted inner membrane, the relatively inflexible outer membrane eventually ruptures, releasing intermembrane proteins, e.g., cytochrome c, present within the inner and outer membranes. Loss of cytochrome c and, or loss of matrix NAD(H) directly through the PTP can cause respiratory inhibition in addition to the uncoupling associated with the flux of protons and other ions through the PTP (Sullivan et al., 2005; Fiskum et al., 2004; Robertson et al., 2009; Mazzeo et al., 2009; Bazil et al., 2010).
Promotion of Mitochondrial Permeability Transition by Oxidative Stress
Oxidative stress is caused by an imbalance between production of reactive oxygen species (ROS) or reactive nitrogen species (RNS) and their detoxification (Wang and Michaelis, 2010). While greater than 99% of the O2 consumed by metabolically active mitochondria is reduced to H2O, up to 1% can be reduced by a one electron transfer producing superoxide anion radical (Kowaltowski et al., 2009).. Superoxide normally either reacts (dismutates) with itself forming hydrogen peroxide (H2O2) or reacts with nitric oxide radical, forming peroxynitrite anion (ONOO−). As shown in Figure 1, metabolism of hydrogen peroxide and other peroxides via mitochondrial glutathione peroxidase and reductase results in oxidation of reduced glutathione. If this oxidation is not matched by NADPH-dependent reduction of oxidized glutathione, then thermodynamically favorable oxidation of protein cysteine sulfhydryl groups occurs due to the lack of their reduction via the thioredoxin / thioredoxin reductase system and possibly other enzymes. Moreover, H2O2 can be reduced by ferrous iron, which is abundant in mitochondria, producing hydroxyl radial, which is possibly the most directly damaging of all biological free radicals reacting with many protein amino acids, DNA and RNA and unsaturated fatty acyl groups. A similar spectrum of targets is exhibited by peroxynitrite and its metabolite nitrogen dioxide, which are best known for generating nitrotyrosines and nitrosocysteines on proteins, including many important mitochondrial metabolic enzymes (Mather and Rottenberg, 2000; Sullivan et al., 2005; Wang and Michaelis, 2010; Fiskum et al., 2004), but also react with unsaturated fatty acids forming bioactive nitro-fatty acids (Groeger and Freeman, 2010).
The probability of mitochondrial PTP opening is modulated both positively and negatively by many factors (Soane et al., 2007). Oxidative stress and abnormally high levels of intramitochondrial Ca2+ are probably the two most important activators (Robertson et al., 2009; Mather and Rottenberg, 2000; Lemasters et al., 2009; Gunter and Pfeiffer, 1990; Halestrap, 2010). These two factors interact synergistically, as reflected by the fact that much lower levels of Ca2+ are required to activate the PTP when mitochondria are oxidatively stressed by exposure to peroxides (Fiskum, 2000). While most studies point toward oxidation of sulfhydryl groups on either CypD or the ANT as promoters of Ca2+-activated MPT, one new study indicates that S-nitrosylation by nitric oxide or one of its metabolites actually inhibits PTP opening (Leite et al., 2010). More work is clearly necessary to determine precisely which sulfhydryl groups on which proteins control PTP opening and what type of oxidative modifications either increase or decrease sensitivity to pore opening.
Inhibition of Mitochondrial Permeability Transition by Bcl-2 Family Proteins
The Bcl-2 family of anti-death proteins is best known for the ability to inhibit mitochondrial-dependent apoptosis, also known as the intrinsic pathway of apoptosis. The simplest explanation for this inhibition is that these proteins heterodimerize with either pro-apoptotic proteins, e.g., Bax, and Bak, or with apoptotic accessory proteins, e.g., truncated Bid and Bim, thereby interfering with their interactions with each other. The interaction among the pro-apoptotic proteins can result in polypeptide oligomerization and megapore formation in the mitochondrial outer membrane, allowing for the release of other pro-apoptotic proteins, e.g., cytochrome c and AIF, from the mitochondria into the cytosol, where they lead to either caspase-dependent or caspase-independent apoptosis, respectively (Kuwana et al., 2002). Bcl-2 and related proteins also protect against necrotic death, possibly by mechanisms unrelated to Bax or Bak-mediated outer membrane pore formation (Soane et al., 2007). One possible mechanism is inhibition of PTP opening. Overexpression of Bcl-2 family proteins, including Bcl-2, Bcl-xl, and Mcl-1, in cell lines inhibits CsA-sensitive mitochondrial permeability transition induced by Ca2+ plus exposure to peroxide (Kowaltowski et al., 2004). This resistance to peroxide-induced PTP opening is associated with resistance to oxidation of NAD(P)H by peroxide (Kowaltowski et al., 2000). While the mechanism by which Bcl-2 and related proteins confers resistance to both peroxide-induced pyridine nucleotide oxidation and Ca2+ release has not been identified, it may be related to the effects of these proteins on mitochondrial production of reactive O2 species. Surprisingly, overexpression of Bcl-2, Bcl-xL, or Mcl-1 all result in a higher level of respiration-dependent mitochondrial ROS (H2O2) production (Kowaltowski et al., 2004). This small but significant increase is accompanied, however, by a large increase in maximal peroxidase activity, which is likely to be responsible for both the resistance to peroxide-induced NAD(P)H oxidation and Ca2+ release by mitochondria from Bcl-2 family overexpressors.
The reason why a small increase in mitochondrial ROS production may lower the sensitivity to oxidative stress, including that which triggers PTP opening, may relate to the control over antioxidant gene expression exerted by the transcriptional activating factor Nrf2. This protein is released from its cytosolic binding partner, KEAP1, when specific cysteine sulfhydryl groups on KEAP1 are oxidized by reaction with electrophiles, e.g., ROS and organic chemicals like the isothiocyanate sulforaphane (Greco and Fiskum, 2010a). Following serine phosphorylation, Nrf2 then translocates to the nucleus where it binds to antioxidant response elements (AREs) and promotes the expression of possibly 100 different genes including antioxidant enzymes like glutathione peroxidase (Kaspar et al., 2009). Thus, the increase in mitochondrial ROS production which accompanies Bcl-2 overexpression may act as a mild stressor and therefore a preconditioning stimulus for expression of antioxidant enzymes including those that provide resistance to redox-regulated PTP opening (Greco and Fiskum, 2010a). This explanation might also explain some of the very early reported effects of Bcl-2 on cellular redox state and resistance to cell death caused by oxidative stress. For instance, Ellerby et al. reported that the expression of Bcl-2 causes a shift in glutathione redox state to a more reduced level, as evidenced by an increase in [GSH] / [GSSH+GSH] ratio, enhancement of protein free thiol groups, and an increased ratio of reduced to oxidized pyridine nucleotides (Ellerby et al., 1996). This shift to a more reduced cellular environment should inhibit PTP opening and also confer resistance to other targets of oxidative stress. It is possible, however, that Bcl-2 could also have direct effects on PTP opening mediated through protein-protein interactions. Cyclosporin A can enhance the ability of Bcl-2 to inhibit truncated Bid-induced cytochrome c release by mitochondria (Eliseev et al., 2009). While this effect is not mediated by PTP opening, the additional fact that Bcl-2 co-immunoprecipitates with cyclophilin D suggests that Bcl-2 could directly inhibit PTP opening, at least under some conditions. Such direct interaction of both Bcl-2 and pro-apoptotic proteins like Bax with PTP components resulting in PTP regulation was also proposed by Narita et al (Narita et al., 1998).
Effects of Aging on Mitochondrial Permeability Transition
The vast majority of research on mitochondrial PTP has used tissue from young adult animals or cultured cells, while very little information about the effects of aging on sensitivity or regulation of PTP opening is available. Mather and Rottenberg demonstrated enhanced susceptibility to PTP activation with excessive Ca2+ uptake by mitochondria isolated from the brains and livers of 20 month old compared to 3 month old mice (Mather and Rottenberg, 2000). They also obtained evidence for similar enhanced sensitivity to PTP opening in intact thymocytes from aged mice, indicating that this effect can be observed under true intracellular conditions. Brown et al observed increased Ca2+-induced osmotic swelling and ROS production in mitochondria isolated from the cerebral cortex or hippocampi but not from cerebella from 25 month old compared to 4 or 13 month old rats; however, no experiments were performed with cyclosporin A or other conditions that would directly implicate PTP activity (Brown et al., 2004). Similarly, LaFrance reported enhanced sensitivity to Ca2+-induced PTP in cortical mitochondria but not striatal mitochondria from 32 month old compared to 24 month old or younger rats (LaFrance et al., 2005).
Evidence also indicates increased PTP activity with aging in mitochondria from tissues other than the brain. For instance, the Ca2+ uptake capacity and the ability to maintain membrane potential during Ca2+ uptake are lower in mitochondria from the hearts of 25 month old compared to 5 month old rats (Petrosillo et al., 2010). This aging effect is associated with increased oxidation of cardiolipin, a mitochondrial inner membrane-selective phospholipid involved in regulating PTP opening. Moreover, treatment of rats with the antioxidant melatonin inhibits both loss of Ca2+ uptake capacity and cardiolipin oxidation, suggesting that age-related mitochondrial oxidative stress is responsible for these alterations. A similar age-associated rise in PTP activity specifically in interfibrillar heart mitochondria was observed with no apparent increase in either cyclophilin D or adenine nucleotide translocase immunoreactivity (Hofer et al., 2009). These and other studies provide significant evidence that cardiac mitochondrial PTP activity increases as animals age and also suggest that sensitivity to inhibition by cyclosporin A may decrease with age (Di and Bernardi, 2005) (Garcia et al., 2009).
Effects of Aging on Regulators of Mitochondrial Permeability Transition
Several lines of research indicate that age-related changes in redox state or oxidative stress could affect PTP opening. Parihar et al reported a substantial decline in resting reduced pyridine nucleotide concentrations cultured hippocampal neurons prepared from aged rats (Parihar et al., 2008). This loss was also reportedly sufficient to limit mitochondrial dehydrogenase-dependent maintenance of redox potentials after exposure to excitotoxic levels of glutamate. They and others also demonstrated a 10 fold decrease in the reduced to oxidized glutathione (GSH/GSSH) ratio in the neurons from aged rats or the brains of aged mice(Pallardo et al., 1998; Parihar et al., 2008). In addition to potentially greater susceptibility of mitochondria to oxidative stress caused by these redox effects, mitochondrial production of ROS increases in aging rat brain and other tissues (Sawada and Carlson, 1987). Sen et al also reported that there is a significant difference in ROS generation in young versus aged rats (Sen et al., 2007). Experiments with flies showed that the rate of mitochondrial oxygen radical and peroxide generation increases approximately 75% with aging (Yan and Sohal, 1998). Taken together, these findings strongly suggest that age-related oxidative stress could be an important factor in increasing the sensitivity of brain mitochondria to PTP opening (Zorov et al., 2009) (Macho et al., 1997; Parihar et al., 2008).
Impaired calcium homeostasis is thought to be important in the degenerative processes of aging as well as playing a key role in the secondary necrotic cell death following an acute ischemic or traumatic event (Crompton, 2004; Fiskum et al., 2008). The calcium hypothesis of aging proposes that early in the aging process, calcium homeostasis in brain cells becomes dysregulated, leading to functional impairment (Toescu et al., 2004; Xiong et al., 2002). There are many ways that abnormally elevated cytosolic Ca2+ can injure cells, including stimulation of mitochondrial Ca2+ uptake, which is normally almost negligible at normal cytosolic Ca2+ concentrations of around 0.1 μM (Nicholls, 2009). Mitochondrial Ca2+ uptake per se is not deleterious unless accumulation over time is sufficient to trigger PTP opening or other forms of mitochondrial injury. While some reports indicate that there is no significant difference in resting intra-neuronal [Ca2+] with aging (Xiong et al., 2002), recovery of cytosolic Ca2+ to baseline after even physiological elevations in Ca2+ is relatively slow in neurons of aged rat brains compared to young brains (Toescu et al., 2004). Mitochondria in aged neurons are also relatively depolarized, which might either explain the slow return of [Ca2+] to baseline or might reflect damage to at least a subset of mitochondria caused by a time-averaged increase in cytosolic [Ca2+] (Xiong et al., 2002) (Toescu and Vreugdenhil, 2010).
In addition to changes in Ca2+ and redox state, which induce the MPT, changes in levels of proteins associated with the PTP occur with aging. Marzetti et al. demonstrated an increase in the ratio between CypD and ANT with aging and postulated that this increase is responsible for higher susceptibility to PTP opening (Marzetti et al., 2008). Other work substantiates a link between mitochondrial levels of CypD and PTP activity during brain development. Eliseev et al. showed with RT-PCR and immunoblots that CypD expression is progressively down-regulated during brain development during early neonatal life (Eliseev et al., 2006). The increased resistance to Ca2+ induced MPT which occurred with maturation was attributed in part to the reduced expression of CypD. It remains to be established, however, whether this same cause-and-effect relationship can explain an increase in PTP opening in mitochondria from the brains of aged rats.
The adenine nucleotide translocase (ANT) is another important protein associated with the PTP that is thought to be affected by the aging process (Yan and Sohal, 1998; Gouspillou G. et al., 2010). ANT is a small but abundant protein in the inner mitochondrial membrane and is responsible for the selective and reversible exchange of ADP for ATP across the mitochondrial inner membrane under physiological conditions (Zorov et al., 2009). One explanation for PTP opening is that abnormally high intramitochondrial Ca2+ causes a conformation change in the ANT resulting in loss of substrate specificity and conversion to the non-specific PTP (Halestrap and Brenner, 2003). As stated earlier, the ANT may also be an integral component of the PTP or a site where sulfhydryl oxidation occurs that promotes PTP opening. It is therefore potentially important that ANT is oxidatively modified during aging in several tissues, including rat liver, heart, and skeletal muscle. Chen et al reported that rat liver mitochondrial ANT is susceptible to and impaired by lipid peroxidation (Chen et al., 1995). Subsequently Girón-Calle and Schmid reported peroxidative modification of heart mitochondrial ANT even under very mild peroxidative conditions (Giron-Calle and Schmid, 1996). Yan and Sohal reported that ANT is the only protein present in mitochondrial membranes that exhibits a detectible, age-associated increase in carbonyl groups and that this corresponds with loss of normal transport activity (Yan and Sohal, 1998). Further work is necessary whether these age-related oxidative modifications to the ANT also occur in the brain and if they actually promote PTP opening.
In addition to changes in PTP associated proteins with age, alterations in membrane lipids may contribute to increased PTP sensitivity. Cardiolipin is a phospholipid that is particularly rich in unsaturated fatty acids and localized almost entirely in the inner mitochondrial membrane (Petrosillo et al., 2008). The ANT is bound to six molecules of cardiolipin, which are necessary for its normal transport activity. Cardiolipin peroxidation and its subsequent effects on ANT structure and function may be a primary cause of impaired ANT activity with aging (Yan and Sohal, 1998; Giron-Calle and Schmid, 1996). Although not yet demonstrated with brain mitochondria, the age-dependent oxidation of cardiolipin observed in heart mitochondria appears responsible for the similar age-dependent increase in PTP activity (Petrosillo et al., 2010).
There has been considerable research on changes in the expression of the Bcl-2 family of proteins with aging in several tissues. It is well established, for instance, that there is a general decreased expression of pro-apoptotic Bcl-2 family proteins in brain mitochondria during early postnatal brain development (Soane et al., 2007). Pollack et al. determined the levels of Bcl-2, Bax and the Bcl-2/Bax ratio in the heart, skeletal muscle and brain of 6 month versus 24 month rats (Pollack et al., 2002). They found that in the brain there is no change in Bcl-2 but a decrease in Bax, resulting in an increase in Bcl-2/Bax ratio. In contrast, the heart exhibits a decrease in Bcl-2 and maintenance of Bax, resulting in the decrease of the Bcl-2/Bax ratio in old versus young rats. Research by Marzetti et al. found that the mitochondrial levels of both anti-apoptotic Bcl-2 and proapoptotic Bax were significantly increased in the gastrocnemius of an aged rat, with no age related change in the Bcl-2/ Bax ratio (Marzetti et al., 2008). While there are no consistent changes among different tissues, there are significant changes in mitochondrial apoptotic proteins that occur with the aging process. By extension, therefore, changes in regulation of the MPT related to a potential decline in mitochondrial Bcl-2 could occur with aging.
As mentioned earlier, the “antioxidant” effects of Bcl-2 and other related mitochondrial anti-death proteins may actually due to cellular stress responses compensating for mild oxidative stress caused by these proteins at the mitochondrial level. Nrf2 is a transcriptional activating factor that often promotes transcription of antioxidant and related genes, and its pharmacologic activation inhibits redox-regulated PTP opening in mitochondria from normal young rats (Greco and Fiskum, 2010b). While there is no information available about the levels of Nrf2 or its transcriptional activating activity in the brains of aged rats, one study found a significant, 30% decline in Nrf2 immunoreactivity in the hearts of 24 month old rats compared to those of 3 month old animals (Jacob et al., 2010). Another study focusing on the effects of high fat diets on cognition found that brain Nrf2 levels and signaling activity were significantly reduced by a high fat diet given to 20 month old mice and were associated with significant elevations of brain protein carbonyl groups (Morrison et al., 2010). It will therefore be important to determine if antioxidant gene expression under the control of Nrf2 in brains from normal aged rats is impaired, whether this in turn promotes sensitivity to brain mitochondrial PTP opening, and whether agents that stimulate Nrf2 dependent gene expression are as mito-protective and cyto-protective in aged rats as they are in normal mature animals.
Conclusions and Future Directions
The mitochondrial membrane permeability transition is a well-studied phenomenon that is not, however, well-characterized at the molecular level. Nevertheless, it is strongly implicated in the pathophysiology of aging-associated neurological disorders and is therefore a potential target for neuroprotective interventions in this age group. Some evidence from animal models indicates that the MPT is more active or at least more sensitive to activation in aged animals, such as rats that are equal to or greater than 24 months old, roughly equivalent to humans greater than 80 years old. At this juncture, most studies point toward conditions, e.g., oxidative stress and Ca2+ dyshomeostasis, as the most important age-related regulators that may promote sensitivity to PTP opening. The finding that overexpression of anti-death proteins, e.g., Bcl-2, inhibit PTP opening and that these proteins can act at the mitochondrial level to slightly elevate ROS production but also stimulate a larger expression of antioxidant proteins could be a clue for the development of effective interventions. Moreover, the fact that Bcl-2 overexpression elevates levels of glutathione and peroxidase activities points toward endogenous genomic responses to sublethal stressors as a potential avenue toward neuroprotection. Pharmacologic stimulation of antioxidant protein expression by activators of the Nrf2 pathway of gene expression increases resistance of redox regulated PTP activity in normal, young rats (Greco and Fiskum, 2010b). However, it has yet to be determined whether these same agents are also effective in aged animals for both increasing mitochondrial resistance to oxidative stress and PTP opening as well as for neuroprotection in aged-associated neurologic disorders and neurodegenerative diseases.
Table 1.
Age-related factors that affect mitochondrial permeability transition
| Factor | Change with Age | References |
|---|---|---|
| Cell calcium homeostasis | Slow recovery after a rise in cytosolic Ca2+ | (Toescu et al., 2004) |
| Redox State | Decline in resting reduced pyridine nucleotide levels; increased ROS production | (Parihar et al., 2008; Sen et al., 2007) |
| Cyclophilin D | Increases | (Marzetti et al., 2008) |
| Adenine Nucleotide Translocase | Oxidative modification | (Giron-Calle and Schmid, 1996) |
| Bcl-2 | Expression changes varying in tissues | (Pollack et al., 2002) |
Reference List
- Bazil JN, Buzzard T, Rundell AE. A bioenergetic model of the mitochondrial population undergoing permeability transition. Journal of Theoretical Biology. 2010 doi: 10.1016/j.jtbi.2010.06.001. In Press, Uncorrected Proof. [DOI] [PubMed] [Google Scholar]
- Brown MR, Geddes JW, Sullivan PG. Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J Bioenerg Biomembr. 2004;36:401–406. doi: 10.1023/B:JOBB.0000041775.10388.23. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Bertrand H, Yu BP. Inhibition of adenine nucleotide translocator by lipid peroxidation products. Free Radic Biol Med. 1995;19:583–590. doi: 10.1016/0891-5849(95)00066-7. [DOI] [PubMed] [Google Scholar]
- Crompton M. Mitochondria and aging: a role for the permeability transition? Aging Cell. 2004;3:3–6. doi: 10.1046/j.1474-9728.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- Di LF, Bernardi P. Mitochondrial function and myocardial aging. A critical analysis of the role of permeability transition. Cardiovasc Res. 2005;66:222–232. doi: 10.1016/j.cardiores.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Eliseev RA, Filippov G, Velos J, Vanwinkle B, Goldman A, Rosier RN, Gunter TE. Role of cyclophilin D in the resistance of brain mitochondria to the permeability transition. Neurobiol Aging. 2006:1532–1542. doi: 10.1016/j.neurobiolaging.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Eliseev RA, Malecki J, Lester T, Zhang Y, Humphrey J, Gunter TE. Cyclophilin D interacts with Bcl2 and exerts an anti-apoptotic effect. J Biol Chem. 2009;284:9692–9699. doi: 10.1074/jbc.M808750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerby LM, Ellerby HM, Park SM, Holleran AL, Murphy AN, Fiskum G, Kane DJ, Testa MP, Kayalar C, Bredesen DE. Shift of the cellular oxidation-reduction potential in neural cells expressing Bcl-2. Journal of Neurochemistry. 1996;67(3):1259–67. 1259–1267. doi: 10.1046/j.1471-4159.1996.67031259.x. [DOI] [PubMed] [Google Scholar]
- Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotrauma. 2000;17:843–855. doi: 10.1089/neu.2000.17.843. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Danilov CA, Mehrabian Z, Bambrick LL, Kristian T, McKenna MC, Hopkins I, Richards EM, Rosenthal RE. Postischemic oxidative stress promotes mitochondrial metabolic failure in neurons and astrocytes. Ann N Y Acad Sci. 2008;1147:129–138. doi: 10.1196/annals.1427.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr. 2004;36:347–352. doi: 10.1023/B:JOBB.0000041766.71376.81. [DOI] [PubMed] [Google Scholar]
- Garcia N, Zazueta C, Martinez-Abundis E, Pavon N, Chavez E. Cyclosporin A is unable to inhibit carboxyatractyloside-induced permeability transition in aged mitochondria. Comp Biochem Physiol C Toxicol Pharmacol. 2009;149:374–381. doi: 10.1016/j.cbpc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Giron-Calle J, Schmid HH. Peroxidative modification of a membrane protein. Conformation-dependent chemical modification of adenine nucleotide translocase in Cu2+/tert- butyl hydroperoxide treated mitochondria. Biochemistry. 1996;35:15440–15446. doi: 10.1021/bi960840j. [DOI] [PubMed] [Google Scholar]
- Gouspillou G, Bourdel-Marchasson I, Rouland R, Calmettes G, Franconi JM, Deschodt-Arsac V, Diolez P. Alteration of mitochondrial oxidative phosphorylation in aged skeletal muscle involves modification of adenine nucleotide translocator. Biochem Biophys Acta. 2010;1797:143–151. doi: 10.1016/j.bbabio.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Greco T, Fiskum G. Neuroprotection through Stimulation of Mitochondrial Antioxidant Protein Expression. J Alzheimers Dis. 2010a;20(Suppl 2):427–37. doi: 10.3233/JAD-2010-100519. [DOI] [PubMed] [Google Scholar]
- Greco T, Fiskum G. Brain mitochondria from rats treated with sulforaphane are resistant to redox-regulated permeability transition. J Bioenerg Biomembr. 2010b;42:491–497. doi: 10.1007/s10863-010-9312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger AL, Freeman BA. Signaling actions of electrophiles: anti-inflammatory therapeutic candidates. Mol Interv. 2010;10:39–50. doi: 10.1124/mi.10.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans. 2010;38:841–860. doi: 10.1042/BST0380841. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003;10:1507–1525. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- Hofer T, Servais S, Seo AY, Marzetti E, Hiona A, Upadhyay SJ, Wohlgemuth SE, Leeuwenburgh C. Bioenergetics and permeability transition pore opening in heart subsarcolemmal and interfibrillar mitochondria: effects of aging and lifelong calorie restriction. Mech Ageing Dev. 2009;130:297–307. doi: 10.1016/j.mad.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob MH, Janner DR, Araujo AS, Jahn MP, Kucharski LC, Moraes TB, Dutra Filho CS, Ribeiro MF, Bello-Klein A. Redox imbalance influence in the myocardial Akt activation in aged rats treated with DHEA. Exp Gerontol. 2010;45:957–963. doi: 10.1016/j.exger.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Fenton RG, Fiskum G. Bcl-2 family proteins regulate mitochondrial reactive oxygen production and protect against oxidative stress. Free Radical Biology & Medicine. 2004;37(11):1845–53. doi: 10.1016/j.freeradbiomed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Vercesi AE, Fiskum G. Bcl-2 prevents mitochondrial permeability transition and cytochrome c release via maintenance of reduced pyridine nucleotides. Cell Death Differ. 2000;7:903–910. doi: 10.1038/sj.cdd.4400722. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- LaFrance R, Brustovetsky N, Sherburne C, Delong D, Dubinsky JM. Age-related changes in regional brain mitochondria from Fischer 344 rats. Aging Cell. 2005;4:139–145. doi: 10.1111/j.1474-9726.2005.00156.x. [DOI] [PubMed] [Google Scholar]
- Leite AC, Oliveira HC, Utino FL, Garcia R, Alberici LC, Fernandes MP, Castilho RF, Vercesi AE. Mitochondria generated nitric oxide protects against permeability transition via formation of membrane protein S-nitrosothiols. Biochim Biophys Acta. 2010;1797:1210–1216. doi: 10.1016/j.bbabio.2010.01.034. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linard D, Kandlbinder A, Degand H, Morsomme P, Dietz KJ, Knoops B. Redox characterization of human cyclophilin D: identification of a new mammalian mitochondrial redox sensor? Arch Biochem Biophys. 2009;491:39–45. doi: 10.1016/j.abb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Macho A, Hirsch T, Marzo I, Marchetti P, Dallaporta B, Susin SA, Zamzami N, Kroemer G. Glutathione depletion is an early and calcium elevation is a late event of thymocyte apoptosis. J Immunol. 1997;158:4612–4619. [PubMed] [Google Scholar]
- Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev. 2008;129:542–549. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Rottenberg H. Aging enhances the activation of the permeability transition pore in mitochondria. Biochem Biophys Res Commun. 2000;273:603–608. doi: 10.1006/bbrc.2000.2994. [DOI] [PubMed] [Google Scholar]
- Mazzeo AT, Beat A, Singh A, Bullock MR. The role of mitochondrial transition pore, and its modulation, in traumatic brain injury and delayed neurodegeneration after TBI. Exp Neurol. 2009;218:363–370. doi: 10.1016/j.expneurol.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H, Tsujimoto Y. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95:14681–14686. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondrial calcium function and dysfunction in the central nervous system. Biochim Biophys Acta. 2009;1787:1416–1424. doi: 10.1016/j.bbabio.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallardo FV, Asensi M, Garcia dlA, Anton V, Lloret A, Sastre J, Vina J. Late onset administration of oral antioxidants prevents age-related loss of motor co-ordination and brain mitochondrial DNA damage. Free Radic Res. 1998;29:617–623. doi: 10.1080/10715769800300671. [DOI] [PubMed] [Google Scholar]
- Parihar MS, Kunz EA, Brewer GJ. Age-related decreases in NAD(P)H and glutathione cause redox declines before ATP loss during glutamate treatment of hippocampal neurons. Journal of Neuroscience Research. 2008;86:2339–2352. doi: 10.1002/jnr.21679. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Matera M, Casanova G, Ruggiero FM, Paradies G. Mitochondrial dysfunction in rat brain with aging - involvement of complex I, reactive oxygen species and cardiolipin. Neurochemistry International. 2008;53:126–131. doi: 10.1016/j.neuint.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Moro N, Paradies V, Ruggiero FM, Paradies G. Increased susceptibility to Ca(2+)-induced permeability transition and to cytochrome c release in rat heart mitochondria with aging: effect of melatonin. J Pineal Res. 2010;48:340–346. doi: 10.1111/j.1600-079X.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- Pollack M, Phaneuf S, Dirks A, Leeuwenburgh C. The Role of Apoptosis in the Normal Aging Brain, Skeletal Muscle, and Heart. Annals of the New York Academy of Sciences. 2002;959:93–107. doi: 10.1111/j.1749-6632.2002.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Rasola A, Sciacovelli M, Pantic B, Bernardi P. Signal transduction to the permeability transition pore. FEBS Lett. 2010;584:1989–1996. doi: 10.1016/j.febslet.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Scafidi S, McKenna MC, Fiskum G. Mitochondrial mechanisms of cell death and neuroprotection in pediatric ischemic and traumatic brain injury. Exp Neurol. 2009;218:371–380. doi: 10.1016/j.expneurol.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Carlson JC. Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech Ageing Dev. 1987;41:125–137. doi: 10.1016/0047-6374(87)90057-1. [DOI] [PubMed] [Google Scholar]
- Sen T, Sen N, Jana S, Khan FH, Chatterjee U, Chakrabarti S. Depolarization and cardiolipin depletion in aged rat brain mitochondria: relationship with oxidative stress and electron transport chain activity. Neurochemistry International. 2007;50:719–725. doi: 10.1016/j.neuint.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Soane L, Kahraman S, Kristian T, Fiskum G. Mechanisms of impaired mitochondrial energy metabolism in acute and chronic neurodegenerative disorders. J Neurosci Res. 2007;85:3407–3415. doi: 10.1002/jnr.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J Neurosci Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27:614–620. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Vreugdenhil M. Calcium and normal brain ageing. Cell Calcium. 2010;47:158–164. doi: 10.1016/j.ceca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- Wang X, Michaelis EK. Selective Neuronal Vulnerability to Oxidative Stress in the Brain. Frontiers in Aging Neuroscience. 2010;2:1–13. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Verkhratsky A, Toescu EC. Changes in mitochondrial status associated with altered Ca2+ homeostasis in aged cerebellar granule neurons in brain slices. J Neurosci. 2002;22:10761–10771. doi: 10.1523/JNEUROSCI.22-24-10761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Sohal RS. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc Natl Acad Sci U S A. 1998;95:12896–12901. doi: 10.1073/pnas.95.22.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res. 2009;83:213–225. doi: 10.1093/cvr/cvp151. [DOI] [PMC free article] [PubMed] [Google Scholar]