Abstract
A stabilized tibia fracture model was used in young (8-week old) and aged (1-year old) mice to define the relative bone regenerative potential and the relative responsiveness of the periosteal progenitor population with aging and PTH 1-34 (PTH) systemic therapy. Bone regeneration was assessed through gene expressions, radiographic imaging, histology/histomorphometry, and biomechanical testing. Radiographs and microCT showed increased calcified callus tissue and enhanced bone healing in young compared to aged mice. A key mechanism involved reduced proliferation, expansion, and differentiation of periosteal progenitor cell populations in aged mice. The experiments showed that PTH increased calcified callus tissue and torsional strength with a greater response in young mice. Histology and quantitative histomorphometry confirmed that PTH increased callus tissue area due primarily to an increase in bone formation, since minimal changes in cartilage and mesenchyme tissue area occurred. Periosteum examined at 3, 5, and 7 days showed that PTH increased cyclin D1 expression, the total number of cells in the periosteum, and width of the periosteal regenerative tissue. Gene expression showed that aging delayed differentiation of both bone and cartilage tissues during fracture healing. PTH resulted in sustained Col10a1 expression consistent with delayed chondrocyte maturation, but otherwise minimally altered cartilage gene expression. In contrast, PTH 1-34 stimulated expression of Runx2 and Osterix, but resulted in reduced Osteocalcin. β-catenin staining was present in mesenchymal chondroprogenitors and chondrocytes in early fracture healing, but was most intense in osteoblastic cells at later times. PTH increased active β-catenin staining in the osteoblast populations of both young and aged mice, but had a lesser effect in cartilage. Altogether the findings show that reduced fracture healing in aging involves decreased proliferation and differentiation of stem cells lining the bone surface. While PTH 1-34 enhances the proliferation and expansion of the periosteal stem cell population and accelerates bone formation and fracture healing, the effects are proportionately reduced in aged mice compared to young mice. β-catenin is induced by PTH in early and late fracture healing and is a potential target of PTH 1-34 effects.
Keywords: Fracture healing, Parathyroid hormone, Periosteum, Wnt-β-catenin signaling, Mesenchymal stem cell
Introduction
Aging impairs the rate and quality of fracture healing and increases the risks of mal-union and non-union. [1-5]. A delay in bone repair is associated with joint stiffness, muscle atrophy, disuse related bone loss, and an increased risk of falling [6-8]. The rate of fractures is highest in the elderly and represents a major societal health care burden. In the United States, management of fractures is among the most costly hospitalization associated medical conditions in the Medicare population [9, 10]. Improved understanding of the causes of impaired bone healing and the development of targeted therapies to enhance bone regeneration is a priority.
Fracture typically results in a highly organized repair process [11]. Bone injury results in proliferation of the periosteal progenitor population with accumulation of the expanded progenitor population along the bone surface. These cells subsequently undergo differentiation into both bone and cartilage tissues [12-14]. Chondrocytes formed at the fracture site hypertrophy, the cartilage matrix undergoes calcification, and the process of endochondral ossification is completed with vascularization and primary bone formation on the cartilage template. Intramembranous ossification occurs in parallel with endochondral ossification and involves the direct differentiation of the periosteal progenitors into osteoblasts and bone formation in the absence of a cartilage template. Fractures are healed when calcified callus tissue unites the bone fragments at the fracture site, although remodeling of the fracture with formation of a more organized bone structure continues after clinical healing [11]. This complex series of cellular, molecular, and tissue events involved in bone regeneration is controlled by growth factors and signaling molecules. Our understanding of the role and integration of these factors during the normal fracture healing process as well as how their alteration or dysregulation impairs fracture healing is currently incomplete.
One such factor is PGE2, a lipid-signaling molecule that is derived from arachidonic acid. The enzyme cyclooxygenase-2 (COX-2) is a key, rate-limiting enzyme involved in the formation of PGE2 [15]. COX-2 expression is absent in the growth plate and Cox-2-/- mice have normal skeletal development. However, COX-2 is highly expressed in early mesenchymal chondroprogenitors and immature chondrocytes in the fracture callus and Cox-2-/- mice have a marked delay in fracture healing [16-18]. In Cox-2-/- mice, mesenchymal stem cells along the periosteum have reduced proliferation, delayed differentiation into cartilage and bone, and delayed vascularization and remodeling during fracture healing [16-18]. PGE2 receptors are G-coupled protein receptors that consist of four isoforms, EP1, EP2, EP3, and EP4. PGE2 stimulates fracture healing through activation of EP2 and/or EP4 [18-21], receptors that both activate Gs, increase cAMP levels, and stimulate PKA signaling [22]. PGE2 signaling through EP2 and EP4 has anabolic effects on bone formation [22, 23]. Interestingly, the PTH1R receptor is also a G-coupled protein receptor. The bone anabolic agent PTH 1-34 similarly enhances bone formation by stimulating Gs alpha and leading to accumulation of cAMP and activation of PKA signaling [24].
Aging is associated with a reduced rate of fracture healing in animal models [20, 25-28]. The fracture callus of aged mice has reduced COX-2 expression and a phenotype similar to Cox-2-/- mice, including smaller callus size, delayed differentiation of cartilage and bone, and a reduced rate of vascularization and remodeling [20, 25-28]. Administration of an EP4 receptor agonist to the fracture sites of aged mice accelerates the rate of fracture healing and corrects the reduced rate of bone repair that occurs in these mice [20]. Since the EP4 receptor stimulates Gs, the findings suggest that activation of the PKA signaling pathway functions to compensate for the reduced fracture healing observed in aged mice [18, 21, 23]. Because of the similar signaling effects of PGE2/EP4 and PTH/PTH1R, we explored the hypothesis that PTH 1-34 might rescue the reduced fracture healing that is observed in aging.
To determine the effect of PTH 1-34, a stabilized tibia fracture model was used in young (8-week-old) and aged (1-year-old) mice. Fracture healing was assessed through characterization of gene expressions, radiographic imaging, histology, and biomechanical testing. The findings establish that the periosteal stem cells from young mice are more responsive to bone injury than those observed in aged mice. The findings further show that PTH 1-34 stimulates the periosteal stem cells in both young and aged mice, however, the anabolic effect of PTH 1-34 is proportionately reduced in aged mice suggesting that there are fundamental differences in the young and aged periosteal stem cell populations. Molecular analysis of fracture healing showed that PTH 1-34 positively regulates β-catenin signaling in osteoblasts in fracture callus in both young and aged mice.
Materials and Methods
Experimental animals
Healthy female C57BL/6, 7- to 8-week-old (young) mice were procured from the Jackson Laboratories (Bar Harbor, ME, USA). C57BL/6, 52- to 54-week-old (aged) female mice were obtained from the National Institutes on Aging (Bethesda, MD, USA). All animal experimental procedures were performed according to the protocol approved by the Laboratory Animal Care and Use Committee of The University of Rochester.
Mouse tibia fracture model
Mice were anesthetized with an intraperitoneal injection (15 μl/g body weight) of 2.5% avertin. A 4mm longitudinal incision was made at the front of the right tibia. A small hole was drilled into the proximal tibial plateau using a 26-gauge needle. A transverse osteotomy was performed with a No. 11 scalpel blade at the proximal diaphysis of the tibia. The fibula was not broken. The tibia fracture was stabilized with an intramedullary pin using a 26G Quincke type spinal needle (BD Medical Systems, Franklin Lakes, NJ). The wound was closed using 5-0 nylon sutures. Mice were kept in cages after recovery from anesthesia, allowing free unrestricted weight bearing, and were given six subcutaneous injections of 2 mg/kg Buprenorphine (Abbot Laboratories, Abbott Park, IL, USA) every 12h to control pain. After surgery, mice were divided into two treatment groups: recombinant human PTH 1-34 (Forteo™; 40μg/kg) and normal saline vehicle treatment. Subcutaneous drug administration was delivered daily until sacrifice or up to 3 weeks. A Faxitron system (Faxitron X-ray, Wheeling, IL, USA) was used to acquire X-ray images at the time of surgery and on days 7, 10, 14, and 21 following surgery until sacrifice.
Micro-computed tomography (microCT)
Fractured tibias were harvested at indicated times and scanned using the VivaCT 40 system (Scanco Medical, Bassersdorf, Switzerland) at high resolution with a voxel size of 12.5 μm to image bone. New bone formation was measured as previously described [20, 29]. An integration time of 300 ms, a current of 145 mA, and an energy setting of 55 kV were used. The threshold was chosen using 2D evaluation of several slices in the transverse anatomic plane so that mineralized callus was identified but surrounding soft tissue was excluded. An average threshold of 250 was optimal and used uniformly for all samples. Next, each sample was contoured around the external callus and along the edge of the cortical bone. All mineralized tissues above threshold between these two boundaries were included. Thus, external soft tissues and cortical bone including the marrow cavity were excluded. Contouring of images was done every 20 axial slices proximally to distally until the callus was not visible. We used 6 animals for each group.
Biomechanical testing
Immediately following microCT imaging of bone, the torsional biomechanical properties of the fractured tibiae were determined using an EnduraTec TestBench™ system (200N.mm torque cell; Bose Corporation, Minnetonka, MN) at a rate of 1°/sec. Ultimate torque, yield torque, torsional rigidity, and toughness were determined for each specimen. We used 6 animals for each group.
Histology and histomorphometric analyses
Fractured tibiae were harvested and processed for histology [20]. Mice were sacrificed at 3, 5, 7, 10, 14, or 21 days after fracture. Tibiae were disarticulated from the knee and trimmed to remove excess muscle and skin. Specimens were placed in 10% neutral buffered formalin for 3 days. The tissues were infiltrated and embedded in paraffin. Alcian blue hematoxylin/orange G staining was done to visualize cartilage and bone, respectively. Tartrate-resistant acid phosphatase (TRAP) staining was used to detect osteoclasts. Histomorphometric analyses were performed using OsteoMeasure™ software (Osteometrics, Inc., Decatur, GA) to determine the area of total fracture callus, bone, cartilage, and mesenchyme (a subtraction of total callus from bone and cartilage tissue) formation in the external fracture callus by tracing. At least three non-consecutive sections were used for histomorphometric analyses and a mean of three represents one sample. Four specimens were included in each group for analyses. The mean from 4 samples was used in statistical analyses to determine the composition of the fracture callus. Cortical bone and internal calluses were excluded from the histomorphometric analyses.
Immunohistochemistry
Immunohistochemical staining for PCNA was performed using a PCNA staining kit (Invitrogen, Carlsbad, CA). The staining procedures were followed as instructed by the manufacturer. To determine the number of PCNA-positive cells, three sections from each fracture sample were used to count the positive cells within the periosteum at a site 1mm proximal to the osteotomized site using 25X magnification. The total number of cells and percentage of PCNA-positive cells in the region of interest were counted, and the thickness of newly formed bone on the external surface of the cortical bone was measured. Three different sections were used from each sample. Three different samples were used for each time point. The mean from 3 samples divided by the average area of the region of interest was used for statistical analyses. Immunostaining for total and active β-catenin, SOX9, and OSX in the fracture callus was performed using an avidin-biotin peroxidase detection system (Vector Lab, Burlingame, CA). The primary antibodies were either anti-total β-catenin rabbit polyclonal antibody (1:50: Cat. # 9562, Cell Signaling Technology, Inc, Danvers, MA ), anti-active β-catenin mouse monoclonal antibody (1:50: Cat. #05-665, Millipore, Temecula, CA), anti-SOX9 (1:500: sc-20095, Santa Cruz Biotechnology, Santa Cruz, CA), and anti-OSX (1:600: ab22552, Abcam, Cambridge, MA). Reactions were visualized with diaminobenzidine as substrate (Vector Lab). The sections were counterstained with hematoxylin.
Real-time RT-PCR analyses
For RNA analyses, mice were sacrificed on days 3, 5, 7, 10, 14, or 21 following surgery. Fracture calluses, approximately 4mm in length, including cortical bone were carefully dissected free of soft tissue. Normal bone was harvested from the tibias of young and aged mice as a day 0 control. Bone marrow was flushed out using phosphate-buffered saline (PBS), and the samples were immediately snap frozen in a liquid nitrogen bath. Frozen tissue samples were homogenized using the TissueLyser system (Qiagen, Hilden, Germany) and mRNA was purified via phase separation using the RNeasy Lipid Tissue Mini Kit (Qiagen). Exactly 1 μg of total RNA per callus was reverse transcribed to make single-stranded cDNA using the SuperScript® III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Quantitative PCR reactions were performed using SyberGreen (Quanta Biosciences, Inc., Gaithersburg, MD) in a RotorGene real-time PCR machine (Corbett Research, Carlsbad, CA). All genes were normalized to β-actin. Data were assessed quantitatively using bootstrap-adjusted t-tests comparing relative levels of transcript expression as a function of time. The following mouse specific primers were used for the assessment: β-actin; 5’-AGATGTGGATCAGCAAGCAG-3’, 5’-GCGCAAGTTAGGTTTTGTCA-3’, Sox-9; 5’-AGGAAGCTGGCAGACCAGTA-3’, 5’-CGTTCTTCACCGACTTCCTC-3’, Col2a1; 5’-ACTGGTAAGTGGGGCAAGAC-3’, 5’-CCACACCAAATTCCTGTTCA-3’, Ihh; 5’- GAGCTTTCCAGGTCATCGAG -3’, 5’- TGATTGTCCGCAATGAAGAG -3’, Col10a1; 5’-ACCCCAAGGACCTAAAGGAA-3’, 5’-CCCCAGGATACCCTGTTTTT-3’, Runx2; 5’-GCTATTAAAGTGACAGTGGACGG-3’, 5’-GGCGATCAGAGAACAAACTAGG-3’, Osterix (Sp7); 5’-GTCAAGAGTCTTAGCCAAACTC-3’, 5’-AAATGATGTGAGGCCAGATGG-3’, Osteocalcin (Bglap); 5’-CTTGGTGCACACCTAGCAGA-3’, 5’-CTCCCTCATGTGTTGTCCCT-3’, CyclinD1 (Ccnd1); 5’- TGGTGAACAAGCTCAAGTGG-3’, 5’-CTGGCATTTTGGAGAGGAAG-3’, Dkk1; 5’- GAGGGGAAATTGAGGAAAGC-3’, 5’-AGCCTTCTTGTCCTTTGGTG-3’, Sost; 5’-CAAGCCTTCAGGAATGATGC-3’, 5’-ACATCTTTGGCGTCATAGGG-3’
Statistical analysis
Data were presented as mean± standard error (SEM). Statistical significance between experimental groups was determined using heteroscedastic t-tests. Normality was confirmed using Shapiro-Wilk tests. For each time-point of each outcome variable, four comparisons were made: aged PTH vs. aged control, and young control vs. each of young PTH, aged PTH, and aged control. The overall Type I error rate across these four comparisons was controlled using the bootstrap method to adjust the P values [30]. Analyses were performed in SAS version 9.3 (Cary, NC), with bootstrap-adjusted P values <0.05 considered statistically significant.
Results
Enhanced bony fracture callus formation by intermittent PTH 1-34 treatment in young and aged mice
Radiographs were obtained 10 and 14 days following fracture. Calcified callus and evidence of bone union were observed in the fractures of young control mice by day 10. In contrast, a radiolucent line persisted in fractures from aged control mice through 14 days following fracture (Fig. 1A). PTH 1-34 treatment enhanced radiographic evidence of fracture callus formation and healing in both young and aged mice (Fig. 1A). MicroCT was performed on limbs harvested at 7, 10, 14, 21, and 42 days following fracture so as to quantify the amount of calcified fracture callus tissue formed (Figs. 1B and 1C). At 7 and 10 days, the volume of calcified callus was significantly lower (0.13-fold and 0.45-fold, respectively; p<0.01) in fractures from aged control mice compared to young mice (Fig. 1C). The temporal difference in the rate of formation and remodeling of the fracture callus in young and aged mice persisted throughout the healing process. Peak calcified callus volume occurred at 10 days in young mice and at 14 days in aged mice. Remodeling occurred progressively after the time of peak callus formation, but was accelerated in young compared to aged mice. At 42 days, fractures in aged mice were still undergoing remodeling resulting in increased calcified callus in aged mice at this point compared to the young mice that had already completed the remodeling process.
Fig. 1. PTH 1-34 enhances bony callus formation following tibia fracture in young and aged mice.
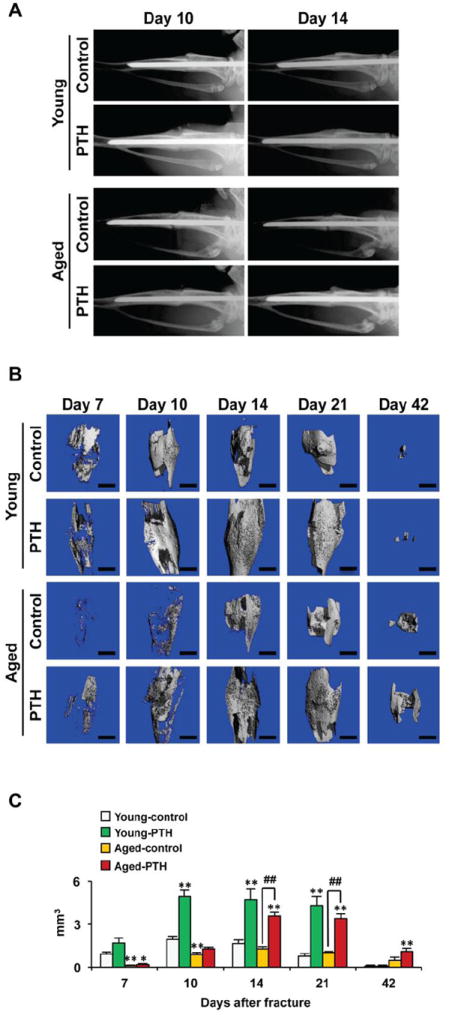
(A) Serial radiographs of representative young and aged mice treated with vehicle (saline) or Teriparatide (PTH 1-34) for 10 and 14 days following fracture. Fracture healing was delayed in aged mice compared to control mice. PTH 1-34 enhanced fracture callus formation in young mice and aged mice. (B) Fractured limbs were harvested and high-resolution microCT scans performed on young (n=6) and aged (n=6) mice treated with vehicle or PTH 1-34 to detect calcified external callus formation. Representative scans are shown for days 7, 10, 14, 21, and 42. (C) External bony callus volume was quantified and data presented as mean± standard error (SEM). Statistical analysis was performed using bootstrap-adjusted t-tests and statistical significance denoted as follows: *p<0.05 and **p<0.01 compared to Young control group, or #p<0.05 and ##p<0.01 for Aged control vs. Aged PTH 1-34 groups.
PTH 1-34 treatment enhanced external calcified callus volume in both young and aged mice (Figs. 1B and 1C). Interestingly, administration of PTH 1-34 did not alter the temporal pattern of the fracture repair process; peak external callus formation occurred in both young and aged mice at 10 and 14 days, respectively, the same as observed in the untreated control mice. The remodeling process also had a similar temporal pattern in the PTH 1-34 treated and vehicle treated control groups. While remodeling was completed in both treated and control young mice by day 42, the aged control and PTH 1-34 treated mice continued to have increased external calcified callus present, and the amount of callus remaining was significantly greater in the aged PTH 1-34 treated mice. However, PTH 1-34 did result in more robust fracture healing with the formation of an increased volume of calcified callus tissues in both young and aged mice. The response to PTH 1-34 occurred earlier in young compared to aged mice, although the magnitude of the effect was similar (Fig. 1C). Thus, while enhanced bone regeneration occurs following PTH 1-34 administration in both young and aged mice, a more robust effect was observed in young mice. The finding that differences in the temporal course and magnitude of bone regeneration persists in young and aged mice treated with PTH 1-34 suggest that there are intrinsic differences in the responsive cell populations of young and aged mice.
The connectivity and mineral density of the calcified fracture calluses were also calculated using microCT (Suppl. Figs. 1A and 1B). PTH 1-34 increased the external fracture callus connectivity density in the healing fractures of both the young and aged mice. Although PTH increased callus volume, it resulted in a slight reduction in the fracture callus mineral density in both young and aged mice. This is best observed at day 21 where a significant reduction in mineral density was noted in PTH 1-34 treated mice. This is consistent with prior reports showing that while PTH 1-34 enhances the deposition of bone matrix, it delays mineralization of the matrix [30].
PTH 1-34 treatment increases biomechanical strength of fracture healing in young and aged mice
Since biomechanical testing is considered a definitive measure of fracture healing, torsion testing was performed on fractured tibiae harvested from control and PTH 1-34 treated young and aged mice at 10, 14, 21, and 42 days. PTH 1-34 treatment increased ultimate torque in the healing fractures of both young mice and aged mice, but the effect was more robust in the young mice (Fig. 2A). The ultimate torque was significantly elevated by PTH 1-34 treatment in young mice compared to their matched vehicle controls at 10, 14, and 21 days. PTH 1-34 also stimulated significant increases in both yield torque and toughness in young mice at 10 and 14 days (Figs. 2B and 2D). In contrast, while PTH 1-34 caused a substantial increase ultimate torque (1.42-fold, day 14), yield torque (1.41-fold, day 14), and toughness in aged mice, statistical significance was not achieved when compared to the aged vehicle control mice (Figs. 2A, 2B, and 2D). PTH 1-34 did not cause a significant difference in the torsional rigidity of either the young or aged mice compared to their respective vehicle controls (Fig. 2C). However, aged mice had reduced torsional rigidity compared to young mice during the early stages of fracture healing (Fig. 2C). At 42 days, the ultimate torque, yield torque, and torsional rigidity were elevated in both vehicle and PTH 1-34 treated aged mice compared to the young vehicle control, consistent with microCT results that showed increased callus tissue due delayed fracture remodeling in the aged mice (Figs. 2A, 2B, and 2D). Altogether the biomechanical testing confirmed that while PTH 1-34 enhances fracture healing, young mice are more responsive to this treatment.
Fig. 2. PTH 1-34 enhances the biomechanical properties of the tibia following fracture in both young and aged mice.
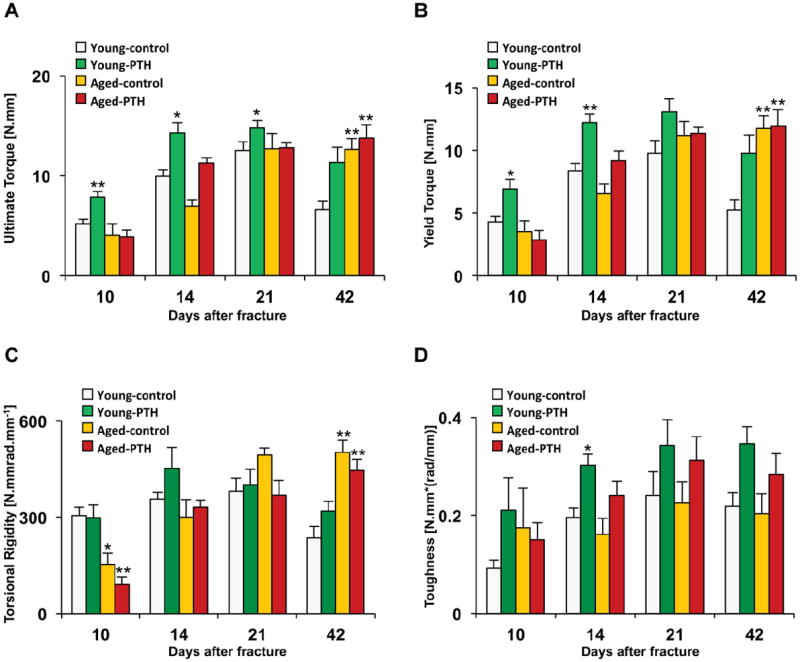
Fractured tibiae were retrieved upon animal sacrifice at 10, 14, 21, and 42 days and tested in torsion at 1°/sec to determine the ultimate torque (A), yield torque (B), torsional rigidity (C), and toughness (D). Data are presented as mean± standard error (SEM). Statistical analysis was performed using bootstrap-adjusted t-tests and statistical significance denoted as follows: *p<0.05 and **p<0.01 compared to Young control group.
PTH 1-34 increases bone, but not cartilage, callus formation in young and aged mice
A detailed temporal analysis of histology and gene expression was performed on fractured tibia so as to determine the cell populations and genes regulated by PTH 1-34 in young and aged mice during bone repair. The histology and histomorphometry complement the microCT analysis by examining both calcified and uncalcified tissues. During fracture healing both bone and cartilage callus formation was delayed in aged mice (Fig. 3A). Quantitative histomorphometry confirmed microCT findings and showed that PTH 1-34 treatment increased the total callus area in both young and aged mice. An increase in total callus area was observed by 5 days in young mice, whereas an increase was first observed in aged mice at 7 days (Fig. 3B). Interestingly, the increase in callus area was due to stimulation of bone/osteoid matrix formation by PTH 1-34 (Fig. 3C). PTH 1-34 treatment significantly increased bone callus area at days 5, 7, 10, 14, and 21 in young mice, and at 7, 10, and 21 days after fracture in aged mice. In contrast, PTH 1-34 significantly increased the cartilage callus area in young mice only at days 14 and 21 and in aged mice only at day 14 (Fig. 3D). The existence of this residual cartilage is likely due to the ability of PTH 1-34 to inhibit chondrocyte terminal differentiation, thereby, suppressing endochondral bone formation. Additionally, peak cartilage callus area in aged mice occurred at day 10 compared to day 7 in young mice consistent with a general delay in endochondral bone formation in aged mice. Mesenchyme area, which consists of undifferentiated periosteal cells and premature osteo- and chondro- progenitor cells, was increased in the fractures of young mice on days 7 and 10 and in aged mice only on day 7 by PTH 1-34 treatment (Fig. 3E). These findings demonstrate that while the administration of PTH 1-34 increases callus tissue, this effect is primarily due to a relative increase in the proportion of bone tissue compared to cartilage tissue. These effects appear related to anabolic effects since PTH 1-34 did not lead to a decrease in osteoclast formation, as evidence by TRAPC staining in the fracture callus in young and aged mice 21 days after fracture (Suppl. Figure 2).
Fig. 3. PTH 1-34 enhances bone formation, but not cartilage formation, following tibia fracture in both young and aged mice.
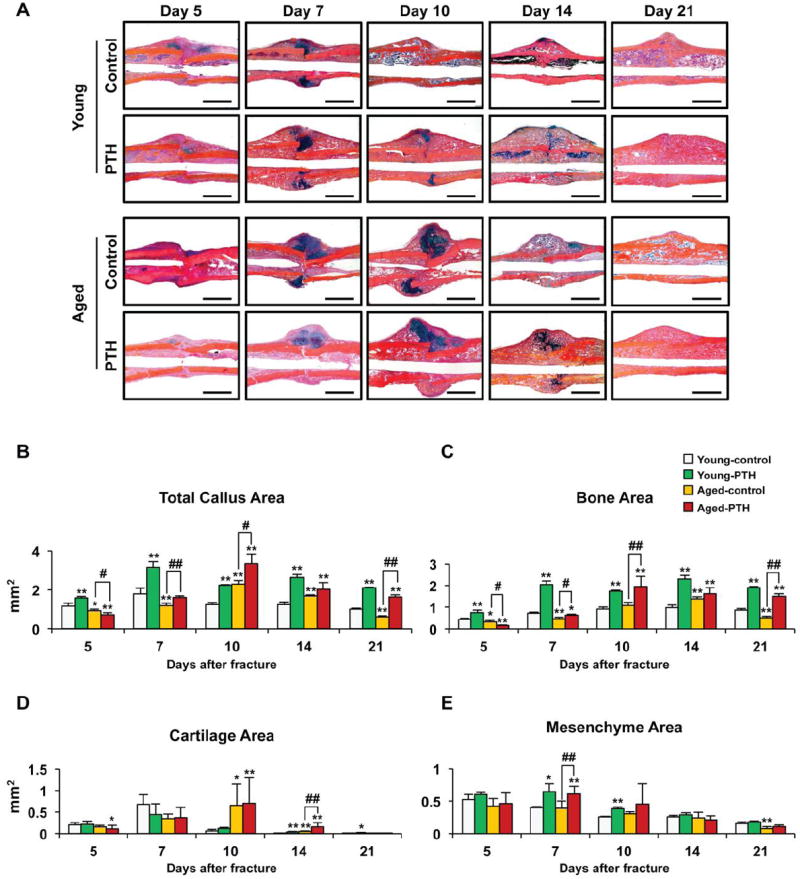
(A) Representative Alcian blue hematoxylin/orange G stained sections from the fractures of young and aged mice treated with saline or PTH 1-34 for 5, 7, 10, 14, or 21 days. PTH 1-34 increased intramembranous bone formation in both young and aged mice, but delayed completion of endochondral bone formation evident by the presence of persistent cartilage at days 10 and 14 in PTH 1-34 treated fractures. Histomorphometry was used to measure total callus area (B), bone area (C), cartilage area (D), and mesenchyme area (E) in the fractures of young (n=4) and aged (n=4) mice treated with saline or PTH 1-34 for 5, 7, 10, 14, or 21 days. Three levels were measured per sample. PTH 1-34 increased total callus, mesenchyme, and bone area, but not cartilage area. Data are presented as mean ± standard error (SEM). Statistical analysis was performed using bootstrap-adjusted t-tests and statistical significance denoted as follows: *p<0.05 and **p<0.01 compared to Young control group, or #p<0.05 and ##p<0.01 for Aged control vs. Aged PTH 1-34 groups.
Chondrocytes and osteoblasts in fracture healing express tissue specific proteins, such as Sox9 and Osterix (Suppl. Figure 3). To determine the effect of PTH 1-34 on progenitor cell fate during fracture healing, we examined and compared the expression levels of chondrogenesis and osteogenesis related genes over time (Fig. 4). Real-time RT-PCR showed that peak levels of Sox9, Col2a1, Ihh, and Col10a1 gene expressions were lower in the fractures of aged mice compared with young mice (Fig. 4). However, similar to our histological findings, PTH 1-34 had a minimal effect on cartilage gene expression in either young or aged mice, except for causing persistence of cartilage related genes Sox9, Col2a1, and Col10a1 at later time points consistent with a delay in the completion of endochondral ossification secondary to PTH 1-34 treatment.
Fig. 4. Molecular characterization of tibia fracture healing in young and aged mice treated with vehicle or PTH 1-34.
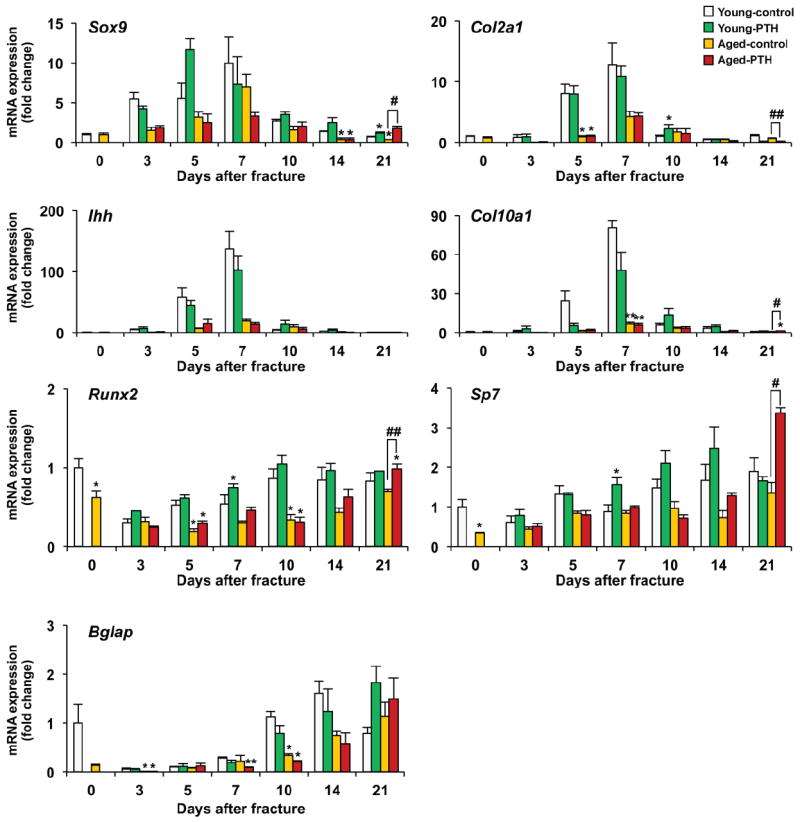
Real-time RT-PCR analyses were performed using total RNA collected from young or aged fracture calluses following vehicle or PTH 1-34 treatment for 3, 5, 7, 10, 14, or 21 days post-fracture. Expression of the chondrogenic genes, Sox9, Col2a1, Ihh, and Col10a1, and osteogenic genes, Runx2, Osterix (Sp7), and Osteocalcin (Bglap) were examined and normalized to β-actin expression. Each time point included at least 4 fracture samples. Data are presented as mean ± standard error (SEM). Statistical analysis was performed using bootstrap-adjusted t-tests and statistical significance denoted as follows: *p<0.05 and **p<0.01 compared to Young control group, or #p<0.05 and ##p<0.01 for Aged control vs. Aged PTH 1-34 groups.
The temporal expression of the osteoblast genes, Runx2, Osterix (Sp7), and Osteocalcin (Bglap) were also examined in the healing fractures in order to assess osteoblast activity (Fig. 4). Fractures in aged mice generally had reduced expression levels of these genes compared to fractures in young mice, consistent with reduced osteoblast differentiation and impaired healing with aging. In young mice PTH 1-34 significantly increased the expression of Runx2 and Osterix in the fracture callus at 7 days with peak levels of expression occurring at days 10 and 14, respectively. In contrast, significant increases and peak expression levels of Runx2 and Osterix were not observed in fracture calluses from aged mice until 21 days of healing. Osteocalcin (a marker of mature osteoblasts) expression was gradually elevated throughout fracture healing and reached a peak level at 21 days in both young and aged mice. Osteocalcin expression was significantly reduced in aged fractures compared to young fractures at 3 and 10 days. PTH 1-34 tended to reduce the expression of Osteocalcin in both young and aged mice between 7 and 14 days. This reduction in Osteocalcin expression is consistent with the known effect of PTH 1-34 on reducing maturation and mineralization of the osteoid matrix [30]. Consistent with a PTH 1-34-dependent reduction in osteoblast maturation was the finding by microCT analysis that PTH 1-34 resulted in a reduction of external bony callus mineralization at 21 days following fracture in both young and aged mice (Suppl Fig. 1B).
PTH 1-34 treatment increases periosteal progenitor cell proliferation and expansion in early fracture healing
Periosteal fracture callus formation from both young and aged mice was examined histologically at days 3, 5, and 7 following fracture and the total number of cells, percentage of proliferating cells as determined by PCNA staining, and the width of the reparative tissue was measured using histomorphometry (Fig. 5). The percentage of cells in the total population undergoing proliferation was similar in young and aged mice (Fig. 5B). PTH 1-34 treatment resulted in a similar magnitude increase in the percent of the cell population undergoing proliferation at days 3, 5, and 7 in young and aged mice. Although not statistically significant, PTH 1-34 treatment resulted in an increase in the total number of cells within the periosteum of both young and aged mice at days 3, 5, and 7 of healing with similar numbers of cells noted regardless of age (Figs. 5A and 5C).
Fig. 5. PTH 1-34 increases proliferation and expansion of the periosteal mesenchymal stem cell population.
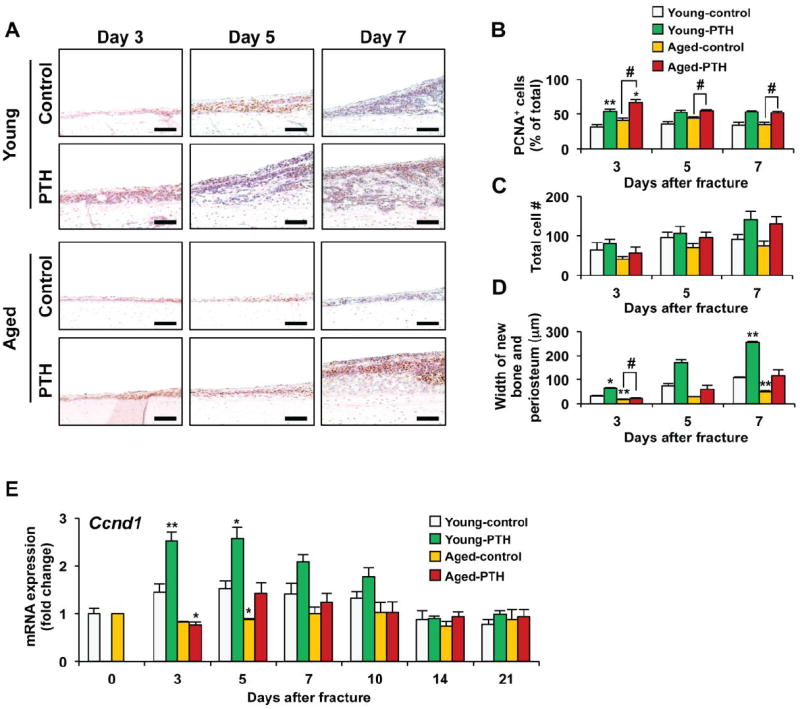
(A) Representative sections of PCNA stained periosteal tissues from young and aged mice treated with vehicle or PTH 1-34 for 3, 5, and 7 days. Brown staining in the nuclei identifies PCNA-positive cells. Scale bar: 100μm. Histomorphometry was used to quantify the percentage of PCNA-positive cells in the periosteal callus (B), the total cell number in the periosteal callus (0.24mm2) (C), and the average width of newly formed periosteal callus tissue (D) in the fractures of young (n=3) and aged (n=3) mice treated with saline or PTH 1-34 for 3, 5, or 7 days. (E) Real-time RT-PCR analysis was performed to determine Cyclin D1 (Ccnd1) expression using total RNA collected from young or aged fracture calluses following vehicle or PTH 1-34 treatment for 0, 3, 5, 7, 10, 14, or 21 days post-fracture. Expression of Cyclin D1 was normalized to β-actin (n=4). Data are presented as mean ± standard error (SEM). Statistical analysis was performed using bootstrap-adjusted t-tests and statistical significance denoted as follows: *p<0.05 and **p<0.01 compared to Young control group, or #p<0.05 and ##p<0.01 for Aged control vs. Aged PTH 1-34 groups.
The most striking result from this set of experiments was the observation that the width of the newly formed periosteal regenerative tissue was greater in young mice compared to old mice at all times examined (Figs. 5A and 5D). The enhanced thickness of the periosteum despite similar total cell numbers suggests that the periosteal cells in young mice produce more matrix and undergo more rapid differentiation. This is consistent with the microCT and histology findings described earlier. PTH 1-34 treatment increased the width of the periosteum in response to the bone injury in both the young and aged mice (Fig. 5D). Thus, treatment of the fractures with PTH 1-34 stimulated proliferation and increased accumulation of the pool of stem cell progenitors able to participate in the regenerative response. The effect was greater in young control and PTH 1-34 treated young mice compared to aged mice.
In order to confirm that PTH 1-34 regulates proliferation and expands the periosteal progenitor cell population, the gene expression of the cell cycle regulator Cyclin D1 (Ccnd1) was measured (Fig. 5E). Cyclin D1 expression levels were significantly lower in the fractures of aged mice at day 5 compared to fractures from young mice. PTH 1-34 increased Cyclin D1 expression in the fractures of both the young and aged mice with significant increases observed at days 3 and 5 in the fractures from young mice. These early effects further suggest that PTH 1-34 has a role in the expansion and accumulation of an increased pool of mesenchymal progenitor cells available for the healing response. The effect on Cyclin D1 expression was more robust in the young mice and the effect was absent at the later time points of fracture healing.
PTH 1-34 stimulates canonical Wnt signaling during fracture healing with aging
β-catenin signaling stimulates proliferation of progenitor cell populations, is known to be important during fracture healing, and is induced in fractures by PTH 1-34 treatment [31]. We performed experiments to compare β-catenin signaling in the periosteal stem cell populations of young and aged mice and the relative responsiveness of such signaling to PTH 1-34. Activation of β-catenin depends upon its stabilization and intracellular accumulation as a result of inhibition of phosphorylation at sites that target it for degradation. Antibodies recognizing specifically this non-phosphorylated, or active, form of β-catenin as well as total β-catenin were used to perform immunohistochemistry in sections from day 5 and 14 fractures [32]. In day 5 fracture calluses, total β-catenin staining was present in both mesenchymal chondroprogenitors and in pre-hypertrophic chondrocytes (Fig. 6A). However, the staining intensity for active β-catenin was greater in the chondro-progenitor population and was more intense in the fractures of young mice compared to aged mice, which had minimal active β-catenin staining. In the day 5 fracture callus there was no apparent increase in either total or active-catenin immunostaining in young or aged mice following PTH 1-34 administration.
Fig. 6. PTH 1-34 regulates Wnt/β-catenin signaling during tibia fracture healing in young and aged mice.
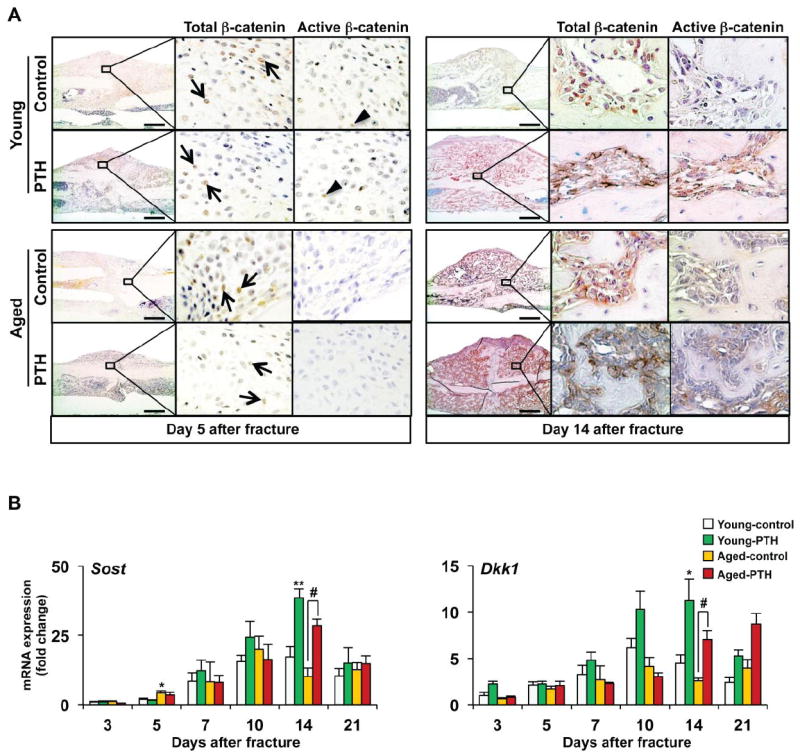
(A) Immunohistochemistry was performed to detect total and active non-phospho-β-catenin in day 5 and 14 tibia fracture calluses in both young and aged mice treated with vehicle or PTH 1-34. Total (arrows) and active (arrowheads) β-catenin staining was present in immature chondrocytes in the callus as well as in osteoblastic cells lining newly formed bone surfaces. PTH 1-34 treatment increased active β-catenin staining in the fracture calluses of both young and aged mice at day 14. (B) Real-time RT-PCR was performed on total RNA to measure the gene expression Sost and Dkk1 during fracture healing in both young and aged mice treated with vehicle or PTH 1-34. Expression was normalized to β-actin (n=4). Data are presented as mean ± standard error (SEM). Statistical analysis was performed using bootstrap-adjusted t-tests and statistical significance denoted as follows: *p<0.05 and **p<0.01 compared to Young control group, or #p<0.05 and ##p<0.01 for Aged control vs. Aged PTH 1-34 groups.
At 14 days, total β-catenin as well as active β-catenin staining was present in osteoblastic cells lining the newly formed bone present in the fracture callus at 14 days from both young and aged fractures (Fig. 5A). PTH 1-34 treatment increased active β-catenin staining in osteoblastic cells in the fracture callus of both young and aged mice.
The Sclerostin (Sost) and Dickkoph-related protein 1 (Dkk1) genes encode proteins that inhibit activation of the Wnt/β-catenin signaling pathway. Antibodies targeting both Sost and Dkk1 have previously been shown to enhance fracture healing in animal models. The expression levels of these two major modulators of Wnt/β-catenin signaling were examined in young and aged mice between days 3 and 14 of fracture healing (Fig. 5B). The expression of Sost and Dkk1 both increased during the course of fracture healing, with the lowest levels of expression observed in day 3 callus tissues and the highest levels observed in 10-14 day fracture calluses. Compared with the levels of expression observed at 3 days, the magnitude of the increase in Sost (17.2-fold in young control at day 14 and 19.8-fold in aged control at day 10) and Dkk1 (5.7-fold in young control at day 10 and 3.5-fold in aged control at day 10) is large. PTH 1-34 did not significantly alter the expression levels of Sost or Dkk1 in either the young or aged mice during the mesenchymal or the chondrogenic phases of fracture healing (3-10 days). However, PTH 1-34 significantly increased the expression of both Sost and Dkk1 in day 14 fracture calluses where the osteogenic phase of healing is predominant. As Sost and Dkk1 are both direct target genes of β-catenin, their increase in expression at this time point is consistent with enhanced β-catenin activity as detected by immunostaining. Thus, increased detection of active β-catenin is observed in osteoblasts/bone forming areas in both young and aged mice treated with PTH 1-34. These findings, along with our histomorphometry and gene expression studies demonstrate that PTH 1-34 has important anabolic effects on osteoblast mediated bone formation that may be facilitated, in part, through enhanced Wnt/β-catenin signaling.
Discussion
PTH 1-34 is a potent anabolic agent in osteoporosis patients [33, 34]. PTH 1-34 increased mesenchymal stem cell proliferation and osteoblast differentiation and results in enhanced bone formation [33]. The clinical effect of PTH is greatest in the spine, where it increases trabecular thickness and number [35]. The overall effect on cortical bone is less since PTH 1-34 causes cortical bone porosity [33, 34]. However, PTH 1-34 also stimulates periosteal bone formation [33, 36, 37]. Human subjects treated with PTH 1-34 for 1 month exhibited a 4-fold increase in periosteal bone formation rates along the outside of the ileum [37]. Thus, patients with osteoporosis have site-specific anabolic responses to PTH 1-34 with anabolic effects primarily in trabecular bone but also along the periosteal surface of bone [33, 35, 37]. Our findings demonstrate that the periosteum is highly responsive to injury and that PTH 1-34 markedly increases the anabolic activity of periosteal mesenchymal stem cells in the setting of bone injury. Furthermore our findings demonstrate that a key mechanisms involved in reduced fracture healing with aging is related to decreased responsiveness of the chondro- and osteo progenitor populations along the periosteal surface following fracture. While PTH 1-34 enhances the proliferation, expansion and differentiation of the progenitor populations, anabolic responses are reduced in aged mice compared to young mice.
Consistent with observations in humans, prior work has established a reduced rate of fracture healing in various animal models with aging [20, 25-27, 38-42]. Aged mice have reduced callus size, delayed cartilage differentiation, a prolonged period of endochondral ossification, and delayed mineralization in healing bone [20, 41]. PTH 1-34 enhances bone repair in animal models. Similar to our findings, others have shown that PTH 1-34 increases the proliferation of mesenchymal progenitor cells in callus tissue during the early phases of fracture healing [43]. Intermittent administration of PTH 1-34 increased the ultimate load, the external callus volume, and bone mineral content (BMC) in 3-month-old rat tibia fractures and the torsional strength, stiffness, BMC, and bone mineral density in a rat closed femur fracture model [44, 45]. In mice, PTH 1-34 increased callus volume, and cartilage formation as well as enhanced the expression of the early osteoblastic marker gene, Osterix [31, 46]. In a mouse allograft transplant model PTH 1-34 resulted in a 2-fold increase in callus formation as well as a significant increase in host-graft integration and torsional strength [29]. In models of compromised bone healing, PTH 1-34 has also been observed to increase bone regeneration. In a model that used 27-month-old mice, PTH 1-34 increased fracture strength, callus volume, and callus mineral content, but a young comparison group was not included in this study [47]. In both normal mice and in mice with ovariectomy, PTH 1-34 enhanced osteotomy healing, caused a 2-3 fold increase in stem cell proliferation, and resulted in a relative increase in osteogenesis and decrease in adipogenesis [48].
However, there are conditions in which the anabolic effects of PTH 1-34 on bone formation are attenuated. In a rat open femur fracture model with periosteal stripping, the anabolic effect of PTH 1-34 treatment is significantly reduced compared to the response observed in closed fractures [49]. Similarly, in a non-injury model with 1, 3, and 13-month-old rats, a one-week treatment with intermittent PTH 1-34 showed a substantially reduced anabolic response in the older animals [50]. In mice treated with glucocorticoids, the anabolic effect of PTH 1-34 on fracture healing was reduced [51]. The decreased responses to PTH 1-34 in glucocorticoid treated rats are consistent with the known inhibitory effect of glucocorticoids on the proliferation of mesenchymal stem cells, including cells in the periosteum [52-55].
Here, we show that while PTH 1-34 increases fracture healing in aging, the effects are attenuated compared to the response observed in young mice. The periosteum is a primary source for stem cell populations in bone regeneration, but undergoes age related changes. [55-57]. The periosteum of aged rats has a significant decrease in both thickness and in the number of Stro1+ progenitor cells in the cambium layer and there is reduced potential for periosteal cells to undergo differentiation [55, 56]. Unlike humans, which have a thick periosteum with an inner cambium layer with progenitor cells and an outer fibrous layer, mice have a thin layer of progenitor cells lining the bone surface [14]. In our experiments, periosteum in aged mice had impaired responsiveness to fracture and to PTH 1-34 administration. In young mice, the width of the periosteum was greater at 3, 5, and 7 days following fracture compared to the periosteal width in aged mice and a concomitant enhanced responsiveness to PTH 1-34 was observed. However, a similar total number of cells as well as a similar percentage of cells undergoing proliferation, as measured by PCNA staining, were observed in the periosteum of young and aged mice. Our findings, therefore, suggest that the tissue differences that develop over time in the periosteum of young and aged mice are primarily related to young mice having accelerated stem cell differentiation into bone forming tissue with increased matrix deposition. This is slightly different than observations previously made in an ex vivo study of rabbit periosteum [58]. In the rabbit, aging results in a decline in the thickness of the periosteum and a reduction in the number of progenitor cells in the cambium layer. In ex vivo culture, periosteal explants had marked reduction in proliferation and in chondrogenesis, but the percentage of cells undergoing proliferation in young and aged periosteal explants remained the same, consistent with our observations [58]. However, analysis of Cyclin D1 gene expression showed increased expression in young mice and enhanced responsiveness to PTH 1-34 administration compared to aged mice. Thus, the enhanced responsiveness, while primarily due to increased differentiation and matrix deposition, likely also includes a more robust increase in stem cell proliferation in young mice.
Since the canonical Wnt signaling pathway is a potent stimulator of bone formation, the anabolic effect of PTH 1-34 on fracture healing might be explained, at least in part, by the crosstalk between the PTH and Wnt signaling pathways [59-61]. In normal bone, PTH induces β-catenin signaling through 1) direct activation of the signaling pathway by association with the LRP5/6 or disheveled receptors; 2) induction of Wnt protein expression; or 3) down-regulation of Sost expression in osteocytes [31, 33, 62-65]. A role for β-catenin in fracture healing is clearly established in studies demonstrating that antibody blockade of SOST and DKK1 result in accelerated fracture healing [66-68]. A non-fracture model using Dmp1-caPthr1 mice suggested that the anabolic effects of PTH 1-34 signaling on periosteal cells is dependent upon β-catenin since the increased bone formation in this model could be blocked in mice that also over-expressed SOST or were deficient in LRP5 [36].
We confirm and extend prior work into the aging model by showing increased β-catenin signaling during fracture repair and demonstrate further induction following PTH 1-34 administration [31]. While we observed that active β-catenin levels are higher in day 5 mesenchyme and cartilage callus tissues of young mice compared to aged mice following bone injury, PTH 1-34 did not enhance active β-catenin immunostaining in cartilage tissues. However, in day 14 callus tissues, PTH 1-34 enhanced active β-catenin staining in osteoblasts. These data are consistent with gene expression and histomorphometry experiments that demonstrate a strong anabolic response in osteoblasts. In contrast, PTH 1-34 did not increase cartilage formation in the fracture callus and resulted in a delay in completion of endochondral ossification. The anabolic effect of PTH 1-34 on fracture healing is not related to the suppression of Sost or Dkk1 since, similar to others, we found that both inhibitors are elevated during fracture healing. Furthermore, we found that PTH 1-34 increases their expression [31]. The findings are surprising in that PTH 1-34 reduces Sost expression in normal bone and Dkk1, while highly expressed in young mice, is minimally expressed in aged mice [33] [68]. Thus, similar to the unique COX-2 expression and signaling observed in bone healing, a unique pattern of Wnt/β-catenin gene expression and cell responses occur in fracture healing that is distinct from that observed in normal bone tissue metabolism [16, 69].
Like other tissues, a fundamental requirement for the repair and regeneration of bone is the proliferation, accumulation, and differentiation of stem cell progenitors populations, including chondro- and osteo-progenitor populations . These findings establish that fracture healing in aging is associated with decreased proliferation and accumulation of periosteal stem cell populations and a subsequent delay in the differentiation of the expanded population of progenitor cells into cartilage and bone. The findings suggest that aging reduces the responsiveness of the stem cell populations to injury and to PTH 1-34 administration. As a preclinical study, this manuscript cannot make any definitive statements regarding the potential role of PTH1-34 in fracture healing in elderly patients. However, PTH 1-34 has already been proven to be anabolic for bone formation in elderly humans. Our findings suggest that PTH 1-34 will have anabolic activity in adult patients, but likely with reduced responses compared to anticipated effects in a young and healthy population. As aging is associated with an increase in fractures, is a risk factor for non-union and other morbidities, and is an increasing demographic group, future work fully characterizing the factors involved in reduced healing is critical. It is also essential that agents that are anabolic for bone repair be evaluated with aging, since this is the group at greatest risk for impaired bone regeneration and is a key target group for improved understanding and treatment of bone regeneration.
Supplementary Material
Highlights.
Histology and imaging showed enhanced fracture healing in young compared to aged mice.
Aged mice have reduced stem cell proliferation and osteoblast differentiation after fracture.
PTH stimulated stem cell proliferation, osteoblast differentiation, and fracture healing.
PTH enhanced bone regeneration in young and aged mice, but with reduced responsiveness with aging.
β-catenin is induced by PTH in early and late fracture healing and is a potential target of PTH.
Acknowledgments
We would like to thank Ryan Tierry, Sarah Mack and Nehal Porecha for their assistance with histological work and Michael Thullen for microCT analyses. This study is supported by National Health Services awards NIH RO1 AR48681 and NIH P50, AR054041.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kiminori Yukata, Email: Kiminori_Yukata@URMC.Rochester.edu.
Chao Xie, Email: Chao_Xie@URMC.Rochester.edu.
Tian-Fang Li, Email: TianFang_Li@URMC.Rochester.edu.
Masahiko Takahata, Email: Masahiko_Takahata@URMC.Rochester.edu.
Sirish Kondabolu, Email: Sirish_Kondabolu@URMC.Rochester.edu.
Xinping Zhang, Email: Xinping_Zhang@URMC.Rochester.edu.
Hani A. Awad, Email: Hani_Awad@URMC.Rochester.edu.
Edward M. Schwarz, Email: Edward_Schwarz@URMC.Rochester.edu.
Christopher A. Beck, Email: Beck@BST.Rochester.edu.
Jennifer H. Jonason, Email: Jennifer_Jonason@URMC.Rochester.edu.
References
- 1.Nilsson BE, Edwards P. Age and fracture healing: a statistical analysis of 418 cases of tibial shaft fractures. Geriatrics. 1969;24:112–7. [PubMed] [Google Scholar]
- 2.Nieminen S, Nurmi M, Satokari K. Healing of femoral neck fractures; influence of fracture reduction and age. Annales chirurgiae et gynaecologiae. 1981;70:26–31. [PubMed] [Google Scholar]
- 3.Hee HT, Wong HP, Low YP, Myers L. Predictors of outcome of floating knee injuries in adults: 89 patients followed for 2-12 years. Acta orthopaedica Scandinavica. 2001;72:385–94. doi: 10.1080/000164701753542050. [DOI] [PubMed] [Google Scholar]
- 4.Gruber R, Koch H, Doll BA, Tegtmeier F, Einhorn TA, Hollinger JO. Fracture healing in the elderly patient. Exp Gerontol. 2006;41:1080–93. doi: 10.1016/j.exger.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Kwong FN, Harris MB. Recent developments in the biology of fracture repair. J Am Acad Orthop Surg. 2008;16:619–25. doi: 10.5435/00124635-200811000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Mangione KK, Craik RL, Palombaro KM, Tomlinson SS, Hofmann MT. Home-based leg-strengthening exercise improves function 1 year after hip fracture: a randomized controlled study. Journal of the American Geriatrics Society. 2010;58:1911–7. doi: 10.1111/j.1532-5415.2010.03076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd BD, Williamson DA, Singh NA, Hansen RD, Diamond TH, Finnegan TP, Allen BJ, Grady JN, Stavrinos TM, Smith EU, Diwan AD, Fiatarone Singh MA. Recurrent and injurious falls in the year following hip fracture: a prospective study of incidence and risk factors from the Sarcopenia and Hip Fracture study. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009;64:599–609. doi: 10.1093/gerona/glp003. [DOI] [PubMed] [Google Scholar]
- 8.Ziden L, Kreuter M, Frandin K. Long-term effects of home rehabilitation after hip fracture - 1-year follow-up of functioning, balance confidence, and health-related quality of life in elderly people. Disability and rehabilitation. 2010;32:18–32. doi: 10.3109/09638280902980910. [DOI] [PubMed] [Google Scholar]
- 9.Becker DJ, Kilgore ML, Morrisey MA. The societal burden of osteoporosis. Current rheumatology reports. 2010;12:186–91. doi: 10.1007/s11926-010-0097-y. [DOI] [PubMed] [Google Scholar]
- 10.Cutler DM, Ghosh K. The potential for cost savings through bundled episode payments. The New England journal of medicine. 2012;366:1075–7. doi: 10.1056/NEJMp1113361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–84. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 12.Yu YY, Lieu S, Lu C, Colnot C. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone. 2010 doi: 10.1016/j.bone.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24:274–82. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR, Rubery PT, Schwarz EM, O’Keefe RJ, Guldberg RE. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res. 2005;20:2124–37. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clinical immunology. 2006;119:229–40. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Schwarz EM, Young DN, Puzas JE, Rosier RN, O’Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–15. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon AM, Manigrasso MB, O’Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002;17:963–76. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 18.Xie C, Liang B, Xue M, Lin AS, Loiselle A, Schwarz EM, Guldberg RE, O’Keefe RJ, Zhang X. Rescue of impaired fracture healing in COX-2-/- mice via activation of prostaglandin E2 receptor subtype 4. Am J Pathol. 2009;175:772–85. doi: 10.2353/ajpath.2009.081099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paralkar VM, Borovecki F, Ke HZ, Cameron KO, Lefker B, Grasser WA, Owen TA, Li M, DaSilva-Jardine P, Zhou M, Dunn RL, Dumont F, Korsmeyer R, Krasney P, Brown TA, Plowchalk D, Vukicevic S, Thompson DD. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6736–40. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, Guldberg R, Drissi H, Puzas JE, Boyce B, Zhang X, O’Keefe RJ. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Miner Res. 2009;24:251–64. doi: 10.1359/jbmr.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka M, Sakai A, Uchida S, Tanaka S, Nagashima M, Katayama T, Yamaguchi K, Nakamura T. Prostaglandin E2 receptor (EP4) selective agonist (ONO-4819.CD) accelerates bone repair of femoral cortex after drill-hole injury associated with local upregulation of bone turnover in mature rats. Bone. 2004;34:940–8. doi: 10.1016/j.bone.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Sugimoto Y, Narumiya S. Prostaglandin E receptors. The Journal of biological chemistry. 2007;282:11613–7. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Thompson DD, Paralkar VM. Prostaglandin E(2) receptors in bone formation. International orthopaedics. 2007;31:767–72. doi: 10.1007/s00264-007-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanyu R, Wehbi VL, Hayata T, Moriya S, Feinstein TN, Ezura Y, Nagao M, Saita Y, Hemmi H, Notomi T, Nakamoto T, Schipani E, Takeda S, Kaneko K, Kurosawa H, Karsenty G, Kronenberg HM, Vilardaga JP, Noda M. Anabolic action of parathyroid hormone regulated by the beta2-adrenergic receptor. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7433–8. doi: 10.1073/pnas.1109036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing Z, Lu C, Hu D, Miclau T, 3rd, Marcucio RS. Rejuvenation of the inflammatory system stimulates fracture repair in aged mice. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28:1000–6. doi: 10.1002/jor.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahl EC, Aronson J, Liu L, Fowlkes JL, Thrailkill KM, Bunn RC, Skinner RA, Miller MJ, Cockrell GE, Clark LM, Ou Y, Isales CM, Badger TM, Ronis MJ, Sims J, Lumpkin CK., Jr Restoration of regenerative osteoblastogenesis in aged mice: modulation of TNF. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:114–23. doi: 10.1359/jbmr.090708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta M, Strube P, Peters A, Perka C, Hutmacher D, Fratzl P, Duda GN. Influences of age and mechanical stability on volume, microstructure, and mineralization of the fracture callus during bone healing: is osteoclast activity the key to age-related impaired healing? Bone. 2010;47:219–28. doi: 10.1016/j.bone.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Meyer MH, Meyer RA., Jr Genes with greater up-regulation in the fracture callus of older rats with delayed healing. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25:488–94. doi: 10.1002/jor.20334. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds DG, Takahata M, Lerner AL, O’Keefe RJ, Schwarz EM, Awad HA. Teriparatide therapy enhances devitalized femoral allograft osseointegration and biomechanics in a murine model. Bone. 2011;48:562–70. doi: 10.1016/j.bone.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Misof BM, Paschalis EP, Blouin S, Fratzl-Zelman N, Klaushofer K, Roschger P. Effects of 1 year of daily teriparatide treatment on iliacal bone mineralization density distribution (BMDD) in postmenopausal osteoporotic women previously treated with alendronate or risedronate. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:2297–303. doi: 10.1002/jbmr.198. [DOI] [PubMed] [Google Scholar]
- 31.Kakar S, Einhorn TA, Vora S, Miara LJ, Hon G, Wigner NA, Toben D, Jacobsen KA, Al-Sebaei MO, Song M, Trackman PC, Morgan EF, Gerstenfeld LC, Barnes GL. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Miner Res. 2007;22:1903–12. doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- 32.Li TF, Chen D, Wu Q, Chen M, Sheu TJ, Schwarz EM, Drissi H, Zuscik M, O’Keefe RJ. Transforming growth factor-beta stimulates cyclin D1 expression through activation of beta-catenin signaling in chondrocytes. J Biol Chem. 2006;281:21296–304. doi: 10.1074/jbc.M600514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baron R, Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. The Journal of clinical endocrinology and metabolism. 2012;97:311–25. doi: 10.1210/jc.2011-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraenzlin ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nature reviews. Endocrinology. 2011;7:647–56. doi: 10.1038/nrendo.2011.108. [DOI] [PubMed] [Google Scholar]
- 35.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 36.Rhee Y, Allen MR, Condon K, Lezcano V, Ronda AC, Galli C, Olivos N, Passeri G, O’Brien CA, Bivi N, Plotkin LI, Bellido T. PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:1035–46. doi: 10.1002/jbmr.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22:495–502. doi: 10.1359/jbmr.070104. [DOI] [PubMed] [Google Scholar]
- 38.Histing T, Garcia P, Holstein JH, Klein M, Matthys R, Nuetzi R, Steck R, Laschke MW, Wehner T, Bindl R, Recknagel S, Stuermer EK, Vollmar B, Wildemann B, Lienau J, Willie B, Peters A, Ignatius A, Pohlemann T, Claes L, Menger MD. Small animal bone healing models: standards, tips, and pitfalls results of a consensus meeting. Bone. 2011;49:591–9. doi: 10.1016/j.bone.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Meyer RA, Jr, Meyer MH, Tenholder M, Wondracek S, Wasserman R, Garges P. Gene expression in older rats with delayed union of femoral fractures. The Journal of bone and joint surgery American. 2003;85-A:1243–54. doi: 10.2106/00004623-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Meyer RA, Jr, Meyer MH, Tenholder M, Wondracek S, Wasserman R, Garges P. Gene expression in older rats with delayed union of femoral fractures. J Bone Joint Surg Am. 2003;85-A:1243–54. doi: 10.2106/00004623-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Lu C, Miclau T, Hu D, Hansen E, Tsui K, Puttlitz C, Marcucio RS. Cellular basis for age-related changes in fracture repair. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2005;23:1300–7. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu C, Hansen E, Sapozhnikova A, Hu D, Miclau T, Marcucio RS. Effect of age on vascularization during fracture repair. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26:1384–9. doi: 10.1002/jor.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakazawa T, Nakajima A, Shiomi K, Moriya H, Einhorn TA, Yamazaki M. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1-34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005;37:711–9. doi: 10.1016/j.bone.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999;14:960–8. doi: 10.1359/jbmr.1999.14.6.960. [DOI] [PubMed] [Google Scholar]
- 45.Alkhiary YM, Gerstenfeld LC, Krall E, Westmore M, Sato M, Mitlak BH, Einhorn TA. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34) J Bone Joint Surg Am. 2005;87:731–41. doi: 10.2106/JBJS.D.02115. [DOI] [PubMed] [Google Scholar]
- 46.Kaback LA, Soung do Y, Naik A, Geneau G, Schwarz EM, Rosier RN, O’Keefe RJ, Drissi H. Teriparatide (1-34 human PTH) regulation of osterix during fracture repair. J Cell Biochem. 2008;105:219–26. doi: 10.1002/jcb.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andreassen TT, Fledelius C, Ejersted C, Oxlund H. Increases in callus formation and mechanical strength of healing fractures in old rats treated with parathyroid hormone. Acta Orthop Scand. 2001;72:304–7. doi: 10.1080/00016470152846673. [DOI] [PubMed] [Google Scholar]
- 48.Nozaka K, Miyakoshi N, Kasukawa Y, Maekawa S, Noguchi H, Shimada Y. Intermittent administration of human parathyroid hormone enhances bone formation and union at the site of cancellous bone osteotomy in normal and ovariectomized rats. Bone. 2008;42:90–7. doi: 10.1016/j.bone.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 49.Tagil M, McDonald MM, Morse A, Peacock L, Mikulec K, Amanat N, Godfrey C, Little DG. Intermittent PTH(1-34) does not increase union rates in open rat femoral fractures and exhibits attenuated anabolic effects compared to closed fractures. Bone. 2010;46:852–9. doi: 10.1016/j.bone.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Friedl G, Turner RT, Evans GL, Dobnig H. Intermittent parathyroid hormone (PTH) treatment and age-dependent effects on rat cancellous bone and mineral metabolism. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25:1454–64. doi: 10.1002/jor.20433. [DOI] [PubMed] [Google Scholar]
- 51.Doyon AR, Ferries IK, Li J. Glucocorticoid attenuates the anabolic effects of parathyroid hormone on fracture repair. Calcified tissue international. 2010;87:68–76. doi: 10.1007/s00223-010-9370-3. [DOI] [PubMed] [Google Scholar]
- 52.Lui JC, Baron J. Effects of glucocorticoids on the growth plate. Endocrine development. 2011;20:187–93. doi: 10.1159/000321244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun P, Cai DH, Li QN, Chen H, Deng WM, He L, Yang L. Effects of alendronate and strontium ranelate on cancellous and cortical bone mass in glucocorticoid-treated adult rats. Calcified tissue international. 2010;86:495–501. doi: 10.1007/s00223-010-9363-2. [DOI] [PubMed] [Google Scholar]
- 54.Hardy R, Cooper MS. Glucocorticoid-induced osteoporosis - a disorder of mesenchymal stromal cells? Frontiers in endocrinology. 2011;2:24. doi: 10.3389/fendo.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turner CH. Aging and fragility of bone. Journal of musculoskeletal & neuronal interactions. 2007;7:342–3. [PubMed] [Google Scholar]
- 56.Fan W, Crawford R, Xiao Y. Structural and cellular differences between metaphyseal and diaphyseal periosteum in different aged rats. Bone. 2008;42:81–9. doi: 10.1016/j.bone.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 57.Seeman E. The periosteum--a surface for all seasons. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2007;18:123–8. doi: 10.1007/s00198-006-0296-6. [DOI] [PubMed] [Google Scholar]
- 58.O’Driscoll SW, Saris DB, Ito Y, Fitzimmons JS. The chondrogenic potential of periosteum decreases with age. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2001;19:95–103. doi: 10.1016/S0736-0266(00)00014-0. [DOI] [PubMed] [Google Scholar]
- 59.Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433:1–7. doi: 10.1016/j.gene.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Kim JB, Leucht P, Lam K, Luppen C, Ten Berge D, Nusse R, Helms JA. Bone regeneration is regulated by wnt signaling. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22:1913–23. doi: 10.1359/jbmr.070802. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4:e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sims NA. Building bone with a SOST-PTH partnership. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:175–7. doi: 10.1002/jbmr.53. [DOI] [PubMed] [Google Scholar]
- 63.Ono N, Nakashima K, Schipani E, Hayata T, Ezura Y, Soma K, Kronenberg HM, Noda M. Constitutively active PTH/PTHrP receptor specifically expressed in osteoblasts enhances bone formation induced by bone marrow ablation. Journal of cellular physiology. 2012;227:408–15. doi: 10.1002/jcp.22986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes & development. 2008;22:2968–79. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romero G, Sneddon WB, Yang Y, Wheeler D, Blair HC, Friedman PA. Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-Catenin signaling and osteoclastogenesis. The Journal of biological chemistry. 2010;285:14756–63. doi: 10.1074/jbc.M110.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li C, Ominsky MS, Tan HL, Barrero M, Niu QT, Asuncion FJ, Lee E, Liu M, Simonet WS, Paszty C, Ke HZ. Increased callus mass and enhanced strength during fracture healing in mice lacking the sclerostin gene. Bone. 2011;49:1178–85. doi: 10.1016/j.bone.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 67.Ominsky MS, Li C, Li X, Tan HL, Lee E, Barrero M, Asuncion FJ, Dwyer D, Han CY, Vlasseros F, Samadfam R, Jolette J, Smith SY, Stolina M, Lacey DL, Simonet WS, Paszty C, Li G, Ke HZ. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:1012–21. doi: 10.1002/jbmr.307. [DOI] [PubMed] [Google Scholar]
- 68.Li X, Grisanti M, Fan W, Asuncion FJ, Tan HL, Dwyer D, Han CY, Yu L, Lee J, Lee E, Barrero M, Kurimoto P, Niu QT, Geng Z, Winters A, Horan T, Steavenson S, Jacobsen F, Chen Q, Haldankar R, Lavallee J, Tipton B, Daris M, Sheng J, Lu HS, Daris K, Deshpande R, Valente EG, Salimi-Moosavi H, Kostenuik PJ, Li J, Liu M, Li C, Lacey DL, Simonet WS, Ke HZ, Babij P, Stolina M, Ominsky MS, Richards WG. Dickkopf-1 regulates bone formation in young growing rodents and upon traumatic injury. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:2610–21. doi: 10.1002/jbmr.472. [DOI] [PubMed] [Google Scholar]
- 69.Xie C, Ming X, Wang Q, Schwarz EM, Guldberg RE, O’Keefe RJ, Zhang X. COX-2 from the injury milieu is critical for the initiation of periosteal progenitor cell mediated bone healing. Bone. 2008;43:1075–83. doi: 10.1016/j.bone.2008.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


