Abstract
The precocial nature of orofacial sensorimotor control underscores the biological importance of establishing ororythmic activity in human infants. The purpose of this study was to assess the effects of comparable doses of three forms of orosensory experience, including a low-velocity spectrally reduced orocutaneous stimulus (NT1), a high-velocity broad spectrum orocutaneous stimulus (NT2), and a SHAM stimulus consisting of a blind pacifier. Each orosensory experience condition was paired with gavage feedings 3x/day for 10 days in the neonatal intensive care unit (NICU). Four groups of preterm infants (N=214), including those with respiratory distress syndrome (RDS), chronic lung disease (CLD), infants of diabetic mothers (IDM), and healthy controls (HI) were randomized to the type of orosensory condition. Mixed modeling, adjusted for gender, gestational age, postmenstrual age, and birth weight, demonstrated the most significant gains in non-nutritive suck (NNS) development among CLD infants who were treated with the NT2 stimulus, with smaller gains realized among RDS and IDM infants. The broader spectrum of the NT2 stimulus maps closely to known response properties of mechanoreceptors in lip, tongue, and oral mucosa and is more effective in promoting NNS development among preterm infants with impaired oromotor function compared to the low-velocity, spectrally reduced NT1 orosensory stimulus.
Keywords: Pulsatile oral stimulation, Power spectrum, non-nutritive suck, CLD, IDM, preterm birth, trigeminal nerve, mechanoreceptors
Introduction
The trigeminal and facial cranial nerve systems attain functionality well before term gestational age (GA) in humans. Toward the end of the 8th week post-menstrual age (PMA), light tactile stimulation of the lips and face evoke a rooting and orienting response (Humphrey, 1970). The precocial nature of orofacial touch sensitivity underscores the biological importance of establishing motor rhythms as evidenced by the presence of sucking, swallowing, and chest wall movements in utero (Popescu et al., 2008). In the infant’s mouth and tongue, there are at least three known types of rapidly-conducting Aβ mechanoreceptors, including Merkel cells, Meissner’s corpuscles, and Ruffini nerve endings which transmit touch, pressure, vibration and motion sense information along trigeminal pathways to the developing thalamus and sensorimotor cortex. Each mechanoreceptor type exhibits a unique response profile. For example, the Ruffini ending is most responsive to slow indentations of the lip and encode position, whereas the Meissner corpuscle is most responsive to rapid changes in skin indentation and pressure (e.g., vibration). Collectively, these mechanoreceptors make it possible for the infant to appreciate a wide range of oral experiences, some of which are presumed to be soothing (e.g., light touch from a caretaker’s finger, stiffness of a pacifier, infant’s fingers, mother’s breast) whereas other unexpected orosensory experiences may lead to maladaptive oral aversion (e.g., orotracheal intubation, nasogastric feeding tube, ventilator, tape on the skin) at a critical period of brain development for ororhythmic pattern formation (Barlow, 2009a; Barlow et al., 2010; Shiao et al., 1995).
The central nervous system and oromotor pattern generation is vulnerable to delay and injury in RDS, CLD, and IDM infants (Barlow 2009b; Khaksar, Jelodar, and Hematian, 2012; Nold and Georgieff, 2004; de Regnier et al., 2007). For example, infants diagnosed with CLD often manifest oromotor dyscoordination, absent or weak suck, poor airway protection, dysphagia, and poor state control (Gewolb and Vice, 2006). Delayed development of NNS is well documented in preterm RDS infants (Poore et al., 2008; Stumm et al., 2008; Estep et al., 2008). The invasiveness of lengthy intubation, oxygen supplementation, and nasogastric feeding procedures associated with prematurity and lung disease cost the baby precious sensory and motor experiences during a critical period of brain development for oromotor pattern generation (Bosma, 1973). Preterm infants of diabetic mothers (IDM) babies exhibit macrosomia but are lethargic when attempting sucking and feeding (de Regnier et al., 2007). A common approach is to provide preterm infants with orotactile stimulation to promote sucking. The dose, skin site, stimulus features (slow vs fast touch, low vs high pacifier stiffness, etc.), vary widely among NICUs, including but not limited to a gloved finger, gentle stroking of the mouth using a finger or cotton swab, silicone pacifier, or a computerized oral entrainment system. Overall, controlled somatosensory stimulation strategies have proven beneficial in developing NNS and oral feeding skills in premature infants (Fucile, Gisel, Lau, 2005; Fucile et al., 2011; Rocha et al., 2007). Recently, a pressure-modulated pacifier was developed to provide tube fed preterm infants with a pulsatile orosensory experience that closely mimics the expected spatiotemporal pattern of non-nutritive suck (Barlow et al., 2008).
Each form of oral somatosensory stimulation has a unique spectral ‘frequency’ signature (power spectrum) that will activate a subset of mechanoreceptor types while leaving other mechanoreceptors in a quiescent state. The relation between orocutaneous stimulus power spectrum and NNS development in preterm infants is unknown. In the present study, we systematically investigate the effects of comparable doses of 3 different types of orosensory experience, including a high-velocity broad spectrum orocutaneous stimulus, a low-velocity spectrally reduced orocutaneous stimulus, and a SHAM condition consisting of a silicone pacifier. Given the exquisite frequency sensitivity of trigeminal mechanoreceptors in orofacial tissues, it is hypothesized that the high-velocity broad spectrum orocutaneous stimulus (with frequency components up to 16 Hz), will be significantly more effective in promoting NNS development among preterm infants compared to either a low-velocity reduced spectrum orocutaneous stimulus (DC to 2 Hz) or the SHAM pacifier condition.
Methods
Participants
A randomized design was used to evaluate the efficacy of pneumatic orocutaneous stimulation on NNS development among 214 newborn infants (116F/98M) distributed among 4 preterm subpopulations, including 55 healthy infants (HI), 35 infants of diabetic mothers (IDM), 38 infants with respiratory distress syndrome (RDS), and 87 infants with chronic lung disease (CLD) (power ≥ .80 at α=.05). Participant characteristics are given in Table 1. The human subjects committees at the Overland Park Regional Medical Center (Overland Park, Kansas), and Stormont-Vail Regional HealthCare (Topeka, Kansas) approved the research protocol for this study. Written informed consent was obtained at each NICU prior to the participants’ enrollment into the study. Staff involved in nursing care of study participants were blinded to treatment condition for the duration of the 2-week intervention protocol. Infants were randomly assigned to one of three conditions, including NT1 (low velocity orocutaneous), NT2 (high velocity orocutaneous), or SHAM pacifier control.
Table 1.
Preterm infant characteristics. Mean (SD) are given for several clinical variables.
| Healthy Infants HI (N=55) | Infants of Diabetic Mothers IDM (N=35) | Respiratory Distress Syndrome RDS (N=38) | Chronic Lung Disease CLD (N=87) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Blind N=20 |
NT1 N=20 |
NT2 N=15 |
Blind N=10 |
NT1 N=14 |
NT2 N=10 |
Blind N=17 |
NT1 N=11 |
NT2 N=10 |
Blind N=39 |
NT1 N=23 |
NT2 N=25 |
|
| Gender (♂:♀) | 9:11 | 6:14 | 10:5 | 6:4 | 8:6 | 4:6 | 10:7 | 7:4 | 7:3 | 20:19 | 15:8 | 14:11 |
|
| ||||||||||||
| GAbirth (wks) | 32.1 (1.9) | 31.7 (1.8) | 31.7 (1.8) | 33.1 (2.9) | 32.1 (1.0) | 31.6 (3.4) | 30.8 (1.7) | 30.9 (2.0) | 31.6 (2.6) | 26.7 (2.1) | 26.1 (1.7) | 27.2 (1.8) |
|
| ||||||||||||
| BW (gms) | 1773.9 (512.3) | 1665.2 (420.2) | 1701 (349.3) | 2167 (809.8) | 2068.8 (519.4) | 2072.9 (956.9) | 1493.9 (307.7) | 1341.7 (378.3) | 1570.9 (671.5) | 894.9 (367.6) | 812.6 (220.3) | 908.6 (244.5) |
|
| ||||||||||||
| PMAstart (wks) | 34.6 (1.4) | 34.5 (1.6) | 34.7 (1.4) | 34.7 (2.5) | 35.2 (2.3) | 35.7 (2.5) | 33.9 (1.7) | 35.1 (1.5) | 35.3 (2.0) | 35.0 (2.0) | 35.5 (2.1) | 35.5 (1.2) |
|
| ||||||||||||
| %POstart | 31 (35) | 23 (32) | 29 (33) | 14 (29) | 26 (30) | 30 (23) | 8 (18) | 20 (24) | 20 (33) | 13 (19) | 8 (13) | 15 (19) |
|
| ||||||||||||
| O2 Hx (days) | 5.1 (8.9) | 2.2 (3.6) | 2.1 (2.6) | 2.4 (3.1) | 6.3 (9.1) | 15.5 (16.6) | 18.0 (12.6) | 15.5 (8.0) | 19.3 (10.2) | 70.8 (26.9) | 73.8 (29.4) | 63.9 (22.8) |
Population 1
HI designates preterm infants (N=55; 20 SHAM, 20 NT1, 15 NT2) with no specific diagnosis who were otherwise medically stable. Inclusion criteria: born between 23 0/7 and 36 6/7 weeks GA, as determined by obstetric ultrasound and clinical examination, minimal or no oxygen history (≤ 5 days of ventilator, CPAP, and nasal cannula).
Population 2
IDM includes neonates born to mothers with diabetes (gestation or other forms) (N=35; 10 SHAM, 14 NT1, 10 NT2). These infants are born with macrosomia but are lethargic when it comes to early neonatal oromotor skills. Inclusion criteria: born between 23 and 40 weeks GA, days on oxygen <28 days [15].
Population 3
RDS infants (N=38; 17 SHAM, 11 NT1, 10 NT2) had an active diagnosis of respiratory distress syndrome as confirmed by X-ray earlier in their hospital stay and required respiratory support. These infants typically have oxygen therapy due to their lungs not being completely developed and/or to surfactant deficiency. Inclusion criteria: born between 23 0/7 and 36 6/7 weeks GA, as determined by obstetric ultrasound and clinical examination, documented oxygen history for treatment of RDS (days on ventilator + CPAP + nasal cannula).
Population 4
CLD includes sicker preterm infants (N=87; 39 SHAM, 23 NT1 treatment, 25 NT2 treatment) with chronic lung disease and occurs primarily in babies who manifest an oxygen dependency at 36 weeks PMA or beyond
Inclusion criteria: born between 23 0/7 and 36 6/7 weeks GA, and days on oxygen > 28 days [15]. Neurological examination included brain ultrasound and/or MRI to document the severity and localization of intraventricular hemorrhage (IVH) or periventricular leukomalacia (PVL) common to CLD infants.
General inclusion criteria: no functional suck and tube-fed at 34 weeks PMA, head circumference within 10–90th percentile of mean for PMA, neurological examination showing no anomalies for PMA: response to light, sound, and spontaneous movements of all extremities, and with stable vital signs (heart rate, blood pressure, age appropriate respiratory rate, and SpO2 > 92%) to allow for NNS. General exclusion criteria: IVH grades III or IV, other intracranial hemorrhage, PVL, necrotizing enterocolitis, neonatal seizures and culture positive sepsis or meningitis at time of testing, chromosomal anomalies or craniofacial malformation, nervous system anomalies, cyanotic congenital heart disease, gastroschisis, omphalocele, diaphragmatic hernia and/or other major gastrointestinal anomalies, or not ready for oral feedings as determined by the health care team.
Pulsed Oral Somatosensory Stimulation (NT1 and NT2) and SHAM Stimulation Conditions
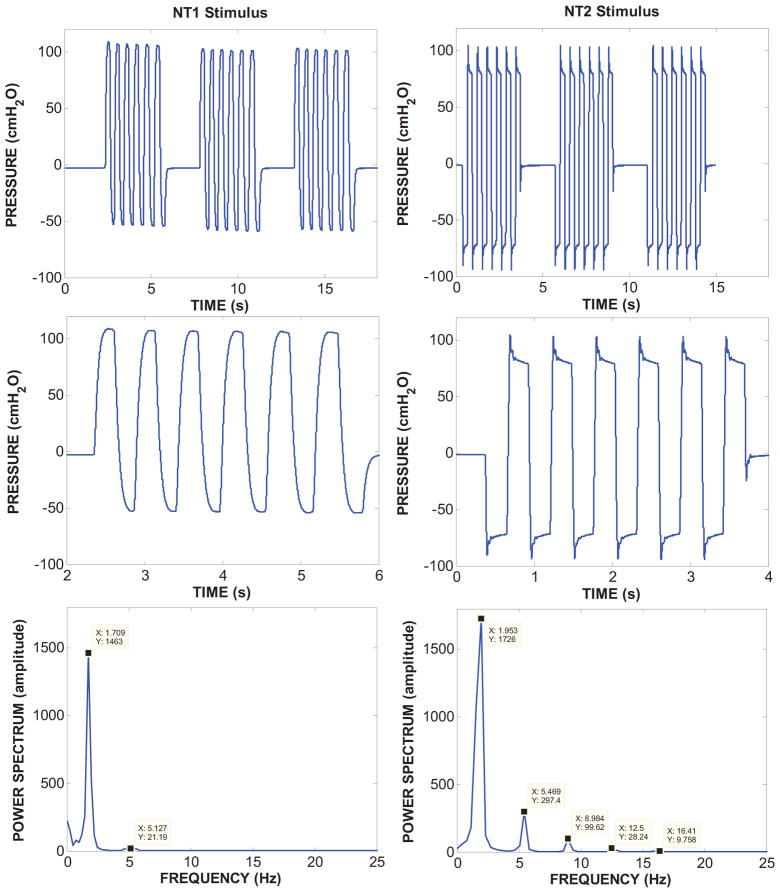
Pulsed orocutaneous stimuli were generated with the NTrainer System® (Innara Health, Inc., Shawnee, Kansas). This device includes a digitally-controlled pneumatic amplifier and handpiece which accepts a regular Philips AVENT Soothie® silicone pacifier (Figure 1). The orocutaneous stimuli were was programmed to mimic the temporal features of an NNS burst (Barlow et al., 2013). Examples of the NT1 and NT2 stimuli with their respective power spectra are shown in Figure 2. The NT1 stimulus waveform has a relatively slow pressure rise/fall time (~145 ms) compared to the brisk NT2 stimulus (~31 ms). The NT2 stimulus has an enhanced power spectrum with a fundamental frequency at 1.95 Hz and significant harmonic energy up to 16.4 Hz. In contrast, the NT1 stimulus has only one harmonic beyond the fundamental frequency. The resultant displacement at the pacifier-tissue interface was approximately 400 μm for the NT1 and NT2 stimuli. The 3-minute orocutaneous stimulation periods included 34 burst-pause trains, and were interleaved with 5.5 minute rest periods during which the pneumatically-charged Soothie pacifier was removed from the mouth (see Table 2). Infants assigned to the SHAM condition were offered the same type of Soothie pacifier minus the pneumatically patterned stimulus. The stimulation regimen was repeated 3 times per day for up to 10 days according to their 3-hour feed cycles, or until the infant attained 90% oral feeds for two consecutive days. Overhead lighting was dimmed in the immediate area to promote eye contact with the neonatal study nurse. Infants were swaddled with limbs at midline, and in a quiet-awake to drowsy state during stimulation (NIDCAP state 3, 4 or 5) (Als, 1995). Observers could not differentiate which babies received the NT1, NT2, or the SHAM condition since the NTrainer System® and pacifier handpiece was used for all NNS stimulation and assessment procedures.
Figure 1.
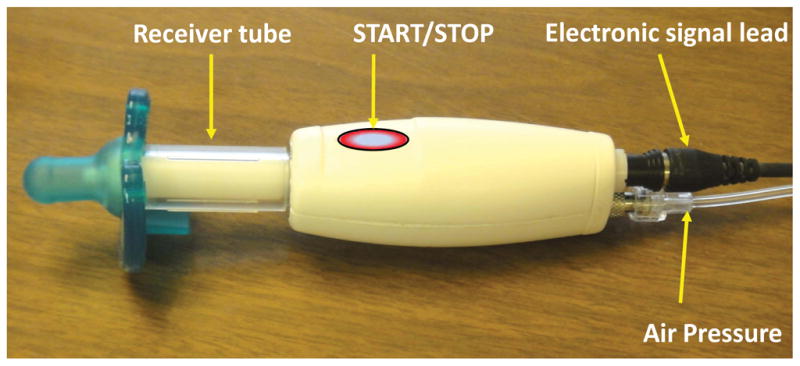
Pneumatic entrainment handpiece (NTrainer System®, Innara Health, Inc., Shawnee, KS USA) with silicone pacifier coupled to a sterilized receiver tube. An air pressure sensor and electronic valve is integrated within the body of the handpiece. Servo-controlled pressure pulses are transmitted and valved through the handpiece into the pacifier to produce pulsatile mechanical stimuli realized as controlled deflections of the surface of the pacifier bulb. The same handpiece is also used to sample the infant’s NNS motor pattern. The START/STOP touch switch allows the user to control automated data acquisition and stimulus control protocols on the host microprocessor.
Figure 2.
NT1 and NT2 pneumatic stimuli and their respective power spectra are shown when coupled to a silicone pacifier. A series of 3 stimulus bursts are shown in the top row. An expanded view of a single pressure waveform stimulus burst is shown in the middle row. The NT1 stimulus is characterized by a relatively long rise/fall time in the intraluminal pressure signal (~145 ms) compared to the much faster NT2 stimulus (~ 31 ms) based on 10–90% intercepts). The brisk NT2 stimulus is reflected in an enhanced power spectrum (bottom row-right panel) which shows a fundamental peak at 1.95 Hz and significant harmonic energy up to 16.4 Hz. The relatively slow NT1 stimulus is limited to a fundamental peak with minimal harmonic energy.
Table 2.
Orofacial stimulation schedule. The pulsed (NT1, NT2) or SHAM ‘blind’ pacifier conditions randomized among study infants are presented during P1, P3, and P5. No oral stimulation is presented during P2 and P4.
|
|
|||||
|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | |
| Experimental | NT-On | NT-Off | NT-On | NT-Off | NT-On |
|
| |||||
| Control | SHAM-On | SHAM-Off | SHAM-On | SHAM-Off | SHAM-On |
|
| |||||
| Duration | 3 min | 5.5 min | 3 min | 5.5 min | 3 min |
|
| |||||
| NG Feed |
|
||||
|
|
|||||
NNS progression
NNS evolution was measured through daily 3-minute digitized recordings of nipple compression pressure completed at the beginning of a gavage feed using the NTrainer System® handpiece and silicone pacifier. During these NNS recording sessions, infants did not receive the pulsed orocutaneous stimulation. The NNS pressure signal was digitized at 3000 samples/sec (16-bits @ 3V full-scale).
Automated NNS digital signal processing and feature extraction
The most active 2-minute period of NNS behavior based on suck cycle count was automatically extracted from each suck assessment data file using a waveform feature extraction algorithm on the NTrainer System®. The NNS pressure waveform was band-pass filtered (0.5 – 20 Hz) to remove low frequency offsets due to tongue/jaw posturing and thermal drift associated with oral contact on the pacifier bulb, and to remove high-frequency jitter. Pressure peaks greater than 1.6 cmH2O were subject to feature extraction criteria, including suck cycle symmetry, cycle duration, and burst identification defined as two or more NNS events occurring within 1200 milliseconds. This algorithm permits objective identification of NNS burst activity distinct from non-NNS mouthing compressions or tongue thrusts against the pacifier. Five measures were objectively extracted from the indexed records of suck, including minute-rates for (1) NNS Bursts where an individual burst includes 2 or more suck cycles, (2) NNS Cycles defined as suck compression cycles with cycle periods less than 1200 milliseconds, (3) Oral Compressions defined as the sum of all pressure events, (4) the proportion of oral compression events specific to NNS activity, designated %NNS, and finally (5) NNS compression pressure expressed in cmH2O.
Advancement to Oral Feeding
Infants were given the Enfamil Grad-U-Feed® nurser when transitioning from NG to oral feeds using standard practice. Infants were allowed to attempt oral feeds as long as their SpO2 ≥ 92% with stable heart rate. Infants exhibiting O2 desaturations, breathing difficulties, spit-ups, postural distress, and bradycardia during oral feed attempts were fed by NG tube. Nutritional content was specific to each infant per physician’s order to satisfy caloric demands. Regarding additives, lipids were given intravenously, not orally. Other additives to the formula via bottle or NG tube included any medication that is indicated ‘by mouth’ (i.e., vitamins, caffeine, Actigall, etc.).
Statistical analysis
The primary endpoint was the longitudinal comparison of NNS performance between three stimulus types (SHAM, NT1, NT2), each consisting of four preterm infant groups (HI, RDS, CLD, IDM). Mixed modeling analyses were conducted to handle interdependency among the performance variables repeatedly measured at multiple time points. Adjusting for the infants’ gender, GA, PMA, and birth weight, mixed models examined (linear) growth over time as well as stimulus type effect and infant group effect via restricted maximum likelihood estimation, which produces unbiased estimates under the conditions of unbalanced sample and/or incomplete data. If the stimulus type effect or infant group effect was significant at 0.05 alpha level, adjusted means were pairwise compared using Bonferroni correction for inflated Type I error. A compound symmetric error covariance structure minimized Akaike Information Criterion and Bayesian Information Criterion and thus was chosen for the mixed models. All analyses were conducted using SAS 9.3 (SAS Institute, 2008).
RESULTS
The high-velocity NT2 orosensory experience yielded significant increases and moderate effects sizes among several NNS performance measures when compared to the SHAM and low-velocity NT1 conditions. These are summarized in the following sections according to the dependent measures for combined and individual preterm groups.
NNS Bursts/minute
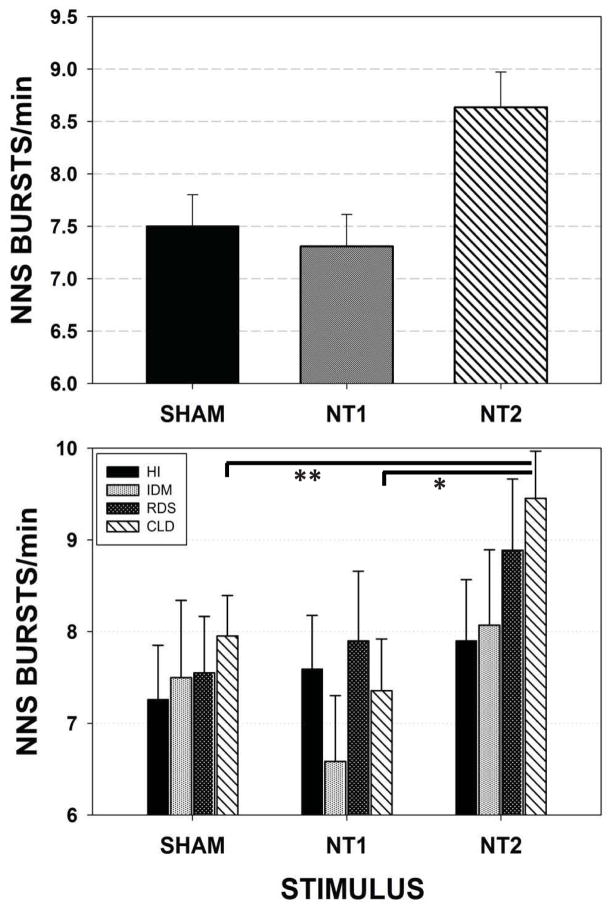
Figure 3 illustrates the adjusted means and standard errors of NNS Bursts/minute. A significant effect for stimulus type (SHAM, NT1, and NT2) when preterm groups are combined (p=.003) is shown in the upper panel. The spectrally enhanced NT2 stimulus yielded greater minute-rates for NNS burst production when compared to either the SHAM (p<.05, d=.40) or NT1 (p<.01, d=.44) conditions. Follow-up analysis (lower panel) among individual preterm groups revealed the most significant gains among CLD infants in NNS Burst production following NT2 stimulation compared to NT1 (p<.01, d=.43) and SHAM (p<.05, d=.35) conditions.
Figure 3.
Mixed model adjusted means for the dependent variable NNS Bursts/minute among the four preterm participant groups combined (upper panel) and pairwise comparisons among individual preterm groups (lower panel). [Error bars represent standard error (SE) of the mean; * p<.01, ** p<.05].
NNS Cycles/minute
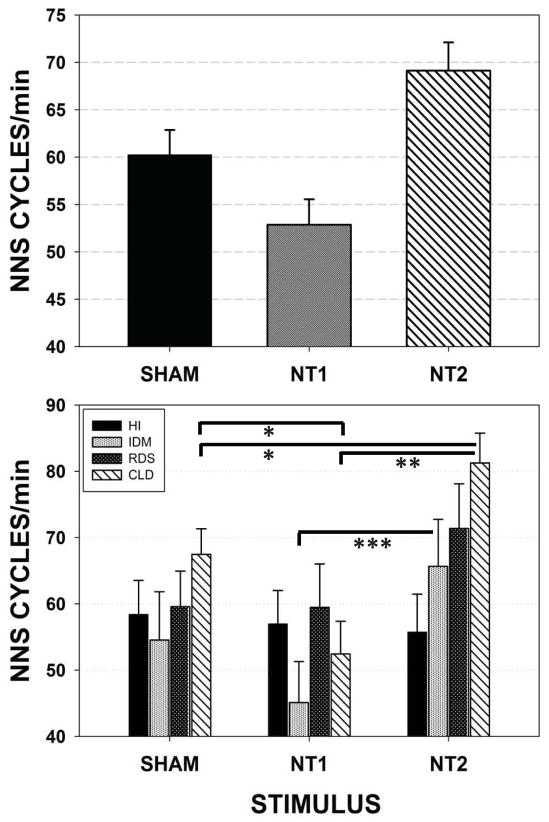
Figure 4 illustrates the adjusted means and standard errors of NNS Cycles/minute. A significant effect for stimulus type (SHAM, NT1, and NT2) when preterm groups are combined (p<.001) is shown in the upper panel. This measure reflects the neonate’s motor ability in generating suck compression cycles that exceed the minimum threshold value of 1.6 cmH2O. NT2 stimulus yielded significantly greater NNS Cycles/minute than did SHAM (p<.05, d=.35) and NT1 (p<.01, d=.61) conditions. NNS Cycles/minute increased from an average of approximately 60.23 NNS cycles/minute in the SHAM condition, to more than 69.13 NNS Cycles/minute in the NT2 condition, representing an increase of 14.78% over the SHAM condition, and 16.83% over the NT1 stimulus condition. Follow-up analysis (lower panel) among individual preterm groups revealed the most significant gains among CLD infants in the minute-rate for NNS Cycle production following NT2 stimulation compared to NT1 (p<.001, d=.68) and SHAM (p<.05, d=.37) conditions. Preterm infants of diabetic mothers (IDM) also showed an increase in the average NNS Cycles/minute between NT1 (45.1) and NT2 (65.7) stimulus conditions.
Figure 4.
Mixed model adjusted means for the dependent variable NNS Cycles/minute among the four preterm participant groups combined (upper panel) and pairwise comparisons among individual preterm groups (lower panel). [Error bars represent SE of the mean; * p<.05, ** p<.001, *** p=.057].
Oral Compression Events/minute
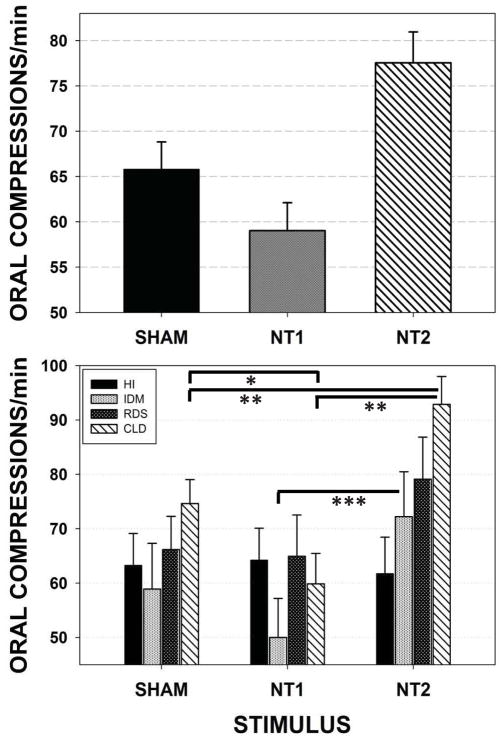
Figure 5 illustrates the adjusted means and standard errors of Oral Compression Events/minute. This oromotor variable reflects both NNS and non-NNS mouthing (e.g., jaw/tongue squeeze, jaw tremor that exceed the minimum threshold value of 1.6 cmH2O) compression behaviors. A significant effect for stimulus type (SHAM, NT1, and NT2) when preterm groups are combined (p<.001) is shown in the upper panel. There are two notable findings in the combined data. First, the NT1 (low-velocity) orosensory input actually reduced Oral Compression Events/minute among preterm infants. Second, NT2 stimulation yielded a significant increase in Oral Compression Events/minute from an average of approximately 65.80 Oral Compression Events/minute in the SHAM condition, to 77.55 Oral Compression Events/minute in the NT2 stimulus condition, representing an increase of 17.86% over the SHAM (p<.05, d=.41), and 19.90% over the NT1 condition (p<.01, d=.60). Follow-up analysis among individual preterm groups revealed the most significant gain among CLD infants in Oral Compression Events/minute production following NT2 stimulation compared to NT1 (p<.001, d=.69) and SHAM (p<.01, d=.43) conditions.
Figure 5.
Mixed model adjusted means for the dependent variable Oral Compressions/minute among the four preterm participant groups combined (upper panel) and pairwise comparisons among individual preterm groups (lower panel). [Error bars represent SE of the mean; * p<.05, ** p<.01, *** p=.088].
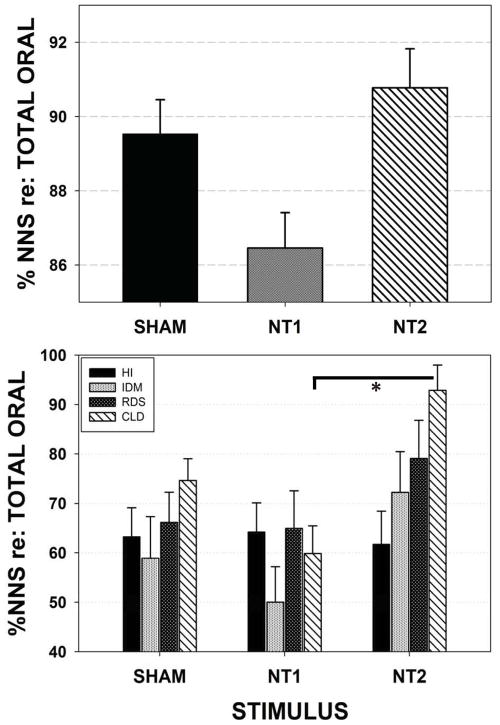
%NNS
Figure 6 illustrates the adjusted means and standard errors (SE) of %NNS. %NNS reflects the proportion of oral mouthing compressions that are part of non-nutritive burst pattern formation, which operationally defined includes at least 2 consecutive suck compression cycles with a cycle period of 1200 milliseconds or less. The measure reflects the efficiency of the neonate’s oromotor pattern generator in producing NNS cycles that exceed the minimum threshold value of 1.6 cmH2O. A significant effect for stimulus type (SHAM, NT1, and NT2) when preterm groups are combined (p=.003) is shown in the upper panel. The NT2 stimulus yielded significantly greater %NNS production than did NT1 (p<.01, d=.46) condition. Follow-up analysis (lower panel) among individual preterm groups revealed the most significant gain among CLD infants in %NNS production following NT2 stimulation compared to NT1 (p<.05, d=.35). The negative effect of NT1 on %NNS production when compared to the SHAM and NT2 conditions is notable, with a difference of 3.6% and 5.0%, respectively.
Figure 6.
Mixed model adjusted means for the dependent variable %NNS events relative to the total number of oral compressions on the instrumented pacifier among the four preterm participant groups combined (upper panel) and pairwise comparisons among individual preterm groups (lower panel). [Error bars represent SE of the mean; * p<.05].
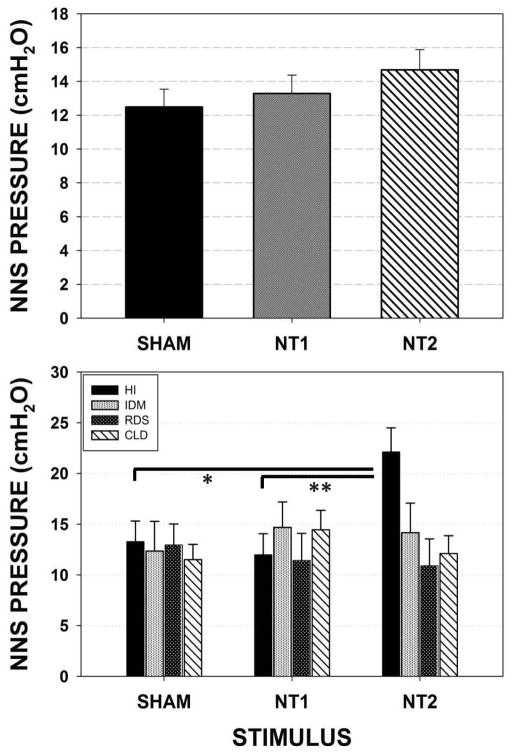
NNS Pressure
A slight increase in NNS pressure was found as one advanced from the SHAM condition to NT1, and to NT2 conditions, however this change did not reach statistical significance (upper panel of Figure 7). Follow-up analysis (lower panel) among individual preterm groups revealed a highly significant increase in NNS pressure for the HI infants between the SHAM and NT2 (p=.007, d=.44), and NT1 versus NT2 (p=.002, d=.49). For the HI infants, NNS pressure increased by 65.17% from 13.28 cmH2O to 22.13 cmH2O between the SHAM and the spectrally enhanced NT2 conditions. No significant increases in NNS pressure were found among IDM, RDS, and CLD preterm infants.
Figure 7.
Mixed model adjusted means for the dependent variable NNS Pressure (cmH2O) on the instrumented pacifier among the four preterm participant groups combined (upper panel) and pairwise comparisons among individual preterm groups (lower panel). [Error bars represent SE of the mean; * p=.007, ** p=.002].
DISCUSSION
The nature of an oral somatosensory intervention designed to promote ororhythmic activity is clearly dependent on its spectral (frequency) characteristics. We discovered that a pulsatile high-velocity NT2 stimulus is significantly more effective than either a pulsed low-velocity NT1 stimulus or a SHAM pacifier in providing preterm neonates with a salient somatosensory experience to facilitate development of the NNS. The most significant gains in NNS performance were observed for CLD and IDM preterm infants. One possible explanation for this finding is that the NT1 stimulus lacks salience to drive trigeminal mechanoreceptive afferents above background levels of neural activity. This may be due to sensory masking resulting from ongoing afferent activity associated with low frequency postural adjustments of the lower face and mouth produced by the infant, including their attempts to produce suck and other orofacial movements. By contrast, the NT2 stimulus, with its broader frequency content, provides the neonate with an enhanced oral somatosensory experience to drive the trigeminal system. We hypothesize that Meissner corpuscles and Merkel cells possess the most suitable frequency and amplitude sensitivities to encode the NT2 oral stimulation in preterm infants. Meissner corpuscles are most sensitive to skin vibrations less than 50 Hz and well matched to encode the NT2 oral stimulus. The Merkel ending is a slow adapting mechanoreceptor with small receptive fields (1–8 mm) on the face (2 receptors/mm2) and high sensitivity to low frequency vibration (~5 to 15 Hz) at micron displacement amplitudes, and thus, well suited for encoding fine form and texture of objects that are touched or manipulated in the mouth (i.e., pacifier). These mechanoreceptors transmit somatosensory cues to the ventroposteromedial nucleus of the thalamus at high conduction velocities (33–75 m/sec at 1-year of age) (Barlow, Finan, Bradford, and Andreatta, 1993; Barlow, Dusick, Finan, Coltart, and Biswas, 2001). The salience of the NT2 stimulus is consistent with experimental evidence on the neurobiology and mechanosensitivity of the human orofacial system (Barlow, 1987; Trulsson & Essick, 2004).
In summary, pulsed oral somatosensory entrainment therapies provided to preterm infants with delayed/disordered oromotor control should incorporate a high-velocity burst-pattern stimulus profile with significant spectral energy from 0 to 16 Hz to maximize NNS development.
Acknowledgments
This study was supported by grants NIH R01 DC003311 (SM Barlow), NIH P30 HD02528, and the Sutherland Foundation. The authors express gratitude to Joy Carlson, NNP and Mimi Burch, MS, for clinical support in patient recruitment and data collection, Drs. Meredith P. Harold and Emily Zimmerman for project support, Kenny Aron for engineering support, and the scores of families who participated in this project. This paper is dedicated to our dear colleague, Anna M. Dusick, MD (1955–2012), who encouraged us more than 20 years ago to pursue studies on oral sensorimotor development in the preterm infant.
Footnotes
Financial Disclosure. None of the authors have a direct financial relation with the manufacturers of the Soothie® pacifier, Honeywell Sensors, nor with the SAS statistical software. Dr. Barlow is the inventor of the NTrainer System® which is registered and licensed by the University of Kansas to Innara Health, Incorporated (Shawnee, Kansas USA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Als H. A manual for naturalistic observation of the newborn (preterm and full term infants) In: Goldson E, editor. Nurturing the premature infant, Developmental Interventions in the Neonatal Intensive Care Nursery. Oxford University Press; New York, NY, USA: 1995. pp. 77–85. [Google Scholar]
- Barlow SM. Mechanical frequency detection thresholds in the human face. Exp Neurol. 1987;96(2):253–261. doi: 10.1016/0014-4886(87)90044-6. [DOI] [PubMed] [Google Scholar]
- Barlow SM. Oral and respiratory control for preterm feeding. Curr Opinion Otolaryngol Head Neck Surg. 2009a;17(3):179–186. doi: 10.1097/MOO.0b013e32832b36fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow SM. Central pattern generation involved in oral and respiratory control for feeding in the term infant. Curr Opinion Otolaryngol Head Neck Surg. 2009b;17(3):187–193. doi: 10.1097/MOO.0b013e32832b312a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow SM, Dusick A, Finan DS, Coltart S, Biswas A. Mechanically evoked perioral reflexes in premature and term human infants. Brain Res. 2001;899(1–2):251–254. doi: 10.1016/s0006-8993(01)02239-9. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Finan DS, Bradford PT, Andreatta RD. Transitional properties of the mechanically evoked perioral reflex from infancy through adulthood. Brain Res. 1993;623(2):181–188. doi: 10.1016/0006-8993(93)91425-r. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Finan DS, Lee J, Chu S. Synthetic orocutaneous stimulation entrains preterm infants with feeding difficulties to suck. J Perinatol. 2008;28(8):541–548. doi: 10.1038/jp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow SM, Lund JP, Estep M, Kolta A. Central pattern generators for speech and orofacial activity. In: Brudzynski SM, editor. Handbook of Mammalian Vocalization. Elsevier; Oxford, UK: 2010. pp. 351–370. [Google Scholar]
- Barlow SM, Urish M, Venkatesan L, Harold M, Zimmerman E. Frequency modulation (FM) and spatiotemporal stability of the sCPG in preterm infants with RDS. Int J Pediatrics. 2012 doi: 10.1155/2012/581538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma JF. Prologue to the symposium. In: Bosma JF, editor. Fourth Symposium on Oral Sensation and Perception. Charles C. Thomas; Bethesda, MD, USA: 1973. p. 7. [PubMed] [Google Scholar]
- deRegnier RA, Long JD, Georgieff MK, Nelson CA. Using event-related potentials to study perinatal nutrition and brain development in infants of diabetic mothers. Dev Neuropsychol. 2007;31:379–396. doi: 10.1080/87565640701229524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep M, Barlow SM, Vantipalli R, Finan D, Lee J. Nonnutritive suck parameters in preterm infants with RDS. J Neonatal Nurs. 2008;14(1):28–34. doi: 10.1016/j.jnn.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S, Gisel E, Lau C. Effect of an oral stimulation program on sucking skill maturation of preterm infants. Dev Med Child Neurol. 2005;47(3):158–162. doi: 10.1017/s0012162205000290. [DOI] [PubMed] [Google Scholar]
- Fucile S, Gisel E, McFarland DH, Lau C. Oral and non-oral sensorimotor interventions enhance oral feeding performance in preterm infants. Dev Med Child Neurol. 2011;53(9):829–835. doi: 10.1111/j.1469-8749.2011.04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewolb IH, Vice FL. Abnormalities in the coordination of respiration and swallow in preterm infants with bronchopulmonary dysplasia. Dev Med Child Neurol. 2006;48(7):595–599. doi: 10.1017/S0012162206001241. 2006. [DOI] [PubMed] [Google Scholar]
- Humphrey T. The development of human fetal activity and its relation to postnatal behavior. Adv Child Devel Behav. 1970;5(C):1–57. doi: 10.1016/s0065-2407(08)60464-4. [DOI] [PubMed] [Google Scholar]
- Khaksar Z, Jelodar G, Hematian H. Cerebrum malformation in offspring of diabetic mothers. Comp Clin Path. 2012;21(5):699–703. [Google Scholar]
- Nold JL, Georgieff MK. Infants of diabetic mothers. Ped Clinics N America. 2004;51(3):619–637. doi: 10.1016/j.pcl.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Poore M, Zimmerman E, Barlow SM, Wang J, Gu F. Patterned orocutaneous therapy improves sucking and oral feeding in preterm infants. Acta Paediatrica. 2008;97(7):920–927. doi: 10.1111/j.1651-2227.2008.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu EA, Popescu M, Wang J, Barlow SM, Gustafson KM. Non-nutritive sucking recorded in utero via fetal magnetography. Physiol Measurement. 2008;29(1):127–139. doi: 10.1088/0967-3334/29/1/009. [DOI] [PubMed] [Google Scholar]
- Rocha AD, Moreira MEL, Pimenta HP, Ramos JRM, Lucena SL. A randomized study of the efficacy of sensory-motor-oral stimulation and non-nutritive sucking in very low birth weight infant. Early Hum Devel. 2007;83:385–388. doi: 10.1016/j.earlhumdev.2006.08.003. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT 9.2 user’s guide. Cary, NC, USA: 2008. [Google Scholar]
- Shiao SYPK, Youngblut JM, Anderson GC, DiFiore JM, Martin RJ. Nasogastric tube placement: effects on breathing and sucking in very-low-birth-weight infants. Nurs Res. 1995;44(2):82–88. [PubMed] [Google Scholar]
- Stumm SL, Barlow SM, Estep M, Lee J, Cannon S, Carlson J, Finan D. Respiratory distress syndrome degrades the fine structure of the non-nutritive suck in preterm infants. J Neonatal Nurs. 2008;14(1):9–16. doi: 10.1016/j.jnn.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trulsson M, Essick GK. Mechanosensation. In: Miles TS, Nauntofte B, Svensson P, editors. Clinical Oral Physiology. Quintessence Books; Copenhagen, Denmark: 2004. pp. 1–33. [Google Scholar]