Abstract
Therapeutic agents that inhibit a single target often cannot combat a multifactorial disease such as cancer. Thus, multi-target inhibitors (MTIs) are needed to circumvent complications such as the development of resistance. There are two predominant types of MTIs, (a) single drug inhibitor (SDIs) that affect multiple pathways simultaneously, and (b) combinatorial agents or multi-drug inhibitors (MDIs) that inhibit multiple pathways. Single agent multi-target kinase inhibitors are amongst the most prominent class of compounds belonging to the former, whereas the latter includes many different classes of combinatorial agents that have been used to achieve synergistic efficacy against cancer. Safe delivery and accumulation at the tumor site is of paramount importance for MTIs because inhibition of multiple key signaling pathways has the potential to lead to systemic toxicity. For this reason, the development of drug delivery mechanisms using nanotechnology is preferable in order to ensure that the MDIs accumulate in the tumor vasculature, thereby increasing efficacy and minimizing off-target and systemic side effects. This review will discuss how nanotechnology can be used for the development of MTIs for cancer therapy and also it concludes with a discussion of the future of nanoparticle-based MTIs as well as the continuing obstacles being faced during the development of these unique agents.’
Keywords: Nanotechnology, Multi-target inhibitors, Multi-drug inhibitors, Nanoliposomes, Cancer therapy
What are Multi-Target Inhibitors (MTIs), and why are they Needed?
During the past several decades, there has been significant progress in treating cancer with chemotherapy, radiation, and surgery [1]. However, traditional methods of cancer treatment are frequently limited because of systemic side effects, the development of resistance, and sub-optimal drug concentrations at the tumor site [2, 3]. Many recent advances in cancer therapy have centered on targeting oncogenes involved in proliferation and survival pathways specific to cancer cells [4–6]. Several targeted therapies that have enjoyed great success are Zelboraf (vemurafenib; formerly known as PLX4032) for treating advanced melanoma [7], monoclonal antibody trastuzumab for human epidermal growth factor receptor 2 (HER2) positive breast cancer [8], imatinib for break cluster region-abelson leukemia (BCR-Abl) positive chronic myelogenous leukemia (CML) and gastrointestinal stromal tumors (GIST) [9,10], and gefitinib and erlotinib for non-small cell lung cancer (NSCLC) [11]. However, in many cases, cancer patients develop resistance when treated with therapies that target single pathways because the multi-genic abnormalities present in cancer cells allow them to circumvent the action of these agents. The ability of advanced melanoma to develop resistance to Zelboraf is a recent example of how tumors can bypass the point of inhibition, leading to disease recurrence and progression [12,13]. Because it is often true that single-target agents cannot combat a multifactorial disease such as cancer, multi-target inhibitors (MTIs) are becoming more and more attractive in cancer therapy as they are often more effective and less prone to resistance development than monotherapies.
Single agent Multi-Target Inhibitors (MTIs)
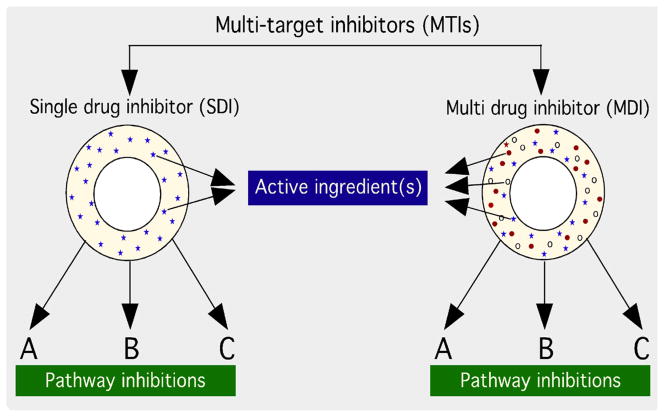
MTIs can be either single drugs that inhibit multiple targets (SDIs), or a combination of multiple agents “Multi-Drug inhibitors” (MDIs) that synergistically inhibit multiple pathways (Figure 1). The most common types of single-drug MTIs are small molecule kinase inhibitors, which are continually being evaluated as new anticancer therapies (Table 1). Small molecule kinase inhibitors are used most commonly as MTIs because deregulation of kinase activity is a major mechanism by which cancer cells evade normal controls regulating cell proliferation and survival [14]. There are roughly 500 kinases in the human kinome, and there are often multiple aberrant kinase pathways involved in a single tumor. Kinase inhibitors are often designed to target oncogenic receptors such as vascular endothelial growth factor receptor (VEGFR), endothelial growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), or they may be designed to inhibit downstream intracellular kinases or pathways such as tyrosine-protein kinase (cSrc) and mitogen activated protein (MAPK) pathways.
Figure 1.
Schematic of multi-target inhibitors (MTI) that affect multiple pathways simultaneously to inhibit cancer cell growth and survival.
Table 1.
Single-agent MTIs currently undergoing preclinical and clinical use.
| Agent | Company | Indication | Targets | References |
|---|---|---|---|---|
| Sorafenib | Onyx/Bayer | RCC, HCC | VEGFR, PDGFR, c-Kit, Raf | [38–41] |
| Nilotinib | Novartis | CML | Bcr-Abl, PDGFR, cSrc, c-Kit | [42] |
| Sunitinib | Pfizer | GIST, RCC | PDGFR, VEGFR, c-Kit, RET, FLT3 | [43] |
| Crizotinib | Pfizer | NSCLC | EML4/ALK, HGFR | [44, 45, 226] |
| Motesanib | Amgen/Takeda | Breast cancer | PDGFR, VEGFR, c-Kit | [46] |
| Vandetanib | Astra Zeneca | Thyroid, NSCLC | EGFR, VEGFR, RET | [30] |
| Lesaurtinib | Cephalon | AML | JAK2, FLT3, Trk | [48, 49] |
| Cabozatinib | Exelixis | Thyroid, solid tumors | VEGFR, MET, c-Kit, FLT3, RET, TEK | [51, 52] |
| Pazopanib | GlaxoSmithKline | RCC, sarcoma | VEGFR, PDGFR, c-Kit | [53] |
Abbreviations: RCC: Renal Cell Carcinoma; HCC: Hepato Cellular Carcinoma; CML: Chronic Myelogenous Leukemia; GIST: Gastro-intestinal Stromal Tumor; NSCLC: Non-small-cell Lung Cancer; AML: Angiomyolipoma; VEGFR: Vascular Endothelial Growth Factor Receptor; PDGFR: Platelet-derived Growth Factor Receptor; cKit: Mast/stem Cell Growth Factor Receptor; cSrc: tyrosine-protein Kinase; Bcr-Abl: Break Cluster Region-Abelson Leukemia; RET: REarranged during Transfection; FLT3: FMS-like Tyrosine Kinase 3; EML4: Echinoderm Microtubule-associated protein-like 4; ALK: Anaplastic Lymphoma Kinase; HGFR: Hepatocyte Growth Factor Receptor; JAK2: Janus Kinase 2; Trk: Tropomyosin Receptor Kinase; TEK: Tyrosine Endothelial Kinase
It is also increasingly apparent that simultaneous inhibition of factors within the tumor microenvironment is necessary and MTIs might be helpful for this application. Hypoxic tumor microenvironment forces tumors to acquire additional vascularization, which requires aberrant vascular endothelial growth factor (VEGF) signaling [15]. Carcinoma cells can recruit macrophages, lymphocytes, and mast cells and form an aberrant paracrine loop, which causes these cells to secrete VEGF and other factors that provide the cancer cells with additional nutrients and vascularization [16]. This additional leaky vasculature also provides an escape route for metastatic cells, which is further exacerbated by macrophage secretion of matrix metalloproteinases (MMPs) [17]. Furthermore, paracrine signaling by carcinoma cells can also lead to fibroblast activation and a subsequent change in the production of growth factors such as platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β) [18].
Fibroblasts can also impact disease progression by secreting MMPs that act on the extracellular matrix (ECM) surrounding the tumor [19]. These types of paracrine loops are well established in many cancers [20–25], and thus the future of cancer therapy relies on targeting pathways present within the tumor cells as well as within the cells of the tumor microenvironment. This method has the potential to be particularly effective, because the microenvironment includes normal stromal cells that do not have the same proclivities towards resistance, which means MTIs affecting neoplastic cells and the microenvironment may further aid in the prevention of resistant disease [26].
The use of rational drug design to inhibit specific kinases in different cancer types has vastly improved cancer therapy [27]. There are a number of examples of multi-target kinase inhibitors currently on the market that inhibit multiple oncogenic pathways within tumors simultaneously, including sorafenib (inhibits MAPK, VEGFR, PDGFR, and mast/stem cell growth factor receptor (cKit) for the treatment of renal cell carcinoma (RCC) and hepatocellular carcinoma (HCC) [28,29], vandetanib (inhibits rearranged during transfection (RET) kinase, VEGFR, and EGFR) for treatment of thyroid tumors [30], and pozopanib (inhibits PDGFR, VEGFR, and cKit) for treatment of RCC and sarcoma [31,32]. When designing these types of agents, it is necessary to understand the structural basis of individual kinases in order to achieve selective inhibition. While there has been success in rational drug design for cancer therapy, only a few pathways are druggable with current chemistries [33,34]. This means that few first-in-class inhibitors enter the market, with established kinase inhibitors continuing to dominate the field.
Multi-Drug Multi-target Inhibitors (MDIs)
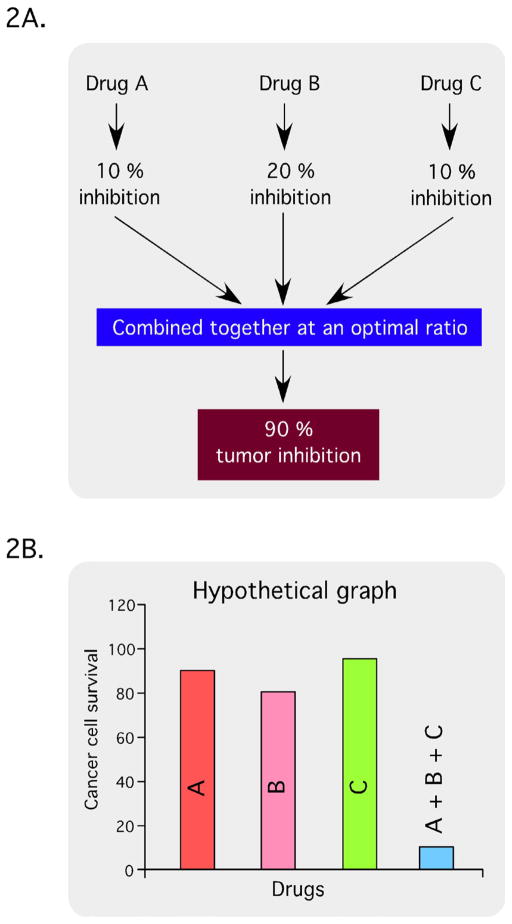
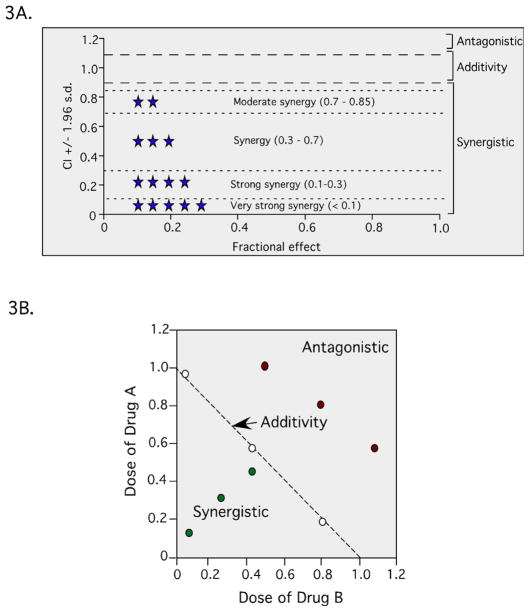
In addition to single-agent MTIs, synergistic drug combinations are also becoming increasingly important in cancer therapy. The hypothesis is that by combining multiple agents, a ratio for optimal inhibition of multiple targets can readily be achieved. Discovery of synergistic drug combinations can be accomplished through the use of drug synergy screens (Figure 2). In the example shown, agent A leads to 10% inhibition, agent B to 20% inhibition and agent C to 10% inhibition. However, in this hypothetical scenario, combining them causes 90% tumor inhibition. At a minimum, compound libraries for drug synergy screens should contain all active pharmaceutical ingredients (APIs) from the United States, Europe, and Japan. Over-the-counter (OTC) drugs, and Generally Recognized As Safe (GRAS) drugs and additives, as these compounds are already approved and can be moved through preclinical and clinical testing more quickly than new chemical entities [35]. When conducting these types of screens, synergy can be demonstrated using the Chou-Talalay method to determine the combination index (CI) using Calcusyn software [36,37]. Combination index values of <0.85 are synergistic, 0.9–1.1 are nearly additive, and >1.1 are antagonistic (Figure 3A), which are illustrated graphically with an isobologram (Figure 3B).
Figure 2.
Schematic of hypothetical tumor inhibition by synergistically acting drug combinations targeting multiple key pathways.
Figure 3.
Schematic of Chou-Talalay method to determine the combination index. (3A) Combination index values of <0.85 are synergistic, 0.9–1.1 are nearly additive and >1.1 are antagonistic, and (3B) a representative isobologram showing hypothetical results that could be interpreted as antagonistic, additive or synergistic.
Current clinical and preclinical development of MTIs
Single drug MTIs (SDIs)
There are a number of drugs currently in the market or in development that are single-agent MTIs. The various types of single-agent MTIs, the companies that market them, protein targets, and the type of cancer treated are shown (Table 1). Sorafenib is a single-agent MTI developed by Onyx and Bayer’s that targets VEGFR, PDGFR, c-Kit, and rapidly accelerated fibrosarcoma (Raf) for the treatment of RCC and HCC [38,39]. It has been shown to improve progression-free survival (PFS) and overall survival (OS) for both indications [40,41]. Novarti’s nilotinib inhibits Bcr-Abl, PDGFR, cSrc, and cKit kinases, and it has been shown to be effective in treating imatinib-resistant chronic CML [42]. Sunitinib is a multi-target tyrosine kinase inhibitor with antitumor activity has been identified as a potent inhibitor of PDGFR, VEGFR, RET, and FMS-like tyrosine kinase 3 (FLT3), and it is being used for imatinib-resistant GIST as well as advanced RCC [43]. Furthermore, CUDC-101 is a novel, small-molecule inhibitor, which simultaneously targets histone deacetylases (HDACs), EGFR, and HER2 in cancer cells. The multi-functional activity of CUDC-101 has the potential to be able to overcome drug resistance and is also currently in phase I clinical development in patients with solid tumors. Crizotinib also inhibits hepatocyte growth factor receptor (HGFR), which is an oncogene implicated in numerous cancers [44,45]. Thus, crizotinib is currently approved for NSCLC, but it is being tested in clinical trials against anaplastic large cell lymphoma, neuroblastoma, and several solid tumors in both adults and children [44,45].
Amgen & Takeda’s motesanib is being evaluated in phase II clinical trials as a first-line therapy against breast cancer. Motesanib is an inhibitor of PDGFR, VEGFR, and cKit, and earlier phase III studies against NSCLC failed to show a significant treatment-related benefit [46]. AstraZeneca’s vandetanib is a selective kinase inhibitor that selectively targets pathways critical for tumor growth and angiogenesis and being used to treat medullary thyroid cancer [30], and also currently in clinical trials with the combination of docetaxel for the treatment of NSCLC [47]. Cephalon’s lestaurtinib is in phase III trials for treatment of acute myeloid leukemia and is an inhibitor of janus kinase 2 (JAK2), FLT3, and tropomyosin receptor kinase (Trk) family kinases [48,49]. Exelixis developed cabozatinib for the treatment of medullary thyroid cancer [50], which inhibits VEGFR, cKit, FLT3, RET, and TEK. It is currently in clinical trials against numerous types of solid tumors [51,52].
GlaxoSmithKline (GSK) has developed pazopanib, a VEGFR, PDGFR, and cKit inhibitor, for the treatment of RCC and soft tissue sarcoma, and it may also be effective against NSCLC and ovarian cancer [53]. E7080 is an orally active multi-targeted kinase inhibitor whose targets include VEGFR, EGFR and PDGFR and was tested on-six human cell lines representing a number of different tumor types. However, E7080 had little effect on tumor cell proliferation but inhibits tumor angiogenesis by targeting endothelial cells. Because all of the aforementioned treatments target many pathways simultaneously, there are often severe systemic side effects when they are used. In addition, actual drug concentrations at the tumor site may be sub-optimal in many cases. With the use of a nanoparticle delivery platform, these agents may have the potential to become more effective while producing fewer side effects.
Multidrug MTIs (MDIs)
Combinatorial therapeutic regiments for cancer therapy have been well documented [54]. Administration of a combined array of therapeutic agents affecting different targets and displaying different toxicity profiles can lead to an improved therapeutic index [55]. While the combination therapy might be more expensive than mono-therapies, its benefits may include substantially reduced treatment failure, decreased mortality and as lower rate of drug resistance development [56]. In addition, combination therapy is also increasingly gaining in importance because it has numerous merits over conventional therapy. These merits include synergistic anticancer effects, reduced individual drug-related toxicity and reversal of drug resistance [57,58].
There are also a number of combination-MTIs on the market or in preclinical and clinical development. In some cases, combination MTIs can act synergistically to kill the cells. Recently, the mammalian target of rapamycin (mTOR) inhibitor everolimus was shown to act synergistically with gemcitabine or paclitaxel for treatment against non-Hodgkin lymphoma (NHL) cell lines [59]. The knockdown of mTOR signaling was shown to enhance the apoptosis-inducing effects of gemcitabine and paclitaxel [59]. In another study, elevated concentrations of paclitaxel were detected in brains of mice when co-administered the P-glycoprotein (P-gp) inhibitors elacridar and tariquidar [60]. Similarly, in a phase I trial, zosquidar co-delivered with daunorubicin and cytarabine increased the anticancer activity of these agents by themselves through inhibition of P-gp expression and activity [61]. Tanabe et al. [62] showed that co-delivery of mitomycin C and methotrexate significantly improved the antitumor effects against breast cancer patients pretreated with taxanes and anthracyclines. Combinations of monoclonal antibodies directed against several tumor-specific oncogenes is another method of treatment that relies on the unique ability of collections of antibodies to target cancer cells and utilize the body’s immune response to kill them through the complement cascade [57]. Expanded uses of examples of combinatorial chemotherapeutic agents are shown in Table 2.
Table 2.
Combination MTIs currently undergoing preclinical and clinical trials.
| Agents | Indication | Reference |
|---|---|---|
| Everolimus + Gemcitabine or Paclitaxel | Non-Hodgkin’s Lymphoma | [59] |
| Daunorubicin + Cytarabine + Zosquidar | Angiomyolipoma | [61] |
| Mitomycin C + Methotrexate + Taxanes | Breast cancer | [62] |
| 5-Fluorouracil + Leucovorin | Colon cancer | [227] |
| Paclitaxel + Carboplatin | Non-small-cell lung cancer | [228] |
| Exemestane + Zoledronic acid | Breast cancer | [229] |
Impediments in the Development of MTIs Overcome through Nanotechnology
The development of MTIs for the treatment of cancer faces numerous hurdles. By using MTIs, the pharmacokinetics of the individual drugs can be coordinated and controlled, leading to optimized therapeutic activity over conventional combination treatments, which presents significant advantages for enhanced cancer chemotherapy [63]. Both types of MTIs (SDIs and MDIs) have advantages and disadvantages. For example, when using single agent MTIs, there is a customary drug development process, a standard new chemical entity intellectual property (IP) position, and easier manufacturing for a single APIs [35]. However, it can be difficult to prevent non-selectivity while achieving good potency against all intended molecular targets. Thus, there cannot be sequenced action time or dose titration when using a single agent MTI.
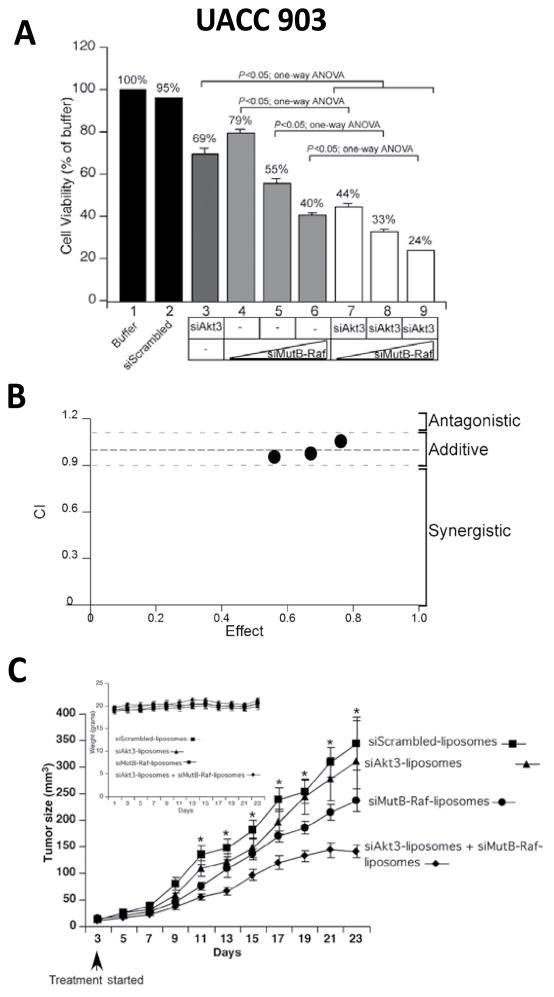
Combining multiple agents makes it much easier to tailor the ratio of the different agents to achieve optimal potency against all intended targets and also allows for sequenced action as well as varied target exposure through the use of immediate or extended release formulations [35]. For example, studies have reported that the phosphatidylinositol-3-kinase and protein kinase B (PI3K/Akt) and MAPK signaling cascades can be additively inhibited in melanoma xenograft using to small interfering ribonucleic acid (siRNA) Akt3 and V600EB-Raf loaded into cationic nanoliposomes (Figure 4). Nanoliposomes were applied topically to tumor-bearing mice that had been pretreated with low-frequency ultrasound using a lightweight four-cymbal transducer array enabling penetration of the nanoliposomal-siRNA complex throughout the epidermal and dermal layers of laboratory-generated or animal skin [64,65]. Nanoliposomal-mediated siRNA targeting of V600EB-Raf and Akt3 resulted in improved synergistic reduction in tumor size in early or invasive cutaneous melanoma compared with inhibition of each target separately with negligible associated systemic toxicity (Figure 4).
Figure 4.
Inhibition of melanoma tumors in an additive manner following treatment with a nanoparticle containing siRNAs directed against two different targets. (4A) SiMutB-Raf and siAkt3 cooperate to reduce anchorage independent growth in cell culture. (4B) SiAkt3 and siMutB-Raf act additively to inhibit cell viability. Calculation of the CI index for the combination of siAkt3 and siMutB-Raf showed additive inhibition of cell viability with CI values between 0.94 and 1.10. (4C) Ultrasound treatment followed by topical application of siAkt3-liposomal complex containingsiMutB-Rafdecreased melanoma development in animal skin [64].
On the other hand, the increased risk of drug-drug interactions when multiple drugs are delivered simultaneously presents a serious challenge to the development of viable MDIs. By modulating mass ratios to an optimal level, these interactions can be effectively minimized while maximizing the therapeutic efficacy of the combined agents [66]. For example, poly (lactide-co-glycolide) microsphere formulations that co-deliver an antisense oligonucleotide and 5-fluorouracil (5-Fu) do not work due to the interaction of 5-FU with the oligodeoxynucleotide [66]. Each individual drug has its own distinct pharmacokinetic profile and the synergistic drug ratio for the “drug cocktails” needs to be controlled and optimized in the laboratory to reduce changes in drug dynamics, and these studies must then be replicated in animal models prior to evaluation in humans [58].
Use of Nanotechnology to Develop MTIs
Both types of MTIs (SDIs & MDIs) have particular sets of strengths and weaknesses, but a common problem is how to achieve successful delivery and accumulation at the tumor site. Current combination therapies are limited because different drug molecules have different pharmacokinetics, biodistribution, and membrane transport properties, which creates complications in dosing and scheduling optimization [67–70]. These challenges have driven clinicians and scientists to investigate various methods of delivering multiple therapeutic agents within a single nanoparticle [71,72]. The goal of nanoparticle-based therapy is to achieve better specificity and optimized pharmacokinetics while delivering multiple therapeutic agents [73,74].
Nanoparticle-based drugs have unique properties such as small size (1–100 nm) and large surface-to-volume ratios [75]. They also benefit from self-assembly, better solubility, increased stability, and natural accumulation in the leaky tumor vasculature [73], all of which can help improve the utility of MTIs. Resistance to initially effective agents can develop because of increased metabolism, mutation of drug targets, circumvention of target pathways, or overexpression of efflux pumps [76]. By creating a nanoparticle with drug(s) that target multiple pathways, the likelihood of developing resistance decreases [77,78].
Types of nanoparticles that could be used as MTIs
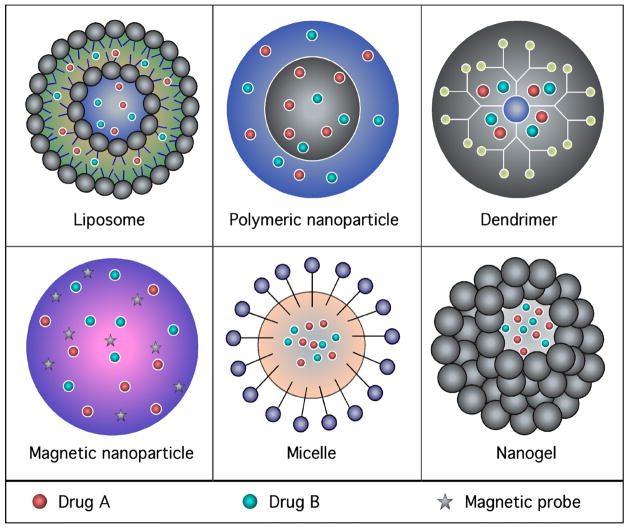
Nanoparticles are being used to circumvent many of the limitations of conventional drug delivery systems used to load single or multiple active ingredients [79–81]. The advantages include (a) delivery systems that can extend drug circulation half-life, (b) increased drug concentration at the tumor site through the passive enhanced permeation and retention (EPR) effect, (c) the ability to modify the ratio-metric dosing based on pharmacological dispositions, and (d) reduced nonspecific uptake [79,82–84]. Therefore, using nanotechnology may allow for a single platform in which multiple genetic or pharmacological agents can be loaded into nanoparticles and synergistically inhibit cancer development as well as overcome the occurrence of resistance [85–89]. Many types of nanotechnology-based therapies have been developed for treating cancers, including nanoliposomes, polymeric nanoparticles, dendrimers, magnetic nanoparticles, micelle, and nanogels (shown in Figure 5) [90–96]. These nanocarriers have been demonstrated to be capable of carrying two or more types of therapeutic payloads while promoting synergy through controlled combinatorial drug delivery. Each platform has its unique strengths and characteristics, which will be discussed briefly [97,98].
Figure 5.
Schematic diagram representing the various types of nanoparticle use to develop MTIs. (5A) Liposome; (5B) Polymeric nanoparticle; (5C) Dendrimer; (5D) Magnetic nanoparticle; (5E) Micelle; (5F) Nanogel.
Nanoliposomes
Nanoliposomes (shown in Figure 5) are an extensively studied drug delivery platform that is currently used in clinical practice, and it has shown promise for improving the solubility of many amphiphilic drugs [92,97,99–101]. Liposomes of certain sizes, typically less than 100–200 nm, can rapidly enter tumor sites from the blood due to EPR effect, but are kept in the bloodstream by the endothelial wall in healthy tissue vasculature [65,102–104]. Furthermore, liposomes can have various molecules attached to the surface. The most common surface modification is PEGylation to make the particle stealthy, in which polyethylene glycol (PEG) is covalently linked to the surface of the liposome [65,91,95,105,106].
PEGylated liposomes are highly stable and lead to improvement in circulation time, enhanced tumor uptake, avoidance of the reticulo-endothelial system, and minimization of toxicity [107–109]. For example, PEGylated-liposomal doxorubicin (Doxil) was characterized by a very long circulating half-life, favorable pharmacokinetic behavior and specific accumulation in tumor tissues compared with conventional liposomal doxorubicin or free doxorubicin [110,111]. Numerous liposomal drug formulations containing chemotherapeutic agents, antisense-oligodeoxynucleotides, siRNA, deoxyribonucleic acid (DNA), or radioactive particles that can target multiple signaling pathways are in various stages of development [71,92,112]. Several examples of combination drug delivery systems based on liposomes are listed in Table 3.
Table 3.
Liposomes for combination therapy.
| Nanocarrier system | Agents | Indication | Status | References |
|---|---|---|---|---|
| Liposome (CPX-351) | Cytarabine + Daunorubicin | Advanced Hematologic Cancer | Phase II | [144] |
| Liposome (CPX-1) | Irinotecan + Floxuridine | Advanced Colorectal Cancer | Phase II | [145, 230] |
| Liposome (CPX-571) | Irinotecan and Cisplatin | Non-small-cell lung cancer | Preclinical | [231] |
| PEG- Liposome | Topotecan + Vincristine | Brain cancer | Preclinical | [138] |
| Liposome | Topotecan + Amlodipine | Leukemia | Preclinical | [232] |
| Liposome | Vincristine + Quinacrine | Leukemia | Preclinical | [233] |
| Liposome | 6-Mercaptopurine + Daunorubicin | Leukemia | Preclinical | [234] |
| Liposome | Paclitaxel + Tariquidar | Ovarian cancer | Preclinical | [235] |
| Transferrin-conjugated PEGylated liposome | Doxorubicin + Verapamil | Leukemia | Preclinical | [134] |
| Trilysinoyl oleylamide(TLO)-based cationic liposomes | siMcl1 + Suberoylanilide hydroxamic acid | Cervical cancer | Preclinical | [126] |
| Nanolipoplex | Taurocholate(LHT7)+ Suberoylanilide hydroxamic acid | Oral cancer | Preclinical | [130] |
| Liposome | PD0325901 +siMcl1 | Cervical cancer | Preclinical | [127] |
| Liposome | Doxorubicin+ Msurvivin T34A plasmid | Lung carcinoma | Preclinical | [128] |
| Liposome | Ceramide+ Sorafenib | Breast Cancer | Preclinical | [124] |
| Liposome | siB-Raf + siAkt3 | Melanoma | Preclinical | [236] |
| PEGylated lipoplex | siBcl-2-lipoplex+ S-1(5-FU) pro-drug | Colorectal cancer | Preclinical | [92] |
| RGD-modified liposomes | Combretastatin A-4 + Doxorubicin | Melanoma | Preclinical | [147] |
| Liposome | Gemcitabine + Tamoxifen | Breast cancer | Preclinical | [148] |
Liposomes containing nucleic acids
Nucleic acid-based nanoliposomes are used when pharmacological agents are not available to target particular oncogenic proteins. There are a large number of mutations and perturbations in cancer cells, but few play a role in disease progression. While there has been some success in designing inhibitors of specific targets, such as the use of vemurafenib against mutant V600EB-Raf protein in melanoma cells [5], few targets are able to be inhibited with current technologies [113], which means that few first in class inhibitors enter the market [114]. Thus, there is great promise for siRNA-based nanoliposomal drug delivery to target these proteins, as this technique can make almost any oncogene a potential therapeutic target.
RNA interference (RNAi) blocks translation of messenger RNAs (mRNAs), thereby reducing oncogene or mutant gene protein levels. Combinations of different siRNAs targeting multiple oncogenes in different pathways may be on the horizon. For drugs such as these, it is an absolute necessity to use a nanotechnology-based method of delivery. Initial studies using localized delivery of RNAi into tumors involved viral vectors to express the siRNAs; however, limitations included several side effects, high production costs, and poor biodistribution. Nanoliposomal delivery of non-viral, less toxic methods of siRNA is being refined [115]. 1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP) is one of the first cationic lipids that was created for use in liposomes for in vivo delivery of siRNAs [116]. These particles have a size of 60–100 nm are PEGylated (mPEG2000-C-DMA), contain cholesterol, a neutral helper lipid, and the ionizable lipid dimethylaminopropane (DLinDMA), which facilitates membrane fusion and is essential for in vivo efficacy of RNAi-based therapeutics [117]. Newer forms of DOTAP with 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxypoly(ethylene glycol)2000] carboxamide (DPPE-PEG2000) and egg phosphatidylcholine (egg-PC) have demonstrated serum bioavailabity for up to 20 hours [118]. Dioleoylphosphatidylcholine (DOPC)-based nanoliposomes are neutral liposomal formulations for siRNA delivery that are used against a variety of targets [119–121].
Liposomes combining nucleic acids and traditional pharmacological agents
Co-delivery of siRNA and chemotherapeutic agents is also another emerging area of nanoliposomal-based combination therapy [122]. For example, a positively charged cationic liposome containing siRNA in combination with doxorubicin effectively inhibits the activity of B cell lymphoma-1 (BCL-1) and multidrug-resistance-associated protein-1 (MRP1) in H69AR lung cancer lines [123]. In addition, combining nanoliposomes containing ceramide (a lipid based Akt inhibitor) with sorafenib has been shown to synergistically decrease melanoma cell growth [124]. Further studies on cancer genomes, at both the tumor and individual cell level, will enable the identification of a complete list of targets and cancer-relevant genes. By combining in-depth analysis of cancer genomes (e.g. the Cancer Genome Atlas) with RNAi technologies, there should be ample room for the growth of siRNA-based nanoliposomal therapeutic agents [125].
A recent report has described the use of trilysinoyl oleylamide (TLO)-based cationic liposomes which effectively co-delivers siMcl-1 and chemotherapeutic drug suberoylanilide hydroxamic acid (SAHA) [126]. In addition, N′,N″-dioleylglutamide-based cationic liposomes (DGL) with mitogen-activated protein/extracellular signal-regulated kinase (MEK) inhibitor PD0325901 encapsulated in lipid layers and siMcl-1 complexed to the DGL [127] has been explored. Combination treatment of PEGylated siBcl-2-lipoplex and S-1(5-FU) pro-drug has been found to exhibit enhanced antineoplastic activity in a human colorectal adenocarcinoma xenograft model [92]. Furthermore, novel fibroblast growth factor receptor (FGFR)-mediated cationic liposomes for co-delivery of doxorubicin and Msurvivin T34A plasmid have been assessed for enhanced cancer chemotherapy [128]. A recent vaccine-based approach with important implications for cancer therapy has been reported in which a liposomal delivery system carries a self-tumoral epitope (HER-2/neu-derived peptide) and CpG oligodeoxynucleotides (CpG ODN) as an adjuvant, which elicits a CD8+ mediated immune response and enhances efficacy [129].
Liposomes containing traditional pharmacological agents
Several nanoliposomes have been created that contain pharmacological agents and other types of compounds. Nanoliposomes containing ceramide and sorafenib have been shown to synergistically decrease melanoma cell growth [124]. Combinatorial approaches aimed at achieving greater synergistic anti-angiogenic effects have been reported by Kim et al. [130], wherein a cationic nanolipoplex has been designed to co-deliver heparin-taurocholate conjugate and SAHA. A novel polymer-lipid hybrid nanoparticle (PLN) formulation has been developed with doxorubicin and the P-gp inhibitor GG918, which can help overcome multidrug-resistant (MDR) breast cancer lines at significantly lower doses than free drugs [131]. Similarly, doxorubicin-mitomycin C co-loaded PLNs were effective in killing MDR breast cancer lines at 20–30-fold lower doses, thus indicating the potential to enhance chemotherapy and reduce the therapeutic limitations of systemic toxicity [132]. Another study Basu et al. revealed that, novel hexadentate-polyD,L-lactic acid-co-glycolic acid polymer chemically conjugated to PD98059 (MEK1 inhibitor) can significantly retard tumor development in xenograft models [133]. Dual doxorubicin-and verapamil-loaded liposomes with surface-conjugated transferrin successfully inhibited the doxorubicin-resistant K562 leukemia tumor cell line with about 5-fold greater potency compared to non-targeted, doxorubicin/verapamil loaded liposomes [134]. Since systemic injection of verapamil can cause serious cardiotoxicity, liposomal delivery of verapamil together with doxorubicin presents a promising approach to reversing cancer drug resistance and minimizing verapamil-related side effects [134]. Furthermore, alginate/bis(2-ethylhexyl) sulfosuccinate (AOT)-alginate nanoparticle-mediated photodynamic therapy using doxorubicin and methylene blue was also able to overcome resistance mechanisms in mammary adenocarcinoma tumor models, resulting in enhanced cytotoxicity against multiple drug resistant tumor cells [135].
In a phase II trial of weekly nab (nanoparticle albumin-bound)- paclitaxel (nab-paclitaxel) (Abraxane®) in combination with gemcitabine, established the activity and manageable toxicity as a first-line therapy of metastatic breast cancer patients [136]. Furthermore, these favorable results provide a rationale for testing nab-paclitaxel, gemcitabine, and an anti-angiogenic agent in future clinical trials [136]. In another study, Zucker et al. [137] developed a PEGylated liposomal formulation containing dual anticancer inhibitors topican and vincristine (LipoViTO), which displayed 91% tumor suppression compared with either liposomal formulations containing only one drug (40%) or a combination of free drugs (30 %) [138]. Furthermore, simultaneous liposomal delivery of quercetin and vincristine was shown to enhance estrogen-receptor-negative breast cancer treatment [139]. In vitro 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assays of this liposomal formulation showed significant synergism, with a combination index of 0.113 and a dose-reduction index value of 115 at ED50 for vincristine [139].
Recently, advances in liposome creation and drug-loading methods have produced specific control over combinatorial drug dosing in liposomes [140–143]. Mayer et al. [143] reported that liposomal drug combinations could be loaded at the required ratios by modifying the liposome synthesis and drug encapsulation process. This technology has yielded several products that are currently in clinical trials. For example, CPX-351 is a 5:1 cytarabine:daunorubicin dual drug loaded liposome that is currently under phase II clinical trial for the treatment of acute myeloid leukemia [144]. In addition, CPX-1, a 1:1 irinotecan:floxouridine liposome is currently under investigation in a phase II trial for colorectal cancer treatment. It exhibited superior anticancer activity in various human tumor xenograft murine models compared with liposomal irinotecan or liposomal floxouridine alone [145]. These liposomal formulations may bring a paradigm shift in clinical treatment by enabling dosage optimization in combination chemotherapy.
Palmitoyl ascorbate-modified liposomes have been described as a promising nanoparticle platform for co-delivery of paclitaxel and ascorbate, which mediates oxidative stress-induced cytotoxicity [146]. In addition, a study involving co-encapsulation of combretastatin A-4 (vascular disrupting agent) and doxorubicin (anticancer agent) suggested that a combinatorial strategy focused on arginyl-glycylaspartic acid (RGD) mediated delivery of drugs may be a promising strategy for cancer treatment [147]. Another ‘mix and match’ combinatorial treatment regimen involving a multidrug carrier (MDC) containing both gemcitabine and tamoxifen for the treatment of breast cancer has also shown good therapeutic potential [148].
Sengupta et al. [149] suggested that a nanocell drug delivery platform could enable a temporal release of multiple drugs. For this type of system, the outer PEGylated lipid envelope first releases an antiangiogenic drug shutting down the tumor vasculature and trapping the inner nanoparticle inside. The inner particle is then free to release its drug(s) targeting additional pathways in the tumor microenvironment. In addition, the liposomes can be functionalized with ligands for tumor-specific receptors, such as transferrin, folate, and integrin [150,151]. Recent advancements report the targeted nanoliposomal delivery of octreotide to the somatostatin receptor in gastric cancer [152]. On the other hand, targeted nanoconjugates are not yet effective anti-cancer agents, because they do not have easily reproducible synthesis and the surface targets display a heterogeneous intra-tumoral distribution making uniform dispersal difficult. An in-depth understanding of liposomal-drug interaction with biological system will lead to the emergence of a novel class of nanoliposomal drug delivery systems with improved anticancer activity, efficacy and safety.
Polymeric nanoparticles
Most polymeric nanoparticles (shown in Figure 5) contain a solid, polymer-filled hydrophobic core that is better suited for water-insoluble MTI payloads [153–156]. These nanoparticles generally have higher stability, subcellular size distribution, controlled/sustained drug-release profiles, and higher loading capacity for poorly water-soluble drugs [157–159]. Polymeric nanoparticles are fabricated from biodegradable natural or synthetic polymers [98]. Several synthetic polymers are approved by the United States Food and drug administration such as poly lactic co glycolic acid (PLGA), and polycaprolactone (PCL). Other natural polymers such as chitosan, polysaccharides, and polypeptides have been investigated extensively for drug delivery and clinical applications, including cardiovascular disease, cancer, vaccines and tissue engineering [160,161]. Furthermore, these polymeric micelle systems can also be used to or concurrently deliver two or more therapeutic MTI modalities such as radiation sensitizers and drugs [79,162].
Milane et al. [163] demonstrated that epidermal growth factor receptor (EGFR)-targeted polymeric nanoparticles loaded with ionidamine and paclitaxel displayed antitumor activity through down regulation of MDR proteins in human breast and ovarian tumor cells. Furthermore, PLGA nanoparticles loaded with vincristine and verapamil triggered MDR reversal activity on MCF-7/ADR cells resistant to vincristine [164]. In another study, Misra and Sahoo [165] demonstrated that the synergistic effect of doxorubicin/curcumin PLGA nanoparticles enhances the cytotoxicity of the drugs in leukemic K562 cells in vitro by overcoming the MDR phenotype.
Dendrimers
Dendrimers (shown in Figure 5) have emerged as another class of drug delivery nanoparticle platform because of their unique properties [166–168]. While these nanoparticles have not received as much attention as liposomes and polymeric nanoparticles for delivery of MTI’s, several efforts have been made to deliver multiple therapeutic agents simultaneously using a dendrimeric platform [92]. Dendrimers are globular, highly branched and synthetic polymers that are characterized by a central inner core surrounded by repetitive layers and an outermost layer of multivalent functional groups [166,167]. The high level of control over the architecture affecting size, shape, density, and surface functionality makes these compounds excellent carriers of MTIs as well as imaging agents through chemical modification of multiple terminal groups [169–172].
Despite the promise of dendrimers, a major constraints for delivery of MTIs is toxicity due to the interaction of surface cationic charges with negatively charged biological membranes in vivo, which can cause membrane nanoholes [173]. Surface engineering can be used to mask the cationic charges, which involves PEGylation, acetylation, and carbohydrate or peptide conjugation. The chemical modifications necessary to overcome the toxicity have been discussed by others [173,174]. The synthesis of dendrimers can be tightly controlled to establish specific size range and branching complexity. Drug could be loaded into the core or branches [175]. The dendritic surface can be further modified with antibodies or ligands to improve targeting and utility of these nanoparticles to carry anti-cancer agents [176].
The unique properties of dendrimers make them a desirable platform for simultaneous delivery of hydrophobic and hydrophilic MTI’s [171,172]. Tekade et al. [177] developed dual drug-loaded polyamidoamine dendrimers loaded with hydrophobic methotrexate and hydrophilic all-trans retinoic acid. These particles exhibited less hemolytic toxicity and enhanced cytotoxicity in HeLa cells compared to free drugs. In another study, Kaneshiro et al. formulated novel nanoglobular dendrimers conjugated with cyclo(-Arg-Gly-Asp-d-Phe-Lys-(cRGDfK) peptide with PEG spacer for co-delivery of doxorubicin (DOX) and siRNA. The siRNA complex of the targeted conjugates resulted in higher gene silencing efficiency in glioblastoma U87-Luc cells and greater efficacy than either agent alone [178]. Other examples of dendrimer-based combination cancer therapy are summarized in Table 4.
Table 4.
Nanoparticles based combination therapy.
| Nanocarrier system | Agents | Indication | Status | Reference |
|---|---|---|---|---|
| RGDfK-G3 Poly-lysine dendrimer | Doxorubicin + siRNA | Glioblastoma | Preclinical | [178] |
| Dendritic PEG | Paclitaxel + Alendronate | Cancer bone metastases | Preclinical | [237] |
| Folate-G5 poly-propyleneimine dendrimer with ethylenediamine core | Methotrexate + all-trans-retinoic acid | Leukemia | Preclinical | [238] |
| G5 PAMAM dendrimer | Antisense-miRNA21 + 5-Fluorouracil | Glioblastoma | Preclinical | [239] |
| Aptamer-G4 PAMAM dendrimer conjugates | Unmethylated CpG-ONTs + Doxorubicin | Prostate | Preclinical | [240] |
| PLGA | Vincristine + Verapamil | Hepatocellular carcinoma | Preclinical | [241] |
| Methoxy PEG-PLGA | Doxorubicin + Paclitaxel | Various cancer | Preclinical | [242] |
| PLGA-PEG-biotin | Paclitaxel + Tariquidar | Various cancer | Preclinical | [243] |
| PLGA-PEG-biotin | Paclitaxel + P-gp siRNA | Various cancer | Preclinical | [244] |
| HPMA-Gem-Dox | Gemcitabine +Doxorubicin | Prostate cancer | Preclinical | [245] |
| HER2 conjugated- GMO-MNPs | Paclitaxel + Rapamycin | Breast cancer | Preclinical | [246] |
| Ac-(AF)6-H5-K15-NH2 (FA32) micelle | Doxorubicin + p53 gene | Hepatocarcinoma | Preclinical | [213] |
Abbrviations: RGD: Arginylglycylaspartic Acid; siRNA: Small Interfering Ribonucleic Acid; miRNA: Micro Ribonucleic Acid; HER2: Human Epidermal Growth Factor Receptor 2; PEG: Polyethylene Glycol; PLGA: Poly(Lactic-co-glycolic Acid); PAMAM: Polyamidoamine; HPMA: Poly(N-(2-hydroxypropyl)methacrylamide); MNP: Magnetic Nanoparticles
Magnetic nanoparticles
Magnetic nanoparticles (MNPs) (shown in Figure 5) are a major class of nanoparticles with the potential to enhance magnetic resonance imaging (MRI), targeted therapy, tissue repair, virus detection, magnetically enhanced transfection, magnetically induced hyperthermia, cell/protein/DNA separation and radiotherapy [179–183]. MNPs are spherical nanocrystals of 10–100 nm in size with an Fe2+ or Fe3+ core surrounded by lipids, liposomes, proteins, polymers, or dextran and surface-coated with non-polymeric stabilizers, providing the opportunity for the smart delivery of therapeutic materials.
Iron oxide MNPs (magnetite, Fe3O4; maghemite, Fe2O3) are extensively used as the core of magnetic nanocarriers due to super paramagnetic properties and biocompatibility [184–186]. Iron oxide provides significant advantages over traditional contrast agents, including high magnetic signal strength, relatively low cytotoxicity, longer lasting contrast enhancement, and improved delineation of tumor margins as well as low sensitivity to the number of surrounding water molecules [187–189]. While copper, cobalt and nickel are also highly magnetic materials, the chemical composition makes them naturally toxic and more susceptible to oxidation. Thus, they have limited utility for the MTIs based applicaitons. In contrast, titanium and iron oxide-based particles are considered significantly less damaging to cells and could have utility of delivery of MTIs [190,191].
Currently, various studies have investigated the potential application of magnetic nano systems for pharmaceutical and biomedical applications [192–194]. Jaemoon et al. developed an anti-HER2 antibody conjugated to multifunctional magneto-polymeric nanohybrids (MMPNs) encapsulated by an amphiphilic block copolymer that showed excellent synergetic effects for inhibition of tumor growth and simultaneous breast cancer imaging [195]. Furthermore, Yu et al. [196] has developed doxorubicin-loaded thermally cross-linked superparamagnetic iron oxide nanoparticles (Dox@TCL-SPION) and demonstrated simultaneous detection of tumors by magnetic resonance imaging and delivery of anticancer drugs via release from nanoparticles. This strategy exhibited exceptional antitumor effects without any systemic toxicity. Kumar et al. [197] synthesized and characterized novel hybrid MNPs containing hyaluronic acid (HA) and iron oxide, and successfully delivered nearly 100 % in HEK293 and A549 cell lines, which is an encouraging development in effective tissue and cell targeting systems. Murali et al. developed MNPs that are loaded with curcumin as the anticancer agent and used them for simultaneous targeting and imaging in breast cancer cell lines [198]. In addition, Shi et al. [200] developed a multifunctional drug delivery system, which is based on covalently attaching genistein into iron oxide (Fe3O4) nanoparticles coated by cross-linked carboxymethylated chitosan (CMCH). Results suggest that these particles improve the inhibitory effects of SGC-7901 in cancer cells relative to the free drug.
Micelle
A micelle composed of numerous amphiphilic surfactant molecules is emerging as a promising class of MTIs anticancer nanoparticles (shown in Figure 5) [200]. A mixture of hydrophobic interactions, electrostatic interactions, metal complexation, and hydrogen bonding of block copolymers drives the process of micellization in aqueous solutions [201]. The core of the micelle can be a storage area for drugs with many different properties. The outer core can be functionalized to improve its drug-like qualities and also to modify its physicochemical characteristics [201]. The fundamental mechanisms of self-assembly, drug loading/release, stability, and intracellular delivery of micellar formulations has been reviewed in the literature [202,203]. However, to translate micellear formulations into widespread clinical applications, a better understanding of the physicochemical properties of this type of nanoparticle is still needed [204].
Polymeric micelles are a subset of micelles comprised of block copolymers consisting of both hydrophilic and hydrophobic monomeric units [205]. The first clinical polymeric micelle formulation received approval in South Korea and is currently undergoing phase II trials in the United States where it is known as Genexol-PM64 (PEG-poly (D,L-lactide)- paclitaxel) [206,207]. A biodegradable cationic nanomicelle based on a triblock copolymer of poly (N,N-dimethylamino-2-ethyl methacrylate)-polycaprolactone-poly(N,N-dimethylamino-2-ethyl methacrylate) (PDMAEMA-PCL-PDMAEMA) was reported by Zhu et al. [208]. This nano-micellar drug delivery system has paclitaxel loaded in its micellar core while siRNA is complexed to the outer PDMAEMA shell of the micelle [208].
A recent report describes a hybrid polymeric micelle consisting of a PEG-phospholipid block copolymer with an envelope containing the anti-angiogenesis agent combretastatin surrounding an inner PLGA nanoparticle bearing the chemotherapeutic agent doxorubicin [149,209]. Furthermore, simultaneous delivery of two chemotherapeutics agents paclitaxel and 17-allylamino-17-demethoxygeldanamycin (17-AAG) was achieved by employing PEG-distearoylphosphatidylethanolamine/tocopheryl polyethylene glycol 1000 (PEG-DSPE/TPGS) mixed micelles [210]. Fan et al. [211] designed multifunctional micellar nanoparticles containing pyrrolidine dithiocarbamate (PDTC) and doxorubicin with the goal of simultaneously delivering the chemotherapeutic agents and bypassing the multidrug resistance proteins. Co-delivery of survivin small hairpin RNA (shRNA) and paclitaxel has been used by Hu et al. for its synergistic cytotoxic effects in ovarian cancer therapy [212]. Another study by Wiradharma et al. [213] reported the production of [Ac-(AF)6-H5-K15-NH2] FA32 micelles with enhanced potential to deliver the hydrophobic anticancer drug doxorubicin and the p53 gene simultaneously.
Nanogels
Nanoscale hydrogels or nanogels are biocompatible, three-dimensional materials consisting of hydrophilic, cross-linked polymer networks that can be loaded with therapeutic agents (shown in Figure 5) [214, 215]. This unique drug delivery system is characterized by a controlled release mechanism, which is dependent on the diffusion coefficient of the drug through the hydrogel network [216]. Hydrogel nanoparticle systems are an important constituent of a new class of drug delivery system popularly known as “intelligent,” or “smart” or “stimuli-responsive” drug delivery systems. The environmental stimuli include changes in pH, temperature or ionic strength [217–219]. While the nanogel matrix bearing the drug is in a collapsed state, stimuli cause the nanogel to swell, leading to an increase in mesh size and change the drug diffusion rate [220,221].
Nanogels have many characteristics that could be associated with an ideal drug delivery platform, including stability, response to biologically relevant stimuli, passive and active targeting, low toxicity, and ease of synthesis [222]. Nanogels can be prepared from polymer precursors or by fabricating them via heterogeneous polymerization of monomers [222]. In the first method of generation, amphiphilic copolymers are allowed to self-assemble in solution, and then the assembly is ‘locked’ via some form of cross-linking [222]. Polymeric nanogels can also be easily functionalized with cell-targeting ligands, and sizes can be controlled for various drug delivery applications [222]. Parenteral delivery of nanogels is possible since they can easily be delivered in a liquid form [223]. Seo et al. [224] recently reported a chemo-immunotherapeutic strategy to target cervical cancer that employs a biodegradable chitosan-based hydrogel that can codeliver chemotherapeutic agent such as doxorubicin, cisplatin, or cyclophosphamide with an immunoadjuvant-granulocyte macrophage colony-stimulating factor (GMCSF).
General Constraints and Challenges in MTIs Nanoparticle Development
Particle size is the most important and most studied factor affecting nanoparticle toxicity and success of MTIs. If nanoparticles are too small (e.g. <10nm), they can pass the blood brain barrier and cause damage. In contrast, particles >100nm do not possess the desired pharmacological properties for MTIs effective drug delivery [225]. The charge and composition of a MTIs nanoparticle can also have a profound impact on its toxicity. Positively charged nanoparticles can be toxic due to hemagglutination and hemolysis of erythrocytes [225]. They can also agglomerate due to intermolecular forces, to further increase toxicity. Methods have been developed to circumvent this potentially serious side effect, involving sonication, use of detergents, and PEGylation of the nanoparticle [225]. MTIs nanoparticle-related toxicities can vary based on each nanoparticle and in these cases more in depth studies to explore the structural properties that induce organ-specific cytotoxicity would be needed [225].
PEGylation to reduce toxicity, improve biodistribution and half-life of the agent can be used to mask toxic nanoparticles. Many studies have conjugated functional groups to nanoparticles in order to improve targeting, biodistribution, and reduce toxicity. In addition to toxicity, the lack of reproducibility of large-scale commercial production is another major drawback to MTIs based nanoparticles development [225].
Conclusion
The use of MTIs for the treatment of cancer should significantly enhance cancer therapy. The key challenge in development of MTIs based nanotherapeutics lies in optimization of drug loading and controlled release of the encapsulated cargo. In order to circumvent these limitations and to optimally pair the nanocarrier and the therapeutic agent, a greater understanding of the interplay between properties of the carrier and the mechanisms of loading is key [216]. The major driving forces for the development of MTI nanotherapeutics include enhanced efficacy, improved drug delivery, and reduced development of recurrent resistant disease.
If successful, MTIs based nanotherapeutics could revolutionize the future of cancer treatment. Single agent MTIs and combinatorial strategies involving synergistically acting multidrug MTIs have numerous advantages over conventional cancer pharmaceutics. Even though different types of nanoparticles are being developed for the delivery of MTIs, nano-liposomal drug delivery platforms are currently the most developed and versatile nanotechnology based on increased clinical approval of liposomal chemotherapeutic agents.
Acknowledgments
NIH grants R01 CA-136667-02, RO1 CA-1138634-02, RO1 CA-127892- 01A and The Foreman Foundation for Melanoma (Robertson GP). Penn State Melanoma Center (Raghavendra Gowda).
References
- 1.Smalley KS, Herlyn M. Towards the targeted therapy of melanoma. Mini Rev Med Chem. 2006;6:387–393. doi: 10.2174/138955706776361402. [DOI] [PubMed] [Google Scholar]
- 2.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 3.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 4.Das Thakur M, Salangsang F, Landman AS, Sellers WR, Pryer NK, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494:251–255. doi: 10.1038/nature11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A, Madhunapantula SV, Gowda R, Berg A, Neves RI, et al. Identification of aurora kinase B and Wee1-like protein kinase as downstream targets of (V600E) B-RAF in melanoma. Am J Pathol. 2013;182:1151–1162. doi: 10.1016/j.ajpath.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans MS, Madhunapantula SV, Robertson GP, Drabick JJ. Current and future trials of targeted therapies in cutaneous melanoma. Adv Exp Med Biol. 2013;779:223–255. doi: 10.1007/978-1-4614-6176-0_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banaszynski M1, Kolesar JM. Vemurafenib and ipilimumab: new agents for metastatic melanoma. Am J Health Syst Pharm. 2013;70:1205–1210. doi: 10.2146/ajhp120260. [DOI] [PubMed] [Google Scholar]
- 8.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 10.Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 11.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 12.Kudchadkar R, Paraiso KH, Smalley KS. Targeting mutant BRAF in melanoma: current status and future development of combination therapy strategies. Cancer J. 2012;18:124–131. doi: 10.1097/PPO.0b013e31824b436e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan RJ, Flaherty KT. Resistance to BRAF-targeted therapy in melanoma. Eur J Cancer. 2013;49:1297–1304. doi: 10.1016/j.ejca.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 15.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 16.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 17.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch CC, Matrisian LM. Matrix metalloproteinases in tumor-host cell communication. Differentiation. 2002;70:561–573. doi: 10.1046/j.1432-0436.2002.700909.x. [DOI] [PubMed] [Google Scholar]
- 20.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 21.Campbell JS, Johnson MM, Bauer RL, Hudkins KL, Gilbertson DG, et al. Targeting stromal cells for the treatment of platelet-derived growth factor C-induced hepatocellular carcinogenesis. Differentiation. 2007;75:843–852. doi: 10.1111/j.1432-0436.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheng N, Bhowmick NA, Chytil A, Gorksa AE, Brown KA, et al. Loss of TGF-beta type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP- and HGF-mediated signaling networks. Oncogene. 2005;24:5053–5068. doi: 10.1038/sj.onc.1208685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coltrera MD, Wang J, Porter PL, Gown AM. Expression of platelet-derived growth factor B-chain and the platelet-derived growth factor receptor beta subunit in human breast tissue and breast carcinoma. Cancer Res. 1995;55:2703–2708. [PubMed] [Google Scholar]
- 24.Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–8142. [PubMed] [Google Scholar]
- 25.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrelli A, Valabrega G. Multitarget drugs: the present and the future of cancer therapy. Expert Opin Pharmacother. 2009;10:589–600. doi: 10.1517/14656560902781907. [DOI] [PubMed] [Google Scholar]
- 27.Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet. 2010;11:685–696. doi: 10.1038/nrg2841. [DOI] [PubMed] [Google Scholar]
- 28.Guida T, Anaganti S, Provitera L, Gedrich R, Sullivan E, et al. Sorafenib inhibits imatinib-resistant KIT and platelet-derived growth factor receptor beta gatekeeper mutants. Clin Cancer Research: an official journal of the American Association for Cancer Res. 2007;13:3363–3369. doi: 10.1158/1078-0432.CCR-06-2667. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 30.Massicotte MH, Borget I, Broutin S, Baracos VE, Leboulleux S, et al. Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: results from a placebo controlled study. J Clin Endocrinol and Metab. 2013;98:2401–2408. doi: 10.1210/jc.2013-1115. [DOI] [PubMed] [Google Scholar]
- 31.Rajendra R, Jones RL, Pollack SM. Targeted treatment for advanced soft tissue sarcoma: profile of pazopanib. Onco Targets Ther. 2013;6:217–222. doi: 10.2147/OTT.S32200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vrdoljak E, Rini B, Schmidinger M, Omrcen T, Torday L, et al. Bisphosphonates and vascular endothelial growth factor-targeted drugs in the treatment of patients with renal cell carcinoma metastatic to bone. Anticancer Drugs. 2013;24:431–440. doi: 10.1097/CAD.0b013e328360335f. [DOI] [PubMed] [Google Scholar]
- 33.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 34.Paolini GV, Shapland RH, van Hoorn WP, Mason JS, Hopkins AL. Global mapping of pharmacological space. Nat Biotechnol. 2006;24:805–815. doi: 10.1038/nbt1228. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann GR, Lehár J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12:34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Berenbaum MC. The expected effect of a combination of agents: the general solution. J Theor Biol. 1985;114:413–431. doi: 10.1016/s0022-5193(85)80176-4. [DOI] [PubMed] [Google Scholar]
- 37.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 38.Smalley KS, Xiao M, Villanueva J, Nguyen TK, Flaherty KT, et al. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene. 2009;28:85–94. doi: 10.1038/onc.2008.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Molecular cancer therapeutics. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 40.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 41.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 42.Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 43.Blay JY, Reichardt P. Advanced gastrointestinal stromal tumor in Europe: a review of updated treatment recommendations. Expert Rev Anticancer Ther. 2009;9:831–838. doi: 10.1586/era.09.34. [DOI] [PubMed] [Google Scholar]
- 44.Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer ther. 2007;6:3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 45.Janoueix-Lerosey I, Schleiermacher G, Delattre O. Molecular pathogenesis of peripheral neuroblastic tumors. Oncogene. 2010;29:1566–1579. doi: 10.1038/onc.2009.518. [DOI] [PubMed] [Google Scholar]
- 46.Scagliotti GV, Vynnychenko I, Park K, Ichinose Y, Kubota K, et al. International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J Clin oncol. 2012;30:2829–2836. doi: 10.1200/JCO.2011.41.4987. [DOI] [PubMed] [Google Scholar]
- 47.Herbst RS, Sun Y, Eberhardt WE, Germonpré P, Saijo N, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11:619–626. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hexner EO, Serdikoff C, Jan M, Swider CR, Robinson C, et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008;111:5663–5671. doi: 10.1182/blood-2007-04-083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 50.Traynor K. Cabozantinib approved for advanced medullary thyroid cancer. Am J Health Syst Pharm. 2013;70:88. doi: 10.2146/news130005. [DOI] [PubMed] [Google Scholar]
- 51.Lee RJ, Smith MR. Targeting MET and vascular endothelial growth factor receptor signaling in castration-resistant prostate cancer. Cancer J. 2013;19:90–98. doi: 10.1097/PPO.0b013e318281e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaishampayan U. Cabozantinib as a novel therapy for renal cell carcinoma. Curr Oncol Rep. 2013;15:76–82. doi: 10.1007/s11912-012-0289-x. [DOI] [PubMed] [Google Scholar]
- 53.Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin oncol. 2009;27:3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 54.Gee JM, Howell A, Gullick WJ, Benz CC, Sutherland RL, et al. Consensus statement. Workshop on therapeutic resistance in breast cancer: impact of growth factor signalling pathways and implications for future treatment. Endocr Relat Cancer. 2005;12(Suppl 1):S1–7. doi: 10.1677/erc.1.01054. [DOI] [PubMed] [Google Scholar]
- 55.Greco F, Vicent MJ. Combination therapy: opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines. Adv Drug Deliv Rev. 2009;61:1203–1213. doi: 10.1016/j.addr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Logman JF, Heeg BM, Botteman MF, Kaura S, van Hout SA. Economic evaluation of zoledronic acid for the prevention of osteoporotic fractures in postmenopausal women with early-stage breast cancer receiving aromatase inhibitors in the UK. Ann Oncol. 2010;21:1529–1536. doi: 10.1093/annonc/mdp560. [DOI] [PubMed] [Google Scholar]
- 57.Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today. 2012;17:1044–1052. doi: 10.1016/j.drudis.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiang CT, Yeh PY, Gao M, Chen CW, Yeh LC, et al. Combinations of mTORC1 inhibitor RAD001 with gemcitabine and paclitaxel for treating non- Hodgkin lymphoma. Cancer Lett. 2010;298:195–203. doi: 10.1016/j.canlet.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Hubensack M, Müller C, Höcherl P, Fellner S, Spruss T, et al. Effect of the ABCB1 modulators elacridar and tariquidar on the distribution of paclitaxel in nude mice. J Cancer Res Clin Oncol. 2008;134:597–607. doi: 10.1007/s00432-007-0323-9. [DOI] [PubMed] [Google Scholar]
- 61.Lancet JE, Baer MR, Duran GE, List AF, Fielding R, et al. A phase I trial of continuous infusion of the multidrug resistance inhibitor zosuquidar with daunorubicin and cytarabine in acute myeloid leukemia. Leuk Res. 2009;33:1055–1061. doi: 10.1016/j.leukres.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 62.Tanabe M, Ito Y, Tokudome N, Sugihara T, Miura H, et al. Possible use of combination chemotherapy with mitomycin C and methotrexate for metastatic breast cancer pretreated with anthracycline and taxanes. Breast Cancer. 2009;16:301–306. doi: 10.1007/s12282-009-0093-0. [DOI] [PubMed] [Google Scholar]
- 63.Tardi PG, Gallagher RC, Johnstone S, Harasym N, Webb M, et al. Coencapsulation of irinotecan and floxuridine into low cholesterol-containing liposomes that coordinate drug release in vivo. Biochim Biophys Acta. 2007;1768:678–687. doi: 10.1016/j.bbamem.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 64.Tran MA, Gowda R, Sharma A, Park EJ, Adair J, et al. Targeting V600EB-Raf and Akt3 using nanoliposomal-small interfering RNA inhibits cutaneous melanocytic lesion development. Cancer Res. 2008;68:7638–7649. doi: 10.1158/0008-5472.CAN-07-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tran MA, Watts RJ, Robertson GP. Use of liposomes as drug delivery vehicles for treatment of melanoma. Pigment Cell Melanoma Res. 2009;22:388–399. doi: 10.1111/j.1755-148X.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hussain M, Beale G, Hughes M, Akhtar S. Co-delivery of an antisense oligonucleotide and 5-fluorouracil using sustained release poly (lactide-co-glycolide) microsphere formulations for potential combination therapy in cancer. Int J Pharm. 2002;234:129–138. doi: 10.1016/s0378-5173(01)00950-4. [DOI] [PubMed] [Google Scholar]
- 67.Vale CL, Tierney J, Bull SJ, Symonds PR. Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma. Cochrane Database Syst Rev. 2012;8:CD003915. doi: 10.1002/14651858.CD003915.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma A, Madhunapantula SV, Gowda R, Berg A, Neves RI, et al. Identification of aurora kinase B and Wee1-like protein kinase as downstream targets of (V600E) B-RAF in melanoma. Am J Pathol. 2013;182:1151–1162. doi: 10.1016/j.ajpath.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forastiere AA, Orringer MB, Perez-Tamayo C, Urba SG, Husted S, et al. Concurrent chemotherapy and radiation therapy followed by transhiatal esophagectomy for local-regional cancer of the esophagus. J Clin Oncol. 1990;8:119–127. doi: 10.1200/JCO.1990.8.1.119. [DOI] [PubMed] [Google Scholar]
- 70.Moreno Garcia V, Basu B, Molife LR, Kaye SB. Combining antiangiogenics to overcome resistance: rationale and clinical experience. Clin Cancer Res. 2012;18:3750–3761. doi: 10.1158/1078-0432.CCR-11-1275. [DOI] [PubMed] [Google Scholar]
- 71.Hu CM, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol. 2012;83:1104–1111. doi: 10.1016/j.bcp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Shapira A, Livney YD, Broxterman HJ, Assaraf YG. Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist Updat. 2011;14:150–163. doi: 10.1016/j.drup.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Grodzinski P, Silver M, Molnar LK. Nanotechnology for cancer diagnostics: promises and challenges. Expert Rev Mol Diagn. 2006;6:307–318. doi: 10.1586/14737159.6.3.307. [DOI] [PubMed] [Google Scholar]
- 74.McNeil SE. Nanotechnology for the biologist. J Leukoc Biol. 2005;78:585–594. doi: 10.1189/jlb.0205074. [DOI] [PubMed] [Google Scholar]
- 75.Singh S, Sharma A, Robertson GP. Realizing the clinical potential of cancer nanotechnology by minimizing toxicologic and targeted delivery concerns. Cancer Res. 2012;72:5663–5668. doi: 10.1158/0008-5472.CAN-12-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 77.Aryal S, Hu CM, Zhang L. Polymeric nanoparticles with precise ratiometric control over drug loading for combination therapy. Mol Pharm. 2011;8:1401–1407. doi: 10.1021/mp200243k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khdair A, Handa H, Mao G, Panyam J. Nanoparticle-mediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance in vitro. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2009;71:2142–22. doi: 10.1016/j.ejpb.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 79.Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today. 2012;17:1044–1052. doi: 10.1016/j.drudis.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 80.Jain KK. Nanotechnology-based drug delivery for cancer. Technol Cancer Res Treat. 2005;4:407–416. doi: 10.1177/153303460500400408. [DOI] [PubMed] [Google Scholar]
- 81.Zhao P, Astruc D. Docetaxel nanotechnology in anticancer therapy. ChemMedChem. 2012;7:952–972. doi: 10.1002/cmdc.201200052. [DOI] [PubMed] [Google Scholar]
- 82.Madani SY, Naderi N, Dissanayake O, Tan A, Seifalian AM. A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int J Nanomedicine. 2011;6:2963–2979. doi: 10.2147/IJN.S16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sultana Y, Maurya DP, Iqbal Z, Aqil M. Nanotechnology in ocular delivery: current and future directions. Drugs Today (Barc) 2011;47:441–455. doi: 10.1358/dot.2011.47.6.1549023. [DOI] [PubMed] [Google Scholar]
- 84.Venkatesan Perumal SB, Shubasis Das, Sen RK, Mandal M. Effect of liposomal celecoxib on proliferation of colon cancer cell and inhibition of DMBA-induced tumor in rat model. Cancer Nano. 2011;2:67–79. doi: 10.1007/s12645-011-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muthu MS, Rajesh CV, Mishra A, Singh S. Stimulus-responsive targeted nanomicelles for effective cancer therapy. Nanomedicine (Lond) 2009;4:657–667. doi: 10.2217/nnm.09.44. [DOI] [PubMed] [Google Scholar]
- 86.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koo H, Huh MS, Sun IC, Yuk SH, Choi K, et al. In vivo targeted delivery of nanoparticles for theranosis. Acc Chem Res. 2011;44:1018–1028. doi: 10.1021/ar2000138. [DOI] [PubMed] [Google Scholar]
- 88.Blanco E, Hsiao A, Mann AP, Landry MG, Meric-Bernstam F, et al. Nanomedicine in cancer therapy: innovative trends and prospects. Cancer Sci. 2011;102:1247–1252. doi: 10.1111/j.1349-7006.2011.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sánchez-Moreno P, Boulaiz H, Ortega-Vinuesa JL, Peula-García JM, Aránega A. Novel drug delivery system based on docetaxel-loaded nanocapsules as a therapeutic strategy against breast cancer cells. Int J Mol Sci. 2012;13:4906–4919. doi: 10.3390/ijms13044906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh S, Sharma A, Robertson GP. Realizing the clinical potential of cancer nanotechnology by minimizing toxicologic and targeted delivery concerns. Cancer Res. 2012;72:5663–5668. doi: 10.1158/0008-5472.CAN-12-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Samarasinghe RM, Gibbons J, Kanwar RK, Kanwar JR. Nanotechnology based platforms for survivin targeted drug discovery. Expert Opin Drug Discov. 2012;7:1083–1092. doi: 10.1517/17460441.2012.719869. [DOI] [PubMed] [Google Scholar]
- 92.Aad G, Abbott B, Abdallah J, Abdelalim AA, Abdesselam A, et al. Search for dilepton resonances in pp collisions at √s=7 TeV with the ATLAS detector. Phys Rev Lett. 2011;107:272002. doi: 10.1103/PhysRevLett.107.272002. [DOI] [PubMed] [Google Scholar]
- 93.Huang Y, He S, Cao W, Cai K, Liang XJ. Biomedical nanomaterials for imaging-guided cancer therapy. Nanoscale. 2012;4:6135–6149. doi: 10.1039/c2nr31715j. [DOI] [PubMed] [Google Scholar]
- 94.Jabir NR, Tabrez S, Ashraf GM, Shakil S, Damanhouri GA, et al. Nanotechnology-based approaches in anticancer research. Int J Nanomedicine. 2012;7:4391–4408. doi: 10.2147/IJN.S33838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumar P, Gulbake A, Jain SK. Liposomes a vesicular nanocarrier: potential advancements in cancer chemotherapy. Crit Rev Ther Drug Carrier Syst. 2012;29:355–419. doi: 10.1615/critrevtherdrugcarriersyst.v29.i5.10. [DOI] [PubMed] [Google Scholar]
- 96.Wang XM1, Liu CM, Zhang CR, Xu XG, Fu SB. [Functional significance of TGF-beta1 signal transduction pathway in oral squamous cell carcinoma] Zhonghua Zhong Liu Za Zhi. 2009;31:28–32. [PubMed] [Google Scholar]
- 97.Heneweer C, Gendy SE, Peñate-Medina O. Liposomes and inorganic nanoparticles for drug delivery and cancer imaging. Ther Deliv. 2012;3:645–656. doi: 10.4155/tde.12.38. [DOI] [PubMed] [Google Scholar]
- 98.Egusquiaguirre SP, Igartua M, Hernández RM, Pedraz JL. Nanoparticle delivery systems for cancer therapy: advances in clinical and preclinical research. Clin Transl Oncol. 2012;14:83–93. doi: 10.1007/s12094-012-0766-6. [DOI] [PubMed] [Google Scholar]
- 99.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30:592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 100.Rouse BT. Liposomes as carriers of antigens as well as other molecules involved in immunity. J Am Vet Med Assoc. 1982;181:988–991. [PubMed] [Google Scholar]
- 101.Dachs GU, Dougherty GJ, Stratford IJ, Chaplin DJ. Targeting gene therapy to cancer: a review. Oncol Res. 1997;9:313–325. [PubMed] [Google Scholar]
- 102.Torchilin VP. Passive and active drug targeting: drug delivery to tumors as an example. Handb Exp Pharmacol. 2010:3–53. doi: 10.1007/978-3-642-00477-3_1. [DOI] [PubMed] [Google Scholar]
- 103.Matsumura Y. Preclinical and clinical studies of anticancer drug-incorporated polymeric micelles. J Drug Target. 2007;15:507–517. doi: 10.1080/10611860701499888. [DOI] [PubMed] [Google Scholar]
- 104.Tarhini AA, Agarwala SS. Novel agents in development for the treatment of melanoma. Expert Opin Investig Drugs. 2005;14:885–892. doi: 10.1517/13543784.14.7.885. [DOI] [PubMed] [Google Scholar]
- 105.Guo P, Haque F, Hallahan B, Reif R, Li H. Uniqueness, advantages, challenges, solutions, and perspectives in therapeutics applying RNA nanotechnology. Nucleic Acid Ther. 2012;22:226–245. doi: 10.1089/nat.2012.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun M, Su X, Ding B, He X, Liu X, et al. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine (Lond) 2012;7:1085–1100. doi: 10.2217/nnm.12.80. [DOI] [PubMed] [Google Scholar]
- 107.Mura S, Couvreur P. Nanotheranostics for personalized medicine. Adv Drug Deliv Rev. 2012;64:1394–1416. doi: 10.1016/j.addr.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 108.Sanna V, Sechi M. Nanoparticle therapeutics for prostate cancer treatment. Nanomedicine. 2012;8(Suppl 1):S31–36. doi: 10.1016/j.nano.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 109.Tiwari M. Nano cancer therapy strategies. J Cancer Res Ther. 2012;8:19–22. doi: 10.4103/0973-1482.95168. [DOI] [PubMed] [Google Scholar]
- 110.Rakowski JA, Ahmad S, Holloway RW. Use of pegylated liposomal doxorubicin in the management of platinum-sensitive recurrent ovarian cancer: current concepts. Expert Rev Anticancer Ther. 2012;12:31–40. doi: 10.1586/era.11.187. [DOI] [PubMed] [Google Scholar]
- 111.Grenader T, Rosengarten O, Isacson R, Plotkin Y, Gabizon A. Pegylated liposomal doxorubicin/carboplatin combination in ovarian cancer, progressing on single-agent pegylated liposomal doxorubicin. World J Clin Oncol. 2012;3:137–141. doi: 10.5306/wjco.v3.i10.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gao Z, Zhang L, Sun Y. Nanotechnology applied to overcome tumor drug resistance. J Control Release. 2012;162:45–55. doi: 10.1016/j.jconrel.2012.05.051. [DOI] [PubMed] [Google Scholar]
- 113.Dancey JE, Bedard PL, Onetto N, Hudson TJ. The genetic basis for cancer treatment decisions. Cell. 2012;148:409–420. doi: 10.1016/j.cell.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 114.Stegh AH. Toward personalized cancer nanomedicine - past, present, and future. Integr Biol (Camb) 2013;5:48–65. doi: 10.1039/c2ib20104f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–3632. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim WJ, Kim SW. Efficient siRNA delivery with non-viral polymeric vehicles. Pharm Res. 2009;26:657–666. doi: 10.1007/s11095-008-9774-1. [DOI] [PubMed] [Google Scholar]
- 117.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 118.Yagi N, Manabe I, Tottori T, Ishihara A, Ogata F, et al. A nanoparticle system specifically designed to deliver short interfering RNA inhibits tumor growth in vivo. Cancer Res. 2009;69:6531–6538. doi: 10.1158/0008-5472.CAN-08-3945. [DOI] [PubMed] [Google Scholar]
- 119.Gray MJ, Van Buren G, Dallas NA, Xia L, Wang X, et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J Natl Cancer Inst. 2008;100:109–120. doi: 10.1093/jnci/djm279. [DOI] [PubMed] [Google Scholar]
- 120.Merritt WM, Lin YG, Spannuth WA, Fletcher MS, Kamat AA, et al. Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J Natl Cancer Inst. 2008;100:359–372. doi: 10.1093/jnci/djn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shahzad MM, Lu C, Lee JW, Stone RL, Mitra R, et al. Dual targeting of EphA2 and FAK in ovarian carcinoma. Cancer Biol Ther. 2009;8:1027–1034. doi: 10.4161/cbt.8.11.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bhavsar D, Subramanian K, Sethuraman S, Krishnan UM. Translational siRNA therapeutics using liposomal carriers: prospects & challenges. Curr Gene Ther. 2012;12:315–332. doi: 10.2174/156652312802083611. [DOI] [PubMed] [Google Scholar]
- 123.Saad M, Garbuzenko OB, Minko T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine (Lond) 2008;3:761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tran MA, Smith CD, Kester M, Robertson GP. Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clin Cancer Res. 2008;14:3571–3581. doi: 10.1158/1078-0432.CCR-07-4881. [DOI] [PubMed] [Google Scholar]
- 125.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shim G, Han SE, Yu YH, Lee S, Lee HY, et al. Trilysinoyl oleylamide-based cationic liposomes for systemic co-delivery of siRNA and an anticancer drug. J Control Release. 2011;155:60–66. doi: 10.1016/j.jconrel.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 127.Kang SH, Cho HJ, Shim G, Lee S, Kim SH, et al. Cationic liposomal codelivery of small interfering RNA and a MEK inhibitor for enhanced anticancer efficacy. Pharm Res. 2011;28:3069–3078. doi: 10.1007/s11095-011-0569-4. [DOI] [PubMed] [Google Scholar]
- 128.Xiao W, Chen X, Yang L, Mao Y, Wei Y, et al. Co-delivery of doxorubicin and plasmid by a novel FGFR-mediated cationic liposome. Int J Pharm. 2010;393:119–126. doi: 10.1016/j.ijpharm.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 129.Li WM, Dragowska WH, Bally MB, Schutze-Redelmeier MP. Effective induction of CD8+ T-cell response using CpG oligodeoxynucleotides and HER-2/neu-derived peptide co-encapsulated in liposomes. Vaccine. 2003;21:3319–3329. doi: 10.1016/s0264-410x(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 130.Kim JY, Shim G, Choi HW, Park J, Chung SW, et al. Tumor vasculature targeting following co-delivery of heparin-taurocholate conjugate and suberoylanilide hydroxamic acid using cationic nanolipoplex. Biomaterials. 2012;33:4424–4430. doi: 10.1016/j.biomaterials.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 131.Wong HL, Bendayan R, Rauth AM, Wu XY. Simultaneous delivery of doxorubicin and GG918 (Elacridar) by new polymer-lipid hybrid nanoparticles (PLN) for enhanced treatment of multidrug-resistant breast cancer. J Control Release. 2006;116:275–284. doi: 10.1016/j.jconrel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 132.Shuhendler AJ, Cheung RY, Manias J, Connor A, Rauth AM, et al. A novel doxorubicin-mitomycin C co-encapsulated nanoparticle formulation exhibits anti-cancer synergy in multidrug resistant human breast cancer cells. Breast Cancer Res Treat. 2009;119:255–269. doi: 10.1007/s10549-008-0271-3. [DOI] [PubMed] [Google Scholar]
- 133.Basu S, Harfouche R, Soni S, Chimote G, Mashelkar RA, et al. Nanoparticle-mediated targeting of MAPK signaling predisposes tumor to chemotherapy. Proc Natl Acad Sci U S A. 2009;106:7957–7961. doi: 10.1073/pnas.0902857106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu J, Lu Y, Lee A, Pan X, Yang X, et al. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J Pharm Pharm Sci. 2007;10:350–357. [PubMed] [Google Scholar]
- 135.Ichiki H, Hamasaki S, Nakasaki M, Ishida S, Yoshikawa A, et al. Relationship between hyperglycemia and coronary vascular resistance in nondiabetic patients. Int J Cardiol. 2010;141:44–48. doi: 10.1016/j.ijcard.2008.11.148. [DOI] [PubMed] [Google Scholar]
- 136.Roy V, LaPlant BR, Gross GG, Bane CL, Palmieri FM North Central Cancer Treatment Group. Phase II trial of weekly nab (nanoparticle albumin-bound)- paclitaxel (nab-paclitaxel) (Abraxane) in combination with gemcitabine in patients with metastatic breast cancer (N0531) Ann Oncol. 2009;20:449–453. doi: 10.1093/annonc/mdn661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zucker D, Andriyanov AV, Steiner A, Raviv U, Barenholz Y. Characterization of PEGylated nanoliposomes co-remotely loaded with topotecan and vincristine: relating structure and pharmacokinetics to therapeutic efficacy. J Control Release. 2012;160:281–289. doi: 10.1016/j.jconrel.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 138.Zucker D, Barenholz Y. Optimization of vincristine-topotecan combination--paving the way for improved chemotherapy regimens by nanoliposomes. J Control Release. 2010;146:326–333. doi: 10.1016/j.jconrel.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 139.Abe K, Abgrall N, Aihara H, Ajima Y, Albert JB, et al. The T2K experiment. Nuclear Instruments & Methods in Physics Research Section a-Accelerators Spectrometers Detectors and Associated Equipment. 2011;659:106–135. [Google Scholar]
- 140.Song S, Wientjes MG, Walsh C, Au JL. Nontoxic doses of suramin enhance activity of paclitaxel against lung metastases. Cancer Res. 2001;61:6145–6150. [PubMed] [Google Scholar]
- 141.Kanzawa F, Koizumi F, Koh Y, Nakamura T, Tatsumi Y, et al. In vitro synergistic interactions between the cisplatin analogue nedaplatin and the DNA topoisomerase I inhibitor irinotecan and the mechanism of this interaction. Clin Cancer Res. 2001;7:202–209. [PubMed] [Google Scholar]
- 142.Raitanen M, Rantanen V, Kulmala J, Helenius H, Grénman R, et al. Supra-additive effect with concurrent paclitaxel and cisplatin in vulvar squamous cell carcinoma in vitro. Int J Cancer. 2002;100:238–243. doi: 10.1002/ijc.10472. [DOI] [PubMed] [Google Scholar]
- 143.Mayer LD, Harasym TO, Tardi PG, Harasym NG, Shew CG, et al. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5:1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 144.Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, et al. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33:129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 145.Harasym TO, Tardi PG, Harasym NL, Harvie P, Johnstone SA, et al. Increased preclinical efficacy of irinotecan and floxuridine coencapsulated inside liposomes is associated with tumor delivery of synergistic drug ratios. Oncol Res. 2007;16:361–374. doi: 10.3727/000000006783980937. [DOI] [PubMed] [Google Scholar]
- 146.Sawant RR, Vaze OS, Rockwell K, Torchilin VP. Palmitoyl ascorbate-modified liposomes as nanoparticle platform for ascorbate-mediated cytotoxicity and paclitaxel co-delivery. Eur J Pharm Biopharm. 2010;75:321–326. doi: 10.1016/j.ejpb.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 147.Zhang YF, Wang JC, Bian DY, Zhang X, Zhang Q. Targeted delivery of RGD-modified liposomes encapsulating both combretastatin A-4 and doxorubicin for tumor therapy: in vitro and in vivo studies. Eur J Pharm Biopharm. 2010;74:467–473. doi: 10.1016/j.ejpb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 148.Cosco D, Paolino D, Cilurzo F, Casale F, Fresta M. Gemcitabine and tamoxifen-loaded liposomes as multidrug carriers for the treatment of breast cancer diseases. Int J Pharm. 2012;422:229–237. doi: 10.1016/j.ijpharm.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 149.Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 150.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yoshizawa T, Hattori Y, Hakoshima M, Koga K, Maitani Y. Folate-linked lipid-based nanoparticles for synthetic siRNA delivery in KB tumor xenografts. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2008;70:718–725. doi: 10.1016/j.ejpb.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 152.Chen CH, Liu DZ, Fang HW, Liang HJ, Yang TS, et al. Evaluation of multi-target and single-target liposomal drugs for the treatment of gastric cancer. Biosci Biotechnol Biochem. 2008;72:1586–1594. doi: 10.1271/bbb.80096. [DOI] [PubMed] [Google Scholar]
- 153.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 154.Mainardes RM, Silva LP. Drug delivery systems: past, present, and future. Curr Drug Targets. 2004;5:449–455. doi: 10.2174/1389450043345407. [DOI] [PubMed] [Google Scholar]
- 155.Parveen S, Sahoo SK. Polymeric nanoparticles for cancer therapy. J Drug Target. 2008;16:108–123. doi: 10.1080/10611860701794353. [DOI] [PubMed] [Google Scholar]
- 156.Cegnar M, Kristl J, Kos J. Nanoscale polymer carriers to deliver chemotherapeutic agents to tumours. Expert Opin Biol Ther. 2005;5:1557–1569. doi: 10.1517/14712598.5.12.1557. [DOI] [PubMed] [Google Scholar]
- 157.Akhter S, Ahmad I, Ahmad MZ, Ramazani F, Singh A, et al. Nanomedicines as cancer therapeutics: current status. Curr Cancer Drug Targets. 2013;13:362–378. doi: 10.2174/1568009611313040002. [DOI] [PubMed] [Google Scholar]
- 158.Abolmaali SS, Tamaddon AM, Dinarvand R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother Pharmacol. 2013;71:1115–1130. doi: 10.1007/s00280-012-2062-0. [DOI] [PubMed] [Google Scholar]
- 159.Men K, Liu W, Li L, Duan X, Wang P, et al. Delivering instilled hydrophobic drug to the bladder by a cationic nanoparticle and thermo-sensitive hydrogel composite system. Nanoscale. 2012;4:6425–6433. doi: 10.1039/c2nr31592k. [DOI] [PubMed] [Google Scholar]
- 160.Lü JM, Wang X, Marin-Muller C, Wang H, Lin PH, et al. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9:325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jain AK, Das M, Swarnakar NK, Jain S. Engineered PLGA nanoparticles: an emerging delivery tool in cancer therapeutics. Crit Rev Ther Drug Carrier Syst. 2011;28:1–45. doi: 10.1615/critrevtherdrugcarriersyst.v28.i1.10. [DOI] [PubMed] [Google Scholar]
- 162.Tang MF, Lei L, Guo SR, Huang WL. Recent progress in nanotechnology for cancer therapy. Chin J Cancer. 2010;29:775–780. doi: 10.5732/cjc.010.10075. [DOI] [PubMed] [Google Scholar]
- 163.Milane L, Duan Z, Amiji M. Development of EGFR-targeted polymer blend nanocarriers for combination paclitaxel/lonidamine delivery to treat multi-drug resistance in human breast and ovarian tumor cells. Mol Pharm. 2011;8:185–203. doi: 10.1021/mp1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Song X, Cai Z, Zheng Y, He G, Cui FY, et al. Reversion of multidrug resistance by co-encapsulation of vincristine and verapamil in PLGA nanoparticles. Eur J Pharm Sci. 2009;37:300–305. doi: 10.1016/j.ejps.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 165.Misra R, Sahoo SK. Coformulation of doxorubicin and curcumin in poly(D,L-lactide-co-glycolide) nanoparticles suppresses the development of multidrug resistance in K562 cells. Mol Pharm. 2011;8:852–866. doi: 10.1021/mp100455h. [DOI] [PubMed] [Google Scholar]
- 166.Walter MV, Malkoch M. Simplifying the synthesis of dendrimers: accelerated approaches. Chem Soc Rev. 2012;41:4593–4609. doi: 10.1039/c2cs35062a. [DOI] [PubMed] [Google Scholar]
- 167.Baker JR., Jr Dendrimer-based nanoparticles for cancer therapy. Hematology Am Soc Hematol Educ Program. 2009 doi: 10.1182/asheducation-2009.1.708. [DOI] [PubMed] [Google Scholar]
- 168.Bai S, Thomas C, Rawat A, Ahsan F. Recent progress in dendrimer-based nanocarriers. Crit Rev Ther Drug Carrier Syst. 2006;23:437–495. doi: 10.1615/critrevtherdrugcarriersyst.v23.i6.10. [DOI] [PubMed] [Google Scholar]
- 169.Kaminskas LM, Boyd BJ, Porter CJ. Dendrimer pharmacokinetics: the effect of size, structure and surface characteristics on ADME properties. Nanomedicine (Lond) 2011;6:1063–1084. doi: 10.2217/nnm.11.67. [DOI] [PubMed] [Google Scholar]
- 170.Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4:130ra46. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wijagkanalan W, Kawakami S, Hashida M. Designing dendrimers for drug delivery and imaging: pharmacokinetic considerations. Pharm Res. 2011;28:1500–1519. doi: 10.1007/s11095-010-0339-8. [DOI] [PubMed] [Google Scholar]
- 172.Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–2237. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 173.Jain K, Kesharwani P, Gupta U, Jain NK. Dendrimer toxicity: Let’s meet the challenge. Int J Pharm. 2010;394:122–142. doi: 10.1016/j.ijpharm.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 174.Hourani R, Kakkar A. Advances in the elegance of chemistry in designing dendrimers. Macromol Rapid Commun. 2010;31:947–974. doi: 10.1002/marc.200900712. [DOI] [PubMed] [Google Scholar]
- 175.Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, Nanjwade VK. Dendrimers: emerging polymers for drug-delivery systems. Eur J Pharm Sci. 2009;38:185–196. doi: 10.1016/j.ejps.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 176.Svenson S, Tomalia DA. Dendrimers in biomedical applications-- reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 177.Tekade RK, Dutta T, Gajbhiye V, Jain NK. Exploring dendrimer towards dual drug delivery: pH responsive simultaneous drug-release kinetics. J Microencapsul. 2009;26:287–296. doi: 10.1080/02652040802312572. [DOI] [PubMed] [Google Scholar]
- 178.Kaneshiro TL, Lu ZR. Targeted intracellular codelivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nanoglobular carrier. Biomaterials. 2009;30:5660–5666. doi: 10.1016/j.biomaterials.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 179.Pablico-Lansigan MH, Situ SF, Samia AC. Magnetic particle imaging: advancements and perspectives for real-time in vivo monitoring and image-guided therapy. Nanoscale. 2013;5:4040–4055. doi: 10.1039/c3nr00544e. [DOI] [PubMed] [Google Scholar]
- 180.Hilger I, Kaiser WA. Iron oxide-based nanostructures for MRI and magnetic hyperthermia. Nanomedicine (Lond) 2012;7:1443–1459. doi: 10.2217/nnm.12.112. [DOI] [PubMed] [Google Scholar]
- 181.Maas ML, Specht JP, Buckwalter KC, Gittler J, Bechen K. Nursing home staffing and training recommendations for promoting older adults’ quality of care and life: Part 1. Deficits in the quality of care due to understaffing and undertraining. Research in gerontological nursing. 2008;1:123–133. doi: 10.3928/19404921-20080401-03. [DOI] [PubMed] [Google Scholar]
- 182.Alexiou C, Tietze R, Schreiber E, Lyer S. [Nanomedicine: Magnetic nanoparticles for drug delivery and hyperthermia - new chances for cancer therapy] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2010;53:839–845. doi: 10.1007/s00103-010-1097-9. [DOI] [PubMed] [Google Scholar]
- 183.Laurent S, Boutry S, Mahieu I, Vander Elst L, Muller RN. Iron oxide based MR contrast agents: from chemistry to cell labeling. Curr Med Chem. 2009;16:4712–4727. doi: 10.2174/092986709789878256. [DOI] [PubMed] [Google Scholar]
- 184.Atmaca H, Karaca B, Uzunoglu S, Karabulut B, Sanli UA, et al. Synergistic Inhibition of Angiogenin by Combined Treatment with Gossypol and Cisplatin in a Human Ovarian Cancer Cell Line, Ovcar-3. Iubmb Life. 2009;61:349–349. [Google Scholar]
- 185.Schwerdt JI, Goya GF, Calatayud MP, Hereñú CB, Reggiani PC, et al. Magnetic field-assisted gene delivery: achievements and therapeutic potential. Curr Gene Ther. 2012;12:116–126. doi: 10.2174/156652312800099616. [DOI] [PubMed] [Google Scholar]
- 186.Hsieh WJ, Liang CJ, Chieh JJ, Wang SH, Lai IR, et al. In vivo tumor targeting and imaging with anti-vascular endothelial growth factor antibody-conjugated dextran-coated iron oxide nanoparticles. Int J Nanomedicine. 2012;7:2833–2842. doi: 10.2147/IJN.S32154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tang C, Russell PJ, Martiniello-Wilks R, Rasko JE, Khatri A. Concise review: Nanoparticles and cellular carriers-allies in cancer imaging and cellular gene therapy? Stem Cells. 2010;28:1686–1702. doi: 10.1002/stem.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 189.Kievit FM, Zhang M. Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Acc Chem Res. 2011;44:853–862. doi: 10.1021/ar2000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cywinska MA, Grudzinski IP. [Modern toxicology of magnetic nanomaterials] Rocz Panstw Zakl Hig. 2012;63:247–256. [PubMed] [Google Scholar]
- 191.Mattheolabakis G, Rigas B, Constantinides PP. Nanodelivery strategies in cancer chemotherapy: biological rationale and pharmaceutical perspectives. Nanomedicine (Lond) 2012;7:1577–1590. doi: 10.2217/nnm.12.128. [DOI] [PubMed] [Google Scholar]
- 192.Namiki Y, Fuchigami T, Tada N, Kawamura R, Matsunuma S, et al. Nanomedicine for cancer: lipid-based nanostructures for drug delivery and monitoring. Acc Chem Res. 2011;44:1080–1093. doi: 10.1021/ar200011r. [DOI] [PubMed] [Google Scholar]
- 193.Liu Y, Miyoshi H, Nakamura M. Nanomedicine for drug delivery and imaging: a promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. Int J Cancer. 2007;120:2527–2537. doi: 10.1002/ijc.22709. [DOI] [PubMed] [Google Scholar]
- 194.Puri A, Blumenthal R. Polymeric lipid assemblies as novel theranostic tools. Acc Chem Res. 2011;44:1071–1079. doi: 10.1021/ar2001843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang J, Lee CH, Ko HJ, Suh JS, Yoon HG, et al. Multifunctional magneto-polymeric nanohybrids for targeted detection and synergistic therapeutic effects on breast cancer. Angew Chem Int Ed Engl. 2007;46:8836–8839. doi: 10.1002/anie.200703554. [DOI] [PubMed] [Google Scholar]
- 196.Yu MK, Jeong YY, Park J, Park S, Kim JW, et al. Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angew Chem Int Ed Engl. 2008;47:5362–5365. doi: 10.1002/anie.200800857. [DOI] [PubMed] [Google Scholar]
- 197.Kumar A, Sahoo B, Montpetit A, Behera S, Lockey RF, et al. Development of hyaluronic acid-Fe2O3 hybrid magnetic nanoparticles for targeted delivery of peptides. Nanomedicine. 2007;3:132–137. doi: 10.1016/j.nano.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 198.Yallapu MM, Othman SF, Curtis ET, Bauer NA, Chauhan N, et al. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int J Nanomedicine. 2012;7:1761–1779. doi: 10.2147/IJN.S29290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shi X, Thomas TP, Myc LA, Kotlyar A, Baker JR., Jr Synthesis, characterization, and intracellular uptake of carboxyl-terminated poly(amidoamine) dendrimer-stabilized iron oxide nanoparticles. Phys Chem Chem Phys. 2007;9:5712–5720. doi: 10.1039/b709147h. [DOI] [PubMed] [Google Scholar]
- 200.Rawat M, Singh D, Saraf S, Saraf S. Nanocarriers: promising vehicle for bioactive drugs. Biol Pharm Bull. 2006;29:1790–1798. doi: 10.1248/bpb.29.1790. [DOI] [PubMed] [Google Scholar]
- 201.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 202.Zheng C, Qiao MX, Yan L, Hu HY, Chen DW. [Recent advances in the study of dendrimers-based drug delivery systems] Yao Xue Xue Bao. 2007;42:924–929. [PubMed] [Google Scholar]
- 203.Blanco E, Kessinger CW, Sumer BD, Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp Biol Med (Maywood) 2009;234:123–131. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li W, Feng S, Guo Y. Tailoring polymeric micelles to optimize delivery to solid tumors. Nanomedicine (Lond) 2012;7:1235–1252. doi: 10.2217/nnm.12.88. [DOI] [PubMed] [Google Scholar]
- 205.Torchilin VP. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell Mol Life Sci. 2004;61:2549–2559. doi: 10.1007/s00018-004-4153-5. [DOI] [PubMed] [Google Scholar]
- 206.Lee KS, Chung HC, Im SA, Park YH, Kim CS, et al. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008;108:241–250. doi: 10.1007/s10549-007-9591-y. [DOI] [PubMed] [Google Scholar]
- 207.Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10:3708–3716. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 208.Zhu C, Jung S, Luo S, Meng F, Zhu X, et al. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA-PCL-PDMAEMA triblock copolymers. Biomaterials. 2010;31:2408–2416. doi: 10.1016/j.biomaterials.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 209.Lee JH, Nan A. Combination drug delivery approaches in metastatic breast cancer. J Drug Deliv. 2012;2012:915375. doi: 10.1155/2012/915375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Katragadda U1, Teng Q, Rayaprolu BM, Chandran T, Tan C. Multi-drug delivery to tumor cells via micellar nanocarriers. Int J Pharm. 2011;419:281–286. doi: 10.1016/j.ijpharm.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fan L, Li F, Zhang HT, Wang YK, Cheng C, et al. Co-delivery of PDTC and doxorubicin by multifunctional micellar nanoparticles to achieve active targeted drug delivery and overcome multidrug resistance. Biomaterials. 2010;31:5634–5642. doi: 10.1016/j.biomaterials.2010.03.066. [DOI] [PubMed] [Google Scholar]
- 212.Hu Q, Li W, Hu X, Hu Q, Shen J, et al. Synergistic treatment of ovarian cancer by co-delivery of survivin shRNA and paclitaxel via supramolecular micellar assembly. Biomaterials. 2012;33:6580–6591. doi: 10.1016/j.biomaterials.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 213.Wiradharma N, Tong YW, Yang YY. Self-assembled oligopeptide nanostructures for co-delivery of drug and gene with synergistic therapeutic effect. Biomaterials. 2009;30:3100–3109. doi: 10.1016/j.biomaterials.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 214.Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Progress in Polymer Science. 2008;33:448–477. [Google Scholar]
- 215.Kabanov AV, Vinogradov SV. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed Engl. 2009;48:5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Phillips MA, Gran ML, Peppas NA. Targeted Nanodelivery of Drugs and Diagnostics. Nano Today. 2010;5:143–159. doi: 10.1016/j.nantod.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 218.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 219.Banerjee S, Sen K, Pal TK, Guha SK. Poly(styrene-co-maleic acid)- based pH-sensitive liposomes mediate cytosolic delivery of drugs for enhanced cancer chemotherapy. Int J Pharm. 2012;436:786–797. doi: 10.1016/j.ijpharm.2012.07.059. [DOI] [PubMed] [Google Scholar]
- 220.Sershen SR, Westcott SL, Halas NJ, West JL. Temperature-sensitive polymer-nanoshell composites for photothermally modulated drug delivery. J Biomed Mater Res. 2000;51:293–298. doi: 10.1002/1097-4636(20000905)51:3<293::aid-jbm1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 221.Konishi M, Tabata Y, Kariya M, Hosseinkhani H, Suzuki A, et al. In vivo anti-tumor effect of dual release of cisplatin and adriamycin from biodegradable gelatin hydrogel. J Control Release. 2005;103:7–19. doi: 10.1016/j.jconrel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 222.Chacko RT, Ventura J, Zhuang J, Thayumanavan S. Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv Drug Deliv Rev. 2012;64:836–851. doi: 10.1016/j.addr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Moya-Ortega MD, Alvarez-Lorenzo C, Concheiro A, Loftsson T. Cyclodextrin-based nanogels for pharmaceutical and biomedical applications. Int J Pharm. 2012;428:152–163. doi: 10.1016/j.ijpharm.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 224.Seo SH, Han HD, Noh KH, Kim TW, Son SW. Chitosan hydrogel containing GMCSF and a cancer drug exerts synergistic anti-tumor effects via the induction of CD8(+) T cell-mediated anti-tumor immunity. Clin Exp Metastasis. 2009;26:179–187. doi: 10.1007/s10585-008-9228-5. [DOI] [PubMed] [Google Scholar]
- 225.Sharma A, Madhunapantula SV, Robertson GP. Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems. Expert Opin Drug Metab Toxicol. 2012;8:47–69. doi: 10.1517/17425255.2012.637916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cui JJ, Tran-Dubé M, Shen H, Nambu M, Kung PP, et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK) J Med Chem. 2011;54:6342–6363. doi: 10.1021/jm2007613. [DOI] [PubMed] [Google Scholar]
- 227.Arkenau HT, Bermann A, Rettig K, Strohmeyer G, Porschen R. 5-Fluorouracil plus leucovorin is an effective adjuvant chemotherapy in curatively resected stage III colon cancer: long-term follow-up results of the adjCCA-01 trial. Ann Oncol. 2003;14:395–399. doi: 10.1093/annonc/mdg100. [DOI] [PubMed] [Google Scholar]
- 228.Berhoune M, Banu E, Scotte F, Prognon P, Oudard S, et al. Therapeutic strategy for treatment of metastatic non-small cell lung cancer. Ann Pharmacother. 2008;42:1640–1652. doi: 10.1345/aph.1L200. [DOI] [PubMed] [Google Scholar]
- 229.Logman JF, Heeg BM, Botteman MF, Kaura S, van Hout BA. Economic evaluation of zoledronic acid for the prevention of osteoporotic fractures in postmenopausal women with early-stage breast cancer receiving aromatase inhibitors in the UK. Ann Oncol. 2010;21:1529–1536. doi: 10.1093/annonc/mdp560. [DOI] [PubMed] [Google Scholar]
- 230.Batist G, Gelmon KA, Chi KN, Miller WH, Jr, Chia SK, et al. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res. 2009;15:692–700. doi: 10.1158/1078-0432.CCR-08-0515. [DOI] [PubMed] [Google Scholar]
- 231.Tardi PG, Dos Santos N, Harasym TO, Johnstone SA, Zisman N, et al. Drug ratio-dependent antitumor activity of irinotecan and cisplatin combinations in vitro and in vivo. Mol Cancer Ther. 2009;8:2266–2275. doi: 10.1158/1535-7163.MCT-09-0243. [DOI] [PubMed] [Google Scholar]
- 232.Li X, Lu WL, Liang GW, Ruan GR, Hong HY, et al. Effect of stealthy liposomal topotecan plus amlodipine on the multidrug-resistant leukaemia cells in vitro and xenograft in mice. Eur J Clin Invest. 2006;36:409–418. doi: 10.1111/j.1365-2362.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 233.Liang GW, Lu WL, Wu JW, Zhao JH, Hong HY, et al. Enhanced therapeutic effects on the multi-drug resistant human leukemia cells in vitro and xenograft in mice using the stealthy liposomal vincristine plus quinacrine. Fundam Clin Pharmacol. 2008;22:429–437. doi: 10.1111/j.1472-8206.2008.00613.x. [DOI] [PubMed] [Google Scholar]
- 234.Agrawal V, Paul MK, Mukhopadhyay AK. 6-mercaptopurine and daunorubicin double drug liposomes-preparation, drug-drug interaction and characterization. J Liposome Res. 2005;15:141–155. doi: 10.1080/08982100500364081. [DOI] [PubMed] [Google Scholar]
- 235.Patel NR, Rathi A, Mongayt D, Torchilin VP. Reversal of multidrug resistance by co-delivery of tariquidar (XR9576) and paclitaxel using long-circulating liposomes. Int J Pharm. 2011;416:296–299. doi: 10.1016/j.ijpharm.2011.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tran MA, Gowda R, Sharma A, Park EJ, Adair J, et al. Targeting V600EB-Raf and Akt3 using nanoliposomal-small interfering RNA inhibits cutaneous melanocytic lesion development. Cancer Res. 2008;68:7638–7649. doi: 10.1158/0008-5472.CAN-07-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Clementi C, Miller K, Mero A, Satchi-Fainaro R, Pasut G. Dendritic poly(ethylene glycol) bearing paclitaxel and alendronate for targeting bone neoplasms. Mol Pharm. 2011;8:1063–1072. doi: 10.1021/mp2001445. [DOI] [PubMed] [Google Scholar]
- 238.Tekade RK, Dutta T, Tyagi A, Bharti AC, Das BC, et al. Surface-engineered dendrimers for dual drug delivery: a receptor up-regulation and enhanced cancer targeting strategy. J Drug Target. 2008;16:758–772. doi: 10.1080/10611860802473154. [DOI] [PubMed] [Google Scholar]
- 239.Ren Y, Kang CS, Yuan XB, Zhou X, Xu P, et al. Co-delivery of as-miR- 21 and 5-FU by poly(amidoamine) dendrimer attenuates human glioma cell growth in vitro. J Biomater Sci Polym Ed. 2010;21:303–314. doi: 10.1163/156856209X415828. [DOI] [PubMed] [Google Scholar]
- 240.Lee IH, An S, Yu MK, Kwon HK, Im SH, et al. Targeted chemoimmunotherapy using drug-loaded aptamer-dendrimer bioconjugates. J Control Release. 2011;155:435–441. doi: 10.1016/j.jconrel.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 241.Song XR, Zheng Y, He G, Yang L, Luo YF, et al. Development of PLGA nanoparticles simultaneously loaded with vincristine and verapamil for treatment of hepatocellular carcinoma. J Pharm Sci. 2010;99:4874–4879. doi: 10.1002/jps.22200. [DOI] [PubMed] [Google Scholar]
- 242.Wang H, Zhao Y, Wu Y, Hu YL, Nan K, et al. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials. 2011;32:8281–8290. doi: 10.1016/j.biomaterials.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 243.Patil Y, Sadhukha T, Ma L, Panyam J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J Control Release. 2009;136:21–29. doi: 10.1016/j.jconrel.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 244.Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2010;31:358–365. doi: 10.1016/j.biomaterials.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lammers T, Subr V, Ulbrich K, Peschke P, Huber PE, et al. Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. Biomaterials. 2009;30:3466–3475. doi: 10.1016/j.biomaterials.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 246.Dilnawaz F, Singh A, Mohanty C, Sahoo SK. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials. 2010;31:3694–3706. doi: 10.1016/j.biomaterials.2010.01.057. [DOI] [PubMed] [Google Scholar]