Abstract
Positive genetic associations of rs6313 (102T/C at exon 1) and rs6311 (-1438A/G) on the 5-hydroxytryptamine (serotonin) 2A receptor gene (HTR2A or 5-HT2A) were reported for alcohol and drug abuse, however, other association studies failed to produce consistent results supporting the susceptibility of the two single nucleotide polymorphisms (SNPs). To clarify the associations of the HTR2A gene with substance use disorders, we performed a meta-analysis based on the genotypes from the available candidate gene association studies of the two SNPs with alcohol and drug abuse from multiple populations. Evidence of association was found for HTR2A rs6313 in all the combined studies (e.g., allelic P = 0.0048 and OR = 0.86, 95% CI 0.77 – 0.95) and also in the combined studies of alcohol dependence (abuse) (e.g., allelic P = 0.0001 and OR = 0.71, 95% CI 0.59 – 0.85). The same association trend was also observed in the SAGE (Study of Addiction: Genetics and Environment) datasets. The meta-analysis supports a contribution of the HTR2A gene to the susceptibility to substance use disorders, particularly alcohol dependence.
Keywords: Meta-analysis, Addiction, Linkage Disequilibrium, Heterogeneity, Synaptic Terminal
Introduction
Alcohol and drug abuse are chronic relapsing disorders characterized by compulsive seeking, abuse, tolerance and physical dependence on the substance in question. Studies have showed consistently that substance use disorders share some common genetic factors that play pivotal roles in the development of the disorders (Cadoret et al. 1995; Fu et al. 2002; True et al. 1999; Xian et al. 2008). Studies also demonstrated that drug dependence clusters in the families with drug-dependent probands (Bierut et al. 1998). For instance, among relatives of controls, the drug dependence rate was 3.5%, whereas among relatives of opioid-dependent individuals, the rate was 20.5% (Saxon et al. 2005). The genetic contribution to vulnerability to develop heroin addiction is 40 – 70%, suggesting that addictions are multifactorial disorders caused by complex interaction of genetic and environmental factors (Gelernter and Kranzler 2009; Kendler et al. 2007; Uhl et al. 2008).
The 5-hydroxytryptamine (serotonin or 5-HT) 2A receptor is one of the subtypes of 5-HT receptors (HTR). The 5-HT 2A receptor (HTR2A or formally 5-HT2A) is a member of the G protein superfamily. Presynaptic HTR2A has been localized on dopaminergic neurons within the ventral tegmental area and nucleus accumbens, suggesting an important role within the brain reward system (Frazer and Hensler 1998). Postsynaptic activation of the receptor also plays a relevant role in substance dependence (Kreek et al. 2005). The HTR2A is related to affectivity, regulation, and pharmacologic effects of antidepressant, anti-anxiety and antipsychotic medications. It may also play a role in cellular development and differentiation, while it is the action site of certain drugs and medications (Gelernter et al. 2006; Glatt et al. 2006; Kendler et al. 2003; Tsuang et al. 1998; van den Bree et al. 1998). It has also been shown that HTR2A and HTR2C antagonists cause attenuation of alcohol intake in both animals and humans (Pandey et al. 1995), and activation of HTR2A can reduce ethanol consumption (Maurel et al. 1999). These data suggested a role for the HTR2A gene as a candidate gene for ethanol and drug related traits.
The HTR2A gene, which contains 62 kilo base pairs, is located on 13q14–q21, the long arm of chromosome 13. Previous association studies focused on two common single nucleotide polymorphisms (SNPs) which are in complete linkage disequilibrium in European populations (Arranz et al. 1998; Saiz et al. 2008). For the MspI polymorphic site at nucleotide 102, although the receptor protein remains unaltered, the SNP rs6313 (102T/C at exon 1) has been intensively studied among the patients with alcohol and heroin addiction. Another SNP rs6311 (-1438A/G) is in the promoter region. Significant associations or noticeable effect sizes were described for the two SNPs with alcohol and (or) heroin dependence. However, other association studies failed to produce consistent results, e.g., some individual studies even reported opposite directions of the risk alleles. The nonreplicability of previous results made it necessary to clarify the contrary results among these individual association studies. Therefore, we combined all the available genotype data of HTR2A rs6313 and rs6311 of multiple populations from candidate gene association studies of substance use disorders via meta-analysis approaches.
Methods
Literature search
The studies included in the meta-analysis were selected from Scopus and the database of Chinese Academic Journals with keywords 'serotonergic receptor', ‘HTR2A’, ‘5-HT2A', 'serotonin receptor', 'association', 'associated', 'drug’, 'substance', 'alcoholism’, 'alcohol’, 'alcoholics', 'heroin’, 'cocaine’, 'opiate', 'opioid', 'opium', and other abbreviations of the gene (i.e., 'HTR2'). Both English and Chinese keywords were used for the Chinese Journals. The references cited in these studies and in published reviews were examined in order to identify additional works not indexed by the databases. The analyzed data cover all identified publications in English and Chinese available up to July 2012.
Inclusion criteria
Eligible studies met all of the following criteria: they (i) were published in peer-reviewed journals and contained original data; (ii) presented sufficient data to calculate the odds ratio (OR) with confidence interval (CI) and P value; (iii) were association studies investigating one or two of the polymorphisms using either case-control or family-based approaches; (iv) described or referenced appropriate genotyping primers, machines and protocols; (v) diagnosed the patients according to the World Health Organization’s International Statistical Classification of Diseases and Related Health Problems (ICD)(World Health Organization), American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM) (American Psychiatric Association) or Chinese classification of mental disorders (CCMD) (Chen 2002) systems; and (vi) used random population or healthy individuals as controls in case-control studies. Authors were contacted in cases where it would be useful to have additional information regarding their studies. The procedure of ‘extended-quality score’ (Li et al. 2006a) was applied to assist the assessment of quality of the association studies.
Statistical analyses
Studies were classified depending on whether they dealt with samples with European ancestries and those with Asian ancestries. A study that contained data from multiple populations, each was considered effectively as an independent study if possible. Data from the case-control and haplotype relative risk (HRR) studies were summarized by two-by-two tables. The studies were statistically combined by the method described in our previous study of HTR2A with schizophrenia (Li et al. 2006b) to join population-based and family-based studies into a single meta-analysis. In particular, from each table a log odds ratio and its sampling variance were calculated. The Cochran’s χ2-based Q statistic test was performed to assess the heterogeneity to ensure that each group of studies was suitable for meta-analysis. A test for funnel plot asymmetry, described by Egger et al. (Egger et al. 1997), was used to assess evidence for publication bias. The test used a linear regression approach to measure funnel plot asymmetry on the natural logarithm of the OR. The larger the deviation of each study from the funnel curve, the more pronounced the asymmetry. Results from small studies will scatter widely at the bottom of the graph, with the spread narrowing among larger studies. The significance of the intercept was evaluated using the T test and the P(T) was used to estimate the significance of publication bias. The “Duval and Tweedie's Trim and Fill” procedure (Duval and Tweedie 2000) was used to impute the number of potentially-missing studies for publication bias.
ORs were pooled using the method of DerSimonian and Laird (DerSimonian and Laird 1986), and 95% CIs were constructed using Woolf’s method (Woolf 1955). The significance of the overall OR was determined using the Z-test. In addition, the studies were also subdivided and re-analyzed according to different populations (European vs. Asian) and different traits (alcohol vs. heroin dependence). Genotypic analyses were carried out under both dominant and recessive models. The classic fail-safe analysis (Rosenthal 1979) was adopted to evaluate the impact of publication bias on the results of meta-analysis. Retrospective analysis was performed to better understand the potential effect of year of publication upon the results. The type I error rate was set at 0.05. The tests were two-tailed. Haplotype construction, counting, and linkage disequilibrium (LD) block defining were performed separately using the HapMap CEPH and Asian samples, as described previously (Li et al. 2011).
Results
The combined search yielded 2,409 references. After discarding the references that clearly did not meet the criteria, the studies were then filtered to ensure conformity with our inclusion criteria. Two studies (Jakubczyk et al. 2011; Wrzosek et al. 2011) were excluded because no data of matched normal controls were described, and one study (Yang et al. 2012) was excluded because the genotype data was same as that described in another study (Yang et al. 2010). Finally, 21 studies, composed of 20 case-control studies and one HRR study (Hill et al. 2002), met our criteria for inclusion. The 21 studies included five studies for European populations (Fehr et al. 2001; Hill et al. 2002; Parsian and Cloninger 2001; Wrzosek et al. 2012), two studies for Hispanic samples (Polina et al. 2009; Saiz et al. 2008), and 14 studies for Asian populations (Cao et al. 2002; Gao et al. 2011; Himei et al. 2000; Hwu and Chen 2000; Lee et al. 2009; Li et al. 2002; Nakamura et al. 1999; Shao et al. 2005; Terayama et al. 2003; Tsunoka et al. 2010; Wang et al. 2001; Yang et al. 2010). Among these studies, eight studies (Cao et al. 2002; Gao et al. 2011; Li et al. 2002; Saiz et al. 2008; Shao et al. 2005; Wang et al. 2001; Yang et al. 2010) investigated heroin dependence or abuse; one study (Tsunoka et al. 2010) investigated methamphetamine dependence; and the other 12 studies (Fehr et al. 2001; Hill et al. 2002; Himei et al. 2000; Hwu and Chen 2000; Lee et al. 2009; Nakamura et al. 1999; Parsian and Cloninger 2001; Polina et al. 2009; Terayama et al. 2003; Wrzosek et al. 2012) investigated alcohol dependence or abuse. The 21 studies included 3,506 cases, 3,556 controls, and 35 families. The demography of these studies is shown in supplementary Table 1. The results for each polymorphism are detailed below.
The rs6313 (102T/C) Polymorphism
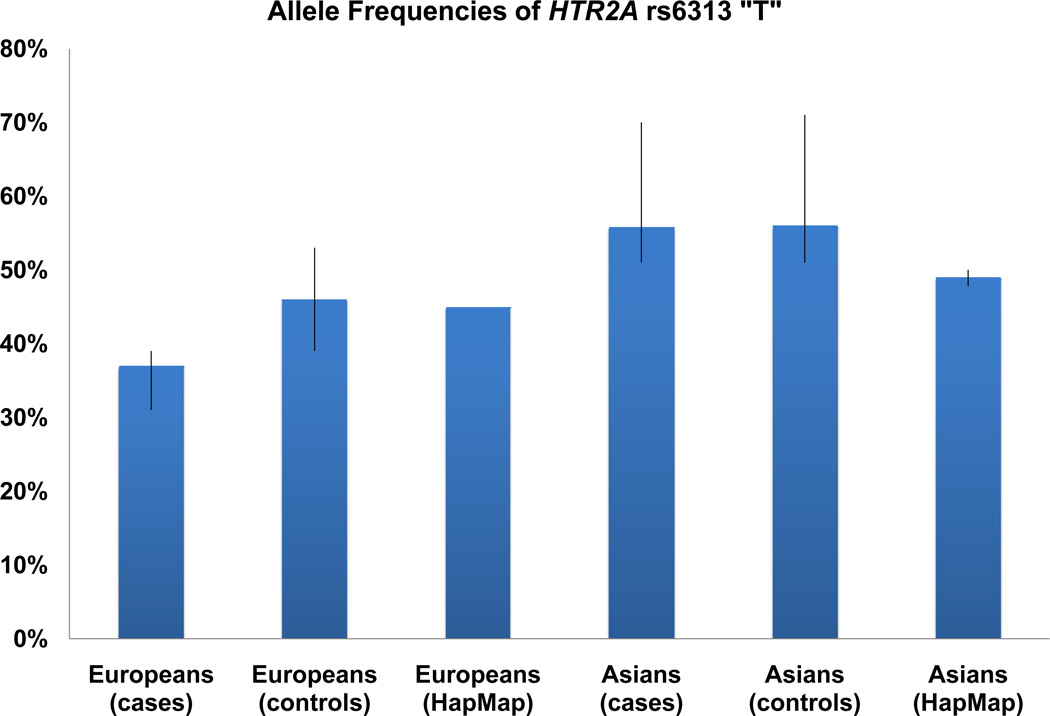
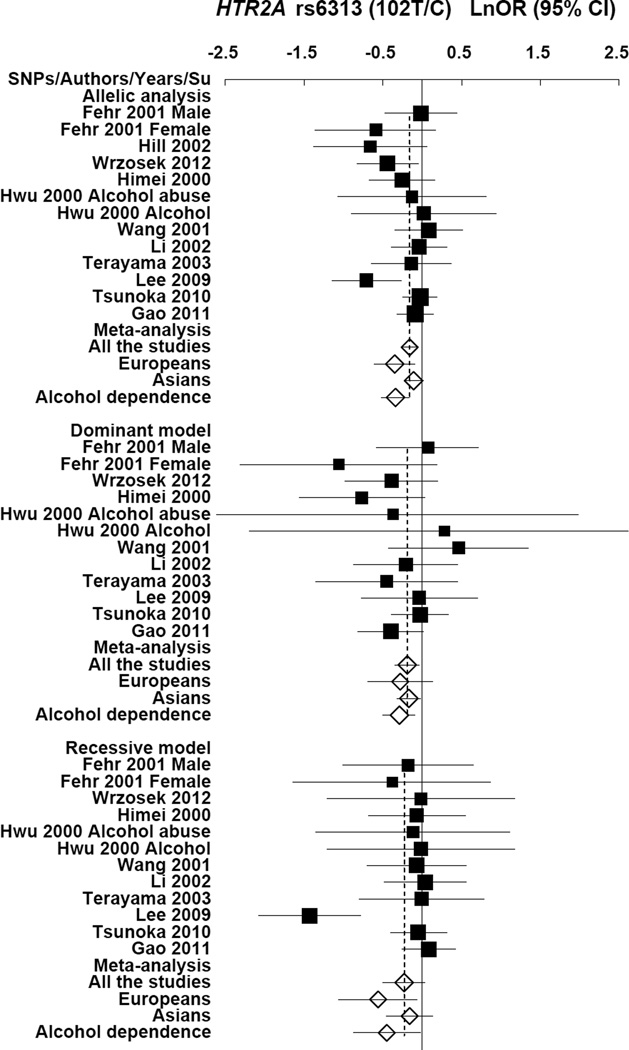
The T allele frequency varied across the samples, being abundant with an average percentage of 46% (39% – 53%) in Europeans controls and 37% (31% – 39%) in cases; and less abundant with an average percentage of 56% (51% – 71%) in Asian controls and 55.9% (51% – 70%) in cases. In the 13 studies, 11 studies showed lower T allele frequency in cases than in controls (or less allele transmissions in families). The meta-analysis of all the combined studies produced an overall allelic P value of 0.0048 (OR = 0.86, 95% CI 0.77 – 0.95) with no evidence for heterogeneity between studies (Table 1). Evidence of significant association was also found in the combined European populations (allelic P = 0.0074 and OR = 0.70 (95% CI 0.54, 0.91)). The combined studies of alcohol dependence (abuse) showed more significant association (allelic P = 0.0001 and OR = 0.71 (95% CI 0.59, 0.85)). Evidence of association was also observed under the dominant ((TT + TC) / CC) and recessive (TT / (TC + CC)) models, e.g., P = 0.0008 and OR = 0.61 (0.46, 0.81) for the combined studies of alcohol dependence (abuse) under the recessive model. The same genetic association trend was also observed in the SAGE (Study of Addiction: Genetics and Environment) datasets of European and African American subjects. The findings are shown for the allelic and genotypic analyses in Table 1 and supplementary Table 2, respectively. The allele frequencies and forest plots of rs6313 are shown in Figures 1 and 2, respectively.
Table 1.
Results of the overall and sub-group allelic analyses
| SNPs / Groups | Studiesa | Samplesb | LnOR (95% CI) | OR (95% CI) | P(Z) | P(Q) |
|---|---|---|---|---|---|---|
| HTR2A rs6313 (102T/C) | ||||||
| All the studies | 13 | 1,407/1,908/35 | −0.16 (−0.26, −0.05) | 0.86 (0.77,0.95) | 0.0048 | 0.23 |
| Europeans | 4 | 326/155/35 | −0.35 (−0.61, −0.09) | 0.7 (0.54,0.91) | 0.0074 | 0.34 |
| Asians | 9 | 1,081/1,753 | −0.11 (−0.23,0.01) | 0.89 (0.79,1.01) | 0.062 | 0.34 |
| Alcohol dependence/abuse | 9 | 715/503/35 | −0.34 (−0.52, −0.17) | 0.71 (0.59,0.85) | 0.0001 | 0.47 |
| Alcohol dependence/abuse (Asians) | 5 | 389/348 | −0.34 (−0.58, −0.1) | 0.71 (0.56,0.91) | 0.0063 | 0.37 |
| All the studies & SAGEc | 15 | 3,098/3,620/35 | −0.11 (−0.19, −0.04) | 0.89 (0.83,0.96) | 0.0023 | 0.29 |
| Europeans & SAGEc | 5 | 1,440/1,437/35 | −0.11 (−0.22, −0.01) | 0.89 (0.8,0.99) | 0.0338 | 0.12 |
| Alcohol dependence/abuse & SAGEc | 11 | 2,406/2,215/35 | −0.14 (−0.23, −0.05) | 0.87 (0.8,0.95) | 0.0013 | 0.15 |
| HTR2A rs6311 (-1438A/G) | ||||||
| All the studies | 12 | 2,760/3,056 | 0.06 (−0.06,0.19) | 1.07 (0.94,1.21) | 0.3263 | 0.0071 |
| Europeans & Hispanic | 3 | 359/623 | 0.42 (0.22,0.61) | 1.51 (1.24,1.85) | 4×10−5 | 0.98 |
| Asians | 9 | 2,401/2,433 | −0.05 (−0.13,0.04) | 0.95 (0.87,1.04) | 0.2957 | 0.41 |
| Heroin dependence/abuse | 7 | 1,770/1,578 | 0.08 (−0.03,0.18) | 1.08 (0.97,1.2) | 0.149 | 0.21 |
| Alcohol dependence/abuse | 4 | 794/676 | 0.12 (−0.27,0.51) | 1.13 (0.76,1.66) | 0.5478 | 0.0016 |
| Alcohol dependence/abuse (Europeans) | 2 | 246/203 | 0.42 (0.15,0.68) | 1.52 (1.16,1.98) | 0.0023 | 0.83 |
| Alcohol dependence/abuse (Asians) | 2 | 548/473 | −0.22 (−0.4, −0.04) | 0.81 (0.67,0.96) | 0.0176 | 0.56 |
| All the studies & SAGEc | 14 | 4,451/4,768 | 0.03 (−0.08,0.13) | 1.03 (0.93,1.14) | 0.6283 | 0.0063 |
, the number of studies included are indicated.
, the number of cases/controls/families. The 35 families included 116 subjects.
, 1,691 cases and 1,712 controls from the Study of Addiction: Genetics and Environment (SAGE) datasets (European and African Americans) were included in the meta-analysis.
P(Z): Z test used to determine the significance of the overall OR. The P values < 0.05 are indicated in red boldfaces.
P(Q): Cochran’s X2-based Q statistic test used to assess the heterogeneity.
P(T): T test used to estimate the significance of publication bias (not shown).
Figure 1.
Frequencies of HTR2A rs6313 T allele. The error bar shows the highest and lowest frequencies in the individual studies anzlyed in the meta-analysis.
Figure 2.
Forest plots of ln (OR) and overall ln (OR) with 95% CI of the allelic and genotypic analyses for HTR2A rs6313. Black squares indicate the ln (OR), with the size of the square inversely proportional to its variance, and horizontal lines represent the 95% CIs. The overall ln (OR) are indicated by the unshaded black diamond.
The rs6311 (-1438A/G) Polymorphism
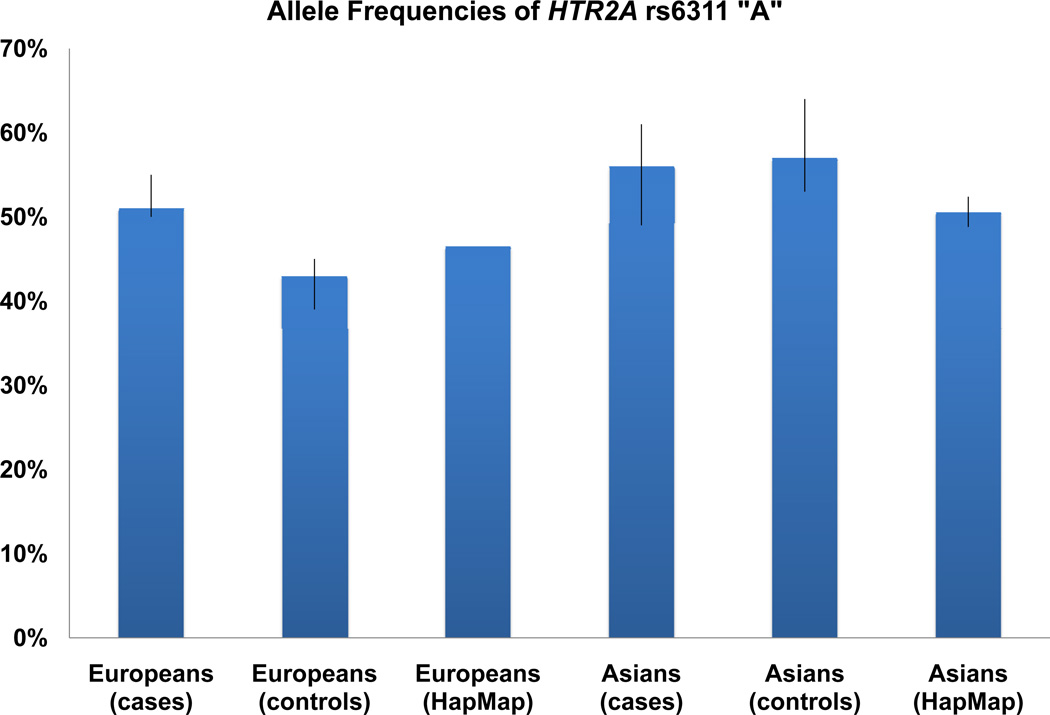
The A allele is the minor allele with an average percentage of 43% (39% – 45%) in the European and Hispanic normal samples and 51% (50% – 55%) in cases, but is the major allele with an average percentage of 57% (53% – 64%) in the Asian normal populations and 56% (49% – 61%) in cases. In the 12 case-control studies, seven studies showed higher A allele frequency in cases than in controls. No evidence of significant association was found when all the studies were combined under the random effects model with heterogeneity between studies. Evidence of heterogeneity was further observed between European and Asian populations (P = 3×10−5), and therefore, we also analyzed the two populations separately. The combined studies of European populations showed an allelic P value of 4×10−5 (OR = 1.51 (1.24, 1.85)), which was also significant under the dominant ((AA + AG) / GG) and recessive (AA / (AG + GG)) models, however, it should be noted that the sample size of the subgroup analysis of Europeans were small. The findings are shown in Table 1 and supplementary Table 2. The allele frequencies and forest plots of rs6311 are shown in Figure 3 and supplementary Figure 1, respectively.
Figure 3.
Frequencies of HTR2A rs6311 A allele.
Publication Bias and Fail-safe Analyses
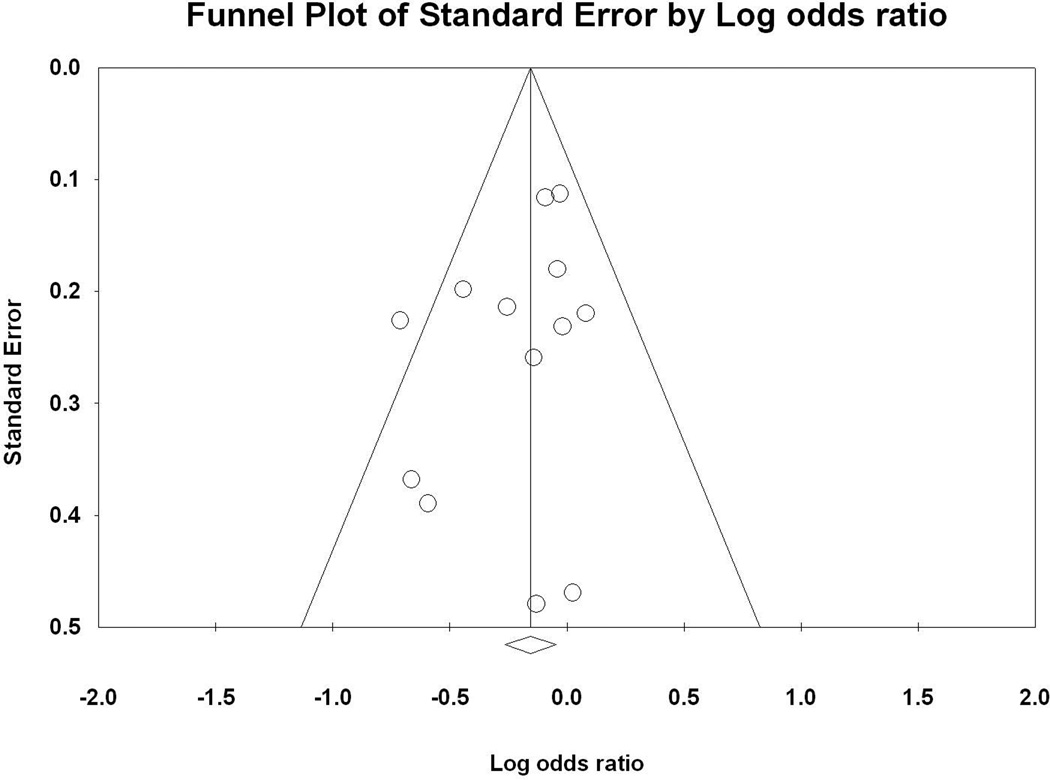
In the present meta-analysis, no evidence of significant publication bias was found (P(T) > 0.05). For rs6313, the classic fail-safe analysis showed that at least 23 and 22 assumed non-significant association studies would be required to bring the P values to > 0.05 for the meta-analysis of all the combined studies and for the meta-analysis of alcohol dependence (abuse), respectively. This finding further supported the significant association observed in rs6313. The funnel plots of standard error by log odds ratio of rs6313 are shown for all the combined studies and the combined studies of alcohol dependence (abuse) in Figure 4 and supplementary Figure 2, respectively.
Figure 4.
Egger’s funnel plots of all the combined studies for HTR2A rs6313.
Sensitivity Analyses
The results of sensitivity analysis showed that no individual study included in the meta-analysis biased the overall significant results significantly. For example, for rs6313, the meta-analysis showed consistency, regardless of the data set removed, the P values was never > 0.035 and never > 0.0051 for all the combined studies and for the studies of alcohol dependence (abuse), respectively. For rs6311, the P values were never > 0.0023 for the Europeans studies, regardless of the data set removed. The results are shown in supplementary Table 3.
Retrospective Analyses
The meta-analysis based on publication years showed that the cumulative results, e.g., the asymptote lines, have not reached stable for rs6313, therefore, more replication studies are still necessary to confirm current findings. The plots of rs6313 are shown for all the combined studies and the studies of alcohol dependence (abuse) in supplementary Figures 3 and 4, respectively.
Discussion
Alcohol and drug abuse constitutes major public health problems. The cost of drug abuse has grown to approximately one trillion dollars per year in the United States (Califano 2007). In this meta-analysis, evidence of association was found for HTR2A rs6313 in all the combined studies (P = 0.0048 and OR = 0.86, 95% CI 0.77 – 0.95) as well as in the combined studies of alcohol dependence (abuse) (P = 0.0001 and OR = 0.71, 95% CI 0.59 – 0.85). For HTR2A rs6311, evidence of association was observed in the combined studies of European populations (allelic P = 4×10−5 and OR = 1.51, 95% CI 1.24 – 1.85), however, considering its limited sample size of the European subjects, more replication association studies are necessary to fully demonstrate the role of rs6311 in substance abuse.
The HTR2C gene was also reported frequently as a candidate gene for ethanol related traits. The potential association of 23Cys/Ser polymorphism (Hinfl) of HTR2C with alcoholism was also analyzed in the present meta-analysis. Our results showed evidence of marginal association (P = 0.045) based on the available genotypes. HTR2A has attracted more attention because it appears to be an important active site for atypical antipsychotic agents (Schmidt et al. 1995), hallucinogens (Marek and Aghajanian 1996), and selective serotonin reuptake inhibitors (Roth et al. 1998). Prior findings suggested that rs6311 might have functional effects on HTR2A expression in the brain and could be responsible for the association of rs6311 and its strongly linked rs6313 with many neuropsychiatric disorders (Lesch et al. 1996; Myers et al. 2007).
Although most disease associated genes show similar effect sizes in different populations, there are still many loci that have unique effects in specific ethnic groups, and those loci identified in one population fail to be replicated in other populations. The population-specific effects could be attributable to different genetic background, unique LD structure or different environmental factors of those ethnic groups. This meta-analysis showed different levels of association results between the two ethnicity populations, e.g., Europeans and Asians for rs6311 (Table 1). The allele frequencies varied among the investigated populations, such as, the rs6311 A allele showed lower frequency in Europeans than Asians. It is well known that gene-trait associations largely depend on the ethnic background of investigated patients and matched controls. Sampling methods and sample heterogeneity may differentiate the results. The power to detect the putative vulnerability alleles might be diminished due to the quality of the samples. For instance, one of the studies (Hill et al. 2002) analyzed in the meta-analysis included a small proportion of Hispanic and African-American subjects.
Substance use disorders are often comorbid with psychiatric disorders, for instance, co-occurrence of alcohol dependence and behavior disorders may differentiate the findings. Some psychiatric disorders and addiction may not have mutual pathogenesis associated with the HTR2A gene, and thus the potential comorbidity might have significantly impaired the possibility to detect strong association signals. Diagnostic classification and ascertainment of the patients and stages of the disorders (Kreek et al. 2005) may further differentiate the association levels, such as, two studies investigated the diagnostic category of alcohol abuse (Hwu and Chen 2000) and heroin abuse (Cao et al. 2002) other than “dependence”. In addition, small sample size may exaggerate population variations. The ethnic and genetic heterogeneity may also explain underlying population-specific gene pathways observed in each population.
It is possible that rs6313 and rs6311 may not contribute directly to the etiology of alcohol and heroin dependence, but it could be assumed that a variant in LD with the SNPs may be responsible for the vulnerability effect. The supplementary Figures 5 and 6 shows the LD plots of the HTR2A gene for the European and Asian ancestries, respectively (the pair-wise LD measures (D’ and r2 values) are shown in supplementary Table 4). Overall, the Asian samples showed a relatively weak LD than the European samples. Because addiction has a complex inheritance mode in which multiple genes exert small effects, the possibility that other genetic variants on this gene or another gene that is in LD with the HTR2A variants should also be investigated. For example, for the two HTR2A SNPs, Thr25Asn and His452Tyr, which cause amino acid replacement, Thr25Asn is located within the same haplotype block as rs6313 and rs6311; and His452Tyr maps in another dense haplotype block; the ESD gene, a genetic marker for retinoblastoma and Wilson’s disease, is very close to the HTR2A gene.
This study also has some limitations. For example, small sample size may not have sufficient power to detect risk variants, particularly when the effects of variants are small. The sample size in this meta-analysis was moderate or small, particularly for subgroup analyses, and therefore, the subgroup results should be interpreted cautiously since they could be influenced by factors such as random genotyping errors from individual association studies. Secondly, two SNPs and multiple phenotype and ethnicity subgroups were analyzed in this meta-analysis (Table 1). Although the subgroup analyses were not independent, and the two SNPs were in very strong LD, multiple comparisons should still be noted. Some less significant associations might not survive when stringent Bonferroni correction was applied.
To conclude, evidence of association was found at the HTR2A gene, particularly for rs6313 with alcohol and heroin dependence. The vulnerability effect of rs6311 warrants further investigation in larger samples. Our findings support a contribution of the HTR2A gene to the susceptibility to alcohol and heroin dependence.
Supplementary Material
Supplementary Figure 1 Forest plots of ln (OR) and overall ln (OR) with 95% CI of the allelic and genotypic analyses for rs6311.
Supplementary Figure 2 Egger’s funnel plots of the combined studies of alcohol dependence (abuse) for rs6313.
Supplementary Figure 3 Retrospective analysis of all the combined studies for - rs6313.
Supplementary Figure 4 Retrospective analysis of the combined studies of alcohol dependence (abuse) for rs6313.
Supplementary Figure 5 Graphical representation of the LD structure of European ancestry. The LD structure, spanning 200 kp covering the HTR2A gene, was constructed using European genotype data of 273 SNPs. The current two polymorphisms are shown in red; other polymorphisms are shown in blue. The HTR2A gene, size 62, 662 bp, is indicated in red. Vertical tick marks above the name indicate the relative genomic position of each SNP. The LD structure represents the pairwise calculation of D’ for each possible combination of SNPs. D’ < 0.5 is shown in white, D’ = 1.0 in dark red, with increasing shades of red representing increasing D’ between the SNPs.
Supplementary Figure 6 Graphical representation of the LD structure of Asian ancestry. The LD structure, spanning 200 kp covering the HTR2A gene, was constructed using Asian genotype data of 249 SNPs.
Acknowledgements
This work was supported by the start-up fund from the University of Vermont, and by the research grants DA12849, DA12690, AA017535, AA12870, and AA11330 from the National Institutes of Health, U.S.A. The datasets used for the replication analyses described in this manuscript were obtained from the database of Genotypes and Phenotypes (dbGaP) through accession number phs000092.v1.p1. Funding support for the Study of Addiction: Genetics and Environment (SAGE) was provided through U01 HG004422, U10 AA008401, P01 CA089392, R01 DA013423, and R01 DA019963. We thank the authors who provided the data related to their individual association studies for this meta-analysis, and thank Drs. Joel Gelernter and Hongyu Zhao for their comments on the manuscripts. We also thank the anonymous reviewers for very helpful comments to improve the manuscript.
Footnotes
Conflict of Interest
None
Electronic-database information
Accession Numbers and URLs for data in this article are as follows:
GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ for genomic structure of HTR2A;
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim for HTR2A;
Genotype data, http://www.hapmap.org/ for HTR2A;
Genome data, http://genome.ucsc.edu/ for HTR2A.
References
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM) Washington, DC: American Psychiatric Press; [Google Scholar]
- Arranz MJ, Munro J, Owen MJ, Spurlock G, Sham PC, Zhao J, Kirov G, Collier DA, Kerwin RW. Evidence for association between polymorphisms in the promoter and coding regions of the 5-HT2A receptor gene and response to clozapine. Mol Psychiatry. 1998;3:61–66. doi: 10.1038/sj.mp.4000348. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, Nurnberger JI, Jr, Porjesz B, Schuckit MA, Reich T. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry. 1998;55:982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. Adoption study demonstrating two genetic pathways to drug abuse. Arch Gen Psychiatry. 1995;52:42–52. doi: 10.1001/archpsyc.1995.03950130042005. [DOI] [PubMed] [Google Scholar]
- Califano JA. High Society: How Substance Abuse Ravages America and What to Do About It. Public Affairs. 2007 [Google Scholar]
- Cao L, Li T, Xu K, Liu X. Association study of heroin dependence and 5-HT2A gene polymorphism-1438A/G. Chin J Drug Depend. 2002;11:266–268. [Google Scholar]
- Chen YF. Chinese classification of mental disorders (CCMD-3): towards integration in international classification. Psychopathology. 2002;35:171–175. doi: 10.1159/000065140. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Schleicher A, Szegedi A, Anghelescu I, Klawe C, Hiemke C, Dahmen N. Serotonergic polymorphisms in patients suffering from alcoholism, anxiety disorders and narcolepsy. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:965–982. doi: 10.1016/s0278-5846(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Frazer A, Hensler JG. Basic Neurochemistry, Molecular, Cellular and medical aspects. Philadephia - New York: Lippincott-Raven Publishers; 1998. Serotonin. [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, True WR, Jacob T, Tsuang MT, Eisen SA. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch Gen Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- Gao F, Zhu YS, Wei SG, Li SB, Lai JH. Polymorphism G861C of 5-HT receptor subtype 1B is associated with heroin dependence in Han Chinese. Biochem Biophys Res Commun. 2011;412:450–453. doi: 10.1016/j.bbrc.2011.07.114. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR. Genetics of alcohol dependence. Hum Genet. 2009;126:91–99. doi: 10.1007/s00439-009-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Wilcox M, Hesselbrock V, Rounsaville B, Poling J, Weiss R, Sonne S, Zhao H, Farrer L, Kranzler HR. Genomewide linkage scan for opioid dependence and related traits. Am J Hum Genet. 2006;78:759–769. doi: 10.1086/503631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Su JA, Zhu SC, Zhang R, Zhang B, Li J, Yuan X, Lyons MJ, Faraone SV, Tsuang MT. Genome-wide linkage analysis of heroin dependence in Han Chinese: results from wave one of a multi-stage study. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:648–52. doi: 10.1002/ajmg.b.30361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EM, Stoltenberg SF, Bullard KH, Li S, Zucker RA, Burmeister M. Antisocial alcoholism and serotonin-related polymorphisms: association tests. Psychiatr Genet. 2002;12:143–153. doi: 10.1097/00041444-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Himei A, Kono Y, Yoneda H, Sakai T, Koh J, Sakai J, Inada Y, Imamichi H. An association study between alcoholism and the serotonergic receptor genes. Alcohol Clin Exp Res. 2000;24:341–342. [PubMed] [Google Scholar]
- Hwu HG, Chen CH. Association of 5HT2A receptor gene polymorphism and alcohol abuse with behavior problems. Am J Med Genet. 2000;96:797–800. doi: 10.1002/1096-8628(20001204)96:6<797::aid-ajmg20>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Jakubczyk A, Wrzosek M, Lukaszkiewicz J, Sadowska-Mazuryk J, Matsumoto H, Sliwerska E, Glass J, Burmeister M, Brower KJ, Wojnar M. The CC genotype in HTR2A T102C polymorphism is associated with behavioral impulsivity in alcohol-dependent patients. J Psychiatr Res. 2011;46:44–49. doi: 10.1016/j.jpsychires.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Lee YS, Choi SW, Han DH, Kim DJ, Joe KH. Clinical manifestation of alcohol withdrawal symptoms related to genetic polymorphisms of two serotonin receptors and serotonin transporter. Eur Addict Res. 2009;15:39–46. doi: 10.1159/000173008. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li D, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006a;15:1995–2002. doi: 10.1093/hmg/ddl122. [DOI] [PubMed] [Google Scholar]
- Li D, Duan Y, He L. Association study of serotonin 2A receptor (5-HT2A) gene with schizophrenia and suicidal behavior using systematic meta-analysis. Biochem Biophys Res Commun. 2006b;340:1006–1015. doi: 10.1016/j.bbrc.2005.12.101. [DOI] [PubMed] [Google Scholar]
- Li D, Zhao H, Gelernter J. Strong association of the alcohol dehydrogenase 1B gene (ADH1B) with alcohol dependence and alcohol-induced medical diseases. Biol Psychiatry. 2011;70:504–512. doi: 10.1016/j.biopsych.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu X, Zhao J, Hu X, Ball DM, Loh el W, Sham PC, Collier DA. Allelic association analysis of the dopamine D2, D3, 5-HT2A, and GABA(A)gamma2 receptors and serotonin transporter genes with heroin abuse in Chinese subjects. Am J Med Genet. 2002;114:329–335. doi: 10.1002/ajmg.10200. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. J Pharmacol Exp Ther. 1996;278:1373–1382. [PubMed] [Google Scholar]
- Maurel S, De Vry J, De Beun R, Schreiber R. 5-HT2A and 5-HT2C/5-HT1B receptors are differentially involved in alcohol preference and consummatory behavior in cAA rats. Pharmacol Biochem Behav. 1999;62:89–96. doi: 10.1016/s0091-3057(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Myers RL, Airey DC, Manier DH, Shelton RC, Sanders-Bush E. Polymorphisms in the regulatory region of the human serotonin 5-HT2A receptor gene (HTR2A) influence gene expression. Biol Psychiatry. 2007;61:167–173. doi: 10.1016/j.biopsych.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Matsushita S, Nishiguchi N, Kimura M, Yoshino A, Higuchi S. Association of a polymorphism of the 5HT2A receptor gene promoter region with alcohol dependence. Mol Psychiatry. 1999;4:85–88. doi: 10.1038/sj.mp.4000474. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Davis JM, Pandey GN. Phosphoinositide system-linked serotonin receptor subtypes and their pharmacological properties and clinical correlates. J Psychiatry Neurosci. 1995;20:215–225. [PMC free article] [PubMed] [Google Scholar]
- Parsian A, Cloninger CR. Serotonergic pathway genes and subtypes of alcoholism: association studies. Psychiatr Genet. 2001;11:89–94. doi: 10.1097/00041444-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Polina ER, Contini V, Hutz MH, Bau CH. The serotonin 2A receptor gene in alcohol dependence and tobacco smoking. Drug Alcohol Depend. 2009;101:128–131. doi: 10.1016/j.drugalcdep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Roth BL, Berry SA, Kroeze WK, Willins DL, Kristiansen K. Serotonin 5-HT2A receptors: molecular biology and mechanisms of regulation. Crit Rev Neurobiol. 1998;12:319–338. doi: 10.1615/critrevneurobiol.v12.i4.30. [DOI] [PubMed] [Google Scholar]
- Saiz PA, Garcia-Portilla MP, Arango C, Morales B, Martinez-Barrondo S, Alvarez C, San Narciso G, Carreno E, Alvarez V, Coto E, Bobes J. Association between heroin dependence and 5-HT2A receptor gene polymorphisms. Eur Addict Res. 2008;14:47–52. doi: 10.1159/000110410. [DOI] [PubMed] [Google Scholar]
- Saxon AJ, Oreskovich MR, Brkanac Z. Genetic determinants of addiction to opioids and cocaine. Harv Rev Psychiatry. 2005;13:218–232. doi: 10.1080/10673220500243364. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Sorensen SM, Kehne JH, Carr AA, Palfreyman MG. The role of 5-HT2A receptors in antipsychotic activity. Life Sci. 1995;56:2209–2222. doi: 10.1016/0024-3205(95)00210-w. [DOI] [PubMed] [Google Scholar]
- Shao C, Jiang K, Li Y, Zhao M, Song L, Xu Y, Lin L, Wang Q, Zhu M, Zhou W. Association study between 5-HT2A receptor gene polymorphism and heroin dependence and cue-elicited heroin craving. Chin J Nerv Ment Dis. 2005;31:321–325. [Google Scholar]
- Terayama H, Takimoto T, Fukunishi I, Itoh M, Iwahashi K. The serotonin-2A receptor polymorphism and clinical symptoms in mood disorders, schizophrenia and alcohol dependence in Japan. Acta Neuropsychiatrica. 2003;15:129–132. doi: 10.1034/j.1601-5215.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Tsunoka T, Kishi T, Kitajima T, Okochi T, Okumura T, Yamanouchi Y, Kinoshita Y, Kawashima K, Naitoh H, Inada T, Ujike H, Yamada M, Uchimura N, Sora I, Iyo M, Ozaki N, Iwata N. Association analysis of GRM2 and HTR2A with methamphetamine-induced psychosis and schizophrenia in the Japanese population. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:639–644. doi: 10.1016/j.pnpbp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Johnson C, Li CY, Contoreggi C, Hess J, Naiman D, Liu QR. Molecular genetics of addiction and related heritable phenotypes: genome-wide association approaches identify "connectivity constellation" and drug target genes with pleiotropic effects. Ann N Y Acad Sci. 2008;1141:318–381. doi: 10.1196/annals.1441.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bree MB, Johnson EO, Neale MC, Pickens RW. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug Alcohol Depend. 1998;52:231–241. doi: 10.1016/s0376-8716(98)00101-x. [DOI] [PubMed] [Google Scholar]
- Wang G, Zhang L, Zhong A, Li N, Yu H, Sun F, Hu C, Wang Y. Association study between 5-HT2A receptor gene polymorphism and heroin-dependent patients. Sichuan Mental Health. 2001;14:101–102. [Google Scholar]
- Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Geneva: WHO; World Health Organization World Health Organization’s International Statistical Classification of Diseases and Related Health Problems (ICD) [Google Scholar]
- Wrzosek M, Jakubczyk A, Matsumoto H, Lukaszkiewicz J, Brower KJ, Wojnar M. Serotonin 2A receptor gene (HTR2A) polymorphism in alcohol-dependent patients. Pharmacol Rep. 2012;64:449–453. doi: 10.1016/s1734-1140(12)70787-9. [DOI] [PubMed] [Google Scholar]
- Wrzosek M, Lukaszkiewicz J, Serafin P, Jakubczyk A, Klimkiewicz A, Matsumoto H, Brower KJ, Wojnar M. Association of polymorphisms in HTR2A, HTR1A and TPH2 genes with suicide attempts in alcohol dependence: a preliminary report. Psychiatry Res. 2011;190:149–151. doi: 10.1016/j.psychres.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian H, Scherrer JF, Grant JD, Eisen SA, True WR, Jacob T, Bucholz KK. Genetic and environmental contributions to nicotine, alcohol and cannabis dependence in male twins. Addiction. 2008;103:1391–1398. doi: 10.1111/j.1360-0443.2008.02243.x. [DOI] [PubMed] [Google Scholar]
- Yang M, Hao W, Wu Z, Wang W, Zhou X, Jiang G, Tang M, Huang J. Association Study of 5-HTR2A-1438A/G, COMTVal158Met, MAOA-LPR, DATVNTR and 5-HTTVNTR Polymorphisms with Cooccurrence Heroin Dependence and Antisocial Personality Disorders in Males. Chinese Journal of Drug Dependence. 2010;19:349–363. [Google Scholar]
- Yang M, Kavi V, Wang W, Wu Z, Hao W. The association of 5-HTR2A-1438A/G, COMTVal158Met, MAOA-LPR, DATVNTR and 5-HTTVNTR gene polymorphisms and antisocial personality disorder in male heroin-dependent Chinese subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:282–9. doi: 10.1016/j.pnpbp.2011.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Forest plots of ln (OR) and overall ln (OR) with 95% CI of the allelic and genotypic analyses for rs6311.
Supplementary Figure 2 Egger’s funnel plots of the combined studies of alcohol dependence (abuse) for rs6313.
Supplementary Figure 3 Retrospective analysis of all the combined studies for - rs6313.
Supplementary Figure 4 Retrospective analysis of the combined studies of alcohol dependence (abuse) for rs6313.
Supplementary Figure 5 Graphical representation of the LD structure of European ancestry. The LD structure, spanning 200 kp covering the HTR2A gene, was constructed using European genotype data of 273 SNPs. The current two polymorphisms are shown in red; other polymorphisms are shown in blue. The HTR2A gene, size 62, 662 bp, is indicated in red. Vertical tick marks above the name indicate the relative genomic position of each SNP. The LD structure represents the pairwise calculation of D’ for each possible combination of SNPs. D’ < 0.5 is shown in white, D’ = 1.0 in dark red, with increasing shades of red representing increasing D’ between the SNPs.
Supplementary Figure 6 Graphical representation of the LD structure of Asian ancestry. The LD structure, spanning 200 kp covering the HTR2A gene, was constructed using Asian genotype data of 249 SNPs.