Abstract
We have studied the actions of helper T lymphocyte-1 and -2 (Th1 and Th2) cells in an acute model of eosinophilic airway inflammation by infusing chicken ovalbumin-specific (OVA-specific) Th1 cells, Th2 cells, or both into unsensitized mice and challenging the mice with an OVA aerosol. OVA challenge after infusion of Th1 cells alone resulted in airway inflammation with lymphocytes and monocytes. Challenge after the infusion of Th2 cells alone resulted in minimal inflammation. In contrast, when Th1 and Th2 cells were transferred together, they cooperated to promote a robust eosinophil-predominant inflammatory response. Th1 cells alone were readily recruited to the airways after challenge, but in the absence of Th1 cells, Th2 cells did not accumulate in the airways. When transferred together, both Th1 and Th2 cells, as well as endogenous eosinophils, were effectively recruited. This recruitment was correlated with increased VCAM-1 expression in the medium- and large-sized vessels of the lung and could be inhibited by treating the mice with neutralizing antibodies to TNF-α or VCAM-1. These data indicate that Th2 cells require signals in addition to antigen for their effective recruitment to the airways. Th1 cells can provide these signals.
Introduction
Immune responses to protein antigens are strongly influenced by the nature of the helper T lymphocyte (Th) subsets participating in the response. Th1 cells are characteristically induced during immune responses to intracellular pathogens such as mycobacteria and viruses and produce the cytokines IFN-γ, lymphotoxin (LT), and IL-2 (1, 2). These cytokines activate macrophages, natural killer (NK) cells, and CD8+ cytolytic T cells and promote immunoglobulin class switching to IgG2a. Th2 cells are characteristically induced during host responses to helminthic parasites and produce the cytokines IL-4, IL-5, IL-10, and IL-13. These cytokines promote mast cell and eosinophil growth and differentiation as well as immunoglobulin class switching to IgG1 and IgE. Once deviation toward a Th1 or Th2 response begins, there is potential for further polarization driven by the Th cells themselves. IFN-γ produced by Th1 cells suppresses proliferation of Th2 cells (2), inhibits IgE production (3), and promotes further Th1 differentiation by inducing IL-12 receptor β2-chain expression on T cells (4) and IL-12 production by macrophages (5). In contrast, IL-4, IL-6, and IL-10 produced by Th2 cells can promote further Th2 differentiation (6, 7) and inhibit macrophage antigen presentation and IL-12 production (8). As a result, Th1 and Th2 responses are often viewed as being mutually exclusive.
Airway inflammation is central in the pathogenesis of asthma. The large and medium airways of patients with asthma demonstrate evidence of chronic inflammation, including leukocyte infiltrates in the bronchial tissue, excessive mucus production, epithelial damage, basement membrane thickening, and smooth muscle hypertrophy (9, 10). The inflammatory infiltrates characteristically contain a substantial population of eosinophils as well as T cells, monocytes, and neutrophils. The degree of eosinophilic inflammation correlates with the level of clinical asthma symptoms (11–13). Mediators released by these inflammatory cells are believed to lead to epithelial damage and airway hyperreactivity, the pathognomonic clinical feature of the disease.
The individual roles of Th1 and Th2 cells in promoting airway inflammation in asthma remain unclear (14, 15). Asthma is associated with atopy and recruitment of eosinophils to the airway, leading to the hypothesis that asthma is driven by a Th2 response to inhaled antigens (16, 17). In support of this, in situ hybridization analysis has demonstrated elevated IL-4 and IL-5 mRNA expression in T cells collected by bronchoalveolar lavage (BAL) from asthmatic patients compared with T cells from normal volunteers (18). In situ hybridization has also shown an increase in IL-5 mRNA in the airways after allergen challenge (13); however, other studies focusing specifically on T cells have found evidence for increased numbers of IFN-γ–producing cells (19) or cells producing both IFN-γ and IL-4 (20). These data, together with the fact that viral respiratory infections remain the leading cause for triggering severe asthma attacks (21, 22), suggest a role for Th1 cells in potentiating asthmatic inflammation.
In mouse models of asthma, the evidence is strong for a role of Th2 cells and the Th2 cytokines IL-4 and IL-5 in promoting airway eosinophilia and hyperreactivity. Airway inflammation is attenuated in mice genetically deficient in CD4+ T cells (23, 24), IL-4 (23–26), or IL-5 (27). Adoptive transfer of allergen-specific Th2 cells has also been shown to promote airway eosinophilia, mucus production, and hyperreactivity (28–30). The role of Th1 cells is less clear. Treatment with IL-12 (25, 31) or IFN-γ (32, 33) or infection with Bacille Calmette-Guérin (BCG) (34) have all been shown to decrease eosinophilic airway inflammation. These data suggest that shifting toward a Th1 response could inhibit airway eosinophilia, although cell types other than Th1 cells could also be involved. Indeed, Th1 and Th2 responses have recently been shown to coexist in the lung (29, 35, 36).
Local expression of vascular adhesion molecules during inflammation is critical for efficient recruitment of leukocytes, including CD4+ Th cells, to sites of inflammation. In allergic airway inflammation, interactions between α4 integrins and the adhesion molecule VCAM-1 are particularly important, although other molecules such as ICAM-1, P-selectin, and E-selectin likely contribute as well (37–39). For T-cell recruitment to the lung, P-selectin, E-selectin, and VCAM-1 all contribute (40), and T cells have been shown to contribute to the induction of these adhesion molecules during inflammation (41). Interestingly, Th1 cells, but not Th2 cells, have been shown to use P-selectin and E-selectin, suggesting that Th1 and Th2 recruitment can be differentially regulated at the level of adhesion-molecule expression (42).
We recently showed that both Th1 and Th2 cells were recruited to the airways after antigen challenge in a mouse model of eosinophilic airway inflammation in which mice were sensitized to chicken ovalbumin (OVA) and then challenged with aerosolized OVA (36). Th1 cells predominated early after the challenge, whereas Th2 cells predominated later in the response. Adoptive transfer of OVA-specific Th1 cells before sensitization increased the inflammatory response, including eosinophil recruitment, whereas passive transfer of Th2 cells induced little change in the airway inflammation. These results suggested that both Th1 cells and Th2 cells might contribute to the eosinophilic airway inflammation, with Th1 cells participating particularly in the early stages of the response. In the current experiments we have modified this experimental model to focus on the role of Th1 and Th2 cells in the acute period, in the first 3 days after challenge via the airway. We transferred OVA-specific Th1 cells, Th2 cells, or both to naive mice and then administered 2 airway challenges with OVA 6–8 hours apart. Interestingly, strong eosinophilic inflammatory responses were seen only when Th1 and Th2 cells were transferred together. Further analysis demonstrated that Th1 cells were efficiently recruited to the airways after antigen challenge, but that Th2 cells were efficiently recruited to the airways only when Th1 cells were also present. The increased recruitment of eosinophils and Th2 cells in the presence of Th1 cells correlated with increased VCAM-1 expression in the large vessels of the lung and could be substantially inhibited by treatment of the mice with either anti–VCAM-1 or anti–TNF-α antibodies. These data indicate that, rather than being counterregulatory, Th1 and Th2 cells can cooperate in this setting to promote eosinophilic airway inflammation.
Methods
Animals.
BALB/c mice were obtained from Harlan Sprague Dawley Inc. (Indianapolis, Indiana, USA). O11.10 TCR transgenic mice (43) crossed with mice deficient in the recombination activating gene RAG-2 were kindly provided by O. Kanagawa (Washington University, St. Louis, Missouri, USA). The DO11.10 transgene and RAG-2 mutation had been backcrossed onto the BALB/c (H-2d, Thy1.2) background for more than 10 generations before their use in these experiments. For selected experiments, C57BL/6 Thy1.1 mice (H-2b; The Jackson Laboratory, Bar Harbor, Maine, USA) were backcrossed 2 generations onto BALB/c mice, selecting for the presence of the Thy1.1 allele and the absence of the H-2b MHC alleles by flow cytometric analysis of peripheral blood leukocytes. The mice were then crossed with BALB/c DO11.10 mice to generate Thy1.1 DO11.10 mice. All the mice used in these experiments were females 4–6 weeks of age. Mice were housed in specific pathogen–free conditions with food and water provided ad libitum, and all experiments were approved by the Washington University institutional committee for the humane use of laboratory animals.
Preparation of differentiated CD4+ T cells.
OVA-specific Th1 and Th2 cells were generated in vitro as described previously (36, 44). Briefly, CD4+ L-selectin+ T cells purified from spleens of RAG-2–deficient DO11.10 TCR transgenic mice by flow cytometry were cultured in T-cell medium (Iscove’s medium supplemented with 10% FCS [HyClone Laboratories, Logan, Utah, USA], 0.01 mM nonessential amino acid mix, 2 mM sodium glutamate, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5.5 μM 2-mercaptoethanol; all from GIBCO BRL [Grand Island, New York, USA]) with irradiated BALB/c splenocytes at a ratio of 1:20 for 3 days in the presence of 0.3 μM OVA (323-339) peptide. For polarization to Th1 cells, IL-12 (2 ng/mL; R&D Systems Inc., Minneapolis, Minnesota, USA) and anti–IL-4 (clone 11B11, 10 μg/mL; courtesy of K.M. Murphy, Washington University) were added. For polarization to Th2 cells, IL-4 (1,000 U/mL; R&D Systems Inc.) and anti–IL-12 (clone TOSH, 10 μg/mL; courtesy of E. Unanue, Washington University) were added. On day 3, a 4-fold excess of medium and IL-2 (40 U/mL; R&D Systems Inc.) were added. On day 7, cells were frozen in FCS containing 10% DMSO. Before transfer into recipient mice, cells were thawed and restimulated with irradiated BALB/c splenocytes and 0.3 μM OVA peptide. Supernatants for ELISA were collected at 48 hours. After 72 hours, the cells were diluted 4-fold in medium with 40 U/mL IL-2. Cells were used for transfers on day 8 or 9 of culture. The cytokine profiles of each batch of cells were confirmed by intracellular cytokine staining or by ELISA using Quantikine ELISA kits (R&D Systems Inc.) according to the manufacturer’s instructions. Cells cultured in this manner are strongly polarized to the Th1 or Th2 phenotype (36). Th1 supernatants typically contain more than 1,000 ng IFN-γ/mL and less than 0.04 ng IL-4/mL. Th2 supernatants contain less than 3 ng IFN-γ/mL and more than 4 ng IL-4/mL. In addition, less than 0.1% of Th1 cells are positive for intracellular IL-5, whereas 30–40% of Th2 cells are positive when analyzed by flow cytometry.
Induction and analysis of airway inflammation.
Mice were anesthetized with Metofane (methoxyflurane; Schering-Plough Animal Health Corp., Union, New Jersey, USA) and injected intravenously with the indicated number of T cells. The next day the mice were placed in a Plexiglas chamber attached to a clinical nebulizer (Ultra-Neb 99; DeVilbiss Health Care Inc., Sommerset, Pennsylvania, USA) and challenged for 20 minutes in the morning and afternoon with an aerosol of 1% (wt/vol) OVA (Grade V; Sigma Chemical Co., St. Louis, Missouri, USA) in sterile PBS. Either 48 or 72 hours later, as indicated, the mice were sacrificed by lethal injection of a mixture of ketamine (100 mg/mL), xylazine (20 mg/mL), and acepromazine (10 mg/mL), and their airways were assessed for inflammation. When indicated, the trachea was cannulated and airway inflammatory cells were obtained by bronchoalveolar lavage (BAL) with four 0.8-mL aliquots of ice-cold 2% FCS in PBS. Red blood cells in the lavage were lysed using ammonium chloride, and nucleated cells were counted by hemacytometer. Differential cell counts were obtained from cytospin preparations of the BAL cells using Wright’s stain.
Immunohistochemistry.
Lungs were inflated with 50% OCT (Tissue-Tek; Sakura Finetek, Torrance, California, USA) in PBS, embedded in OCT, frozen on dry ice, and then stored at –70°C for later use. Cryosections from the lungs were cut, air-dried, and then fixed in acetone for 10 minutes. Cyanide-resistant eosinophil peroxidase activity was detected by incubating the slides with Sigma Fast DAB (diaminobenzidine) tablets (Sigma Chemical Co.), dissolved in PBS with 1.6 mg/mL potassium cyanide (KCN). After the DAB was rinsed off, the slides were incubated for 15 minutes in blocking solution (PBS with 0.5% Tween-20 and 3% normal goat serum) containing 4 drops/mL avidin, followed by 15 minutes in blocking solution containing 4 drops/mL biotin (both from Vector Laboratories, Burlingame, California, USA). Then the slides were incubated for 1 hour at room temperature with tissue culture supernatant from the rat hybridomas MK2.7 (anti-mouse VCAM-1) or HB-233 (anti-mouse ICAM-1) (American Type Culture Collection, Rockville, Maryland, USA). After washing with PBS containing 0.5% Tween (PBS/Tween), the slides were incubated with biotinylated mouse anti-rat κ and λ (Sigma Chemical Co.) at 1:200 dilution for 1 hour. After washing again in PBS/Tween, the slides were incubated with the ABC-AP reagents (Vector Laboratories) per the manufacturer’s instructions. After washing, the stain was developed using Vector Red reagents (Vector Laboratories), with levamisole added as an inhibitor of endogenous alkaline phosphatase. After the red developed, the slides were counterstained with methyl green and air-dried, and coverslips were applied.
Transfer of fluorescently labeled cells.
On day 8 or 9 of culture, live T cells were collected by centrifugation over a layer of Histopaque 1119 (Sigma Chemical Co.). Cells were then labeled with fluorescent dyes— either PKH26 (red) or PKH67 (green) (Sigma Chemical Co.)— according to the manufacturer’s instructions. The cells were transferred to anesthetized recipient mice by intravenous injection. One day later, the mice were challenged with an aerosol of 1% OVA in the morning and afternoon. After an additional 3 days, the mice were sacrificed, and their lungs were inflated and frozen as described previously. Cryosections were cut, air-dried, and observed directly by fluorescence microscopy.
Flow cytometric analysis of intracellular cytokines in BAL cells.
BAL cells were cultured at 37°C in T-cell medium for 6 hours in the presence of 2 μM monensin with or without 10 ng/mL PMA and 1 μM ionomycin (all from Sigma Chemical Co.). CD4+ cells were marked with allophycocyanin anti-CD4 (PharMingen, San Diego, California, USA), and transferred DO11.10 T cells were stained with biotinylated anti-clonotype antibody KJ1-26 (43) and streptavidin-CyChrome (PharMingen). The cells were then washed, fixed for 30 minutes in 4% paraformaldehyde in PBS, permeabilized with 0.1% saponin, and stained with phycoerythrin–anti-IFN-γ and either FITC–anti-IL-4 or FITC–anti-IL-5 (all from PharMingen). Anti-CD4 and anti–IFN-γ were used at 1:100 dilution; anti–IL-4 and anti–IL-5 were used at 1:50. One fourth of each sample was analyzed using a FACScalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, California, USA). The forward-scatter and side-scatter properties of the cells were used to exclude dead cells from analysis. For experiments using Thy1.1 Th1 cells and Thy1.2 Th2 cells, BAL cells were stained with allophycocyanin–anti-CD4, biotinylated KJ1-26 with streptavidin-CyChrome, and FITC–anti-Thy1.2 and were analyzed by flow cytometry. Th1 cells were detected as CD4+ KJ1-26+ Thy1.2- cells, whereas Th2 cells were detected as CD4+ KJ1-26+ Thy1.2+ cells.
Treatment of mice with neutralizing antibodies.
Mice were anesthetized with Metofane and injected intravenously with PBS containing hamster anti-mouse IFN-γ (clone H22; 500 μg), hamster anti-mouse TNF-α (clone TN3; 500 μg), rat anti-mouse VCAM-1 (clone MK2.7; 1 mg), or hamster anti–bacterial glutathione-S-methyl transferase (clone PIP; 500 μg) as a negative control. H22, TN3, and PIP were all hamster IgG antibodies and were kindly provided by R. Schreiber (Washington University). MK2.7 was purchased from Southern Biotechnology Associates (Birmingham, Alabama, USA).
Statistical analyses.
Statistical significance was determined using a 2-tailed Student’s t test.
Results
Th1 and Th2 cells cooperate to promote eosinophilic inflammation.
To evaluate potential roles of Th1 and Th2 cells in promoting eosinophilic airway inflammation, 107 OVA-specific DO11.10 Th1 cells, 107 Th2 cells, or 107 of each were transferred by intravenous injection to groups of unsensitized BALB/c mice. Control mice received an intravenous injection of sterile PBS alone. The following day, the mice received 30-minute challenges of nebulized 1% OVA in PBS, both in the morning and in the afternoon. Three days after the challenge, the mice were sacrificed and their airways were evaluated for inflammation by BAL and histology.
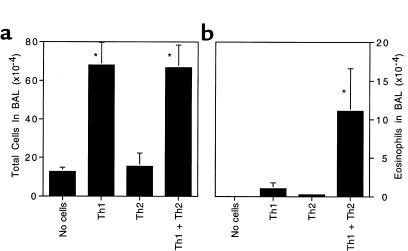
Without transfer of antigen-specific T cells, unsensitized mice showed few inflammatory cells and no eosinophils in their airways 3 days after challenge (Figure 1, a and b). Naive mice that received 107 Th1 cells before challenge with OVA aerosol mounted a dramatic inflammatory response, but the inflammation consisted primarily of mononuclear cells with only rare eosinophils observed. Interestingly, transfer of 107 Th2 cells did not support significant inflammation over background. In contrast, when Th1 and Th2 cells were transferred together, OVA challenge induced a strong eosinophilic inflammatory response. Similar results were obtained when 5 × 106 Th1 cells were mixed with 5 × 106 Th2 cells before intravenous transfer (data not shown). In fact, these patterns of cellular recruitment were observed across a broad range of numbers of transferred cells, from 106 to 2.5 × 107 cells of each Th phenotype (data not shown). These results indicate that Th1 and Th2 cells can cooperate to generate eosinophilic inflammation.
Figure 1.
Synergy between Th1 and Th2 cells promotes eosinophilic airway inflammation. Here, 107 OVA-specific DO11.10 Th1 cells, 107 Th2 cells, or 107 of each were passively transferred to recipient mice by intravenous injection. The next day, in the morning and again in the afternoon, the mice were challenged with an aerosol of 1% OVA in sterile PBS. Three days after the challenge, the mice were sacrificed and BAL cells were collected. Shown is the average number of cells in each sample ± SD (n = 4 mice per group). (a) Total BAL nucleated cells. (b) BAL eosinophils. Similar results were obtained in 4 separate experiments. *Significantly different from all other groups (P < 0.05).
Presence of Th1 cells results in increased recruitment of Th2 cells.
Th1 and Th2 cells have been reported to use distinct adhesion molecules for attachment to the vascular endothelium and for transmigration into tissues (42, 45). In addition, Th1 and Th2 cells have been shown to express different sets of chemokine receptors (46–49). To determine if Th1 and Th2 cells exhibit differential trafficking patterns within the lung after antigen challenge, we performed passive transfer experiments with DO11.10 Th1 and Th2 cells labeled with the fluorescent, membrane-intercalating dyes PKH67 (green) or PKH26 (red). After labeling, 107 cells were transferred to unsensitized BALB/c mice, and the mice were challenged with an OVA aerosol. Three days after challenge, the mice were sacrificed and frozen sections of the lungs were evaluated for the presence and location of fluorescent cells.
When infused into naive mice, Th1 and Th2 cells exhibited different trafficking patterns in the lung after the antigen challenge. Th1 cells were found clustered around vessels and airways throughout the lung tissue (Figure 2a). In contrast, Th2 cells were observed only as scattered individual cells. They did not cluster around the airways and vessels in the same manner as the Th1 cells (Figure 2b). This pattern was not simply due to decreased survival of the Th2 cells after transfer because, after aerosol challenge, numerous Th2 cells could be found in the paratracheal lymph nodes (not shown). In striking contrast to these observations, when Th2 cells were transferred together with Th1 cells, antigen challenge induced the recruitment of substantial numbers of both Th1 and Th2 cells. The Th2 cells were present in dense clusters around the airways and medium-and large-sized vessels of the lung in a pattern similar to that seen for the Th1 cells (Figure 2, c and d). In addition, numerous eosinophils were detected in close proximity to the areas of Th1 and Th2 cell clustering (Figure 2, e and f). Neither cell type accumulated in the lungs in the absence of antigen challenge.
Figure 2.
Th1 cells promote recruitment of Th2 cells to the airways after the challenge. Cultured T cells (107) were labeled with the fluorescent dyes PKH26 (red) or PKH67 (green) and transferred to recipient mice. In the morning and again in the afternoon of the next day, the mice were challenged with an aerosol of 1% OVA in sterile PBS. Three days after the challenge, lung tissue was collected and frozen. Fluorescent cells in the tissue were detected directly by fluorescence microscopy of air-dried 10-μm sections. Where indicated, eosinophils in the tissue were detected by virtue of the cyanide-resistant peroxidase activity in their granules. For detection of eosinophils, lung sections were fixed in acetone and then incubated in a DAB solution containing 1.6 mg/mL KCN. Shown are examples of lung tissue from recipients of (a) 107 PKH26-labeled Th1 cells, (b) 107 PKH26-labeled Th2 cells (c–f), 107 PKH67-labeled Th1 cells, and 107 PKH26-labeled Th2 cells. (c) Single exposure demonstrating Th1 cells. (d) Single exposure revealing Th2 cells in the same section as c. (e) Double exposure demonstrating Th1 and Th2 cells in close proximity to one another. (f) Detection of eosinophils (brown DAB staining) in the same section as e. Similar results were seen in 2 separate experiments.
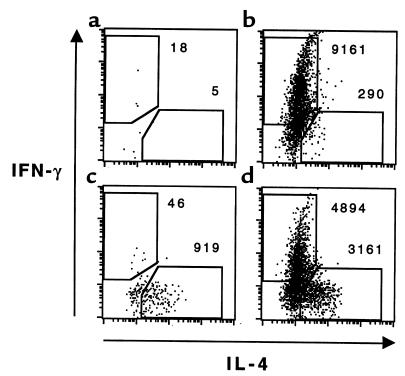
To eliminate the possibility that the labeling dyes were affecting the migration of the transferred cells, we confirmed these results using the anti-clonotype antibody KJ1-26 and intracellular cytokine staining followed by flow cytometry to identify the DO11.10 transgenic Th1 and Th2 cells in the BAL. We transferred 107 Th1 cells, 107 Th2 cells, or 5 × 106 of each to groups of 4 wild-type BALB/c mice. The mice were challenged and BAL cells were collected 3 days later. Cells from groups of identically treated mice were pooled and cultured for 6 hours in T-cell medium containing monensin, PMA, and ionomycin. The cells were stained for CD4 and the transgenic DO11.10 TCR (43), as well as for intracellular IFN-γ as a marker for Th1 cells and IL-4 as a marker for Th2 cells. When 107 Th1 cells were transferred, more than 9.2 × 103 CD4+ KJ1-26+ IFN-γ+ were recovered (Figure 3). When the same number of Th2 cells was transferred, only 9.2 × 102 CD4+ KJ1-26+ IL-4+ cells were recovered in the BAL. In contrast, when only 5 × 106 Th2 cells were transferred together with 5 × 106 Th1 cells, substantial numbers of both IFN-γ+/IL-4– (4.9 × 103) and IFN-γ–/IL-4+ (3.2 × 103) cells were recovered in the BAL. These data, along with the results of transfer of fluorescently labeled cells, support the hypothesis that, over this time course, Th2 cells alone are poorly recruited to the airways. Th1 cells, in contrast, are competent for recruitment. Furthermore, Th1 cells can induce alterations in the lung microenvironment that then potentiate Th2 cell recruitment.
Figure 3.
Flow cytometric analysis of Th1 and Th2 cell recruitment to the airways after challenge. Groups of 4 mice received (a) no transferred cells, (b) 107 DO11.10 Th1 cells, (c) 107 DO11.10 Th2 cells, or (d) 5 × 106 Th1 cells plus 5 × 106 Th2 cells. The mice were challenged, and BAL cells were collected as described in Figure 1. Cells from each group were pooled and then stimulated for 6 hours with PMA and ionomycin in the presence of monensin. Aliquots were stained with anti-CD4 and the anti-clonotypic antibody KJ1-26 to mark the transferred transgenic T cells. The cells were then fixed, permeabilized, and stained for intracellular IFN-γ and IL-4 as markers for Th1 and Th2 differentiation before analysis by flow cytometry. IFN-γ and IL-4 staining are shown for cells within the CD4+ KJ1-26+ gate. The numbers of cytokine-producing Th1 (IFN-γ+ IL-4–) and Th2 (IFN-γ– IL-4+) cells are indicated. Similar results were seen in 3 separate experiments.
Adoptively transferred Th1 and Th2 cells modulate expression of adhesion molecules in the lung.
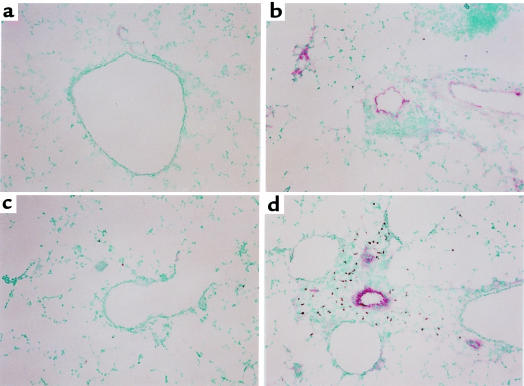
To determine whether transfer of Th cells resulted in changes in adhesion-molecule expression, we analyzed expression of the adhesion molecules ICAM-1 and VCAM-1 in the lungs after cell transfer and challenge. Th1 cells, Th2 cells, or both were transferred to mice, and the next day the mice were challenged with an aerosol of 1% OVA as described previously. Lung tissue was collected 48 hours later and analyzed by immunohistochemistry for ICAM-1 and VCAM-1 expression. ICAM-1 was detected at high levels in unchallenged animals and was induced to even higher levels in animals that had received Th1 cells (data not shown). ICAM-1 expression was strongest in the alveoli and was not seen in the larger vessels that were surrounded by inflammatory infiltrates. Thus, it seems unlikely that ICAM-1 mediates the increased recruitment of Th2 cells or eosinophils seen after antigen challenge. In contrast, VCAM-1 was expressed only at low levels in mice receiving no transferred cells and in recipients of Th2 cells alone (Figure 4, a and c); however, in recipients of Th1 cells, VCAM-1 was induced strongly in the medium- and large-sized vessels of the lungs (Figure 4b). When Th1 and Th2 cells were transferred together, VCAM-1 induction was even stronger, and eosinophils could be seen in the tissue adjacent to the VCAM-1–positive vessels (Figure 4d). This suggests that Th1 cells promote Th2 cell recruitment and subsequent eosinophil recruitment through induction of endothelial VCAM-1.
Figure 4.
Th1 and Th2 cells cooperate to induce high levels of endothelial VCAM-1 expression and tissue eosinophilia. Mice received transferred T cells and were challenged as described in Figure 1. Two days after the challenge, the mice were sacrificed and lung tissue was collected and frozen. Sections of tissue were then cut and stained for the presence of VCAM-1 (red) and eosinophils (brown) as described in Methods. Shown are representative sections of lung from mice that received (a) no transferred cells, (b) Th1 cells, (c) Th2 cells, or (d) Th1 and Th2 cells. Similar results were seen in 4 separate experiments.
TNF-α and VCAM-1 contribute to Th-induced eosinophilic inflammation.
The preceding experiments suggest that Th1 cells supply a factor that supports the recruitment of Th2 cells into the airway and that VCAM-1 may contribute to this process. To identify Th1 products that contribute to this synergy, we assessed the effects of neutralizing anti–IFN-γ, anti–TNF-α, and anti–VCAM-1 mAb’s on eosinophil and Th2 cell recruitment. Naive BALB/c mice were treated with 107 Thy1.1 DO11.10 Th1 and 107 Thy1.2 DO11.10 Th2 cells by intravenous infusion. The next day, groups of 4 unsensitized mice were injected intravenously with 500 μg of control antibody (anti–glutathione-S-methyl transferase PIP), anti–IFN-γ (H22), anti–TNF-α (TN3), or anti–VCAM-1 (MK2.7). The mice were then challenged with an aerosol of 1% OVA in the morning and afternoon. The day after the challenge, the mice receiving the anti–VCAM-1 antibody were injected with a second dose of antibody. All of the mice were sacrificed on the second day after the challenge, and BAL cells were collected. Anti-TNF-α– and anti-VCAM-1–treated mice had significantly decreased airway eosinophilia, whereas mice that received anti–IFN-γ antibody showed increased airway eosinophilia compared with the control antibody–treated animals (Figure 5a). Similar results were seen in 2 separate experiments.
Figure 5.
Neutralization of IFN-γ, TNF-α, or VCAM-1 alters eosinophil and Th2 cell recruitment. Mice received 107 Thy1.1 Th1 cells and 107 Thy1.2 Th2 cells by intravenous injection. On the following day, groups of 4 mice received injections of anti–glutathione-S-methyl transferase (control antibody), anti–TNF-α, anti–VCAM-1, or anti–IFN-γ antibodies and then were challenged with an aerosol of OVA as described in Figure 1. The day after the challenge, the anti-VCAM-1–treated group received an additional injection of antibody. Two days after the challenge, the mice were sacrificed and BAL cells were collected and counted. (a) The number of eosinophils in the BAL. Shown are the average values ± SEM for 2 pooled experiments. (b) The ratio of transferred Th2 to Th1 cells in the BAL. An aliquot of the BAL cells from each animal was stained for CD4, KJ1-26, and Thy1.2 and analyzed by flow cytometry. The average ratio of CD4+KJ1-26+Thy1.2+ to CD4+KJ1-26+Thy1.2– cells is shown. *Significantly different from control antibody–treated group (P < 0.05).
Recruitment of OVA-specific Th1 and Th2 cells to the lungs was also assessed by flow cytometry using allotypic markers present on the transferred cells (Thy1.1 for Th1 and Thy1.2 for Th2). BAL cells were stained with anti-CD4, KJ1-26, and anti-Thy1.2, and then analyzed by flow cytometry. Transferred Th2 cells were CD4+ KJ1-26+ Thy1.2+ and transferred Th1 cells were CD4+ KJ1-26+ Thy1.2–. There was a clear correlation between the degree of eosinophil inflammation in the lungs and the degree of Th2 recruitment (Figure 5, a and b). Mice treated with anti–IFN-γ had higher proportions of Th2 cells relative to Th1 cells in their airways, whereas mice treated with anti–TNF-α or anti–VCAM-1 had lower proportions of Th2 cells recruited to their airways.
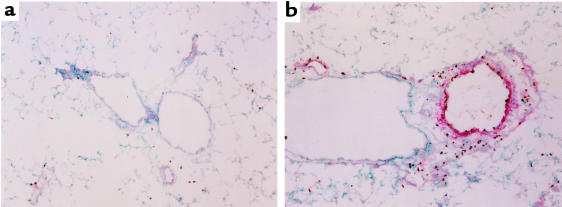
Finally, we assessed the effect of neutralizing TNF-α antibodies on vascular VCAM-1 expression. Mice treated with anti–TNF-α antibody showed greatly reduced VCAM-1 staining and decreased tissue eosinophilia when compared with mice treated with the control antibody (Figure 6). Together, these results suggest that Th1 cells promote VCAM-1 expression and subsequent Th2 and eosinophil recruitment through a pathway using TNF-α.
Figure 6.
Neutralizing antibody against TNF-α inhibits VCAM-1 expression and tissue eosinophilia. Mice were treated as described in Figure 5. After BAL cells were harvested, the lung tissue was collected and frozen. Sections of lung were then stained for the presence of VCAM-1 (red staining) and eosinophils (brown staining). (a) Section from a mouse treated with anti–TNF-α, and (b) section from a mouse treated with anti–glutathione-S-methyl transferase (control antibody). Similar results were seen in 2 separate experiments.
Discussion
We have taken advantage of a defined adoptive transfer system to investigate the ability of Th1 and Th2 cells to induce lung inflammation after aerosol challenge with their cognate antigen. In these experiments in vitro differentiated OVA–specific DO11.10 Th1 and Th2 cells were transferred to naive, unsensitized BALB/c recipients 1 day before challenge with an aerosol of 1% OVA in PBS. Three days later, the mice were sacrificed and their lungs were analyzed for evidence of inflammation. In this short-term transfer model, the contributions to the inflammatory process from specific antibodies or host T cells were expected to be minimal. In this system, Th1 cells, when transferred alone, promoted an antigen-dependent inflammatory response consisting primarily of lymphocytes and mononuclear phagocytes. Strikingly, Th2 cells, when transferred alone, were unable to induce airway inflammation; however, when Th1 and Th2 cells were transferred together, they cooperated to induce eosinophilic inflammation (Figure 1).
In this model, the differential activity of Th1 and Th2 cells in promoting inflammation and their cooperative interactions in generating eosinophilic inflammation appear to be based, in part, on the different tissue-trafficking capabilities of Th1 and Th2 cells (Figures 2 and 3). After airway challenge, Th1 cells were efficiently recruited to the lungs, perhaps through P-selectin and E-selectin. In contrast, Th2 cells, lacking the appropriate ligands for binding to P-selectin and E-selectin, were poorly recruited to the lungs, and their pattern of recruitment was diffuse unless Th1 cells were also present. These results suggest that Th1 cells induce changes in the lung environment, such as alterations in chemokine or adhesion-molecule expression, that enhance recruitment of Th2 cells to the airways. Indeed, Th1 cell recipients challenged with OVA showed increased expression of a number of chemokines, including RANTES, MIP1-α, MIP-1β, MCP-1, eotaxin, and lymphotactin (data not shown). Th2 cells have been reported to express the cognate receptors for each of these chemokines (50). Thus, these chemokines are candidates for the mediators that underlie the cooperative interactions between Th1 and Th2 cells.
Th1 cells also induced changes in adhesion-molecule expression in the lung. There were modest increases in the expression of ICAM-1, but both baseline and induced expression were localized to the smallest airways at sites that were not prominently involved in the tissue inflammation. The induction of VCAM-1 on the endothelium of medium to large vessels within the lung was more dramatic (Figure 4). VCAM-1 has been reported to be critical for the development of airway inflammation in various asthma models (37, 39, 40). A synergistic effect of TNF-α and IL-4 on VCAM-1 induction has also been reported (51). Thus, it is particularly interesting that in our experiments, induction of VCAM-1 was strongest when both Th1 and Th2 cells were present.
The enhancement of Th2 cell recruitment to the airways by Th1 cells may involve a direct interaction between the Th1 cells and the lung tissue or may be mediated by secreted products of the Th1 cells. We investigated possible contributions of soluble Th1 mediators by using neutralizing antibodies against IFN-γ or TNF-α to inhibit the actions of these cytokines during challenge of mice that had received both Th1 and Th2 cells (Figure 5). Consistent with other reports, neutralizing IFN-γ actually increased airway eosinophilia and Th2 recruitment (32, 33, 52). In contrast, neutralizing TNF-α or blocking VCAM-1 decreased Th2 and eosinophil recruitment, with neutralization of TNF-α itself leading to decreased VCAM-1 expression (Figure 6). These data suggest that TNF-α provides an important signal initiating Th2 recruitment in this model. Of interest, OVA-specific DO11.10 Th1 cells release only modestly higher amounts of TNF-α compared with DO11.10 Th2 cells (400 ng/mL vs. 300 ng/mL in tissue culture supernatants 3 days after restimulation with OVA). However, because Th1 cells are recruited efficiently to the lungs soon after OVA aerosol challenge and Th2 cells are not, the effective local production of TNF-α in the lung is likely to be much higher from Th1 cells than from Th2 cells. It remains possible that host parenchymal cells are induced to contribute biologically significant amounts of TNF-α after Th1 transfer. In addition, the fact that anti–TNF-α antibodies did not completely inhibit the eosinophil response suggests that other factors besides TNF-α may be involved. Other Th1 cell mediators that may contribute to the enhancement of Th2 recruitment include lymphotoxin, either in its soluble homotrimer or membrane heterotrimer form (53), or IL-2, which has been shown to cause eosinophilia (54) and to induce TNF-α expression in the lung (55). In preliminary experiments, however, treatment with anti–IL-2 antibody did not interfere with the Th1+Th2–induced eosinophilia (data not shown).
Cohn et al. have also observed the activities of adoptively transferred DO11.10 Th1 and Th2 cells in naive recipients challenged with OVA (28). In their experiments, transfer of Th2 cells alone could promote eosinophilic airway inflammation after multiple aerosol challenges over a period of 7–10 days. In their system, IL-4 production from the Th2 cells was important in the recruitment of the Th2 cells to the airways. In the absence of IL-4, inflammation could be restored by intratracheal instillation of TNF-α. These results underscore the importance of Th2 cells and TNF-α in eosinophilic airway inflammation. We have observed very similar results to these when, instead of using our short-term protocol, we have administered aerosol challenges on 7 consecutive days to recipients of Th2 cells. In this setting, Th2 recipients develop eosinophilic lung inflammation (data not shown). However, in these experiments and those of Cohn et al., the length of the experiment makes it likely that activated endogenous T cells and anti-OVA antibodies participate in the ultimate response.
Recently, Hansen et al. reported experiments using adoptive transfer of Th1, Th2, and Th0 cell lines derived from long-term culture of OVA-specific DO11.10 splenocytes (35). With these cell lines, Th2 and Th0 cells could promote airway eosinophilia in SCID mice after 2 intranasal challenges, whereas Th1 cells promoted a mononuclear inflammatory reaction without eosinophils. Differences were not observed in the recruitment of Th1 cells compared with Th2 cells. When Th1 and Th2 cells were transferred together, the Th1 cells were unable to inhibit the eosinophilic response in the airways. We have not defined the reasons for the differences between our results and those of Hansen et al. (35), but we suspect they are because of differences in the T-cell culture conditions, the method of antigen delivery, or the use of SCID mice as recipients. Katz and colleagues have shown in a mouse model of diabetes that adoptively transferred Th2 cells, which are unable to induce islet inflammation in immunocompetent NOD mice, cause an inflammatory infiltrate with eosinophilia leading to diabetes when transferred to NOD.scid mice (56, 57). This suggests that T cells behave differently in vivo when they are transferred to T cell–deficient mice compared with normal mice.
In summary, our data show that Th2 cells on their own are inefficiently recruited to the airways after antigen challenge and are ineffective at promoting eosinophilic airway inflammation. Th1 cells, rather than serving as suppressors of eosinophilic airway inflammation, can actually cooperate with Th2 cells by altering the lung microenvironment to enhance Th2 and eosinophil recruitment. Specifically, Th1 cells induce chemokine production and VCAM-1 expression in a TNF-α–dependent fashion. These data should not be interpreted to indicate that Th1 cells are unique in this regard. We are testing whether other inflammatory stimuli in the lung, particularly those that induce local TNF-α expression, can potentiate Th2 cell recruitment in a similar fashion.
Our data suggest a possible mechanism whereby viral infections could exacerbate asthma. Atopic patients could have a repertoire of circulating allergen-specific Th2 cells yet remain relatively symptom-free as long as the degree of inflammation in the lungs was below the threshold necessary for efficient Th2 cell recruitment. Virus-specific Th1 cells responding to a respiratory tract infection could alter the local lung environment sufficiently to increase Th2 recruitment. Once in the lung tissues, Th2 cells could respond to inhaled allergens by secreting IL-4, IL-5, and other mediators, thereby triggering an asthma attack. It will be interesting to see if the cooperation we have observed is duplicated in model systems with virus-specific Th1 cells and allergen-specific Th2 cells.
Acknowledgments
We thank Osami Kanagawa for providing the DO11.10/RAG-2–/– mice; Robert Schreiber and Kathy Sheehan for providing the PIP, H22, and TN3 mAb’s; Ken Murphy for the 11B11 mAb and for helpful discussions; and Emil Unanue for the TOSH anti–IL-12 mAb and for helpful discussions. This work was supported in part by National Institutes of Health grants AI-34580 and HL-56419 (to D.D. Chaplin) and Medical Scientist Training Program grant T32 GM07200 (to D.A. Randolph). D.D. Chaplin is an Investigator of the Howard Hughes Medical Institute.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 3.Finkelman FD, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 4.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12Rβ2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh CS, et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh CS, Heimberger AB, Gold JS, O’Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 9.Boushey HA, Fahy JV. Basic mechanisms of asthma. Environ Health Perspect. 1995;103(Suppl. 6):229–233. doi: 10.1289/ehp.95103s6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotran, R.S., Robbins, S.L., and Kumar, V. 1994. Pathologic basis of disease. W.B. Saunders Co. Philadelphia, PA. 1400 pp.
- 11.Bousquet J, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JW, Djukanovic R, Howarth PH, Holgate ST. Lymphocyte activation in bronchoalveolar lavage and peripheral blood in atopic asthma. Am Rev Respir Dis. 1992;145:958–960. doi: 10.1164/ajrccm/145.4_Pt_1.958. [DOI] [PubMed] [Google Scholar]
- 13.Bentley AM, et al. Increases in activated T lymphocytes, eosinophils, and cytokine mRNA expression for interleukin-5 and granulocyte/macrophage colony-stimulating factor in bronchial biopsies after allergen inhalation challenge in atopic asthmatics. Am J Respir Cell Mol Biol. 1993;8:35–42. doi: 10.1165/ajrcmb/8.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Holtzman MJ, Sampath D, Castro M, Look DC, Jayaraman S. The one-two of T helper cells: does interferon-γ knock out the Th2 hypothesis for asthma? Am J Respir Cell Mol Biol. 1996;14:316–318. doi: 10.1165/ajrcmb.14.4.8600934. [DOI] [PubMed] [Google Scholar]
- 15.Borish L, Rosenwasser L. TH1/TH2 lymphocytes: doubt some more. J Allergy Clin Immunol. 1997;99:161–164. doi: 10.1016/s0091-6749(97)70090-3. [DOI] [PubMed] [Google Scholar]
- 16.Kay AB. T cells as orchestrators of the asthmatic response. Ciba Found Symp. 1997;206:56–67. [PubMed] [Google Scholar]
- 17.Kline JN, Hunninghake GW. T-lymphocyte dysregulation in asthma. Proc Soc Exp Biol Med. 1994;207:243–253. doi: 10.3181/00379727-207-43813a. [DOI] [PubMed] [Google Scholar]
- 18.Robinson DS, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 19.Krug N, et al. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol. 1996;14:319–326. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 20.Krouwels FH, et al. Cytokine production by T-cell clones from bronchoalveolar lavage fluid of patients with asthma and healthy subjects. Eur Respir J Suppl. 1996;22:95s–103s. [PubMed] [Google Scholar]
- 21.Corne JM, Holgate ST. Mechanisms of virus induced exacerbations of asthma. Thorax. 1997;52:380–389. doi: 10.1136/thx.52.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busse WW, Gern JE, Dick EC. The role of respiratory viruses in asthma. Ciba Found Symp. 1997;206:208-13; discussion 213–219. doi: 10.1002/9780470515334.ch13. [DOI] [PubMed] [Google Scholar]
- 23.Corry DB, et al. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brusselle GG, et al. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994;24:73–80. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 25.Kips JC, et al. Importance of interleukin-4 and interleukin-12 in allergen-induced airway changes in mice. Int Arch Allergy Immunol. 1995;107:115–118. doi: 10.1159/000236947. [DOI] [PubMed] [Google Scholar]
- 26.Coyle AJ, et al. Interleukin-4 is required for the induction of lung Th2 mucosal immunity. Am J Respir Cell Mol Biol. 1995;13:54–59. doi: 10.1165/ajrcmb.13.1.7598937. [DOI] [PubMed] [Google Scholar]
- 27.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997;186:1737–1747. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Xia Y, Nguyen A, Feng L, Lo D. Th2-induced eotaxin expression and eosinophilia coexist with Th1 responses at the effector stage of lung inflammation. J Immunol. 1998;161:3128–3135. [PubMed] [Google Scholar]
- 30.Kaminuma O, et al. Successful transfer of late phase eosinophil infiltration in the lung by infusion of helper T cell clones. Am J Respir Cell Mol Biol. 1997;16:448–454. doi: 10.1165/ajrcmb.16.4.9115756. [DOI] [PubMed] [Google Scholar]
- 31.Gavett SH, et al. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwamoto I, Nakajima H, Endo H, Yoshida S. Interferon gamma regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J Exp Med. 1993;177:573–576. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lack G, et al. Nebulized IFN-gamma inhibits the development of secondary allergic responses in mice. J Immunol. 1996;157:1432–1439. [PubMed] [Google Scholar]
- 34.Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998;187:561–569. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175–183. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randolph DA, Carruthers CJL, Szabo SJ, Murphy KM, Chaplin DD. Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma. J Immunol. 1999;162:2375–2383. [PubMed] [Google Scholar]
- 37.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145–1154. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chin JE, et al. Airway recruitment of leukocytes in mice is dependent on α4-integrins and vascular cell adhesion molecule-1. Am J Physiol. 1997;272:L219–L229. doi: 10.1152/ajplung.1997.272.2.L219. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalo JA, et al. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines, and adhesion receptors. J Clin Invest. 1996;98:2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolber FM, et al. Endothelial selectins and α4 integrins regulate independent pathways of T lymphocyte recruitment in the pulmonary immune response. J Immunol. 1998;161:4396–4403. [PubMed] [Google Scholar]
- 41.Horie Y, et al. Lymphocytes mediate TNFα—induced endothelial cell adhesion molecule expression: studies on SCID and RAG-1 mutant mice. J Immunol. 1997;159:5053–5062. [PubMed] [Google Scholar]
- 42.Austrup F, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 43.Murphy KM, Heimberger AB, Loh YD. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh CS, Macatonia SE, O’Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maly P, et al. The α(1, 3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 46.Bonecchi R, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin S, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward SG, Bacon K, Westwick J. Chemokines and T lymphocytes: more than an attraction. Immunity. 1998;9:1–11. doi: 10.1016/s1074-7613(00)80583-x. [DOI] [PubMed] [Google Scholar]
- 51.Iademarco MF, Barks JL, Dean DC. Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-alpha in cultured endothelial cells. J Clin Invest. 1995;95:264–271. doi: 10.1172/JCI117650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coyle AJ, et al. Mice lacking the IFN-γ receptor have impaired ability to resolve a lung eosinophilic inflammatory response associated with a prolonged capacity of T cells to exhibit a Th2 cytokine profile. J Immunol. 1996;156:2680–2685. [PubMed] [Google Scholar]
- 53.Chaplin DD, Fu Y-X. Cytokine regulation of secondary lymphoid organ development. Curr Opin Immunol. 1998;10:289–297. doi: 10.1016/s0952-7915(98)80167-2. [DOI] [PubMed] [Google Scholar]
- 54.Wardlaw AJ. Eosinophils in the 1990s: new perspectives on their role in health and disease. Postgrad Med J. 1994;70:536–552. doi: 10.1136/pgmj.70.826.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson JA, et al. The role of cytokines, adhesion molecules, and chemokines in interleukin-2-induced lymphocytic infiltration in C57BL/6 mice. J Clin Invest. 1996;97:1952–1959. doi: 10.1172/JCI118627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 57.Pakala SV, Kurrer MO, Katz JD. T helper 2 (Th2) T cells induce acute pancreatitis and diabetes in immune-compromised nonobese diabetic (NOD) mice. J Exp Med. 1997;186:299–306. doi: 10.1084/jem.186.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]