Abstract
Colonic bacteria liberate large quantities of the highly toxic gases hydrogen sulfide (H2S) and methanethiol (CH3SH). The colonic mucosa presumably has an efficient means of detoxifying these compounds, which is thought to occur through methylation of H2S to CH3SH and CH3SH to dimethylsulfide (CH3SCH3). We investigated this detoxification pathway by incubating rat cecal mucosal homogenates with gas containing H2S, CH3SH, or CH3SCH3. Neither CH3SH nor CH3SCH3 was produced during H2S catabolism, whereas catabolism of CH3SH liberated H2S but not CH3SCH3. Thus, H2S and CH3SH are not detoxified by methylation to CH3SCH3. Rather, CH3SH is demethylated to H2S, and H2S is converted to nonvolatile metabolites. HPLC analysis of the homogenate showed the metabolite to be primarily thiosulfate. Analysis of cecal venous blood obtained after intracecal instillation of H235S revealed that virtually all absorbed H2S had been oxidized to thiosulfate. The oxidation rate of H2S by colonic mucosa was 10,000 times greater than the reported methylation rate. Conversion to thiosulfate appears to be the mechanism whereby the cecal mucosa protects itself from the injurious effects of H2S and CH3SH, and defects in this detoxification possibly could play a role in colonic diseases such as ulcerative colitis.
Introduction
The colonic bacteria produce large quantities of hydrogen sulfide (H2S) and methanethiol (CH3SH), highly toxic compounds with LD50’s for rodents that are on the same order of magnitude as cyanide. Given the very high exposure of the colonic mucosa to H2S and CH3SH, local tissue damage would be expected if the mucosa did not possess an efficient detoxification mechanism. The possibility that sulfide might be injurious to colonic mucosa was first proposed by Roediger and Nance (1), who postulated that excess sulfide production could play an etiological role in ulcerative colitis.
A variety of high-molecular-weight thiols are detoxified in a methylation reaction catalyzed by thiol S-methyltransferase (2–4). Recent literature assumes that a similar mechanism is used in the detoxification of the low-molecular-weight thiols H2S and CH3SH (5–8). Such methylation would result in the conversion of H2S to CH3SH, and then CH3SH to dimethylsulfide (CH3SCH3), a relatively nontoxic compound. However, the reported rate of methylation of H2S by rat colonic mucosa is only about 10–13 mol/min per milligram of protein (8). This activity appears to be many orders of magnitude less than that required to metabolize H2S released in the colon, which we found to occur at a rate of about 10–7 mol/min in the rat cecum (9).
In the process of incubating H2S and CH3SH with rat cecal mucosa, we observed that both gases disappeared far more rapidly than could be accounted for by reported methylation rates. The present report describes in vitro and in vivo experiments demonstrating that the colonic mucosa possesses a very efficient, but largely unrecognized, system to metabolize H2S and CH3SH. This system proceeds, not by methylation, but rather by demethylation of CH3SH to H2S and oxidation of H2S primarily to thiosulfate.
Methods
Tissues.
Cecal and hepatic tissues were obtained from male Sprague-Dawley rats (300–400 g) while the animals were under pentobarbital anesthesia. The cecum was first cleansed of luminal debris by rinsing with isotonic saline, and the mucosa was then scraped off using the edge of a glass microscope slide. Tissue samples were maintained on ice until homogenized in an ice-cold buffer solution in a ratio of 1 part tissue to 15 parts buffer (wt/vol). Homogenization was performed with a Duall grinder (Kontes, Vineland, New Jersey, USA) with a Teflon pestle, using 8–10 strokes. Except when noted, studies were carried out using RPMI buffer, which has a pH of 7.4 when equilibrated with 5% CO2. This buffer contains a variety of electrolytes, including 0.4 mM sulfate. In addition, some studies were carried out in 0.1 M PBS (pH 7.0) or a buffer system containing 1.15% KCl, 5 mM Tris-HCl (pH 7.8), and 0.475 mM of the methyl donor S-adenosylmethionine, a milieu used in previously published studies (7) designed to assess thiol S-methyltransferase activity.
Incubation studies.
A 32-μL volume of homogenate or buffer was added to 20-mL polypropylene syringes. (Preliminary studies showed that both H2S and CH3SH reacted rapidly with glass and most plastic surfaces, but had only minimal reactivity with polypropylene.) Ten milliliters of gas containing H2S, CH3SH, and CH3SCH3, either as a mixture of the 3 gases or each gas singly, was then added to the syringes, which were sealed with stopcocks. In some experiments, H235S or CH335SH was also added to the gas space. In most studies, the bulk gas was nitrogen containing 5% CO2; however, some O2 was consistently present in these incubations, because of the atmosphere in the dead spaces of the syringe and stopcock, and in the buffer that was not deoxygenated. The PO2 of the gas space averaged 7 mmHg in these experiments. In selected studies, the influence of a higher PO2 on the reaction rate was studied using air as the gas space, and the influence of a very low PO2 was determined using rigorously deoxygenated reagents and a glove box. The role of thiol S-methyltransferase in H2S metabolism was investigated by comparing the rate of H2S disappearance in the presence or absence of the thiol S-methyltransferase inhibitor S-adenosyl-L-homocysteine (2 mM).
The syringes were incubated at 37°C. In studies in which the metabolism of the sulfur gases was determined by assessment of disappearance from the gas space, 1-mL aliquots of gas were removed for analysis at 30 and 60 minutes of incubation. In experiments where metabolism of the sulfur gases was quantitated by incorporation of the radioactive gas into the homogenate, the incubation was carried out for 5 minutes. All gas in the syringe was then rapidly expelled, and the syringe was immediately placed in boiling water for 1 minute to stop the reaction.
In vivo studies.
The in vivo metabolism of H235S and CH335SH was investigated as follows. Under pentobarbital anesthesia, the cecum was cannulated via the terminal ileum using a catheter (1.52-mm internal diameter). The catheter was secured with a ligature around the ileum. All fecal material in the cecum was flushed into the more distal colon by an infusion of isotonic saline and gentle stripping. The cecal-colonic junction was then ligated. Two milliliters of nitrogen containing H235S or CH335SH was infused into the isolated cecum. The concentrations of sulfur-containing gases in this infusate ranged from 24,000 to 68,000 ppm, and contained about 1 μCi of radioactivity. For the 1-minute period after the cecal instillation of gas, blood was constantly withdrawn from a vein draining the cecum; then a blood sample was obtained from the heart. The gas space was removed from the cecum for analysis, the rat was sacrificed, and then a sample of cecal mucosa was obtained.
Synthesis of H235S and CH335SH.
Because neither of these gases is commercially available, and their shelf-life is only a few days, it was necessary to synthesize these compounds on a daily basis as follows. Freshly passed rat feces were homogenized in a blender (Eberbach Corp., Ann Arbor, Michigan, USA) using 1 part feces to 4 parts PBS (pH 7.0). Fifty microcuries of [35S]sodium sulfate, [35S]cysteine, or [35S]methionine was added to 10 mL of homogenate, and the mixture was incubated in a sealed 50-mL polypropylene syringe along with 40 mL of nitrogen. The gas space was removed at 24 hours, and H235S and CH335SH were isolated by their differential adsorption to MTO-Tenax-TA (80/100 mesh; Supelco, Bellefonte, Pennsylvania, USA). The gas space was passed through a glass column containing Tenax (43 mm × 5 mm; 150 mg) maintained on dry ice. Both gases were completely adsorbed at this temperature. H2S, CH3SH, and CH3SCH3 were differentially desorbed by heating the column from 25°C to 100°C as nitrogen was passed through the column. The purity of the sulfur gas preparations was confirmed by gas chromatography.
Gas chromatography.
Samples (0.30 mL) were analyzed for H2S, CH3SH, and CH3SCH3 using a gas chromatograph (model 5890; Hewlett-Packard, Palo Alto, California, USA) equipped with a Teflon column (8 ft × 0.125 in, packed with Chromosil 330 [Supelco], maintained at 80°C with a flow rate of 20 mL/m) and a sulfur chemiluminescence detector (model 355; Sievers Instruments, Boulder, Colorado, USA), which is specific for sulfur-containing gases. The identity of the gases was initially verified using gas chromatography/mass spectroscopy, and subsequently by measuring retention times. The gases were quantitated by comparison of peak areas with the areas of authentic standards.
Radioactivity and protein measurements.
The radioactivity in the gas space was determined by adding 1 mL of gas to 0.3 mL of 0.2 N hyamine contained in a 5-mL polypropylene syringe. (Preliminary studies showed that both H2S and CH3SH were avidly taken up by hyamine.) The hyamine (0.1 mL) was then added to 10 mL of Ultima Gold (Packard Instrument Co., Meriden, Connecticut, USA) and radioactivity determined by scintillation counting (Packard Instrument Co., Downers Grove, Illinois, USA). The radioactivity of the homogenates was determined after treatment with 1 N HCl to drive off dissolved H2S and CH3SH (0.3 mL of 1 N HCl was added to 32 μL of homogenate). The acidified homogenate (0.1 mL) was added to 10 mL of Ultima Gold, and radioactivity was determined by scintillation counting. The protein content of the tissue homogenates was determined by the Coomassie protein assay (Pierce Chemical Co., Rockford, Illinois, USA), using BSA as a standard.
HPLC.
The nonvolatile metabolites produced during incubation of tissue with H235S and CH335SH were identified by HPLC (model C-R3A Chromatopac; Shimadzu Corp., Kyoto, Japan) run at 2 mL/min and 2,000 psi, using an anion-ion exchange column (IonPac AS16; Dionex Corp., Salt Lake City, Utah, USA) and a conductivity monitor (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA) for mass measurements. For radioactivity measurements, the column eluate was collected in 2.0-mL fractions in individual scintillation vials, and the radioactivity of these fractions was determined as described above. The retention times of the unknowns were compared with retention times of authentic standards.
Calculation of metabolism.
In studies where metabolic rate was determined from the fall in concentration of the sulfur compound in the gas space, metabolism was assumed to equal the disappearance rate of the compound when incubated with the tissue homogenate minus the disappearance rate observed with the buffer control. In studies where metabolic rate was determined by incorporation of radioactivity into the homogenate, metabolism was calculated from the radioactivity of the tissue-containing homogenate minus that of the buffer control.
Results
Disappearance of H2S, CH3SH, and CH3SCH3 during incubation with cecal mucosa and liver.
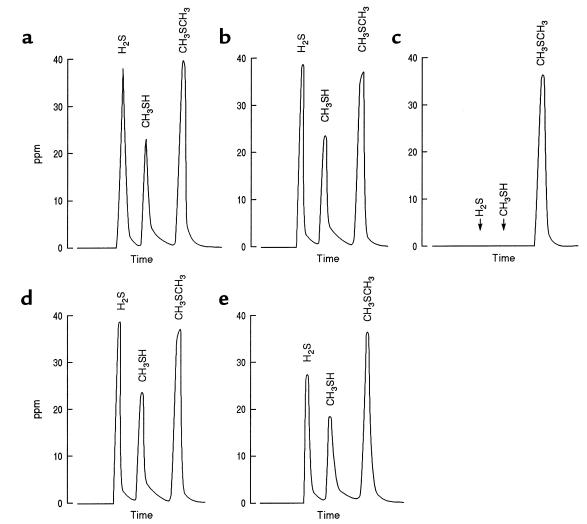
Figure 1 shows typical gas-chromatographic patterns of aliquots of the gas space obtained 60 minutes after gas containing a mixture of H2S, CH3SH, and CH3SCH3 (∼40 ppm of each gas) was incubated with buffer or a homogenate of rat cecal mucosa or liver. A minimal decrease in the concentrations of the test gases was observed during incubation with buffer (Figure 1b). During incubation with cecal tissue, the change in CH3SCH3 concentration was similar to that observed with buffer, whereas both H2S and CH3SH were entirely eliminated from the gas space during the 60-minute incubation (Figure 1c). Heating the tissue homogenate at 100°C for 5 minutes before the incubation abolished the ability of the tissue to metabolize H2S or CH3SH (Figure 1d). As shown in Figure 1e, hepatic tissue metabolized H2S and CH3SH, but at a much slower rate than was observed with cecal mucosa, and there was no detectable metabolism of CH3SCH3.
Figure 1.
Gas-chromatographic tracings showing the concentration of sulfur-containing gases in the gas space before and after a 60-minute incubation of homogenates with gas containing H2S, CH3SH, and CH3SCH3. The peak heights indicate the approximate concentrations of the gases at time 0 (a) and after incubation with buffer (b), cecal mucosal homogenate (c), heat-denatured cecal mucosal homogenate (d), and liver homogenate (e). Note the complete removal of H2S and CH3SH by nondenatured cecal mucosa with minimal alteration in CH3SCH3 concentration (c).
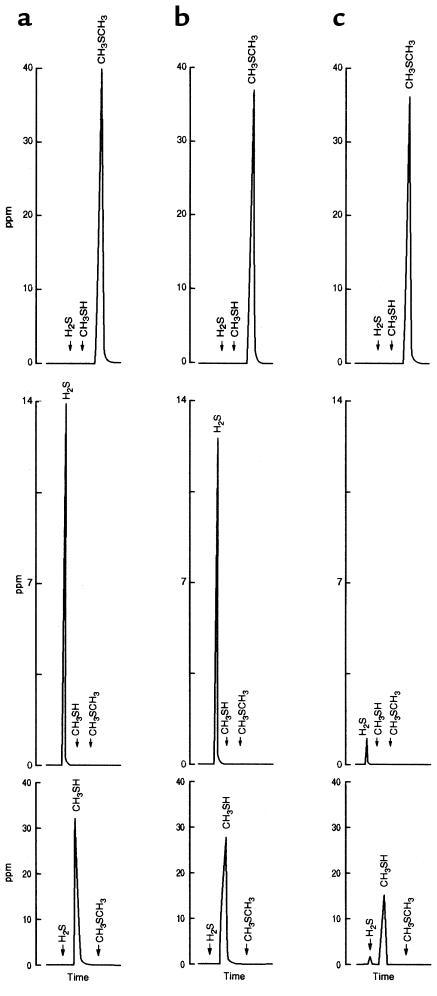
Figure 2 shows the gas-chromatographic tracings of the gas space obtained during incubation of cecal mucosa or buffer with H2S, CH3SH, or CH3SCH3. The minimal disappearance of CH3SCH3 during incubation with cecal mucosa was similar to that with incubation with buffer (Figure 2, top). Thus, the failure to observe an increase in concentration of CH3SCH3 when incubated as a mixture with H2S and CH3SH (Figure 1) was not due to a fortuitous balance between production and catabolism, but rather to an inability of the tissue to produce (or catabolize) CH3SCH3.
Figure 2.
Gas-chromatographic tracings showing the concentration of sulfur-containing gases in the gas space before and after incubation of cecal homogenate with gas containing CH3SCH3 (top), H2S (middle), and CH3SH (bottom). Tracings denoted a were obtained at time 0, whereas those denoted b and c are the results obtained after a 10-minute incubation with buffer and cecal mucosal homogenates, respectively. Note the inability of the mucosa to metabolize CH3SCH3 (top), the absence of CH3SH or CH3SCH3 production during the metabolism of H2S by cecal mucosa (middle), and the appearance of H2S, but not CH3SCH3, during the metabolism of CH3SH by cecal mucosa (bottom).
During the incubation of cecal homogenate with H2S neither CH3SH nor CH3SCH3 appeared (Figure 2, middle). The metabolism of CH3SH by mucosa was not associated with the appearance of CH3SCH3, but consistently resulted in the transient appearance of a small H2S peak (Figure 2, bottom). Similar results were obtained when phosphate buffer or a 1.15% KCl buffer system containing the methyl donor S-adenosylmethionine was used. Figure 3 shows the lack of production of CH3SH or CH3SCH3 during the metabolism of H2S by a cecal homogenate supplemented with S-adenosylmethionine.
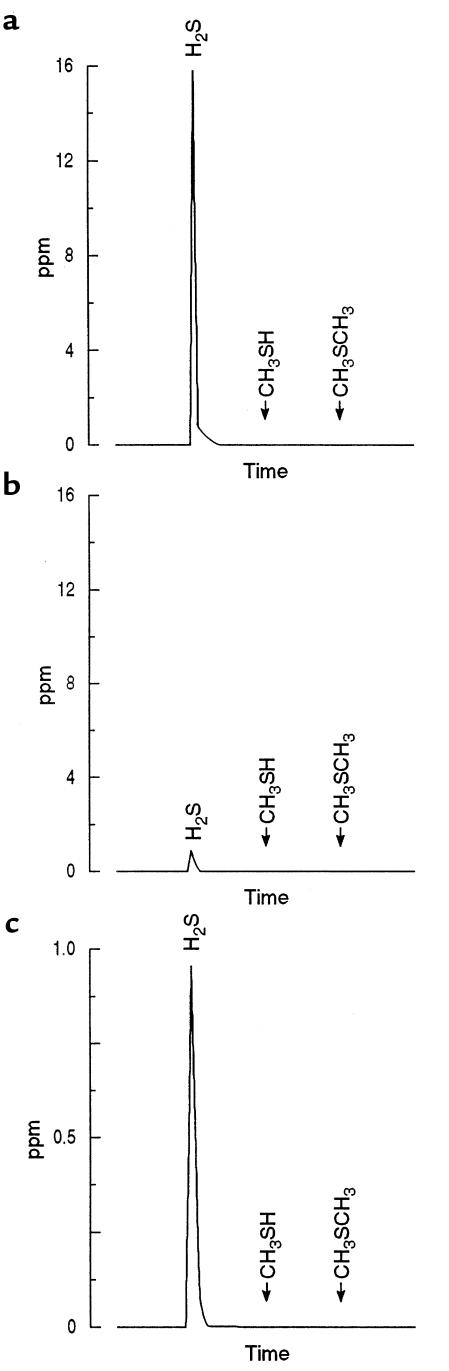
Figure 3.
Gas-chromatographic tracings show the concentrations of sulfur-containing gases in the gas space before and after a 60-minute incubation of cecal homogenate with a gas space initially containing H2S. In contrast to the studies shown in Figures 1 and 2, in this study the buffer was supplemented with the methyl donor S-adenosylmethionine. Similarly attenuated measurements of the sulfur gas concentrations present at 0 and 60 minutes are shown in a and b, respectively. Most of the H2S had been metabolized by 60 minutes, with no evidence of CH3SH or CH3SCH3 production (b). (c) The same recording shown in b, with the attenuation reduced by 16-fold. Even with 16-fold “magnification,” no CH3SH nor CH3SCH3 evolution could be identified.
To exclude the possibility that methylation of H2S to CH3SH was being obscured by extremely rapid metabolism of CH3SH in the experiments shown in Figure 2, cecal homogenate was incubated with gas containing a low concentration of H235S and a relatively high concentration of CH3SH to “trap” CH335SH released during metabolism of H235S. Disappearance of the H2S was not associated with the appearance of radioactivity in the CH3SH peak. In contrast, when a low concentration of CH335SH and a relatively high concentration of H2S were incubated with cecal mucosa, more than 50% of the radioactivity of the metabolized CH335SH was found in the H2S peak.
Influence of PO2 and an inhibitor of thiol S-methyltransferase on reaction rates.
Compared with the reaction rates observed in our standard assay (PO2 ∼8 mmHg), at ambient PO2 the metabolism of H2S and CH3SH by cecal mucosa was increased by 40% and 103%, respectively, and at very low PO2 the metabolic rates were reduced by 36% and 78%, respectively. The presence of the thiol S-methyltransferase inhibitor adenosyl-L-homocysteine did not inhibit H2S metabolism by cecal mucosa (the rate with inhibitor/rate without inhibitor ratio averaged 1.03 ± 11 in 3 studies).
Quantitation of metabolic rate.
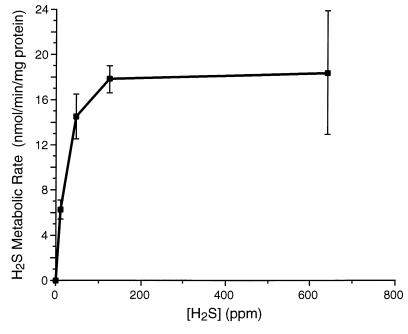
The rates of metabolism of H2S and CH3SH, as determined by disappearance rate from the gas space during the first 30 minutes of the study, averaged 1.8 ± 0.13 and 1.7 ± 0.13 nmol/min per milligram of protein, respectively. Interpretation of these metabolic rates was complicated, because the concentration of substrate, which initially was well below saturating, decreased markedly during the 30-minute incubation. To more accurately assess the rate of H2S metabolism, measurements were made of the rate at which 35S was incorporated into cecal or liver homogenates as nonvolatile metabolites during a 5-minute incubation with H235S. Figure 4 shows a plot of the rate at which cecal mucosa incorporated H235S into nonvolatile metabolites in the homogenate versus H2S concentration in the gas phase. Saturation was observed at a gas space H2S concentration of about 150 ppm. The Vmax of this reaction was calculated to be 21 nmol/min per milligram of protein, and the Km was about 25 ppm. At an H2S concentration of 150 ppm, the rate of H2S metabolism by hepatic homogenates averaged 2.5 nmol/min per milligram of protein.
Figure 4.
Plot of disappearance rate of H2S from gas space versus H2S concentration in the gas space during incubation with cecal homogenate. Maximal rate of metabolism was about 21 nmol/min per milligram of tissue.
Metabolic products of H235S metabolism by the cecal mucosa and liver.
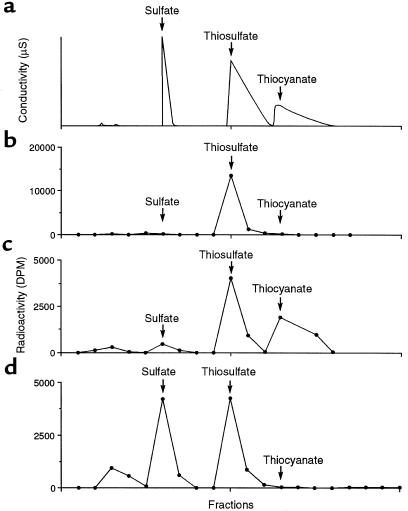
In the in vitro studies, H235S or CH335SH was incubated with cecal mucosa, and the reaction was allowed to continue until neither sulfur gas nor CH3SCH3 was detectable by gas chromatography. At this time, scintillation counting of the gas space showed no radioactivity, indicating that no volatile metabolite had been formed. The radioactivity was quantitatively recovered in the homogenate. Figure 5 shows the HPLC elution patterns of authentic sulfate, thiosulfate, and thiocyanate, as well as the radioactivity elution patterns of cecal or hepatic homogenates after incubation with H235S. Virtually all the radioactivity (average: 94%) in the cecal homogenates eluted coincident with thiosulfate (Figure 5b). Similar results were observed when CH3SH was metabolized by cecal mucosa (data not shown). When the incubation mixture contained 1 mM cyanide, the fraction of the radioactivity eluting as thiosulfate decreased, and radioactivity now appeared coincident with the thiocyanate peak (Figure 5c). Homogenates of hepatic tissue converted approximately equal amounts of the radioactivity to sulfate (mean: 42%) and thiosulfate (mean: 47%).
Figure 5.
HPLC patterns of 35S in homogenates of cecal mucosa or liver after incubation with H235S. (a) The elution pattern of authentic standards of sulfate, thiosulfate, and thiocyanate. (b–d) The radioactivity in sulfate, thiosulfate, and thiocyanate peaks after incubation of with H235S cecal mucosa without cyanide (b) or with cyanide (c), or after incubation of liver tissue without cyanide (d).
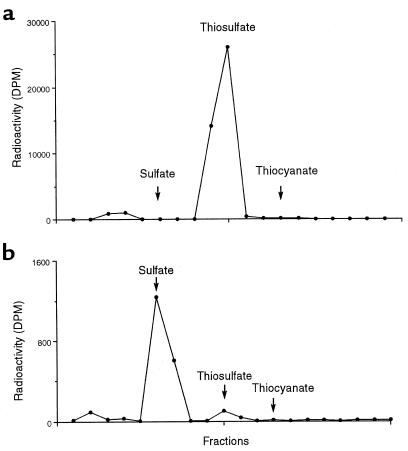
In the in vivo studies, 1 minute after H235S infusion into the cecum, an average of only 6% of the radioactivity remained in the cecum. Figure 6a shows the HPLC elution pattern of serum radioactivity of cecal blood obtained over a 1-minute period after infusion of H235S into the cecum. Virtually all the radioactivity eluted with thiosulfate. When the serum was acidified and equilibrated with air, no radioactivity was liberated into the gas space, indicating that no detectable H235S or any other volatile sulfur-containing compound was present in the blood. HPLC analysis of serum obtained from blood collected by cardiac puncture at 1 minute showed most of the radioactivity to be present as sulfate (Figure 6b).
Figure 6.
HPLC patterns of plasma radioactivity after infusion of H235S into the rat cecum. (a) The radioactivity of cecal blood collected during the first minute after H235S infusion. (b) The radioactivity of systemic blood obtained at 1 minute.
Discussion
The acute toxicity of H2S and CH3SH appears to result, like that of cyanide, from the inhibition of cytochrome oxidase. In vitro measurements have shown that H2S actually is a slightly more potent inhibitor of cytochrome oxidase than is cyanide (10).
Whereas the danger of externally administered H2S and CH3SH is well recognized, there has been less appreciation of the potential problems that could result from endogenous exposure to these compounds. The maximum environmental H2S exposure allowed by government regulations is an atmospheric concentration of 10 ppm for 10 minutes, and evacuation of the facility is required if the concentration reaches 50 ppm, a level that is injurious to both the eyes and the lungs (11). We have observed H2S concentrations in excess of 1,000 ppm in gas samples taken from the rat cecum (9). If inhaled, an H2S concentration of 1,000 ppm induces paralysis, coma, and death within minutes (11).
Most available information on the mechanisms involved in fecal H2S and CH3SH production has been derived from studies of fecal homogenates or cultures of fecal bacteria. Fecal H2S production may result from the action of sulfate-reducing bacteria, a genera of organisms that use sulfate as an electron acceptor during the dissimulation of various compounds, including H2. In the process, sulfate is reduced to sulfide. However, sulfur-containing compounds of endogenous origin, such as mucin, taurocholate, and cysteine, are converted to H2S far more efficiently by human fecal homogenates than sulfate (12). Thus, these endogenous sources of sulfur could be a major substrate for the bacterial production of H2S in the colon, as opposed to sulfate, which is derived from the diet. Supplementation of human fecal homogenates with sulfate also increases CH3SH production; however, methionine is a much better substrate for the bacterial production of this gas (12).
Concentrations of H2S and CH3SH in human flatus collected by rectal tube ranged from 0.01 to 30 ppm (13). Fecal specimens passed by healthy controls released H2S and CH3SH at mean rates of about 7 and 5 μL/h per gram of dry weight, respectively (12), and rat fecal specimens released these gases at approximately the same rate. To more accurately assess the intracolonic production of H2S and CH3SH, we carried out studies on rats fitted with chronically implanted cecal cannulae that provided access to the unperturbed cecum (9). When 3 mL of nitrogen was instilled into the cecum, H2S and CH3SH accumulated in the gas phase at rates of roughly 2.6 and 0.096 μL/min, respectively. When expressed as gas release per gram of fecal material, this rate of H2S release was about 30 times greater than that observed with rat feces passed per rectum. It should be noted that the observed accumulation rate of the H2S and CH3SH in cecal gas represents the net of liberation minus absorption/destruction by the colonic mucosa. To assess this absorption/destruction rate, nitrogen containing H2S, CH3SH, H2, and CH4 was rapidly infused into the rat cecum, and gas was collected at the rectum. Over 95% of the H2S and CH3SH disappeared during the 3- to 4-minute transit time through the colon, a disappearance rate that was more than 20 times more rapid than that observed for H2 or CH4. Studies in which H2S labeled with 35S was infused into the cecum showed that about 95% of the H2S was turned over each minute (9). Thus, conclusions drawn from studies of flatus composition, fecal gas production, or even cecal gas composition all yield dramatic underestimates of the true exposure of the colon to the sulfur-containing gases.
Although indirect, measurements of methane excretion possibly offer a means of estimating sulfide production in the human colon. The vast majority of H2 released by fecal bacteria is consumed by other bacteria (14). The major H2-consuming organisms are methanogens and sulfate-reducing bacteria, which use H2 to reduce CO2 to methane and sulfate to sulfide, respectively (15, 16). In these reactions, 4 mol of H2 are used in the process of reducing 1 mol of CO2 or sulfate. Because methanogens and sulfate-reducing bacteria compete for H2, human fecal samples generally contain high concentrations of just 1 of these 2 types of bacteria (16). Because methanogens in the human colon produce methane solely through the oxidation of H2 released by other organisms, the excretion of methane by subjects with a predominantly methanogenic flora should provide an indirect estimate of the rate of sulfide production in subjects with predominantly sulfide-reducing flora. High excretors of methane eliminate up to 50 mmol of this gas per day (17), suggesting that nonmethanogenic subjects may dispose of similar quantities of H2 through the production of up to 50 mmol of H2S per day. This value is a minimal estimate, because sulfate can be reduced by a number of reactions other than the oxidation of H2. The potential toxicity of 50 mmol of H2S is appreciable. For example, given an LD50 of H2S for mice of about 0.02 mmol (administered over 1 hour), 50 mmol would be sufficient to kill roughly 1,250 mice.
The enormous amounts of H2S and CH3SH produced in the colon almost certainly would produce local tissue damage (and possibly systemic toxicity) if colonic tissue did not have an efficient means of detoxifying these compounds. Most of the literature on this topic suggests that this detoxification occurs by methylation, a reaction that would convert H2S to CH3SH and CH3SH to CH3SCH3 (5–8). Our initial goal was to measure the rate of methylation by incubating cecal mucosal homogenates with H2S, CH3SH, and CH3SCH3, as a mixture (Figure 1) or singly (Figure 2). These experiments showed that cecal tissue rapidly metabolized H2S and CH3SH, but had no discernible ability to metabolize CH3SCH3. If H2S and CH3SH were being converted to CH3SCH3 (as would necessarily be the case with methylation), the metabolism of H2S and CH3SH should result in the appearance of CH3SCH3. However, no such CH3SCH3 production was observed during the rapid elimination of H2S and CH3SH by cecal mucosa. Similar results were observed with several different buffer preparations, including a buffer milieu used in measurements of thiol S-methyltransferase activity that contained a marked excess of the methyl donor S-adenosylmethionine. Additional evidence against the existence of appreciable methylation of H2S and CH3SH was the finding that metabolism of these gases was not inhibited by the addition of S-adenosyl-L-homocysteine, an inhibitor of S-methyltransferases, to the homogenates (18).
Experiments using H235S showed that neither CH335SH nor CH335SCH3 was detectable during H235S metabolism. However, CH335SH metabolism resulted in the production of labeled H2S, but not CH3SCH3. When the metabolism of H235S and CH335SH was allowed to proceed to completion, no radioactive metabolite was found in the gas space, and the 35S of both labeled compounds was quantitatively recovered in the liquid homogenate. Thus, in contrast to the previous assumption that H2S and CH3SH are detoxified by methylation to the volatile metabolite CH3SCH3, we found that CH3SH was demethylated to H2S, and H2S was disposed of by a reaction that converted this gas to a nonvolatile metabolite.
Studies using HPLC were carried out to identify the end products of H235S and CH335SH metabolism by cecal mucosal homogenates. These studies showed that the vast bulk of the 35S in the homogenate eluted coincident with thiosulfate, and most of the remainder eluted with sulfate. We took advantage of the naturally occurring rhodanese activity of the cecal mucosa to obtain chemical evidence that the labeled peak eluting with thiosulfate was truly thiosulfate. Rhodanese catalyzes the reaction of thiosulfate and cyanide to form thiocyanate. When mucosa was incubated with H235S and cyanide, an appreciable fraction of the material, tentatively identified as [35S]thiosulfate, was converted to a compound that eluted coincident with thiocyanate.
To determine if our in vitro observation of H2S and CH3SH oxidation to thiosulfate accurately reflected in vivo metabolism, studies were carried out in the intact rat. HPLC studies of cecal venous blood obtained immediately after H235S instillation into the cecum showed that virtually all plasma radioactivity was present as [35S]thiosulfate. In contrast, analysis of systemic blood showed that radioactivity was present as both thiosulfate and sulfate, indicating that the liver or some other organ converted thiosulfate to sulfate. Surprisingly, when rats were sacrificed several minutes after the cecal instillation of H235S, virtually no [35S]thiosulfate remained in the cecal mucosa. Thiosulfate has been widely used as an indicator of extracellular fluid volume because of its very slow penetrance of tissue membranes (19). The rapid release of thiosulfate from the cecal mucosa suggests that these cells may possess a thiosulfate secretory mechanism.
An assay using an excess of 35S-labeled H2S was developed to quantitate the rate of tissue metabolism of H2S to nonvolatile metabolites. The rate of metabolism of H2S by cecal tissue, which averaged 21 nmol/min per milligram of protein, was about 8 times that of hepatic tissue. This 8:1 activity ratio contrasts with reported measurements of thiol S-methyltransferase activity, which showed roughly equal activity in colonic mucosa and liver (8).
The reported rate of methylation of H2S by colonic mucosa (8), 127 fmol/min per milligram of protein, is only about 1/10,000 the rate of H2S oxidation observed in the present study. In contrast to the methylation rate, the oxidation of H2S observed in the present study is of the order of magnitude required to metabolize all H2S produced in the rat cecum, and hence could protect the cecal mucosa from the injurious potential of this compound.
It has been proposed that sulfide toxicity may be important in the pathogenesis of ulcerative colitis. The initial evidence in this regard was the demonstration that experimental exposure of colonic tissue to sulfide caused inhibition of butyrate use, a defect similar to that observed in colonic mucosal biopsies obtained from subjects with ulcerative colitis (20, 21). Subsequent studies showed that the colons of patients with ulcerative colitis may be exposed to unusually high quantities of sulfide, as there are higher-than-normal numbers and activity of sulfate-reducing bacteria in feces of subjects with ulcerative colitis (22). In addition, feces of subjects with ulcerative colitis released H2S about 4 times faster than did feces of healthy controls (12).
Incubation of cecal mucosa with H2S and low concentrations of cyanide resulted in the conversion of thiosulfate to thiocyanate. This observation could explain the well-documented observation that cigarette smoking is beneficial in ulcerative colitis. Smoking increases blood cyanide levels, and the colon has been shown to extract cyanide from blood (23). This cyanide could enhance the removal of thiosulfate from the colonic mucosa, and thus facilitate the metabolism of sulfide to thiosulfate.
The demonstration that cecal mucosa possesses a highly developed enzyme system to detoxify H2S and CH3SH could have important clinical implications. To date, studies of the potential pathogenic role of H2S and CH3SH in ulcerative colitis have primarily focused on the finding of increased fecal production of these compounds. It also is possible that defective metabolism of H2S or CH3SH, either genetic or acquired, could allow mucosal accumulation of these compounds, with resultant tissue injury. In addition to the acute injury that results from inhibition of cytochromes, these thiols can react with sulfhydryl-containing compounds to form persulfides (protein-SH + HS– → protein-S-SH + H+ + 2e– or protein-S-S-protein + HS– → protein-S-SH + protein-SH). These reactions not only alter protein function, but might change antigenicity. Thus, tissue damage caused by the thiols could result in the chronic, immune-mediated processes that have been demonstrated in ulcerative colitis. Unlike many hypotheses concerning the pathogenesis of ulcerative colitis, the concept of defective mucosal detoxification of H2S and/or CH3SH is readily testable — by analysis of colonic mucosal biopsies obtained through colonoscopy.
Acknowledgments
Supported in part by the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK 13309-25.
References
- 1.Roediger WEW, Nance S. Selective reduction of fatty acid oxidation in colonocytes: correlation with ulcerative colitis. Lipids. 1990;25:646–652. doi: 10.1007/BF02536016. [DOI] [PubMed] [Google Scholar]
- 2.Bremer J, Greenberg DM. Enzymatic methylation of foreign sulfhydryl compounds. Biochim Biophys Acta. 1961;46:217–224. [Google Scholar]
- 3.Keith RA, Otterness DM, Kerremans AL, Weinshilboum RM. S-methylation of D- and L-penicillamine by human erythrocyte membrane thiol methyltransferase. Drug Metab Dispos. 1985;83:669–676. [PubMed] [Google Scholar]
- 4.Woodson LC, Dunnette JH, Weinshilboum RM. Pharmacogenetics of human thiopurine methyltransferase: kidney-erythrocyte correlation and immunotitration studies. J Pharmacol Exp Ther. 1982;222:174–181. [PubMed] [Google Scholar]
- 5.Roediger WEW, Moore J, Babidge W. Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Dig Dis Sci. 1997;42:1571–1579. doi: 10.1023/a:1018851723920. [DOI] [PubMed] [Google Scholar]
- 6.Roediger WEW, Babidge W, Millard S. Methionine derivatives diminish sulphide damage to colonocytes: implications for ulcerative colitis. Gut. 1996;39:77–81. doi: 10.1136/gut.39.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitcher MCL, Beatty ER, Harris RM, Waring RH, Cummings JH. Sulfur metabolism in ulcerative colitis investigation of detoxification enzymes in peripheral blood. Dig Dis Sci. 1998;43:2080–2085. doi: 10.1023/a:1018867516575. [DOI] [PubMed] [Google Scholar]
- 8.Weisiger RA, Pinkus LM, Jakoby WB. Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulfide. Biochem Pharmacol. 1980;29:2885–2887. doi: 10.1016/0006-2952(80)90029-5. [DOI] [PubMed] [Google Scholar]
- 9.Suarez FL, Furne J, Springfield J, Levitt MD. Production and elimination of sulfur-containing gases in the rat colon. Am J Physiol. 1998;274:G727–G733. doi: 10.1152/ajpgi.1998.274.4.G727. [DOI] [PubMed] [Google Scholar]
- 10.Nicholls P. The effect of sulphide on cytochrome aa3. Isoteric and allosteric shifts of the reduced alpha-peak. Biochim Biophys Acta. 1975;396:24–35. doi: 10.1016/0005-2728(75)90186-3. [DOI] [PubMed] [Google Scholar]
- 11.Ellenhorn, M.J. 1997. Respiratory toxicology. In Ellenhorn’s medical toxicology: diagnosis and treatment of human poisoning. 2nd edition. M.J. Ellenhorn, S. Schonwald, G. Ordog, and J. Wasserberger, editors. Williams & Wilkins. Baltimore, MD. 1489–1493.
- 12.Levine J, Ellis CJ, Furne JK, Springfield J, Levitt MD. Fecal hydrogen sulfide production in ulcerative colitis. Am J Gastroenterol. 1998;93:83–87. doi: 10.1111/j.1572-0241.1998.083_c.x. [DOI] [PubMed] [Google Scholar]
- 13.Suarez FL, Springfield J, Levitt MD. Identification of gases responsible for the odour of human flatus and evaluation of a device purported to reduce this odour. Gut. 1998;43:100–104. doi: 10.1136/gut.43.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strocchi A, Levitt MD. Factors affecting hydrogen production and consumption by human fecal flora. The critical roles of hydrogen tension and methanogenesis. J Clin Invest. 1992;89:1304–1311. doi: 10.1172/JCI115716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson GR, Macfarlane GT, Cummings JH. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J Appl Bacteriol. 1988;65:103–111. doi: 10.1111/j.1365-2672.1988.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 16.Christl SU, Gibson GR, Cummings JH. Role of dietary sulphate in the regulation of methanogenesis in the human large intestine. Gut. 1992;33:1234–1238. doi: 10.1136/gut.33.9.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christl SU, Murgatroyd PR, Gibson GR, Cummings JH. Production, metabolism, and excretion of hydrogen in the large intestine. Gastroenterology. 1992;102:1269–1277. [PubMed] [Google Scholar]
- 18.Borchardt RT, Cheng CF. Purification and characterization of rat liver microsomal thiol methyltransferase. Biochim Biophys Acta. 1978;522:340–353. doi: 10.1016/0005-2744(78)90068-2. [DOI] [PubMed] [Google Scholar]
- 19.Cardozo RH, Edelman IS. The volume of distribution of thiosulfate as a measure of the extracellular fluid. J Clin Invest. 1952;31:280–290. doi: 10.1172/JCI102604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roediger WEW, et al. Sulphide impairment of substrate oxidation in rat colonocytes: a biochemical basis for ulcerative colitis? Clin Sci. 1993;85:623–627. doi: 10.1042/cs0850623. [DOI] [PubMed] [Google Scholar]
- 21.Chapman MAS, et al. Butyrate oxidation is impaired in the colonic mucosa of sufferers of quiescent ulcerative colitis. Gut. 1994;35:73–76. doi: 10.1136/gut.35.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson GR, Cummings JH, Macfarlane GT. Growth and activities of sulphate-reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiol Excol. 1991;86:103–112. [Google Scholar]
- 23.Clemedson CJ, Sorbo B, Ullberg S. Autoradiographic observations on injected S35-thiocyanate and C14 cyanide in mice. Acta Physiol Scand. 1960;48:382–389. doi: 10.1111/j.1748-1716.1960.tb01871.x. [DOI] [PubMed] [Google Scholar]