Abstract
Context and Objective: Chagas disease is considered a worldwide emerging disease; it is endemic in Mexico and the state of Coahuila and is considered of little relevance. The objective of this study was to determine the seroprevalence of T. cruzi infection in blood donors and Chagas cardiomyopathy in patients from the coal mining region of Coahuila, Mexico.
Design and Setting: Epidemiological, exploratory and prospective study in a general hospital during the period January to June 2011.
Methods: We performed laboratory tests ELISA and indirect hemagglutination in three groups of individuals: 1) asymptomatic voluntary blood donors, 2) patients hospitalized in the cardiology department and 3) patients with dilated cardiomyopathy.
Results: There were three levels of seroprevalence: 0.31% in asymptomatic individuals, 1.25% in cardiac patients and in patients with dilated cardiomyopathy in 21.14%.
Conclusions: In spite of having detected autochthonous cases of Chagas disease, its importance to local public health remains to be established as well as the details of the dynamics of transmission so that the study is still in progress.
Keywords: Chagas disease; American trypanosomiasis; Trypanosoma cruzi; Chagasic cardiomyopathy; Coal mining region; Serology; Seroprevalence, ELISA
Abstract
Contexto e Objetivo: A doença de Chagas é mundialmente considerada uma doença emergente, é endêmica no México e no estado de Coahuila e considerada de pouca relevância. O objetivo do estudo foi determinar a soroprevalência da infecção pelo T. cruzi em doadores de sangue e cardiomiopatia chagásica em pacientes da região carbonífera de Coahuila, México.
Desenho e Local: Estudo epidemiológico, exploratório e prospectivo em um hospital geral no período de janeiro a junho de 2011.
Métodos: Foram realizados testes de laboratório ELISA e hemoglutinação indireta em três grupos de indivíduos: 1) doadores de sangue voluntários assintomáticos, 2) pacientes internados na área de cardiologia e 3) pacientes com cardiomiopatia dilatada.
Resultados: Foram achados três níveis de soroprevalência: 0,31% em indivíduos doadores de sangue assintomáticos, 1,25% em pacientes cardiopatas e, em pacientes com cardiomiopatia dilatada 21,14%.
Conclusão: Detectamos casos autóctones de doença de Chagas em área considerada não endêmica. Deve ser determinada sua importância na saúde pública regional e local, para estabelecer os detalhes do mecanismo de transmissão. O estudo ainda está em desenvolvimento.
INTRODUCTION
Chagas disease or American trypanosomiasis is a neglected public health problem in Latin America33. The causative agent of the disease is the protozoan Trypanosoma cruzi (Chagas 1909) (Kinetoplastida: Trypanosomatidae), which is a flagellate haemoparasite. The life cycle of the pathogen involves two hosts corresponding to an insect and to a vertebrate. Parasites are transmitted by haematophagous bugs. The vector is an insect of the family Reduviidae, and the subfamily Triatominae. The main route of infection of T. cruzi to humans is during defecation after blood-feeding. Nevertheless, other mechanisms for transmission have been documented e.g. blood transfusions from T. cruzi-infected individuals37, transplacental route5,27, organ transplantation20,40, breast feeding11,12, laboratory accidents15,18, skinning wild animals14 and eating undercooked parasitized meat or consuming drink contaminated with triatomine feces44.
The importance of Chagas disease in Mexico was highlighted in the national seroprevalence studies reported by VELASCO-CASTREJON in 199246 and more recently by NOVELO-GARZA in 201032. In both studies it was shown that central and southern Mexico had the highest prevalence rates for the presence of positive antibodies against T. cruzi. The states with the highest frequencies were Chiapas and Oaxaca with 5.0% to 4.5% respectively, whereas the mean national seroprevalence was estimated in 1.6%.
For the state of Coahuila, the seroprevalence rate, was found to range from 0.1% to 0.6%46. Knowledge of Chagas Disease in Coahuila is virtually nonexistent and data on prevalence, incidence, transmission or vector species in the region have been rarely been referenced8.
Because very little is known about Chagas disease in the north of Mexico and especially in the state of Coahuila, in January 2011 we established a research protocol. Its main aim was to provide evidence about the prevalence of infection, and to determine the population and potential risk factors, as well as to conduct entomological investigations to identify potential vectors. During the first phase of this research protocol, we established a primary objective which was to determine the prevalence of antibodies against Trypanosoma cruzi that occurs in the coal mining region of Coahuila. The studied population was divided into three groups: (1) asymptomatic blood donors, (2) patients admitted to the cardiology department and (3) patients with dilated cardiomyopathy.
METHODS
The work was carried out with blood donors and patients of the Hospital General de Zona No. 24 of the Mexican Institute of Social Security in Nueva Rosita, Coahuila. This hospital is the main centre of medical services in the coal mining region of Coahuila and serves a population of 140,000 inhabitants. Medical facilities possess 82 hospital beds and an average of 6,000 discharges per year.
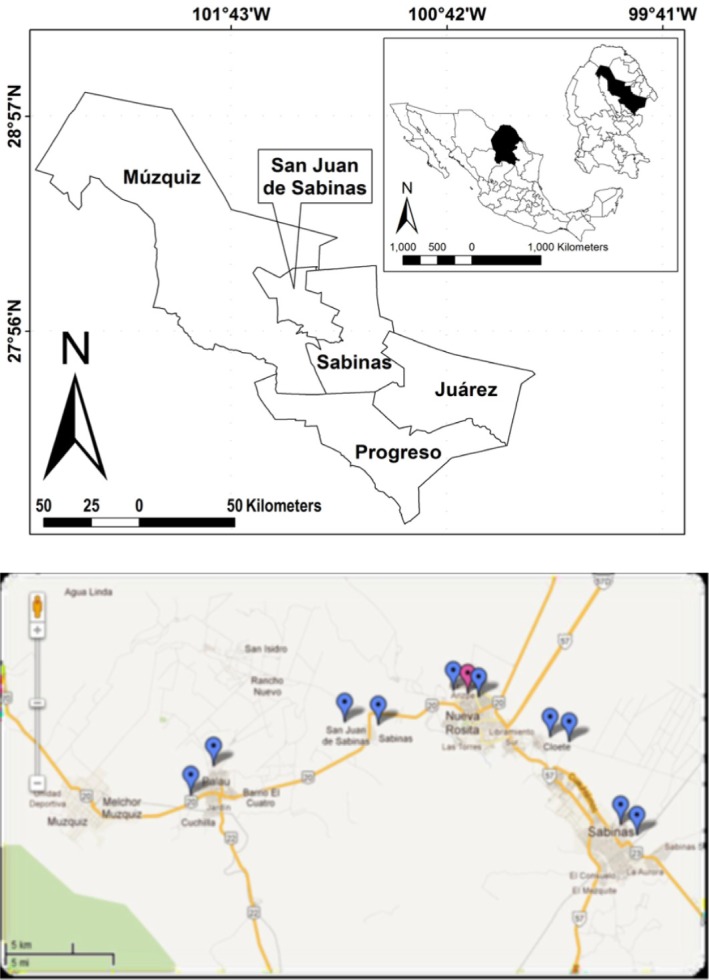
The coal mining region of Coahuila is made up of five municipalities which are: Sabinas, San Juan de Sabinas, Múzquiz, Juárez and Progreso. The coal mining region is located between latitude 27°51′36″ - 28°59′24″ N and longitude 101°07′12-101°14′24″ W and 380 meters above the sea. It has a semi-arid climate which means that it is very hot in summer and cold in winter17.
From January through June of 2011 samples were taken in three groups of individuals to determine the presence of antibodies to Trypanosoma cruzi: (1) blood donors, (2) patients admitted to the cardiology department and (3) patients with dilated cardiomyopathy. We included all persons who attended as volunteer blood donors, those who were admitted to the cardiology hospital department and those reported in the clinical diagnosis as dilated cardiomyopathy patients (I42.0 key of the International Classification of Diseases tenth edition)34 in the period indicated. The only exclusion criterion was refusal to participate in the study. The samples of each individual of these groups were analyzed to determine the presence of antibodies to T. cruzi. The positive cases underwent an epidemiological study that included blood samples taken from house cohabitants. Informed consent was requested. The study was approved by the Local Bioethics Committee under the registration number 2012- 506-25.
The blood donor group included 1615 asymptomatic individuals who came voluntarily to donate blood to the blood bank of the same hospital and covered eligibility criteria according to the corresponding Mexican Official Standard (Norma Oficial Mexicana NOM-003-SSA2-1993, “Provision of human blood and blood components for therapeutic purposes”).30
The second group was composed of patients admitted to the cardiology hospital department in the same period, these included a total of 160 people with various diseases requiring hospital treatment.
The third group was made up of patients with a diagnosis of dilated cardiomyopathy. Through the clinical file we found 14 patients with this diagnosis. These patients were visited at their homes, and in coordination and support of a public health team, blood samples were taken from those individuals.
Blood samples were obtained from these patients by a puncture in peripheral blood, serum separated by centrifugation at 1200 xg for 10 minutes, aliquoted in Eppendorf tubes and frozen at -20 °C until analysis.
Two tests were used for the determination of antibodies:
Enzyme linked immunoassay (Biokit - ELISA ChagasWerfen, Barcelona, Spain) is an immunoassay method in which microtiter wells are coated with four recombinant antigens representing immunodominant epitopes of T. cruzi. This test is based on the detection of antibody responses to four complementary immunodominant epitopes that were discovered by serologic expression cloning, by using sera from infected patients. These epitopes are expressed as a single recombinant protein, called Therapeuticf, consisting of 101 amino acids, including the amino acid hexahistidine tag used for purification. This protein is expressed in an E. coli expression vector and is purified to a single band on SDS page gels36. This procedure was carried out according to Biokit's specifications. The study has a sensitivity of 100% and a specificity of 99.24% according to the manufacturer13,35.
Indirect haemagglutination (HI), also known as reverse passive hemagglutination with Chagatest R (Wiener Laboratory, Rosario, Argentina), based on the property of producing antibodies specific agglutination in the presence of red blood cells sensitized with the corresponding antigens. The procedure was carried out according to the manufacturer's specifications. Titers of 1:16 were considered positive29,45.
The enzyme-linked immunoassay was used as a screening test. Those samples that were positive in the first instance were subsequently analyzed by indirect hemagglutination as a confirmatory test. In accordance with the actual guidelines31, the confirmation of the diagnosis of Chagas disease is established by at least two different positive serologic tests. No other tests were carried out.
In the cases that were found positive samples with both tests, we requested them to answer a questionnaire to elaborate on an epidemiological study that included medical history, history of blood transfusion, travel to endemic areas, chest radiographs, electrocardiogram, housing data, risk activities, photographic identification of triatomines and blood sampling from cohabitants. The medical history included questions on alimentary habits.
In positive cases, a search of triatomines at their home premises was conducted. Triatomine bugs were sought within and around the houses.
RESULTS
The study population had the following characteristics: a total of 1615 volunteer donors whose ages ranged between 18 and 65 years old. It was found that 88% were men (n = 1421) and the remaining 12% were women (n = 194), the average age of the sample was 37 years. Patients hospitalized in the cardiology department were 56 women (35%) and 104 men (65%), with a mean age of 69.4 years old. In the group of patients with dilated cardiomyopathy there were 14 cases, including eight women (54%) and six men (46%) with a mean age of 60.9 years old. Only one of the sampled cohabitants was found as positive, a five year old girl. All positive cases were reactive for both ELISA and HAI.
The results of this study are summarized in Table 1. A total of 1615 asymptomatic individuals were analyzed as potential blood donors, five were positive. Out of 160 patients admitted to the cardiology department we found two positive cases. Finally, in the third group of 14 patients with dilated cardiomyopathy, we found that three of them were positive. There was a positive sample derived from the study of co-inhabitants. Seroprevalence levels were found to be 0.31%, 1.25% and 21.14% in asymptomatic individuals, cardiac patients and in patients with dilated cardiomyopathy, respectively. Table 2 shows the individual characteristics of positive cases. Additionally, the geographic distribution of cases in the study area is shown in Figure 1.
Table 1. Seroprevalence results found in the coal mining region of Coahuila in relation with the different groups analyzed.
| Origin | Samples | Positive samples | Seroprevalence (%) |
|---|---|---|---|
| Blood tranfusion center | 1615 | 5 | 0.31 |
| Cardiology Hospital Service | 160 | 2 | 1.25 |
| Cardiomyopathy | 14 | 3 | 21.14 |
Table 2. Main epidemiological characteristics of positive cases with T. cruzi positive serological results Coahuila coal mining region.
| Progressive number | Age | Sex | Detection | Diagnosis | Previous blood transfusions | Travel to endemic zones | Recognition of triatomines |
|---|---|---|---|---|---|---|---|
| 1 | 42 | M | Transfusion center | Asymptomatic | Negative | Negative | Negative |
| 2 | 53 | M | Transfusion center | Asymptomatic | Negative | Negative | Negative |
| 3 | 35 | M | Transfusion center | Asymptomatic | Negative | Negative | Negative |
| 4 | 26 | M | Transfusion center | Asymptomatic | Negative | Negative | Negative |
| 5 | 25 | M | Transfusion center | Asymptomatic | Negative | Negative | Negative |
| 6 | 76 | M | Hospital | Cardiac Failure | Negative | Negative | Negative |
| 7 | 60 | F | Hospital | Cardiac Failure | Negative | Negative | Negative |
| 8 | 76 | M | Home | Dilated Cardiomyopathy | Negative | Negative | Negative |
| 9 | 60 | F | Home | Dilated Cardiomyopathy | Positive 2 years ago | Negative | Negative |
| 10 | 61 | F | Home | Dilated Cardiomyopathy | Negative | Negative | Negative |
| 11 | 5 | F | Home | Asymptomatic | Negative | Negative | Negative |
Fig. 1. Above, a map showing the five municipalities that comprised the coal mining regions in the state of Coahuila. The insert in the upper right corner, represents (black filled shapes) the location of the state of Coahuila in Mexico, as well as the proportion of the coal mining regions in the state of Coahuila. Below, geographical location of Chagas disease cases in the coal mining region of Coahuila in the period January to June 2011. The image was taken from Google Earth®. The blue balloons correspond to the location of the cases. The red balloon represents a positive case found during the epidemiological study.
The epidemiological survey of positive cases revealed that none had been born outside the studied area nor had any traveled to an endemic area of American trypanosomiasis. The houses of individuals who tested positive were built of block and cement. The questionnaire that included a question on alimentary habits did not reveal any answers that may indicate oral contamination. At the time of our entomological survey, no triatomines were found inside or around the home premises. Only one of the positive cases of dilated cardiomyopathy had had a blood transfusion two years before this study and corresponded to a 60-year-old female who has lived in this area all her life. The transfusion was conditioned by anemia secondary to metrorrhagia.
Socioeconomic status is about average lower middle class, none were considered to be living in a state of poverty. Positive cases were questioned about risk activities such as going to camps, sleeping outdoors, as well as the consumption of wild animals and so forth and the responses were all negative. People were also shown actual-size pictures of triatomines for identification and we recorded that none of them were able to recognize the vector correctly.
DISCUSSION
The northeast region of Mexico (states of Coahuila, Nuevo León and Tamaulipas) is usually not considered as part of the endemic area of Chagas disease in Mexico and for these reasons the disease has largely been neglected by the health sector. In a study carried out by GALAVÍZ-SILVA et al. (2009)13 a seroprevalence of 2.8 % antibodies against T. cruzi was found in blood donors in a hospital of the state of Nuevo León. Regarding the state of Coahuila, there has been virtually no diagnosis in Chagas for the past 20 years. The national study of VELASCO-CASTREJON et al. in 1992 in a sample of 1976 people, found a prevalence of 0.1 to 1:32 dilution using hemagglutination and indirect immunofluorescence46. To the best of our knowledge, no other study has been carried out in the state of Coahuila, and therefore this paper represents the first evidence that there is a suggested risk of infection of T. cruzi among the human population. The work of NOVELO-GARZA, in 2010 revised the responses to antibodies against T. cruzi using the ELISA test carried out among blood donors in the Mexican Social Security Institute and reported that in a population of 230,074 they found a seroprevalence of 0.406 %. For the state of Coahuila that National survey included 4611 persons of which 10 were positive for an overall rate of 0.217 in the state. If the state is divided into north and south regions, leaving the towns of Monclova, Nueva Rosita and Piedras Negras as north, then it can be seen that this estimate increases to 0.37 %, which would be a similar estimate to that found in this study. In the city Nueva Rosita which is the center of the coal mining region, the seroprevalence was found to be 0.77 %, and this corresponds to a single case in a sample of only 129 people32.
The laboratory diagnosis of Chagas disease depends on the clinical stage. It is known that in acute cases it is only possible to identify the etiologic agent, whereas an indeterminate and chronic diagnosis is based on the presence of antibodies against the parasite protozoon T. cruzi in sera of infected individuals. These antibodies are mostly detected using different serological tests, the most widely used are the indirect hemagglutination (IHA), enzyme-linked immunosorbentassay (ELISA) and indirect immunofluorescence (IIF), due to the easy implementation, low cost and good results in terms of specificity and sensitivity. Based on several protocols, it is considered to be a case of Chagas disease when the blood samples of an individual give positive results from at least two different serologic tests21,38,43.
Most recently the polymerase chain reaction has been used to detect T. cruzi DNA in tissues samples from necropsies and additionally, with this technique it has been possible to determine the number of copies as a way to establish the parasite load in these patients22. The use of molecular techniques to confirm the diagnosis of Chagas disease in a daily clinical practice is still out of reach of most health institutions in Mexico6. In our study, confirmation of positive samples by molecular techniques was not considered at this stage because the main objective was to detect seroprevalences in a particular population using the resources provided by the hospital of Instituto Mexicano del Seguro Social (IMSS).
In the present study we found a seroprevalence of 0.31%, 1.25% and 21.14% for the blood donor (asymptomatic) group, hospitalized cardiac patients and patients with dilated cardiomyopathy respectively. These figures show that the coal mining region of Coahuila is an area in which there is circulation of T. cruzi although traditionally it was considered non-endemic region. This data also suggests that Chagas disease may represent a cause for cardiomyopathy. It is likely that many cases are not recognized by the health institutions. Nonetheless, our entomological surveys were limited; we found that in those communities there is basically no knowledge about the vectors and/or transmission mechanisms. Lack of knowledge on vectors or transmission mechanisms represents a shortcoming in the implementation of any prevention program. As was previously mentioned in the results, none of the positive cases were able to identify the insect vector and we did not found triatomine bugs during the search inside and around their houses. The failure to find triatomines in the houses does not necessarily indicate their absence. It is possible that insects might not have been properly detected during searches or that perhaps they have been conducted at a time of the year when their presence is scarce. It is also possible to consider that there is a transmission cycle well outside houses, which may be conditioned by the migration of vectors between suburban and wild environments24. We suggest that more systematic and thorough entomological studies are required to evaluate the transmission and risk potential of triatomine vectors occurring in the region.
An extradomiciliary cycle of T. cruzi can be carried out and maintained in vertebrates other than man, including domestic animals such as pet dogs3. In the United States of America (USA), and particularly in the state of Texas, several vertebrate species (armadillos, coyotes, raccoons, opossums and rats of the genus Neotoma) have been documented as having tested positive for the infection with T. cruzi 42. Until recently, in the USA only seven indigenous cases of Chagas disease have been reported (four in Texas, one in Tennessee, one in California and one in Louisiana)4,10. In addition to the above cases, CANTLEY et al., cited a study “The United Sates Trypanosoma cruzi Infection Study” (USTC), which made possible infection screening of blood donors and found that an initial sample of 29 million, 1084 tested positive for antibodies T. cruzi and after performing exclusion criteria for a follow-up study, it was determined that 15 indigenous cases were confirmed by tests conducted by the Centers for Disease Control (CDC) and also added a case from the state of Mississippi. In the above-mentioned study there were 15 new autochthonous Chagas cases7. These new cases certainly indicate that the prevalence of infection with T. cruzi in the USA may in fact be an underestimation of the actual disease prevalence and we suggest that something similar may well be happening in northern Mexico, where Chagas disease is still considered unimportant by national health programs.
It should be noted that the U.S.-Mexico border region, shares some socio-cultural aspects, such as significant migratory movement and the presence of some common parasitic diseases, including Chagas disease16. Furthermore, it has been reported that in Texas there have been seven major species of triatomine19 of which Triatoma gerstaeckeri, Triatoma lecticularia and Triatoma sanguisuga are considered fairly common and have extensive geographic distributions including many northern states in northeastern Mexico42. Many Texas counties that are bordering Mexico have records of the presence of bugs infected with T. cruzi 19. It remains to be seen which other triatomine species exist in the state of Coahuila and what the population abundance, seasonality, infection rate and vectorial capacity are. Studies conducted in Nuevo Leon, a Mexican State next to Coahuila, reported T. cruzi infection in collected domestic and wild Triatoma gerstaeckeri made in municipality of General Teran23,26. A recent study carried out by our research group, reported the presence of native triatomines T. gerstaeckeri and T. rubida in the north of Coahuila near to the coal region, which may represent another risk factor for local Chagas disease infection25.
Regarding the disease, at this stage it can be said that there are several elements that indicate the presence of autochthonous cases of Chagas. First of all, we have the confirmation of eleven positive samples by two different serological tests and their clinical histories. Secondly, the fact that none of the individuals who tested positive had travelled to high endemicity areas, restricts the possibility of acquiring the infection elsewhere. Thirdly, we have shown in another publication the presence of two triatomine bugs in Coahuila (i.e. T. gerstaeckeri and T. rubida), which have been recognized as species of medical importance in the USA. Fourthly, there are some paleoparasitological reports2,39 that demonstrate that ancient mummies (aged circa 1,000 years) found by the Rio Grande (Rio Bravo) border between Coahuila and Texas were indeed infected with T. cruzi. All the above evidence led us to believe that there is an as yet unraveled transmission cycle of T. cruzi in the region and that therefore, more detailed studies are required to fully assess the impact and magnitude of risk to the human population.
Recognizing the problem of Chagas disease by health authorities and inhabitants of this particular region is a very important aim to be achieved and nowadays much work has yet to be carried out in these areas. There is also the problem of the treatment since there is no consensus about the correct or optimal management of indeterminate and chronic cases1. Until now there is no effective treatment for the indeterminate and chronic cases. Although there are studies on developing alternative autologous stem cell treatment and experimental drugs, its management has been limited to the treatment of clinical presentation, e.g. heart failure or arrhythmias28. It is important at the time of detection of indeterminate cases the decision of treatment in order to prevent the chronic condition leading to high costs and mortality in health47.
CONCLUSION
In this study, we report the presence of autochthonous cases of Chagas disease in the coal zone of Coahuila, which highlights the importance of screening studies and finding cases more frequently and with greater geographic coverage. It is very important to disseminate information about the disease, the parasite and its insect vector between inhabitants living in risk areas. With extensive preventative actions the epidemiological costs will be lower and patient expectations about treatment will be better. Therefore we stress the urgent need to continue with more studies to determine the extent of the Chagas problem in the state and in the northeastern region of the country.
ACKNOWLEDGEMENTS
The first author is grateful for the support given to CONACyT through a scholarship for doctoral studies # 392195. We would also like to thank the staff of the General Hospital No. 24 of the Mexican Social Security Institute in Nueva Rosita, Coahuila for the facilities granted.
REFERENCES
- 1.Amunárriz M, Quito S, Tandazo V, Lopez M. Seroprevalencia de la enfermedad de Chagas en el cantón Aguarico, Amazonia ecuatoriana. Rev Panam Salud Pública. 2010;28:25–9. doi: 10.1590/s1020-49892010000700004. [DOI] [PubMed] [Google Scholar]
- 2.Araujo A, Jansen AM, Reinhard K, Ferreira LF. Paleoparasitology of Chagas disease: a review. Mem Inst Oswaldo Cruz. 2009;104((Suppl I)):9–16. doi: 10.1590/s0074-02762009000900004. [DOI] [PubMed] [Google Scholar]
- 3.Bear CB, Pye G, Steurer JF, Rodríguez R, Campman R, Peterson AT, et al. Chagas disease in a domestic transmission cycle in Southern Texas, USA. Emerg Infect Dis. 2003;9:103–5. doi: 10.3201/eid0901.020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis. 2009;49((5)):e52–4. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 5.Bern C, Verastegui M, Gilman RH, Lafuente C, Galdos-Cardenas G, Calderon M, et al. Congenital Trypanosoma cruzi transmission in Santa Cruz Bolivia. Clin Infect Dis. 2009;49:1167–74. doi: 10.1086/648070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brasil PE, De Castro L, Hasslocher-Moreno AM, Sangenis LHC, Braga JU, ELISA versus PCR for diagnosis of chronic Chagas disease: systematic review, meta-analysis BMC Infect Dis. 2010;10:337. doi: 10.1186/1471-2334-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantley PT, Stramer SL, Townsend RL, Kamel H, Ofafa K, Todd CW, et al. The United States Trypanosoma cruzi infection study: evidence for vector-borne transmission of the parasite that causes Chagas disease among United States blood donors. Transfusion. 2012;52:1922–30. doi: 10.1111/j.1537-2995.2012.03581.x. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Reyes A, Pickering-López JM. Chagas disease in Mexico: an analysis of geographical distribution during the past 76 years: a review. Mem Inst Oswaldo Cruz. 2006;101:345–54. doi: 10.1590/s0074-02762006000400001. [DOI] [PubMed] [Google Scholar]
- 9.Diaz JH. Recognizing and reducing the risks of Chagas disease (American Trypanosomiasis) in travelers. J Travel Med. 2008;15:184–95. doi: 10.1111/j.1708-8305.2008.00205.x. [DOI] [PubMed] [Google Scholar]
- 10.Dorn P, Perniciario L, Yabsley MJ, Roelling DM, Balsamo G, Diaz J, et al. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis. 2007;13:605–7. doi: 10.3201/eid1304.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira CS, Martinho PC, Amato-Neto V, Cruz RR. Pasteurization of human milk to prevent transmission of Chagas disease. Rev Inst Med Trop Sao Paulo. 2001;43:161–2. doi: 10.1590/s0036-46652001000300008. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira CS, Amato-Neto V, Gakiya E, Bezerra RC, Rodriguez-Alarcon RS. Microwave treatment of human milk to prevent transmission of Chagas disease. Rev Inst Med Trop Sao Paulo. 2003;45:41–2. doi: 10.1590/s0036-46652003000100008. [DOI] [PubMed] [Google Scholar]
- 13.Galavíz-Silva L, Molina-Garza DP, Gonzalez-Santos MA, Mercado-Hernandez R, González-Galaviz JR, Rosales-Encina JL, et al. Update on seroprevalence of anti-Trypanosoma cruzi antibodies among blood donors in northeast Mexico. Am J Trop Med Hyg. 2009;81:404–6. [PubMed] [Google Scholar]
- 14.Hanford EJ, Zhan FB, Lui Y, Giordano A. Chagas disease in Texas: recognizing the significance and implications of evidence on the literature. Soc Sci Med. 2007;65:60–79. doi: 10.1016/j.socscimed.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 15.Herwaldt BL. Laboratory acquired parasitic infection from accidental exposures. Clin Microbiol Rev. 2001;14:659–88. doi: 10.1128/CMR.14.3.659-688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotez P, Bottazzi ME, Dumonteil E, Valenzuela JG, Kamhawi S, Ortega J, et al. Texas and Mexico: sharing a legacy of poverty and neglected tropical diseases. PLoS Negl Trop Dis. 2012;6((3)): doi: 10.1371/journal.pntd.0001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Instituto Nacional de Estadística y Geografía Mexico en cifras. Available from: http://www.inegi.org.mx/sistemas/mexicocifras/default.aspx.
- 18.Kinoshita-Yanaga AT, Toledo MJO, Araujo SM, Vier BP, Gomes ML. Accidental infection by Trypanosoma cruzi follow up by the polymerase chain reaction: case report. Rev Inst Med Trop Sao Paulo. 2009;51:295–8. doi: 10.1590/s0036-46652009000500011. [DOI] [PubMed] [Google Scholar]
- 19.Kjos SA, Snowden KF, Olson JG. Biogeography and Trypanosoma cruzi infection prevalence of Chagas disease vectors in Texas, USA. Vector Borne Zoonotic Dis. 2009;9:41–9. doi: 10.1089/vbz.2008.0026. [DOI] [PubMed] [Google Scholar]
- 20.Kun H, Moore A, Mascola L, Stevrer F, Lawrence G, Kubak B, et al. Transmission of Trypanosoma cruzi by heart transplantation. Clin Infect Dis. 2009;48:1534–40. doi: 10.1086/598931. [DOI] [PubMed] [Google Scholar]
- 21.López-Antuñano JF, Rangel-Flores H, Ramos C. Diagnosis of Chagas' disease. Rev Latinoam Microbiol. 2000;42:121–9. [Google Scholar]
- 22.Marcon GEB, Albuquerque DM, Batista AM, Andrade PD, Almeida EA, Guariento ME, et al. Trypanosoma cruzi: parasite persistence in tissues in chronic chagasic Brazilian patients. Mem Inst Oswaldo Cruz. 2011;106:85–91. doi: 10.1590/s0074-02762011000100014. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Ibarra JA, Galaviz-Silva L, Lara-Campos C, Trujillo-Garcia C. Distribución de los triatominos asociados al domicilio humano en el municipio de General Terán, Nuevo León, Mexico. Southwest Entomol. 1992;17:261–5. [Google Scholar]
- 24.Martinez-Ibarra JA, Grant-Guillen Y, Morales-Corona ZY, Haro-Rodriguez S, Ventura-Rodriguez LV, Nogueda-Torres B, et al. Importance of species of Triatominae (Heteroptera: Reduviidae) in risk of transmission of Trypanosoma cruzi in Western Mexico. J Med Entomol. 2008;45:476–82. doi: 10.1603/0022-2585(2008)45[476:iosoth]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Tovar JG, Rodríguez-Rojas JJ, Arque-Chunga W, Lozano-Rendon JA, Ibarra-Juárez LA, Dávila-Barboza A, et al. Nuevos registros geográficos y notas de infección de Triatoma gerstaeckeri (Stål) y Triatoma rubida (Uhler) (Hemiptera: Reduviidae: Triatominae) en Nuevo Leon y Coahuila, México. Acta Zool Mex. 2013;29:227–33. [Google Scholar]
- 26.Molina-Garza ZJ, Rosales-Encina JL, Galaviz-Silva L, Molina-Garza D. Prevalencia de Trypanosoma cruzi en triatominos silvestres de Nuevo León. Salud Pública Mex. 2007;49:37–44. doi: 10.1590/s0036-36342007000100006. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz J, Portús M, Cortachan M, Fumadó V, Gascon J. Congenital Trypanosoma cruzi infection in a non-endemic area. Trans R Soc Trop Med Hyg. 2007;101:1161–2. doi: 10.1016/j.trstmh.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Murator C, Baranchuk A. Current and emerging therapeutic options for the treatment of chronic chagasic cardiomyopathy. Vasc Health Risk Manag. 2010;6:593–601. doi: 10.2147/vhrm.s8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neal RA, Miles RA. Indirect haemagglutination test for Chagas' disease, with a simple method for survey work. Rev Inst Med Trop Sao Paulo. 1970;12:325–32. [PubMed] [Google Scholar]
- 30.Norma Oficial Mexicana NOM-003-SSA2-1993, “Para la disposición de sangre humana y sus componentes con fines terapéuticos”. Available from: http://www.salud.gob.mx/unidades/cdi/nom/003ssa23.html.
- 31.Noma Oficial Mexicana NOM-032-SSA2-2012, Para la vigilancia epidemiológica, prevención y control de las enfermedades transmitidas por vector. Available from: http://www.salud.gob.mx/unidades/cdi/nom/032ssa202.html. [Google Scholar]
- 32.Novelo-Garza B, Benitez-Arvizu G, Pena-Benitez A, Galvan-Cervantes J, Morales Rojas A. Detección de Trypanosoma cruzi en donadores de sangre. Rev Med Inst Mex Seguro Soc. 2010;48:139–44. [PubMed] [Google Scholar]
- 33.Organización Mundial de la Salud Reporte del grupo de trabajo científico sobre la enfermedad de Chagas. Copyright © World Health Organization on behalf of the Special Programme for Research and Training in Tropical Diseases. Actualización 2007. 2005. Available from: http://whqlibdoc.who.int/hq/2007/TDR_SWG_09_spa.pdf.
- 34.Organización Panamericana de la Salud . Clasificación estadística internacional de enfermedades y problemas relacionados con la salud. 10a rev. v. 3. Washington: OPS; 1995. (Publ Cient. no. 554) [Google Scholar]
- 35.Otani MM, Vinelli E, Kirchhoff LV, del Pozo A, Sands A, Veracauteren G, et al. WHO comparative evaluation of serologic assays for Chagas disease. Transfusion. 2009;49:1076–82. doi: 10.1111/j.1537-2995.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- 36.Persing D. Update on testing for Chagas disease. 74th Meeting Blood Products Advisory Committee. At Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Silver Spring, Maryland; 2002 September 12. :p. 262–5. Available from: http://www.fda.gov/OHRMS/DOCKETS/ac/02/transcripts/3892t1.doc.
- 37.Ponce C. Transfusion transmission of Chagas disease in Honduras and other Central American countries. Medicina (B Aires) 1999;59((Suppl 2)):135–7. [PubMed] [Google Scholar]
- 38.Ramos-Ligonio A, Ramirez-Sánchez ME, Gonzalez-Hernandez JC, Rosales-Encina JL, Lopez-Monteon A. Prevalencia de anticuerpos contra Trypanosoma cruzi en donadores de sangre del IMSS, Orizaba, Veracruz, México. Salud Pública Méx. 2006;48:13–21. doi: 10.1590/s0036-36342006000100004. [DOI] [PubMed] [Google Scholar]
- 39.Reinhard K, Fink TM, Skiles J. A case of megacolon in Rio Grande Valley as a possible case of Chagas Disease. Mem Inst Oswaldo Cruz. 2003;98((Suppl 1)):165–72. doi: 10.1590/s0074-02762003000900025. [DOI] [PubMed] [Google Scholar]
- 40.Riarte A, Luna C, Sabatiello R, Sinagra A, Schiavelli R, De Rissio A, et al. Chagas' disease in patients with kidney traansplants: 7 years of experience, 1989-1996. Clin Infect Dis. 1999;29:561–7. doi: 10.1086/598634. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Guillen MC, Bernabe C, Guegan JF. Tibayrenc M, Velazquez-Rojas M, Martinez-Munguia J, et al. High prevalence anti-Trypanosoma cruzi antibodies, among blood donors in the state of Puebla, a non-endemic area of Mexico. Mem Inst Oswaldo Cruz. 2002;97:947–52. doi: 10.1590/s0074-02762002000700004. [DOI] [PubMed] [Google Scholar]
- 42.Sarkar S, Strutz SE, Frank DM, Rivaldi CL, Sissel B, Sanchez-Cordero V. Chagas disease risk in Texas. PLoS Negl Trop Dis. 2010;4((10)): doi: 10.1371/journal.pntd.0000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sosa-Jurado F, Zumaquero-Ríos JL, Reyes PA, Cruz-Garcia A, Guzman-Bracho C, Monteon VM. Factores bióticos y abióticos que determinan la seroprevalencia de anticuerpos contra Trypanosoma cruzi en el municipio de Palmar de Bravo, Puebla, México. Salud Pública Mex. 2004;46:39–48. doi: 10.1590/s0036-36342004000100006. [DOI] [PubMed] [Google Scholar]
- 44.Toso AM, Vial FU, Galanti N. Transmisión de la enfermedad de Chagas por vía oral. Rev Med Chil. 2011;139:258–66. [PubMed] [Google Scholar]
- 45.Vega-Chirinos S, Naquira-Velarde C. Manual de procedimientos de laboratorio para el diagnóstico de la tripanosomiasis americana (enfermedad de Chagas)(Serie de Normas Tecnicas 26) Lima: Instituto Nacional de Salud;; 2006. [Google Scholar]
- 46.Velasco-Castrejon O, Valdespino JL, Tapia-Conyer R, Salvatierra B, Guzman-Bracho C, Magos C, et al. Seroepidemiología de la enfermedad de Chagas en México. Salud Pública Mex. 1992;34:186–96. [PubMed] [Google Scholar]
- 47.Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, et al. Long-term cardiac outcomes of treating chronic Chagas disease with Benznidazole versus no treatment. Ann Intern Med. 2006;144:724–34. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]


