Abstract
Dengue is currently a major public-health problem. Dengue virus (DENV) is classified into four distinct serotypes, DENV 1-4. After 28 years of absence, DENV-4 was again detected in Brazil in 2010 in Roraima State, and one year later, the virus was identified in the northern Brazilian states of Amazonas and Pará, followed by Rio de Janeiro and São Paulo. In Minas Gerais, the first confirmed case of DENV-4 occurred in the municipality of Frutal in 2011 and has now been isolated from a growing number of patients. Although DENV-2 is associated with the highest risk of severe forms of the disease and death due to the infection, DENV-4 has also been associated with severe forms of the disease and an increasing risk of hemorrhagic manifestations. Herein, the first fatal case of confirmed DENV-4 in Brazil is reported. The patient was an 11-year-old girl from the municipality of Montes Claros in northern Minas Gerais State, Brazil. She had idiopathic thrombocytopenic purpura as a comorbid condition and presented with a fulminant course of infection, leading to death due to hemorrhagic complications. Diagnosis was confirmed by detection of Dengue-specific antibodies using IgM capture enzyme-linked immunosorbent assay and semi-nested RT-PCR. Primary care physicians and other health-care providers should bear in mind that DENV-4 can also result in severe forms of the disease and lead to hemorrhagic complications and death, mainly when dengue infection is associated with coexisting conditions.
Keywords: Dengue, Risk factors, Dengue serotype 4, Epidemiology, Minas Gerais
Abstract
Dengue é atualmente um importante problema de saúde pública. O vírus da dengue (DENV) é classificado em quatro sorotipos distintos, DENV 1-4. Após 28 anos de ausência, o DENV-4 foi detectado novamente no Brasil em 2010 no Estado de Roraima, e um ano depois, o vírus foi identificado em outros estados do norte do país, Amazonas e Pará, seguido pelos estados do Rio de Janeiro e São Paulo. Em Minas Gerais, o primeiro caso confirmado de DENV-4 ocorreu no município de Frutal em 2011 e, desde então, o sorotipo foi isolado em um número crescente de pacientes. Apesar do DENV-2 estar associado a um maior risco de formas graves e morte, o DENV-4 também tem sido associado a casos graves e a risco aumentado de manifestações hemorrágicas. Neste relato, descrevemos o primeiro caso fatal confirmado por DENV-4 no Brasil. A paciente era uma menina de 11 anos do município de Montes Claros, no norte de Minas Gerais, Brasil. Apresentava púrpura trombocitopênica idiopática e evoluiu de forma fulminante durante a infecção por dengue, com óbito associado a complicações hemorrágicas. O diagnóstico foi confirmado pela detecção de anticorpos IgM específicos para dengue, por método imunoenzimático, e por semi-nested RT-PCR. Médicos e outros profissionais de saúde devem estar cientes que infecções por DENV-4 também podem resultar em formas graves da doença com complicações hemorrágicas e óbito, principalmente em pacientes com comorbidades.
INTRODUCTION
Dengue is currently a major public health problem, having become the most significant vector-borne viral disease worldwide. Approximately two billion people live in risk areas, consisting primarily of tropical and subtropical developing countries11. The number of countries reporting dengue virus (DENV) cases has increased dramatically in the past decades as a reflection of the expanding habitat of the vector Aedes spp., the poorly planned urbanization of many cities in developing countries, an increased number of susceptible human hosts, and the rapid spread of DENV serotypes through global human travel networks26.
DENV is classified into four distinct serotypes (DENV 1-4). DENV-4 was not detected in Brazil for 28 years after the first clinical and laboratory reports of dengue fever cases in Roraima State during 1981-198222. Since then, no additional cases have been reported in the country until the reemergence of DENV-4 in Boa Vista, Roraima State, in 20101,18,27. Following those first recent detections of DENV-4 in Brazil, the virus was identified in the northern Brazilian states of Amazonas and Pará20. In the Southeast, the first episode of the disease occurred in the state of Rio de Janeiro in 201119. The first isolations of DENV-4 in São Paulo occurred in February and March of 2011, and both patients (a 31-year-old woman and a 49-year-old man) made complete recoveries23.
The four serotypes of dengue virus have been reported in Minas Gerais State. In Minas Gerais, DENV-4 was previously reported and confirmed in the municipality of Frutal in 2011 (unpublished observations). In 2012, DENV-4 was isolated from a growing number of patients in various counties in the state of Minas Gerais. Up to July 2013, there are no published reports of severe DENV-4 cases. To the best of our knowledge, we herein present the first fatal case of confirmed DENV-4 infection in Brazil. The patient lived in Montes Claros, a municipality located in the northern region of the state (16°44′S 43°51′W; 361,971 inhabitants).
CASE REPORT
An 11-year-old girl with a past relevant medical history of idiopathic thrombocytopenic purpura presented on the 26th of December 2012 complaining of a two-day fever, retro-orbital pain, arthralgia and malaise. Upon examination, she was febrile (39.5 °C), with a maculopapular rash and oropharyngeal hyperemia. The initial blood hematocrit results and platelet count were unrevealing, the patient did not have low platelet counts or plasma leakage (Table 1). She was treated with analgesics (acetaminophen) and oral hydration. The initial suspicion was dengue. The following day, she returned to the hospital with persistent fever and received dipyrone, paracetamol and amoxicillin due to a presumptive diagnosis of bacterial pharyngitis. On the fifth day of symptoms, she was readmitted to the hospital with a high axillary temperature (41 °C), and the disease evolved with dizziness, dyspnea, hiccups and eight episodes of vomiting combined with nasal and gingival bleeding. In the emergency room, resuscitation was initiated with an isotonic crystalloid solution combined with a vasopressor (dopamine). She was transferred to the intensive care unit with severe systemic signs of refractory shock. Upon a physical examination, she had an enlarged palpable liver. Due to altered consciousness and respiratory distress, the patient required orotracheal intubation. Five units of platelets, 1200 mL of fresh frozen plasma and 300 mL of red blood cells were transfused due to a low platelet count and low hematocrit (Table 1).
Table 1. Laboratory data.
| Date | 26/12/2012 | 29/12/2012 (19:09) | 29/12/2012 (21:47) |
|---|---|---|---|
| Hemoglobin (g/dL) | 12.6 | 8.6 | 6.7 |
| Hematocrit (%) | 38.2 | 25.3 | 19.8 |
| White blood cells (x103/mL) | 3.8 | 30.6 | 36.2 |
| Neutrophils % | 74 | 47 | 44 |
| Bands % | 8 | 33 | 36 |
| Lymphocytes (%) | 13 | 16 | 15 |
| Monocytes (%) | 5 | 3 | 4 |
| Eosinophils | 0 | 1 | 1 |
| Platelet count (x103/mL) | 163 | 32 | 42 |
| INR* | 2.11 | ||
| Sodium (mEq/L) | 136 | 134 | |
| Potassium (mEq/L) | 4.3 | 5.2 | |
| Urea (mg/dL) | 64 | 42 | |
| Creatinine (mg/dL) | 2.32 | 1.57 |
INR: International normalized ratio.
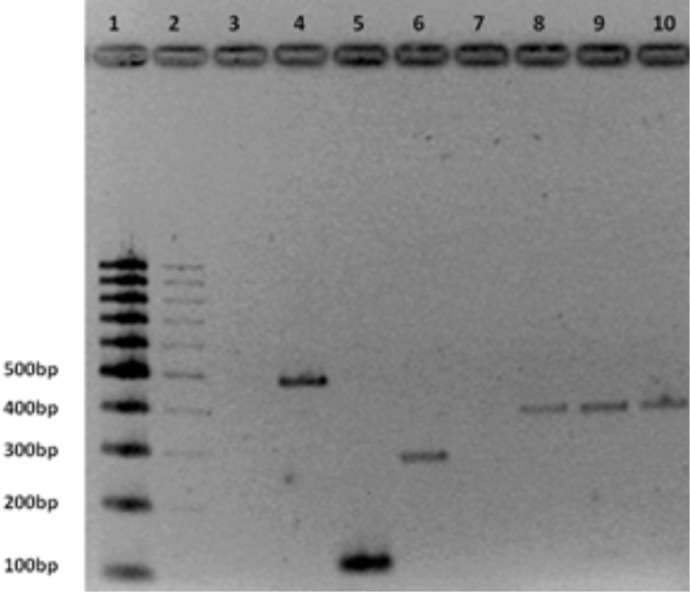
Despite intensive care, the patient deteriorated and became hypotensive, hypothermic and died of hemorrhagic shock, possibly secondary to gastrointestinal bleeding, on the sixth day after the start of symptoms. Further history revealed that all of her family had a dengue-like clinical picture (myalgia, fever and rash). Her mother had symptom onset on December 13th, her father on the 15th and her grandmother on the 20th. There were no recent trips and no contact with natural waters, tick bites or excrement from rats or other animals. Serological tests for yellow fever, hepatitis A and B, leptospirosis and rickettsia were negative. Blood cultures isolated no bacteria. Dengue-specific antibodies were detected in the plasma using an IgM and IgG capture enzyme-linked immunosorbent assay (PanBio, Brisbane, Australia). The sample was collected on the 29th of December 2012, on the fifth day of symptoms. DENV-4 was confirmed by conventional semi-nested reverse transcriptase polymerase chain reaction (semi-nested RT-PCR), which was performed according to LANCIOTTI et al. 13 (Fig. 1).
Fig. 1. Agarose (2%) gel analysis of semi nested RT-PCR products from Dengue virus. Lane 1: 100bpDNA ladder (Bio Labs); Lane 3: negative control; Lane 4: positive control DENV-1 (482bp); Lane 5: positive control DENV-2 (119bp); Lane 6: positive control DENV-3 (290bp); Lane 8 and 9: sample patient (392bp); Lane 10: positive control DENV-4 (392bp).
DISCUSSION
This is the first published case of a confirmed fatal DENV-4 infection in Brazil. Although numerous prospective studies have considered DENV-2 as the dengue virus with the highest risk for severe forms of the disease and death, DENV-4 has also been associated with severe forms of the disease17 and an increasing risk of hemorrhagic manifestations12. Among 29 laboratory-confirmed cases of dengue hemorrhagic fever during an outbreak in Puerto Rico in 1986, DENV-4 was isolated from eight patients7. In India, two cases of severe DENV-4 were reported in 2009 and 2010, one of them fatal4. In Brazil, up to July 2013, there are no published reports of severe dengue cases or death caused by DENV-4.
Serotype is not the only factor that influences dengue severity. Several epidemiologic studies have shown that the risk of severe disease is higher during a secondary dengue virus infection6,10,28. Our patient had positive dengue IgM and IgG antibodies on the fifth day of symptoms. VAZQUEZ et al., found that Panbio Dengue IgM and IgG assays are highly accurate in classifying dengue infection types (primary or secondary infections). According to the author, patients with positive IgM and IgG antibodies could accurately have been classified as having a secondary infection based on the Panbio assay29. A secondary dengue infection may partially explain the severity of the case report here.
Age has also been associated with severity and poor prognosis. Previous studies have pointed out that children younger than 14 years old were at a higher risk of severe dengue9,21 but these findings were not replicated by other researchers. The current WHO30 and the Brazilian guidelines2 for dengue management consider, respectively, infancy and children younger than two years old as groups requiring special care. This topic still remains open and needs further studies to define the exact age group at the highest risk.
Apart from serotype, secondary infection, age and a presence of comorbidities are reported as risk factors for severe dengue. To name a few, sickle cell anemia, autoimmune diseases, asthma, hypertension, uremia and diabetes mellitus have already been linked to poorer outcome and severe forms of the disease3,5,8,16. Their presence renders a more complex dengue management and increases the risk of complications30. Idiopathic thrombocytopenic purpura or immune thrombocytopenic purpura in children is an immune-mediated thrombocytopenia, usually benign, with an average recovery time of three months. A small number of children will have recurrent acute thrombocytopenia24. In 1997, RODRÍGUEZ-ANGULO et al. reported the case of a 35-year-old man with immune thrombocytopenic purpura who developed dengue with hemorrhagic manifestations; the authors suggested that dengue could have exacerbated the chronic immune process producing more severe thrombocytopenia and bleeding25.
In this report, age, the presence of immune thrombocytopenic purpura and the hemorrhagic manifestations (nasal, gingival and intestinal bleeding) might have contributed to the poor prognosis and death. There was no evidence of plasma leakage and hemorrhagic shock was the probable mechanism of death, as indicated by an abrupt drop in the hemoglobin count and massive digestive bleeding. It is difficult to define exactly if dengue infection was the determinant factor for the patient's death. In our opinion, dengue was the underlying cause of death as the infection initiated the train of events leading to the death. So the patient might have died from dengue instead of dying with dengue.
Despite the negative blood cultures, a diagnosis of concomitant bacterial infection cannot be discarded in the present case. LEE et al. have reported concurrent bacteremia in 5.5% of the patients with severe dengue14. According to the author, patients with bacteremia had higher frequencies of gastrointestinal bleeding and altered consciousness, as observed in our report. The leukocytosis may have been secondary to a bacterial infection; nevertheless, leukocytosis can also occur in the presence of hemorrhagic shock as a consequence of stressful stimuli. LEE et al. observed leukocytosis in six of nine patients, who died from dengue, suggesting that massive bleeding and bacteremia were the major causes of leukocytosis in dengue patients15. Regardless of the cause, the authors have suggested that leukocytosis is associated with poor prognosis in dengue patients and this laboratory abnormality could be useful as a warning sign15. The authors still suggest that, especially in dengue patients with altered consciousness and leukocytosis, antibiotics should be empirically initiated until a bacterial infection can be excluded.
In Brazil, the dengue hyperendemicity and recent circulation of DENV-4 may partially explain the increase in severe cases and associated deaths. This case shows that primary care physicians and other healthcare providers should bear in mind that any serotype, including DENV-4, can cause severe forms of disease and lead to hemorrhagic complications and death, mainly when dengue infection is associated with coexisting conditions.
ACKNOWLEDGMENTS
We would like to thank the staff of the Ezequiel Dias Foundation for their laboratorial support and the Minas Gerais Department of Health for epidemiological data. This report was partially supported by the Brazilian National Dengue Control Program.
REFERENCES
- 1.Acosta PO, Maito RM, Granja F, Cordeiro JS, Siqueira T, Cardoso MN, et al. Dengue virus serotype 4, Roraima State, Brazil. Emerg Infect Dis. 2011;17:1979–80. doi: 10.3201/eid1710.110776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasil . Dengue: diagnóstico e manejo clínico - adulto e criança. 4° ed. Brasília: Ministério da Saúde; 2011. Ministério da Saúde. Secretaria de Vigilância em Saúde. Diretoria Técnica de Gestão. [Google Scholar]
- 3.Bravo J, Guzmán MG, Kouri GP. Why dengue haemorrhagic fever in Cuba? 1. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS) Trans R Soc Trop Med Hyg. 1987;81:816–20. doi: 10.1016/0035-9203(87)90041-1. [DOI] [PubMed] [Google Scholar]
- 4.Cecilia D, Kakade MB, Bhagat AB, Vallentyne J, Singh A, Patil JA, et al. Detection of dengue-4 virus in Pune, Western India after an absence of 30 years - its association with two severe cases. Virol J. 2011;8:46. doi: 10.1186/1743-422X-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunha RV, Schatzmayr HG, Miagostovich MP, Barbosa AM, Paiva FG, Miranda RM, et al. Dengue epidemic in the State of Rio Grande do Norte, Brazil, in 1997. Trans R Soc Trop Med Hyg. 1999;93:247–9. doi: 10.1016/s0035-9203(99)90008-1. [DOI] [PubMed] [Google Scholar]
- 6.Díaz A, Kourí G, Guzmán MG, Lobaina L, Bravo J, Ruiz A, et al. Description of the clinical picture of dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) in adults. Bull Pan Am Health Organ. 1988;22:133–44. [PubMed] [Google Scholar]
- 7.Dietz V, Gubler DJ, Ortiz S, Kuno G, Casta-Vélez A, Sather GE, et al. The 1986 dengue and dengue hemorrhagic fever epidemic in Puerto Rico: epidemiologic and clinical observations. P R Health Sci J. 1996;15:201–10. [PubMed] [Google Scholar]
- 8.Figueiredo MA, Rodrigues LC, Barreto ML, Lima JW, Costa MC, Morato V, et al. Allergies and diabetes as risk factors for dengue hemorrhagic fever: results of a case control study. PLoS Negl Trop Dis. 2010;4:e699. doi: 10.1371/journal.pntd.0000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzmán MG, Kouri GP, Bravo J, Valdes L, Vazquez S, Halstead SB. Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis. 2002;6:118–24. doi: 10.1016/s1201-9712(02)90072-x. [DOI] [PubMed] [Google Scholar]
- 10.Guzmán MG, Kourí G, Martínez E, Bravo J, Riverón R, Soler M, et al. Clinical and serologic study of Cuban children with dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) Bull Pan Am Health Organ. 1987;21:270–9. [PubMed] [Google Scholar]
- 11.Halstead SB. Dengue. Lancet. 2007;370:1644–52. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 12.Kumaria R. Correlation of disease spectrum among four dengue serotypes: a five years hospital based study from India. Braz J Infect Dis. 2010;14:141–6. [PubMed] [Google Scholar]
- 13.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–51. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee IK, Liu JW, Yang KD. Clinical characteristics and risk factors for concurrent bacteremia in adults with dengue hemorrhagic fever. Am J Trop Med Hyg. 2005;72:221–6. [PubMed] [Google Scholar]
- 15.Lee IK, Liu JW, Yang KD. Fatal dengue hemorrhagic fever in adults: emphasizing the evolutionary pre-fatal clinical and laboratory manifestations. PLoS Negl Trop Dis. 2012;6:e1532. doi: 10.1371/journal.pntd.0001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MS, Hwang KP, Chen TC, Lu PL, Chen TP. Clinical characteristics of dengue and dengue hemorrhagic fever in a medical center of southern Taiwan during the 2002 epidemic. J Microbiol Immunol Infect. 2006;39:121–9. [PubMed] [Google Scholar]
- 17.Loroño Pino MA, Farfán Ale JA, Rosado Paredes EP, Kuno G, Gubler DJ. Epidemic dengue 4 in the Yucatán, México, 1984. Rev Inst Med Trop Sao Paulo. 1993;35:449–55. doi: 10.1590/s0036-46651993000500011. [DOI] [PubMed] [Google Scholar]
- 18.Naveca FG, Souza VC, Silva GA, Maito RM, Granja F, Siqueira T, et al. Complete genome sequence of a dengue virus serotype 4 strain isolated in Roraima, Brazil. J Virol. 2012;86:1897–8. doi: 10.1128/JVI.06731-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueira RM, Eppinghaus AL. Dengue virus type 4 arrives in the state of Rio de Janeiro: a challenge for epidemiological surveillance and control. Mem Inst Oswaldo Cruz. 2011;106:255–6. doi: 10.1590/s0074-02762011000300001. [DOI] [PubMed] [Google Scholar]
- 20.Nunes MR, Faria NR, Vasconcelos HB, Medeiros DB, Silva de Lima CP, Carvalho VL, et al. Phylogeography of dengue vírus serotype 4, Brazil, 2010-2011. Emerg Infect Dis. 2012;18:1858–64. doi: 10.3201/eid1811.120217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ooi EE, Goh KT, Wang DN. Effect of increasing age on the trend of dengue and dengue hemorrhagic fever in Singapore. Int J Infect Dis. 2003;7:231–2. doi: 10.1016/s1201-9712(03)90057-9. [DOI] [PubMed] [Google Scholar]
- 22.Osanai CH, Travassos da Rosa AP, Tang AT, do Amaral RS, Passos AD, Tauil PL. Surto de dengue em Boa Vista, Roraima. Nota prévia. Rev Inst Med Trop Sao Paulo. 1983;25:53–4. [PubMed] [Google Scholar]
- 23.Rocco IM, Silveira VR, Maeda AY, Silva SJ, Spenassatto C, Bisordi I, et al. First isolation of dengue 4 in the state of São Paulo, Brazil, 2011. Rev Inst Med Trop Sao Paulo. 2012;54:49–51. doi: 10.1590/s0036-46652012000100009. [DOI] [PubMed] [Google Scholar]
- 24.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–93. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez-Angulo EM, Sosa-Muñoz J, García-Miss MR, Farfán-Ale JA, Loroño-Pino MA. A case of autoimmune thrombocytopenic purpura and dengue. Rev Invest Clin. 1997;49:47–9. [PubMed] [Google Scholar]
- 26.Simmons CP, Farrar JJ, Nguyen VV, Wills B. Dengue. N Engl J Med. 2012;366:1423–32. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 27.Temporão JG, Penna GO, Carmo EH, Coelho GE, do Socorro Silva Azevedo R, Teixeira Nunes MR, et al. Dengue virus serotype 4, Roraima State, Brazil. Emerg Infect Dis. 2011;17:938–40. doi: 10.3201/eid1705.101681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, et al. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–72. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 29.Vazquez S, Hafner G, Ruiz D, Calzada N, Guzman MG. Evaluation of immunoglobulin M and G capture enzyme-linked immunosorbent assay Panbio kits for diagnostic dengue infections. J Clin Virol. 2007;39:194–8. doi: 10.1016/j.jcv.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 30.WHO . Geneve: World Health Organization; 2012. Handbook for clinical management of dengue. [Google Scholar]


