Abstract
The immune system has the greatest potential for the specific destruction of tumours with no toxicity to normal tissue and for long-term memory that can prevent cancer recurrence. The last 30 years of immuno-oncology research have provided solid evidence that tumours are recognised by the immune system and their development can be stopped or controlled long term through a process known as immunosurveillance. Tumour specificity of the immune response resides in the recognition of tumour antigens. Viral proteins in tumours caused by viruses and mutated proteins from oncogenes or other genes, as well as nonmutated but abnormally expressed self proteins found on all tumours, have been shown to be good antigens and good targets for immunosurveillance. In many cancers, however, malignant progression is accompanied by profound immune suppression that interferes with an effective antitumour response and tumour elimination. Initially, most of the escape from immunosurveillance was ascribed to changes in the tumour cells themselves (loss of tumour antigens, loss of human leukocyte antigen molecules, loss of sensitivity to complement, or T cell or natural killer (NK) cell lysis), making them a poor target of an immune attack. However, it has become clear that the suppression comes from the ability of tumours to subvert normal immune regulation to their advantage. The tumour microenvironment can prevent the expansion of tumour antigen-specific helper and cytotoxic T cells and instead promote the production of proinflammatory cytokines and other factors, leading to the accumulation of suppressive cell populations that inhibit instead of promote immunity. The best described are regulatory T cells and myeloid-derived suppressor cells. Great conceptual and technical advances in the field of immuno-oncology over the past 30 years have provided us with the knowledge and techniques to develop novel immunotherapeutic approaches for the treatment of cancer. These include methods that enhance tumour immunity by blocking inhibitory pathways and inhibitory cells in the tumour microenvironment (e.g. antibodies against cytotoxic T-lymphocyte-associated antigen-4, programmed death 1 or its ligand programmed death ligand 1, or low-dose chemotherapy). Of equal importance, they include methods that can enhance the specificity of antitumour immunity by inducing the expansion of T cells and antibodies directed to well-defined tumour antigens (e.g. cancer vaccines, potent adjuvants, immunostimulatory cytokines). Even as monotherapies, these approaches are having a substantial impact on the treatment of some patients with advanced, previously untreatable, malignancies. Most exciting of all, these successes provide a rationale to expect that used in various combinations or earlier in disease, current and future immunotherapies may transform cancer treatment, improving a prognosis for many patients.
Keywords: antitumour, immune suppression, immunosurveillance, immunotherapy, tumour microenvironment, tumour-associated antigens
introduction
Most people, and scientists are no exception, measure the passing of time by acknowledging substantial events from the past and looking towards future accomplishments. Using this ‘Janus’ principle, anniversaries that celebrate substantial events or are reminders of what remains to be done can be used to monitor the progress of scientific research. One reminder of the need for progress was recently marked by the 40th anniversary of the US National Cancer Act, a Senate Bill enacted on 23 December 1971 that strengthened the authority of the National Cancer Institute and provided it with new resources to create the National Cancer Program. The US National Cancer Act was prepared and passed in recognition of the serious problem that this lethal disease was posing with its ever-increasing frequency and apparent incurability. The expectation was that an increased understanding of the basic scientific nature of the cancer would be the best road to finding a cure. The bill also recognised the timeliness of this effort, coinciding both with rapid developments in many scientific disciplines and technological advances that appeared close to allowing the biological complexity of the cancer cell to be resolved.
Forty years later, despite many brilliant discoveries around the world in fields as diverse as genetics and molecular biology, virology, chemistry, pharmacology and others, cancer continues to elude cures. However, the pace of scientific discovery and technological developments continues to increase and, as a result, the picture of cancer is being redrawn. Immunology, long considered not to be a critical discipline for understanding cancer, has provided important new clues to cancer biology and for the first time, immune-based therapy is a focus for pharmaceutical companies developing anticancer drugs.
Until recently, investigations into the nature of cancer focused strictly on the cancer cell and on cancer as a genetic disease. This is perfectly illustrated in the widely cited and highly popular paper by Hanahan and Weinberg [1] published in 2000 that, after reviewing a large body of cancer research, proposed six consensus characteristics (hallmarks) that could be used to define a cell as cancerous. The hallmarks comprised the capacity to sustain proliferative signaling, to resist cell death, to induce angiogenesis, to enable replicative immortality, to activate invasion and metastasis, and to avoid growth suppressors.
A decade later, and with increased emphasis on studying cancer as a systemic disease, there is a new understanding that cancer is not one disease, but many different diseases. Therefore, to understand cancer fully, studies must move their focus from the cancer cell to the host and the microenvironment in which the cancer grows; a very important component of which is the immune system. As a result, a new picture of cancer is emerging and, in 2011, four additional hallmarks were proposed. Two of these highlight the newly recognised dual interaction between cancer and the immune system: first, the ability to avoid immune destruction which results in acute inflammation and cancer elimination, and secondly, the potential for chronic inflammation that promotes tumour growth rather than elimination [2, 3].
interactions between the immune system and cancer
Evidence has been accumulating since the middle of the last century, first from animal models and later from studies in cancer patients, that the immune system can recognise and reject tumours. The goal of tumour immunology has been to understand the components of the immune system that are important for tumour immunosurveillance and tumour rejection to understand how, when, and why they fail in cases of clinical disease. Immunotherapy, which involves strengthening the cancer patient's immune system by improving its ability to recognise the tumour or providing a missing immune effector function, is one treatment approach that holds promise of a life-long cure [4].
Studies of cancer–immune system interactions have revealed that every known innate and adaptive immune effector mechanism participates in tumour recognition and control [5]. The first few transformed cells are detected by NK cells through their encounter with specific ligands on tumour cells. This leads to the destruction of some transformed cells and the uptake and processing of their fragments by macrophages and dendritic cells. In turn, these macrophages and dendritic cells are activated to secrete many inflammatory cytokines and present tumour cell-derived molecules to T- and B cells. Activation of T- and B cells leads to the production of additional cytokines that further promote activation of innate immunity and support the expansion and production of tumour-specific T cells and antibodies, respectively. The full power of the adaptive immune system leads to the elimination of remaining tumour cells and, importantly, to the generation of immune memory to specific tumour components that will serve to prevent tumour recurrence.
Effectors of adaptive immunity, such as CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies, specifically target tumour antigens; i.e. molecules expressed in tumour cells, but not in normal cells. Tumour antigens are normal cellular proteins that are abnormally expressed as a result of genetic mutations, quantitative differences in expression, or differences in posttranslational modifications [5]. In tumour types that have a well-documented viral origin, such as cervical cancer, caused by the human papillomavirus [5], or hepatocellular carcinoma caused by the hepatitis B virus [6], viral proteins can also serve as tumour antigens and targets for antitumour immune response [7].
The first indication that tumours carried molecules distinct from those on the normal cell of origin was derived from immunising mice with human tumours and selecting antibodies that recognised human tumour cells but not their normal counterparts. The major question was whether some, or all, of these molecules would also be recognised by the human immune system. 2011 was an important anniversary for human tumour immunology, marking 20 years since the publication by van der Bruggen et al. [8] that described the cloning of MAGE-1, a gene that encodes a human melanoma antigen recognised by patient's antitumour T cells. This was not a mutant protein; its recognition by the immune system was due to the fact that it was only expressed by transformed, malignant cells and, with the exception of testicular germ cells, was not expressed in normal adult tissue. Many similar discoveries followed, with each new molecule providing a better understanding of what might be good targets for different forms of cancer immunotherapy. Tumour antigens have been tested as vaccines, as targets for monoclonal antibodies, and as targets for adoptively transferred cytotoxic T cells. There is a wealth of publications from preclinical studies targeting these antigens and results from phase I/II clinical trials. Recently, these studies were critically reviewed and a list of tumour antigens with the largest body of available data compiled [9]. The goal was to encourage faster progress in the design, testing, and approval of immunotherapeutic reagents that incorporate or target the most promising antigens.
Antitumour immune responses in animal models and cancer patients have contributed to the resurgence of the immunosurveillance theory; albeit one that has been modified to encompass different observed outcomes. Instead of defining immunosurveillance as the process by which cancer is recognised and eliminated and a diagnosis of cancer to represent the failure of this process, it is now recognised that in different individuals and with different cancers, the process can have at least three different but related outcomes: elimination, equilibrium, and escape [10]. A highly immunogenic tumour in a highly immunocompetent individual will result in optimal stimulation of the innate immune system leading to the production of highly immunostimulatory cytokines, acute inflammation, activation of a large number of T- and B cells, and prompt elimination of the arising tumour. With a less immunocompetent individual and/or less immunogenic tumour, however, there might not be a complete elimination leading to the survival of some cancer cells that nevertheless remain under immunosurveillance. Over a prolonged period of time, the slow growth of the tumour would be accompanied by repeated activation of the immune system and elimination of some tumour cells, followed by further cycles of tumour regrowth and immune-mediated destruction. This period, when the tumour is present but not yet a clinical disease, is known as equilibrium. The equilibrium phase could be life-long, thus mimicking elimination, or be disturbed by changes in the tumour that allow it to avoid immunosurveillance or changes in the immune system that weaken its capacity for tumour surveillance. Either change ultimately leads to tumour escape (Figure 1).
Figure 1.
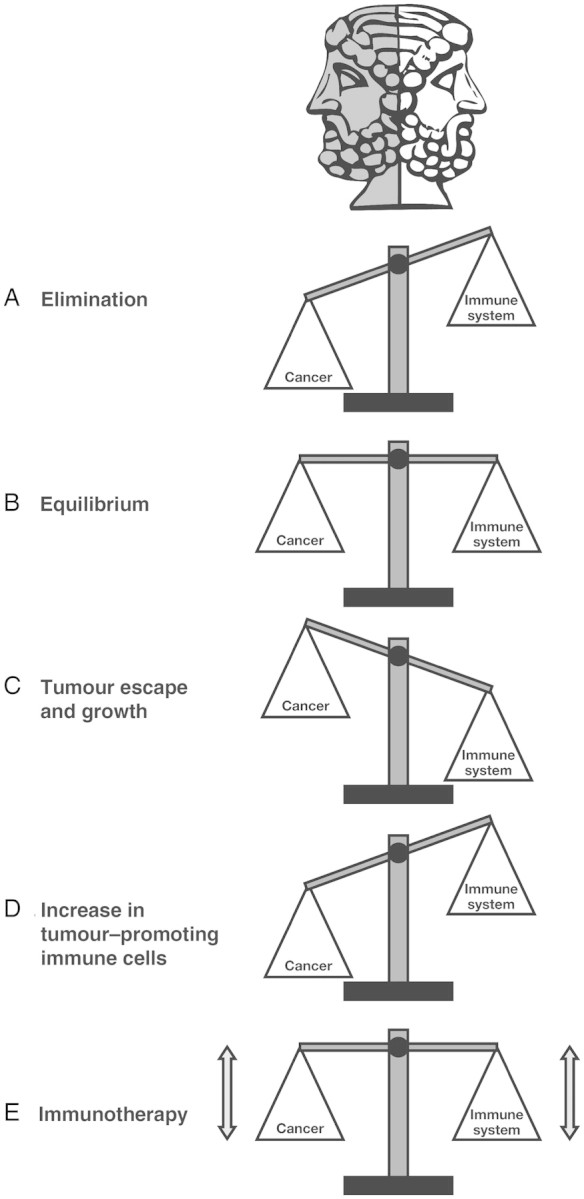
Janus was the Roman god of beginnings and transitions, depicted as two-faced since he looks to the future and the past. In the same way that the Janus principle can be used to illustrate the past accomplishments and future opportunities for scientific progress, the two faces could also be used to represent two sides of the same story; in this case, immune function/tumour rejection and immune dysfunction/tumour promotion. Via the process of immunosurveillance, the immune system can specifically identify and eliminate tumour cells on the basis of their expression of specific antigens (A). However, in cases where the immune system is not able to completely eliminate the cancer, a state of equilibrium develops whereby the tumour does not progress or further metastasize (B). Eventually, if the immune response fails to completely eliminate the tumour, cancer cells that can resist, avoid, or suppress the antitumour immune response are selected, leading to the tumour escape and a progressively growing tumour (C). In addition, infiltration of tumours by inflammatory immune cells can result in a state of chronic inflammation that maintains and promotes cancer progression and suppresses the innate anticancer immune response (D). The aim of immunotherapy is to modulate tumour immunity to change the ongoing immune response from tumour-promoting to tumour-rejecting, thus providing durable and adaptable cancer control (E).
To date, most studies of tumour/immune system interactions have been performed after cancer has been diagnosed, i.e. in the escape phase of immunosurveillance. This particular phase is characterised by an increase in previously unknown immunosuppressive cells, such as regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC), immunosuppressive cytokines derived from Treg, MDSC, and tumour cells and poorly functional effector T cells expressing molecules capable of preventing T-cell activation [11–13].
immunotherapy: old and new
In the past, immunotherapy was referred to as ‘passive’ (e.g. the infusion of preformed immune effectors, such as antibodies, cytokines, or activated T cells, NK cells, or lymphokine-activated killer cells), presumably acting directly on the tumour and independent of the immune system or ‘active’ (e.g. vaccines), designed to activate and therefore be dependent on the patient's immune system.
However, with increased understanding of the importance of multiple immune effector mechanisms for tumour elimination and of the immunosuppressive forces that influence these mechanisms in the tumour microenvironment, it has since become clear that both passive and active immunotherapies depend on the patient's immune system for long-term tumour control or complete tumour elimination.
By directly targeting specific antigens expressed by cancer cells, anticancer monoclonal antibodies are a well-established class of immunotherapeutic agent; more than a dozen of which have been approved by the Food and Drug Administration as standard treatment of several different cancers, including trastuzumab for breast cancer and retuximab for B-cell lymphoma [4]. Although the mechanism of their direct antitumour action has been well studied and is clearly responsible for transient remissions in patients receiving this therapy, cure rates are still very low. The potential of these antibodies is drastically undermined by their administration relatively late in the disease course, when the patient's immune system is largely compromised. Under more optimal conditions, antibody treatment might result not only in the direct cytostatic or cytotoxic effect on the tumour cell, but also in the loading of antibody-bound tumour antigens onto antigen presenting cells (APC) in the tumour microenvironment. The resultant cross-presentation to antitumour T- and B cells could result in additional antibodies to these antigens being produced, and propagation of the immune response at the tumour site would maintain tumour elimination long after the infused monoclonal antibody is gone. Not only would the response change from a monoclonal antibody against a single epitope to a polyclonal response to multiple epitopes, thus avoiding antigen-negative tumour escape, but the effector T-cell response would also generate memory.
The same scenario could be predicted for adoptively transferred T cells. Unlike antibodies, transferred T cells persist longer and may provide a memory response [14]; however, as long as the memory response is restricted to one clone, or a limited number of clones, then antigen-negative tumours will be able to escape. In addition, cancer vaccines encounter large numbers of immunosuppressive Treg and MDSC in circulation, as well as immunosuppressive cell-derived soluble products that flood the lymph nodes, preventing maturation of APCs and activation of T cells. Even when vaccines are delivered in the context of ex vivo matured and activated dendritic cells, their ability to activate T cells is compromised by the high-level expression of various molecules on T cells that block this process.
The scenarios proposed above present a rather bleak picture of the potential of immunotherapy to achieve the cure for cancer that has eluded standard therapy [15]. Interestingly, failures of some standard therapies are beginning to be ascribed to their inability to activate the patient's immune system [16]. However, rather than seeing the picture as a deterrent, it should be considered as a road map, providing at least two major directions for new developments in immunotherapy.
The first direction is to continue using the old classes of immunotherapy that target the cancer directly, but to use them in combination with therapies that target the immune system in the tumour microenvironment, such as cytokines, suppressors of Treg or MDSC activity, or antibodies that modulate T-cell activity. The recently approved antibody, ipilimumab, which acts to sustain cytotoxic T-cell activity by augmenting T-cell activation and proliferation, is one example of such an immunomodulatory agent [17].
The other direction is to use immunotherapies, both old and new, for preventing cancer in individuals at high risk [18]. Studies of the tumour microenvironment are providing information about immunosurveillance of tumours from early premalignant lesions to more advanced dysplastic lesions to cancer. At each step, tumour-derived and immune system-derived components have a unique composition that will have distinct effects on immunotherapy. Because these premalignant microenvironments are less developed and immunosuppression is less entrenched, it should be easier to modulate towards the elimination of abnormal cells.
The lessons learnt from past accomplishments suggest that in the future, well-designed immunotherapies, administered at the right stage of tumour progression, have the potential to significantly change the ongoing immune response in the tumour microenvironment from tumour-promoting to tumour-rejecting (Figure 1).
disclosure
The author declares no conflicts of interest.
references
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Chow MT, Moller A, Smyth MJ. Inflammation and immune surveillance in cancer. Semin Cancer Biol. 2011 doi: 10.1016/j.semcancer.2011.12.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 5.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 6.Chang MH. Cancer prevention by vaccination against hepatitis B. Recent Results Cancer Res. 2009;181:85–94. doi: 10.1007/978-3-540-69297-3_10. [DOI] [PubMed] [Google Scholar]
- 7.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 8.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 9.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 11.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 12.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox BA, Schendel DJ, Butterfield LH, et al. Defining the critical hurdles in cancer immunotherapy. J Transl Med. 2011;9:214. doi: 10.1186/1479-5876-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37:473–484. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lollini PL, Cavallo F, Nanni P, et al. Vaccines for tumour prevention. Nat Rev Cancer. 2006;6:204–216. doi: 10.1038/nrc1815. [DOI] [PubMed] [Google Scholar]


