Abstract
Background:
The effect of high-legume hypocaloric diet on metabolic features in women is unclear. This study provided an opportunity to find effects of high-legume diet on metabolic features in women who consumed high legumes at pre-study period.
Methods:
In this randomized controlled trial after 2 weeks of a run-in period on an isocaloric diet, 42 premenopausal women with central obesity were randomly assigned into two groups: (1) Hypocaloric diet enriched in legumes (HDEL) and (2) hypocaloric diet without legumes (HDWL) for 6 weeks. The following variables were assessed before intervention and 3 and 6 weeks after its beginning: Waist circumference (WC), systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting serum concentrations of triglyceride (TG), high density lipoprotein cholesterol, fasting blood sugar (FBS), insulin, homeostasis model of insulin resistance (HOMA-IR), alanine aminotransferase (ALT) and aspartate aminotransferase (AST). We used multifactor model of nested multivariate analysis of variance repeated measurements and t-test for statistical analysis.
Results:
HDEL and HDWL significantly reduced the WC. HDEL significantly reduced the SBP and TG. Both HDEL and HDWL significantly increased fasting concentration of insulin and HOMA-IR after 3 weeks, but their significant effects on insulin disappeared after 6 weeks and HDEL returned HOMA-IR to basal levels in the subsequent 3 weeks. In HDEL group percent of decrease in AST and ALT between 3rd and 6th weeks was significant. In HDWL group percent of increase in SBP, DBP, FBS and TG between 3rd and 6th weeks was significant.
Conclusions:
The study indicated beneficial effects of hypocaloric legumes on metabolic features.
Keywords: Central obesity, hypocaloric diet, legumes, metabolic syndrome, premenopausal women
INTRODUCTION
Obesity is epidemic in the world[1] and its prevalence has increased significantly.[2] Obesity, especially central obesity is associated with excess deaths in the population.[3] The prevalence of central obesity in Iran is 53.6%.[4] Previous studies provided strong evidence that the metabolic problems of central obesity such as insulin resistance, hypertension, hypertriglyceridemia, low high density lipoprotein cholesterol (HDL-C) and steatosis are marked in South Asians including Iranians at lower amounts of total body fat compared to whites.[5] These differences can be interpreted by high amount of central adipose tissue in South Asians.[5] Healthy foods are protective factor for metabolic syndrome.[6] Legumes are one of the healthy and inexpensive foods. They are high in phytochemicals, fibre, protein, minerals and vitamins. Most of the researches that have investigated the effect of legume consumption on metabolic features studied soybeans rather than non-soybean legumes. The effects of soy bean on metabolic features are well-known.[7] In Iran low amounts of soy beans are consumed, while non-soy legume such as white, red and wax beans, chickpeas, cowpea, lentils and split peas are conventional foods. Anderson and Major in 2002 performed a meta-analysis on secondary outcomes of eleven clinical trials and showed consumption of non-soy legumes was associated with increasing of HDL-C and decreasing of triglyceride (TG) and weight.[8] After Anderson and Major meta-analysis several randomized controlled trials (RCTs) were studied the effects of legumes on metabolic features. Zhang et al. tested the effects of legume on biomarkers of insulin resistance among males in isocaloric and hypocaloric diets. Despite isocaloric diet, in hypocaloric period of intervention, mean body weight, body mass index (BMI), Levels of serum TG, C-peptide and fasting plasma glucose, insulin and C-reactive protein were significantly reduced.[9] In Hermsdorff study, systolic blood pressure (SBP) was improved only with the legume-based hypocaloric diet compared to calorie-restricted legume-free diet.[10] Inconsistent with Zhang et al. study, Crujeiras et al. and Hartman et al. showed baseline and endpoint values of insulin, C-peptide and glucose were not statistically different after following hypocaloric and isocaloric diets with or without legumes.[11,12] Even Hartman et al. showed high-legume diet increased fasting blood sugar (FBS) compared to legume-less diet.[12] Due to paradoxical results this study was planned. The present research takes advantages of higher consumption of non-soy legume among participants at pre-study period in comparison with other similar researches.[13,14,15,16] In Iran, the eating of non-soy legume is more common than western countries. The mean consumption of non-soy legume among Iranians is nearly 3 servings/week compared to 2 servings/week in US and Europe.[13,14,15,16] The average intake of non-soy legume in subjects of current study compared with previous trials was approximately triple.[10,12] To the best of our knowledge, this is the first study that investigates the role of high-legume hypocaloric diet on metabolic features exclusively among women.
METHODS
Study design and participants
The study was approved by the Ethics Committee of Tabriz University of Medical Sciences (Tabriz, Iran) and registered at www.irct.ir (irct ID: irct138712101720N1). Written informed consent was achieved from all selected participants.
The sample size for each intervention group was calculated regarding to the studies conducted on women with central obesity.[17,18] With a 1 – α=95% and 1 – β=95%, the maximum sample size was obtained from waist circumference (WC) marker via the formula:
n = aσ2 ϕ2 / ∑ti2 = 16.49 16
in which a = 4, σ2 = 59.9, ϕ2 (the indicator curve) =2.5 and ∑ti2 = 36.46.
Finally, samples for each group were calculated to be 16 participants.
The study was a RCT with a 2 week pre-trial period and a 6 week trial period. After advertising in local newspapers, 257 pre-menopausal women were eligible to enter the study.
Inclusion criteria were: Pre-menopausal women aged 20-50 years, WC > 88 cm, no involvement in weight-loss programs and maintenance of a stable weight during the previous 6 months (±2 kg).
Exclusion criteria were: Treatment with insulin or oral hypoglycemic agents, anti-hypertensive drugs or anti-lipemic drugs; any secondary cause of hypertension or hyperglycemia; consumption of mineral or vitamin supplements or antacids containing calcium or magnesium; untreated hypothyroidism; psychiatric disorders; cancer; systemic, hepatic, renal, pulmonary, or cardiovascular disease; infectious or inflammatory disease; alcoholism; smoking; and legume intolerance. Figure 1 shows the flowchart of the participants of the study.
Figure 1.
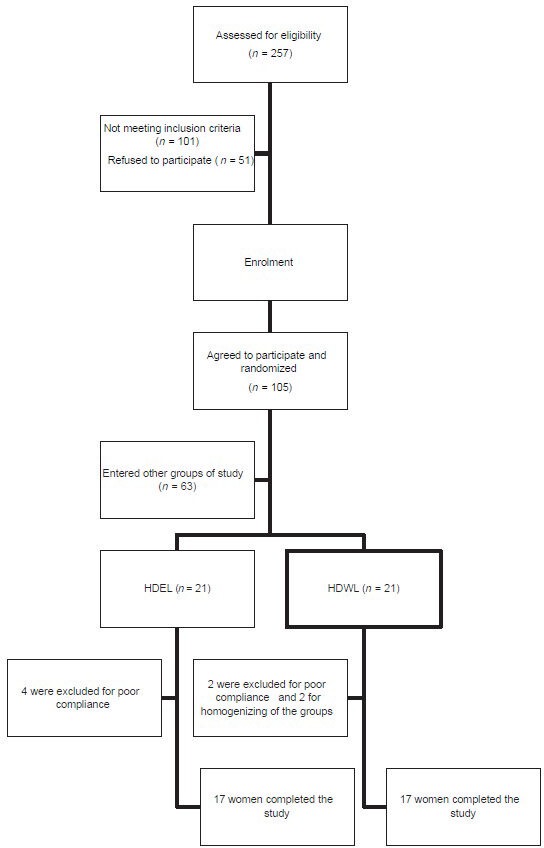
Flowchart for enrolment of participants. HDEL=Hypocaloric diet enriched in legumes, HDWL=Hypocaloric diet without legumes
Diets
The energetic needs were calculated individually by the formula from the Food and Nutrition Board of the Institute of Medicine.[19] The subjects consumed an isocaloric diet for 2 weeks in the run-in period. In the intervention period, intervention group ate hypocaloric diet enriched in legumes (HDEL) (which included 1 cup/day of cooked non-soy legumes including white, red and wax beans, chickpeas, cowpea, lentils and split peas instead of meat) and control group ate hypocaloric diet without legumes (HDWL). Participants in HDWL group increased consumption of animal proteins (meat, poultry, fish, egg or cheese) as much as 2 servings/day (60 g) instead of legumes and reduced consumption of fats as much as 2 servings/day to compensate increased intake of animal fats. The amount of cereals in daily diet of both intervention groups was equivalent but participants in HDEL group were prescribed to consume 2 servings of cereals as legumes. The macronutrient content of both diets was 55% carbohydrate, 30% fat and 15% protein. In the intervention period, all of subjects in both groups were prescribed a hypocaloric diet (500-kcal less than their isocaloric needs). Diets were given individually. Participants were being visited every week for 20-30 min. The nutritionist explained the advantages of diets for the participants and trained participants how to write “food diaries.” Each participant had to write her 3-day physical-activity and diet records before the run-in period as well as before, in the middle and at the end of the intervention period. Participant compliance was evaluated by weekly visits and evaluating the 3-day food diaries. Subjects who did not complete ≥80% of the planned diets for 2 consecutive weeks were excluded from the study (n = 6).
Study procedures
We planned a run-in period to getting detailed information about the study population and to standardize macronutrient consumption. The true isocaloric needs of some of the participants were different from the amount calculated in the formula from the Food and Nutrition Board at the Institute of Medicine. Among individuals eligible to enter the study, only those who maintained their weight at the end of the run-in period were chosen. After the run-in period on an isocaloric diet for 2 weeks, subjects were randomly allocated to two intervention groups for 6 weeks: (1) HDEL and (2) HDWL. For allocation of the participants, a computer-generated list of random numbers was used.
We repeated random allocation several times to obtain most homogenous groups. The dependent variables were measured before, in the middle and at the end of the intervention. Subjects were asked not to vary their common physical activities during the study.
Measurements
All measurements were carried out by the unchanged investigator and the unchanged tool in the first and follow-up evaluations. WC was measured (to the nearest 0.1 cm) at the narrowest point without pressure to the body surface by the light clothing using a tape measure.
After a 12-h fast, blood samples were taken. Samples were centrifuged at 500 ×g for 10 min at 4°C and the serum separated. All parameters except insulin were measured on the day of blood collection. Serum was frozen at –80°C until it was analyzed for insulin.
Levels of fasting blood glucose (FBG), HDL-C and TG were measured enzymatically (ParsAzmoun, Tehran, Iran). Plasma levels of insulin were measured by a human insulin enzyme-linked immunosorbent assay test kit[20] (Diaplus, San Francisco, CA, USA) according to manufacturer instructions. Insulin resistance was calculated on the basis of the homeostasis model assessment of insulin resistance (HOMA-IR).[21] Both alanine aminotransferase (ALT) and aspartate aminotransferases (AST) were measured by International Federation of Clinical Chemistry method without adding prydoxal phosphate (Pars Azmoon kit, Tehran, Iran).
Inter- and intra-assay coefficients of variation were 1.19 and 1.28% for FBG, 1.8 and 0.73% for HDL, 1.04 and 1.47% for TG, 8 and 8% for insulin, 3.08 and 6.22% for ALT and 4.40 and 3.25% for AST, respectively.
Confounding factors was obtained by questionnaires. According to this information “Chronic dieters” were distributed among the groups. Participants were classified into three levels of education (did not obtain a high-school diploma, obtained a high-school diploma and university graduates); income (no income, < US$350/month and > US$350/month); family income (< US$350/month, US$350-700/month and > US$700/month); and overweight subjects and the metabolic syndrome in the family (any relative, first-degree relative and second-degree relative). Overweight was defined as (BMI) >25 kg/m2. Metabolic syndrome was defined according to criteria set by the Adult Treatment Panel III.[22]
Statistical analysis
Two ways were applied for statistical analyses. In the first way, we used multifactor model of nested multivariate analysis of variance (MANOVA) repeated measurements by Minitab Package (v13) as followed:
Variation of dependent variables = Intra-individual variation + variation because of hypocaloric diet or time + variation because of legumes or diet (time) + variation because of legumes * time + error.
In this method, we also controlled the effect of WC:
Variation of dependent variables = Intra-individual variation + variation because of hypocaloric diet or time + variation because of legumes or diet (time) + variation because of legumes * time + error + (B1 * WC).
In the model described above, “Error” represents the random changes during the study. “B1” is regression co-efficient. “B1 * WC” represents the effect of WC on dependent variables. In this model, the concurrency of analyses instead of multiple comparisons minimized the probability of false-positive results.
In second way, we used a paired t-test or its non-parametric equivalent (Wilcoxon test) for comparing the amount of variables in different times within groups. Furthermore, we used an independent t-test or the Mann–Whitney U-test for comparing the percentage changes in variables during different times (T3–T1, T2–T1 and T3–T2) in the HDWL group with a change in the HDEL group. Histograms were used to recognize normal distributions. These analyzes were conducted using SPSS 13.0 (SPSS, Chicago, IL, USA).
We used Chi-square test and independent t-test to find significant differences in baseline values among two intervention groups. For appropriate variables, we merged subclasses of variables and then used the Chi-square test. Two-tailed P < 0.05 was considered to be significant. All values expressed as means ± standard error.
RESULTS
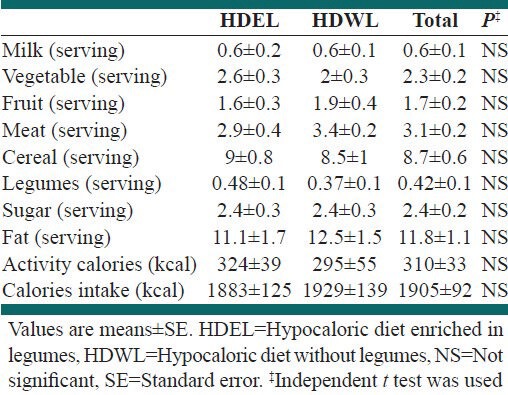
The general characteristics of the groups are shown in Table 1. Food intake of the groups, calorie intake and calories expended in activities before the run-in period are shown in Table 2. The mean consumption of fruit and milk in both groups was low. There were no differences in food intake between the groups before the run-in period.
Table 1.
Baseline characteristics of the groups
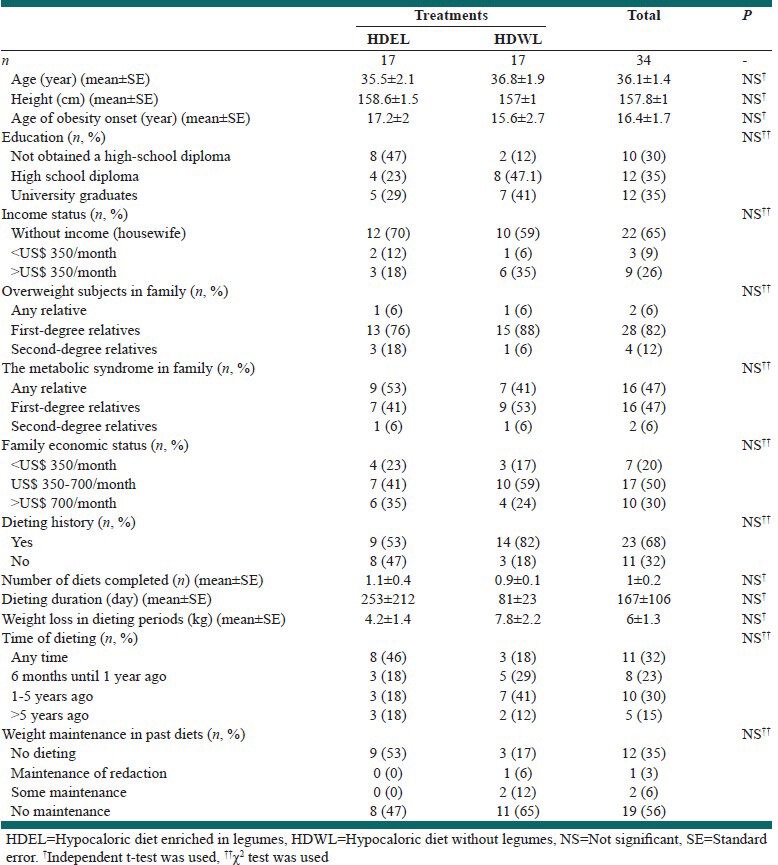
Table 2.
Intake of food, calorie intake and calories expended in activity before the run-in period
The effect of interventions on metabolic features using multifactor model of nested MANOVA repeated measurements are outlined in Table 3. There were no significant differences among basal (before intervention) measurements in two groups [not shown in Table 3].
Table 3.
Effect of interventions on metabolic features by nested MANOVA repeated measurements of multi-factor model
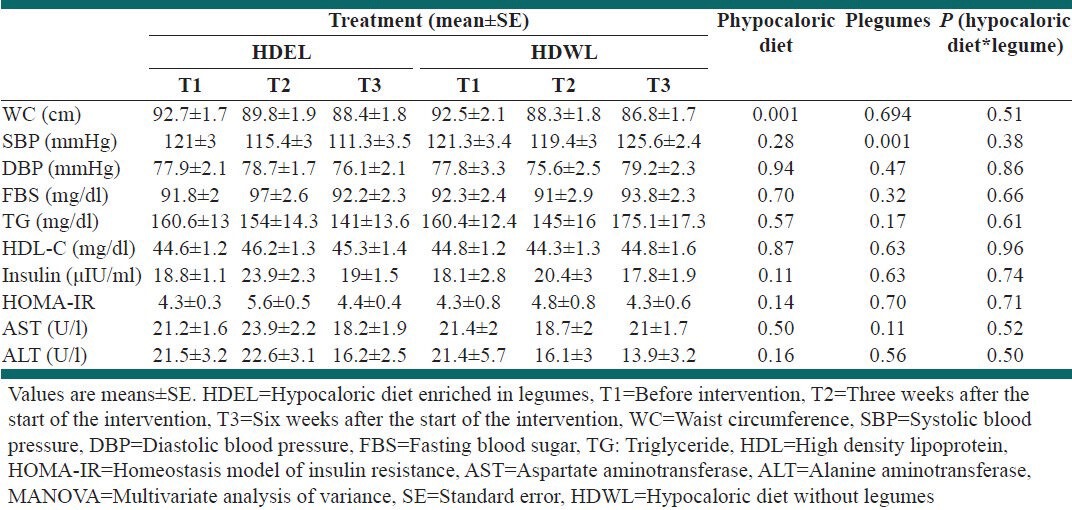
After 6 weeks of intervention the following results were obtained by repeated measurements of MANOVA [Table 3]: (1) HDEL and HDWL significantly reduced the WC (P = 0.001). (2) HDEL significantly reduced the SBP (P = 0.001). This significant effect maintained after adjusting for weight and/or waist. (3) There was not shown any significant effects on diastolic blood pressure (DBP), FBS, TG, HDL-C, Insulin, HOMA-IR, AST and ALT in this model.
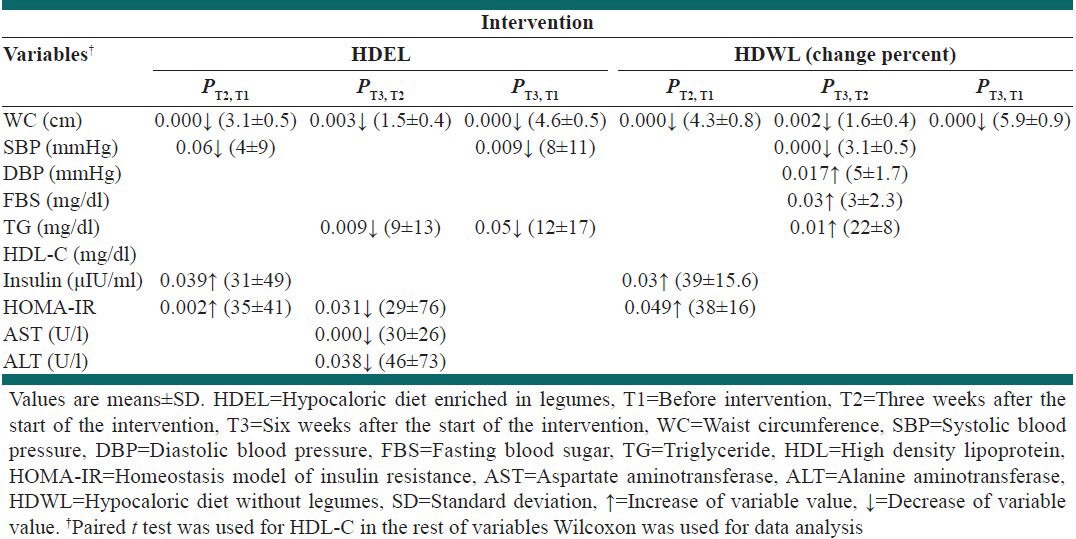
With Wilcoxon or paired t-test, the following results were obtained (paired t-test was used only about HDL-C) [Table 4]: (1) HDEL and HDWL reduced WC in 6 weeks (4.6%, P = 0.000; 5.9%, P = 0.000, respectively); (2) HDEL decreased SBP after 3 and 6 weeks (4%, P = 0.06; 8%, P = 0.009); (3) In HDWL group percent of increase in SBP, DBP, FBS and TG between 3rd and 6th weeks was significant (6.2%, P = 0.005; 5%, P = 0.017; 3%, P = 0.03; 22%, P = 0.01); (4) In HDEL group percent of decrease in TG between 3rd and 6th weeks and 1st and 6th weeks was significant (9%, P = 0.009; 12%, P = 0.05); (5) Both HDEL and HDWL significantly increased fasting concentration of insulin after 3 weeks (HDEL: 31%, P = 0.039; HDWL: 39%, P = 0.03), but their significant effects disappeared after 6 weeks.; (6) Both HDEL and HDWL significantly increased HOMA-IR in the 1st 3 weeks (HDEL: 35%, P = 0.002; HDWL: 38%, P = 0.049) but HDEL returned it to basal levels in the subsequent 3 weeks (29%, P = 0.031); (7) In HDEL group percent of decrease in AST and ALT between 3rd and 6th weeks was significant (AST: 30%, P = 0.000; ALT: 46%, P = 0.038).
Table 4.
Effect of interventions on metabolic features with in groups
With an independent t-test or Mann–Whitney U-test we obtained the following results.(Mann–Whitney U-test was used for comparing the percentage changes in WC and AST during T3 and T1, the percentage changes in SBP during T2 and T1 and the percentage changes in TG and AST during T3 and T2. Independent t-test was used in the rest of variables): (1) Both HDEL and HDWL increased HOMA-IR in the 1st 3 weeks of intervention. Percent of HOMA-IR change in the 1st 3 weeks of intervention in HDWL group was marginally (P = 0.072) less than HDEL group. (2) HDEL increased HDL-C and HDWL decreased it after 3 weeks, the HDEL group had a marginally increased HDL-C compared with that in the HDWL group (P = 0.058). (3) HDEL decreased TG and HDWL increased it after 6 weeks, the HDEL group had a significantly decreased TG compared with that in the HDWL group (P = 0.021). This difference was made in second half of the study. (4) HDEL decreased SBP and HDWL increased it after 6 weeks, the HDEL group had a significantly decreased SBP compared with that in the HDWL group (P = 0.003). This difference was made in second half of the study.
DISCUSSION
This clinical trial explored the effects of high-legume hypocaloric diet on metabolic features among a population of women with central obesity. Men and women have different responses to some exposures on cardiometabolic risk factors due to their physiological differences in sex hormones. Previous RCTs were conducted on men or men/women participants. This study was the first exclusively female study of this type. In this study legumes significantly reduced the SBP. This finding was shown in Hermsdorff and Papanikolaou study, too.[10,23] Legumes are commonly rich in fiber, calcium, potassium and magnesium and low in sodium.[24] Meta-analysis showed that increasing fiber consumption as much as approximately 17 g/day will decrease SBP by 1.15 mmHg and DBP by 1.65 mmHg[25] The mechanisms contributed to hypotensive effects of high-fiber foods are uncertain and several factors may be involved.[26] In epidemiologic studies, High consumption of calcium, potassium and magnesium and low consumption of sodium have been associated with reduced metabolic risks.[27,28] The Dietary Approaches to Stop Hypertension (DASH) clinical trial was a milestone study in treatment and prevention of hypertension.[29] The diet was rich in legumes, vegetables, fruits, vegetables and whole grains. The DASH diet significantly reduced blood pressure.[29] The follow-up clinical trial studied the effects of sodium intake as part of the DASH diet and showed a low sodium intake as part of the DASH diet decreased SBP by 8.9 mmHg and DBP by 4.5 mmHg.[30] These studies suggest that high consumption of legumes may have a beneficial effect on blood pressure.
In this study legumes had beneficial effects on TG compared to legume-less diet in consistent with Anderson and Major meta-analysis and Zhang et al. study.[8,9] Probably legumes decreased TG due to high fiber and specific protein content. Sandström et al. indicated pea fiber reduced fasting and postprandial serum TG concentrations in healthy people.[31] Lasekan et al. showed pea proteins significantly decreased blood TG in rats.[32] In Boualga et al. study proteins of lentil and chickpea reduced TG more than casein in growing rats.[33]
In HDEL group percent of decrease in AST and ALT between 3rd and 6th weeks was significant. No effect of legumes in the 1st 3 weeks of the study and its beneficial effects in subsequent 3 weeks represent probability of beneficial effects of legumes on hepatic function in long period. Recent studies have indicated that liver enzymes are correlated with insulin resistance and cardiovascular diseases.[34] Due to blood liver enzymes levels as a new component of metabolic syndrome and their association with insulin resistance, our study provides new evidence for the benefits of consuming a specific food, like legumes, for women with central obesity.
In consistent with most of previous studies in healthy or obese participants, legumes had not beneficial effects on FBS, insulin and HOMA-IR.[10,35,36,37,38,39,40] However, studies on diabetic or insulin resistant participants showed beneficial effects of legumes on insulin resistance parameters.[9,41] Another reason contributed to beneficial effects of legumes on insulin resistant parameters in Zhang et al. study can be the high amount of legumes in their hypocaloric diet.[9] The amount of legumes in legumes enriched hypocaloric diet of Zhang et al. study (3.8 servings/day) was higher than all of the previous RCTs. Furthermore, we showed in HDWL group percent of increase in FBS between 3rd and 6th weeks were significant and after enhancement of HOMA-IR in 1st 3 weeks in both groups only HDEL returned it to basal levels in the subsequent 3 weeks. These results represent probability of beneficial effects of legumes on insulin resistance in long period.
HDEL and HDWL significantly reduced the WC and legumes had no advantage in 3 and 6 weeks.
In HDWL group percent of increase in SBP, DBP, FBS and TG between 3rd and 6th weeks was significant. These findings confirmed beneficial effects of legumes on metabolic features and showed that omitting of legumes from diet may have harmful effects on metabolic features and increase the cardiovascular risk. In HDWL group participants stopped their usual intake of legumes and replaced it and some of diet liquid fats with animal proteins and fats. Probably the effect of this change on increasing TG is more than lowering effect of hipocaloric diet.
In our study, legumes marginally increased HDL-C compared to legume-less diet only in 1st 3 weeks of the intervention. Hirshberg et al. exhibited a small positive correlation between pulses intake and HDL-C.[42] Short-term effect of legumes on HDL-C levels can be contributed to their specific proteins. Lasekan et al. represented in rats that pea proteins significantly increased HDL-in 4 weeks.[32] In consistent with Zahradka study,[36] HDEL had no advantage in increasing HDL-C compared to HDWL in 6 weeks but Abet et al. showed legumes reduced HDL-C.[35] Probably inconsistent result of Abet et al. study was created because of different amount of fat in their interventional diet.
In this study, the mean consumption of legume in pre-study period was 2.94 servings/week compared to 1serving/week in Hermsdorff study[10] and 1.3 servings/week in Zhang et al. and Hartman et al. study.[9,12] In fact, the pre-study consumption of legume in our study was almost 3 times more than pre-study legume consumption in previous RCTs. In Crujerias and Hermsdorff studies the legumes consumption even after intervention reached to the pre-study level of the current study.[10,11] The beneficial effects of different doses of legumes such as 4 serving/week in Hermsdroff study,[10] 2 servings/d in current study, 3 servings/d in Hartman et al. and Zhang et al. studies,[9,12] on BP, TG, HDL-C and liver enzymes and more beneficial effects of 3.8 servings/d in Zhang et al. study[9] not only on motioned metabolic features but also on C-peptide and fasting plasma glucose and insulin indicates probably there are liner relationship between the legumes consumption and SBP and fasting blood TG, HDL-C, liver enzymes, glucose, insulin and C-peptide. Legumes beneficial effects on these parameters did not reach to a plateau.
To the best of our knowledge, this is the first research studied a high-legume hypocaloric diet exclusively in women. The advantage of current research was the particular population of our research which their mean usual intake of non-soy legumes was nearly threefold of usual intakes in preceding RCTs.[10,12] This study offered an opportunity to discover the effects of high-legume diet on metabolic features in subjects with high basal intake of legumes. The present study had two limitations: First, The subjects’ explanations for leaving the research were not assessed in the current study. Second, Intervention diets had inevitable differences in animal protein and fat content in addition to legumes content and some of observed results could be related to this diversity.
CONCLUSIONS
HDEL significantly reduced the SBP and TG. Both HDEL and HDWL significantly increased fasting concentration of insulin and HOMA-IR after 3 weeks, but their significant effects on insulin disappeared after 6 weeks and HDEL returned HOMA-IR to baseline levels in the subsequent 3 weeks. In HDEL group percent of decrease in AST and ALT between 3rd and 6th weeks was significant. In HDWL group percent of increase in SBP, DBP, FBS and TG between 3rd and 6th weeks was significant. The study indicated beneficial effects of hypocaloric diets on central obesity and legumes on blood pressure, metabolic features and hepatic function. Long-term studies for approving these results are necessary.
ACKNOWLEDGMENTS
The authors would like to thank Tabriz University of medical sciences, Nutrition Research Center and Liver and Gastrointestinal Disease Research Center for their support and also thank the participants of this study for their enthusiastic support.
Footnotes
Source of Support: This work was supported by Tabriz University of medical sciences (Grant no. 5.4.8491), Nutrition Research Center (Grant no. 5.71.2419) and Liver and Gastrointestinal Disease Research Center (Grant no. GT-660)
Conflict of Interest: None declared.
REFERENCES
- 1.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 2.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 3.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esteghamati A, Meysamie A, Khalilzadeh O, Rashidi A, Haghazali M, Asgari F, et al. Third national Surveillance of Risk Factors of Non-Communicable Diseases (SuRFNCD-2007) in Iran: Methods and results on prevalence of diabetes, hypertension, obesity, central obesity, and dyslipidemia. BMC Public Health. 2009;9:167. doi: 10.1186/1471-2458-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol. 2007;36:220–5. doi: 10.1093/ije/dyl245. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, et al. American Heart Association Nutrition Committee. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 7.Omoni AO, Aluko RE. Soybean foods and their benefits: Potential mechanisms of action. Nutr Rev. 2005;63:272–83. doi: 10.1111/j.1753-4887.2005.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JW, Major AW. Pulses and lipaemia, short- and long-term effect: Potential in the prevention of cardiovascular disease. Br J Nutr. 2002;88:S263–71. doi: 10.1079/BJN2002716. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Lanza E, Mitchell D, Mentor-Marcel R, Colburn N, Hartman T. A legume enriched diet facilitates weight loss and improves biomarkers of insulin resistance and inflammation. FASEB J. 2010;24:931–4. [Google Scholar]
- 10.Hermsdorff HH, Zulet MÁ, Abete I, Martínez JA. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr. 2011;50:61–9. doi: 10.1007/s00394-010-0115-x. [DOI] [PubMed] [Google Scholar]
- 11.Crujeiras AB, Parra D, Abete I, Martínez JA. A hypocaloric diet enriched in legumes specifically mitigates lipid peroxidation in obese subjects. Free Radic Res. 2007;41:498–506. doi: 10.1080/10715760601131935. [DOI] [PubMed] [Google Scholar]
- 12.Hartman TJ, Albert PS, Zhang Z, Bagshaw D, Kris-Etherton PM, Ulbrecht J, et al. Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J Nutr. 2010;140:60–7. doi: 10.3945/jn.109.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aranceta J. Spanish food patterns. Public Health Nutr. 2001;4:1399–402. doi: 10.1079/phn2001227. [DOI] [PubMed] [Google Scholar]
- 14.Ghaemi-Hashemi SA, Clarke JA, Margen S. Benefits of the Middle Eastern food model on women's hormonal balance. J Am Diet Assoc. 1998;98:A25. [Google Scholar]
- 15.Ayatollahi SM. Nutritional assessment of lactating women in Shiraz in relation to recommended dietary allowances. East Mediterr Health J. 2004;10:822–7. [PubMed] [Google Scholar]
- 16.McCrory MA, Hamaker BR, Lovejoy JC, Eichelsdoerfer PE. Pulse consumption, satiety, and weight management. Adv Nutr. 2010;1:17–30. doi: 10.3945/an.110.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005;183:308–15. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137:992–8. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]
- 19.Washington, DC: National Academies Press; 2002. Institute of Medicine FaNB. Dietary Reference Intake for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. [DOI] [PubMed] [Google Scholar]
- 20.Alpha B, Cox L, Crowther N, Clark PM, Hales CN. Sensitive amplified immunoenzymometric assays (IEMA) for human insulin and intact proinsulin. Eur J Clin Chem Clin Biochem. 1992;30:27–32. doi: 10.1515/cclm.1992.30.1.27. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 23.Papanikolaou Y, Fulgoni VL., 3rd Bean consumption is associated with greater nutrient intake, reduced systolic blood pressure, lower body weight, and a smaller waist circumference in adults: Results from the National Health and Nutrition Examination Survey 1999-2002. J Am Coll Nutr. 2008;27:569–76. doi: 10.1080/07315724.2008.10719740. [DOI] [PubMed] [Google Scholar]
- 24.Anderson JW, Smith BM, Washnock CS. Cardiovascular and renal benefits of dry bean and soybean intake. Am J Clin Nutr. 1999;70:464S–474. doi: 10.1093/ajcn/70.3.464s. [DOI] [PubMed] [Google Scholar]
- 25.Lee YP, Puddey IB, Hodgson JM. Protein, fibre and blood pressure: Potential benefit of legumes. Clin Exp Pharmacol Physiol. 2008;35:473–6. doi: 10.1111/j.1440-1681.2008.04899.x. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JW, Hanna TJ. Impact of nondigestible carbohydrates on serum lipoproteins and risk for cardiovascular disease. J Nutr. 1999;129:1457S–66. doi: 10.1093/jn/129.7.1457S. [DOI] [PubMed] [Google Scholar]
- 27.He J, Ogden LG, Vupputuri S, Bazzano LA, Loria C, Whelton PK. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA. 1999;282:2027–34. doi: 10.1001/jama.282.21.2027. [DOI] [PubMed] [Google Scholar]
- 28.Ascherio A, Rimm EB, Hernán MA, Giovannucci EL, Kawachi I, Stampfer MJ, et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation. 1998;98:1198–204. doi: 10.1161/01.cir.98.12.1198. [DOI] [PubMed] [Google Scholar]
- 29.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 30.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 31.Sandström B, Hansen LT, Sørensen A. Pea fiber lowers fasting and postprandial blood triglyceride concentrations in humans. J Nutr. 1994;124:2386–96. doi: 10.1093/jn/124.12.386. [DOI] [PubMed] [Google Scholar]
- 32.Lasekan JB, Gueth L, Khan S. Influence of dietary golden pea proteins versus casein on plasma and hepatic lipids in rats. Nutr Res. 1995;15:71–84. [Google Scholar]
- 33.Boualga A, Prost J, Taleb-Senouci D, Krouf D, Kharoubi O, Lamri-Senhadji M, et al. Purified chickpea or lentil proteins impair VLDL metabolism and lipoprotein lipase activity in epididymal fat, but not in muscle, compared to casein, in growing rats. Eur J Nutr. 2009;48:162–9. doi: 10.1007/s00394-009-0777-4. [DOI] [PubMed] [Google Scholar]
- 34.Sato KK, Hayashi T, Nakamura Y, Harita N, Yoneda T, Endo G, et al. Liver enzymes compared with alcohol consumption in predicting the risk of type 2 diabetes: The Kansai Healthcare Study. Diabetes Care. 2008;31:1230–6. doi: 10.2337/dc07-2184. [DOI] [PubMed] [Google Scholar]
- 35.Abete I, Parra D, Martinez JA. Legume-, fish-, or high-protein-based hypocaloric diets: Effects on weight loss and mitochondrial oxidation in obese men. J Med Food. 2009;12:100–8. doi: 10.1089/jmf.2007.0700. [DOI] [PubMed] [Google Scholar]
- 36.Zahradka P, Guzman R, Weighell W, Wright B, Baldwin A, Louis S, et al. Increased consumption of legumes improves arterial stiffness in peripheral vascular disease independent of blood pressure, weight and serum cholesterol. FASEB J. 2009;23:212–3. [Google Scholar]
- 37.Bourdon I, Olson B, Backus R, Richter BD, Davis PA, Schneeman BO. Beans, as a source of dietary fiber, increase cholecystokinin and apolipoprotein b48 response to test meals in men. J Nutr. 2001;131:1485–90. doi: 10.1093/jn/131.5.1485. [DOI] [PubMed] [Google Scholar]
- 38.McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care. 2004;27:538–46. doi: 10.2337/diacare.27.2.538. [DOI] [PubMed] [Google Scholar]
- 39.Nestel P, Cehun M, Chronopoulos A. Effects of long-term consumption and single meals of chickpeas on plasma glucose, insulin, and triacylglycerol concentrations. Am J Clin Nutr. 2004;79:390–5. doi: 10.1093/ajcn/79.3.390. [DOI] [PubMed] [Google Scholar]
- 40.Panagiotakos DB, Tzima N, Pitsavos C, Chrysohoou C, Papakonstantinou E, Zampelas A, et al. The relationship between dietary habits, blood glucose and insulin levels among people without cardiovascular disease and type 2 diabetes; the ATTICA study. Rev Diabet Stud. 2005;2:208–15. doi: 10.1900/RDS.2005.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venn BJ, Mann JI. Cereal grains, legumes and diabetes. Eur J Clin Nutr. 2004;58:1443–61. doi: 10.1038/sj.ejcn.1601995. [DOI] [PubMed] [Google Scholar]
- 42.Hirshberg S, Fernandes J, Lofgren I. Dietary associations with chronic disease risk factors; legumes, MUFA and PUFA. FASEB J. 2010;24:323–4. [Google Scholar]


