Abstract
Background:
Eurycoma longifolia Jack (ElJ) has been shown to elevate serum testosterone and increased muscle strength in humans. This study investigated the effects of Physta® a standardized water extract of ElJ (400 mg/day for 6 weeks) on testosterone: epitestosterone (T:E) ratio, liver and renal functions in male recreational athletes.
Methods:
A total of 13 healthy male recreational athletes were recruited in this double blind, placebo-controlled, cross-over study. The participants were required to consume either 400 mg of ElJ or placebo daily for 6 weeks in the first supplementation regimen. Following a 3 week wash-out period, the participants were requested to consume the other supplement for another 6 weeks. Mid-stream urine samples and blood samples were collected prior to and after 6 weeks of supplementation with either ElJ or placebo. The urine samples were subsequently analyzed for T:E ratio while the blood samples were analyzed for liver and renal functions.
Results:
T:E ratio was not significantly different following 6 weeks supplementation of either ElJ or placebo compared with their respective baseline values. Similarly, there were no significant changes in both the liver and renal functions tests following the supplementation of ElJ.
Conclusions:
Supplementation of ElJ i.e. Physta® at a dosage of 400 mg/day for 6 weeks did not affect the urinary T:E ratio and hence will not breach any doping policies of the International Olympic Committee for administration of exogenous testosterone or its precursor. In addition, the supplementation of ElJ at this dosage and duration was safe as it did adversely affect the liver and renal functions.
Keywords: Doping, Eurycoma longifolia Jack, liver function, renal function, testosterone: epitestosterone ratio
INTRODUCTION
Eurycoma longifolia Jack (ElJ) or commercially known as Tongkat Ali in Malaysia, Pasak Bumi in Indonesia, Piak or Tung Saw in Thailand and Cay Ba Binh in Vietnam is a popular medicinal plant in the family of simaroubaceae.[1] ElJ is a shrub tree that grows up to 10 m in height and has long pinnate leaves that are green in color.[2] ElJ is highly demanded due to its tremendous health benefits and thus ElJ preparations are currently available in the health food market in the form of raw crude powder where the root is dried and grinded without involving any other chemical processing procedures.[3] ElJ is also available in the form of capsules which contain raw crude powder or standardized ElJ extract which is prepared by separating the active ingredients and adjusting the preparations to a defined content of a constituent and subsequently concentrating it to a standard level.[3] For instance, Physta® is a proprietary freeze-dried water extract of ElJ root which is commercially available. ElJ is also available as an additive brewed with coffee and canned as processed drinks.[4,5]
Chemical compounds that have been isolated particularly from the root of ElJ include eurycomanone, eurycomanol, eurycomalactone, canthine-6-one alkaloid, 9-hydroxycanthine-6-one, 14.15 β-hydroxyklaineanone, phenolic components, tannins, quanissoids and triterpenes.[6,7,8,9] These compounds have demonstrated to possess medicinal values such as anticoagulant for complications during child birth,[10] aphrodisiac,[11,12,13] antimalarial,[14,15,16] antibacterial,[17] anticancer,[18] anxiolytic[19] and antiulcer.[20] In addition, ElJ has been reported to have antioxidative properties due to its high concentrations of superoxide dismutase.[21] ElJ is also popular for its aphrodisiac property due to its ability to stimulate the production or action of androgen hormones, especially testosterone. Hence, it can be used as an alternative for testosterone replacement therapy[2] and for the treatment of osteoporosis in androgen-deficient males.[22] Human trials have also demonstrated that ElJ supplementation increased the level of testosterone.[23,24] The precise mechanism for its androgenic effect is still unclear but these data have indicated the potential of ElJ as a natural booster to serum testosterone.
It is a well-known fact that the use of testosterone to enhance athletic performance is prohibited in sports. In order to reveal such an abuse in any sportsman or sportswoman, urine tests have been endorsed. In normal healthy individuals, testosterone: Epitestosterone (T:E) are produced in the ratio of 1:1. Thus, it is assumed that the urinary T:E ratio increases in athletes taking exogenous compounds containing testosterone. According to the guidelines of World Anti-Doping Agency in 2004, if an athlete has a urinary T:E ratio of >4:1, he/she is deemed to have consumed excessive amount of substance that contains testosterone and this urine sample will be submitted to isotopic ratio mass spectrometry (IRMS) for the determination of the 13C/12C ratio. The IRMS test on the urine samples will be able confirm the illegal usage of a banned substance because exogenous compounds contain less 13C than their endogenous homologue 12C.[25]
With all these documented properties of ElJ, particularly its antioxidative and testosterone enhancing properties, it is surprising to note that studies on the effects of ElJ on sports performance are scarce. However, several studies have demonstrated that ElJ supplementation did not seem to improve sports performance.[26,27,28] Acute supplementation of ElJ in the form of a herbal drink at a low dosage (0.7 ± 0.1 mg/trial) consumed half an hour before the experimental trials did not enhance endurance cycling performance of young trained cyclists in a thermoneutral environment.[26,27] Similarly, Muhamad, et al.,[28] reported that supplementation of ElJ at a dosage of 150 mg/day for 7 days also did not indicate any beneficial effect on endurance running capacity and selected physiological responses of recreational athletes in the heat. However, Hamzah and Yusof[29] have reported that supplementation of water soluble extracts of ElJ at a dosage of 100 mg/day for 5 weeks increased fat free mass, muscle strength and size in healthy adult males. Since data on the effectiveness of ElJ supplements on sports performance is still inconclusive, future studies with supplementation of ElJ at a higher dosage and for a longer duration may be warranted. Nevertheless, the two main concerns for future studies that are designed to investigate the effects of ElJ supplementation on human sports performance are: (1) Will ElJ supplementation at a higher dosage lead to toxicity and (2) will ElJ supplementation at a higher dosage result in any increase in the urinary T:E ratio above the limit set by International Olympic Committee (IOC).
To the best of our knowledge, until date, there is no scientific data on the effects of a high dosage and prolonged ElJ supplementation on urinary T:E ratio, liver and renal functions. Thus, the objective of this study was to investigate the effects of a water extract of ElJ supplemented at a dosage of 400 mg/day for 6 weeks on T:E ratio, liver and renal functions in male recreational athletes.
METHODS
A total of 13 healthy male recreational athletes (age: 29.0 ± 5.5 years; VO2 max: 51.7 ± 6.8 ml/kg/min) were recruited in this double blind, placebo-controlled, cross-over study [Table 1]. Participants were screened to ensure that they had no history of renal or liver disease and were requested to refrain from consuming any other nutritional products that contain ElJ except those provided by the researchers. The participants were required to consume either 400 mg of Physta®, a standardized water extract of ElJ, manufactured at Phytes Biotek Sdn Bhd or placebo (maltodextrin) daily for 6 weeks as the first supplementation regimen. Following a 3 week wash-out period, the participants were requested to consume the other supplement for another 6 weeks. The order of these two supplementation regimens was randomized.
Table 1.
Physical characteristics and physiological profiles of the participants
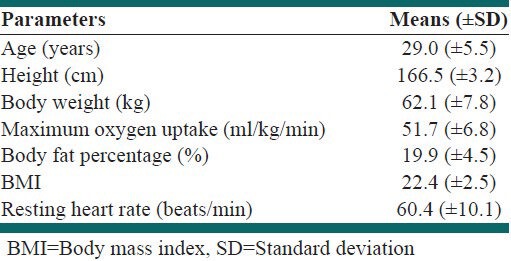
Prior to and after 6 weeks of supplementation with ElJ and after 6 weeks of supplementation with placebo, participants reported to the Sports Science laboratory for collection of urine and blood samples for subsequent analysis of urinary T:E ratio and renal and liver functions respectively. Each participant was required to collect a mid-stream 20-30 ml urine sample into a sterile specimen collection jar and the urine sample was subsequently transferred into storage tubes. A volume of 2 ml of blood was also collected from each participant through a 5 ml sterile syringe for the determination of renal and liver function tests. Both the urine and blood samples were kept frozen at –20°C for later analysis of T:E ratio and renal and liver function tests respectively.
The determination of T:E ratio was performed by the staff of the Doping Control Centre, Universiti Sains Malaysia, Penang, a National Association of Testing Authorities, Australia-accredited laboratory. The quantification of T:E ratio was determined by gas chromatography–mass spectrometry (GC/MS). The free and conjugated T and E were extracted by solid phase extraction using the Nexus (Varian) column combined with liquid-liquid extraction using tert-butyl methyl ether, followed by hydrolysis using enzyme β-glucuronidase from Esherichia coli K12 (Roche Diagnostics Mannheim, Germany); extracts were then derivatized with activated N-methyl-N-trimethylsilyl-trifluoroacetamide. The calibration of T was performed using concentration levels of between 2 and 80 ng/mL while the values for E was between 2 and 20 ng/mL. Selected ion monitoring mode with electron ionization on the GC/MS was used for both identification and quantification of both T and E. The standard renal and liver function tests were performed by the staff of Gribbles Pathology (M) Pvt. Ltd., a Laboratory Accreditation Scheme of Malaysia-accredited laboratory. The liver function test included the determination of total protein, albumin, bilirubin, alkaline phosphatase, gamma glutamyl transpeptidase, alanine amino transferase and aspartate amino transferase while the renal function test included sodium, potassium, chloride, urea, creatinine, uric acid, calcium and phosphate.
Statistical analysis
Statistical analyzes were performed using the Statistical Package for Social Sciences (SPSS version 18.0). Paired t-test was used to determine the differences between the dependent variables: T:E ratio, liver and renal function tests prior to and after the intervention period. Statistical significance was set at P < 0.05.
RESULTS
Prior to the supplementation regimen, urinary T:E ratio were 0.69:1 and 0.84:1 in the placebo and ElJ groups respectively [Figure 1]. Following the 6 weeks of supplementation period, this ratio were 0.63:1 and 0.74:1 in the placebo and ElJ groups respectively. There were no statistical differences between groups at baseline and at post-intervention. In addition, were also no significant changes in these values from baseline and after 6 weeks of supplementation with either ElJ or placebo. Thus, the urinary T:E ratios of the participants prior to and after both the supplementation regimens were below the threshold limit of 4:1 set by IOC.
Figure 1.
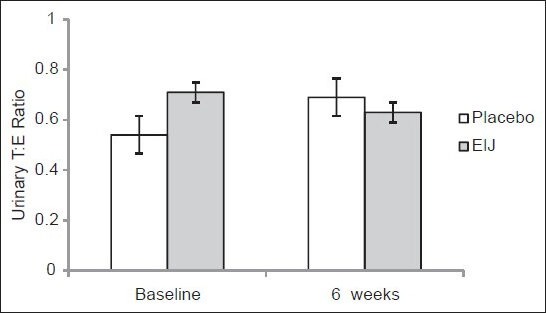
Urinary testosterone:Epitestosterone ratio at baseline and following 6 weeks of supplementation with Eurycoma longifolia Jack or placebo
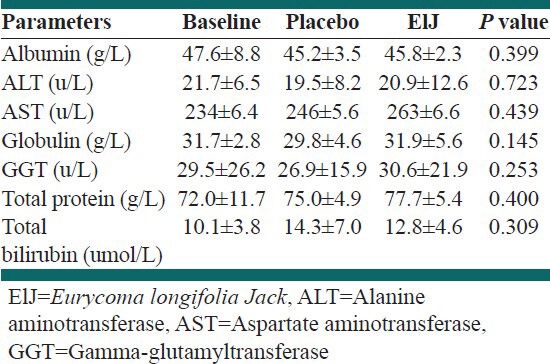
The concentrations of the enzymes for the liver function test at baseline were 47.6 g/L, 21.7 u/L, 23.4 u/L, 31.7 g/L, 29.5 u/L, 72.0 g/L, 10.1 umol/L for albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), globulin, gamma-glutamyltransferase (GGT), total protein and total bilirubin respectively [Table 2]. After the 6 weeks supplementation regimen, these concentrations were 45.2 g/L versus 45.2 g/L; 19.5 u/L versus 20.9 u/L, 24.6 u/L versus 26.3 u/L, 29.8 g/L versus 31.9 g/L, 26.9 u/L versus 30.6 u/L, 75.0 g/L versus 77.7 g/L, 14.3 umol/L versus 12.8 umol/L for ALT, AST, GGT, total protein and total bilirubin in the placebo and ElJ groups respectively. There was no statistical significance between the levels of these enzymes at post intervention compared with the baseline levels in both groups.
Table 2.
Liver function test values at baseline and 6 weeks after supplementation
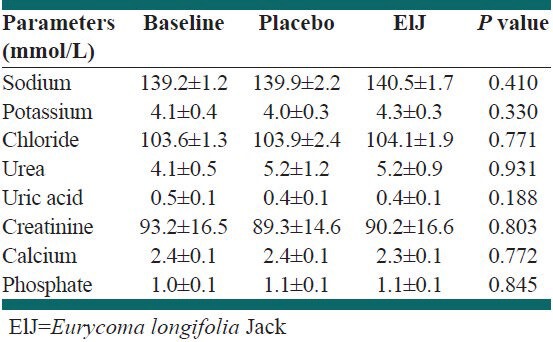
The concentrations of the parameters for the renal function test at baseline were 139.2 mmol/L, 4.1 mmol/L, 103.6 mmol/L, 4.1 mmol/L, 0.5 mmol/L, 93.2 umol/L, 2.4 mmol/L and 1.0 mmol/L for sodium, potassium, chloride, urea, uric acid, creatinine, calcium and phosphate respectively [Table 3]. After the 6 weeks supplementation regimen, these concentrations were 139.9 versus 140.5 mmol/L, 4.0 versus 4.3 mmol/L, 103.9 versus 104.1 mmol/L, 5.2 versus 5.2 mmol/L, 0.4 versus 0.4 mmol/L, 89.3 versus 90.2 umol/L, 2.4 versus 2.3 mmol/L and 1.1 versus 1.2 mmol/L for sodium, potassium, chloride, urea, uric acid, creatinine, calcium and phosphate respectively. There was no statistical significance between the levels of these parameters at post intervention compared with the baseline levels in both groups.
Table 3.
Renal function test values at baseline and 6 weeks after supplementation
DISCUSSION
The urinary T:E ratio of the ElJ group after the supplementation regimen was below the cut-off point of 4:1 by the IOC Medical Commission. Thus, the findings of this investigation indicated that ElJ supplementation for 6 weeks at a dosage of 400 mg/day would not induce a T:E ratio that was prohibited by the IOC Medical Commission. In addition, this ElJ supplementation regimen did not result in any adverse effects on the liver and renal functions of the participants.
Tambi, et al.,[24] have shown that ElJ (Physta®) improved the concentration of serum testosterone in hypogonadic men (men with low serum testosterone levels) and hence concluded that ElJ has potential as a natural substitute to testosterone. The ergogenic effect of ElJ in men has been demonstrated by Hamzah and Yusof[29] when they reported that water-soluble extract of ElJ (100 mg/day for 5 weeks) increased fat free mass, muscular strength and size. However, subsequent but limited studies did not seem to substantiate the ergogenic properties of ElJ on sports performance.[26,28]
Acute supplementation of ElJ at a low dosage (0.67 mg of ElJ per trial) did not improve endurance cycling capacity among young cyclists in a thermoneutral environment.[26] Subsequently, we increased the dosage and duration of ElJ to 150 mg/day for 7 days on recreational athletes.[28] However, the results of this study also did not seem to provide any beneficial effect of ElJ supplementation on endurance running performance. Since the supplementation of ELJ showed ergogenic benefits in studies by Hamzah and Yusoff[29] with supplementation of 100 mg over 5 weeks, whereas in subsequent studies by Ooi, et al.[26] and Muhamad, et al.,[28] with shorter supplementation period (0.67 mg ELJ for ½ h and 150 mg ELJ for 7 days respectively) did not, it is possible that the duration and dosage of supplementation was not sufficient to affect performance. It has been reported that ElJ in the form of water soluble extract i.e. Physta® is non-toxic even at a high dose of 600 mg to the liver function, renal function, hematological profile, lipid profile and immune function in healthy males.[21] Similarly, our present data also indicated that supplementation of ElJ at 400 mg/day for 6 weeks did not induced toxicity to the liver and renal functions among the participants. Hence, higher dosage and longer duration of supplementation of ElJ in humans may be warranted to evaluate its ergogenic property.
CONCLUSIONS
Prolonged ElJ supplementation i.e. Physta® at 400 mg/day for 6 weeks did not affect urinary T:E ratio and hence will not breach doping policies of the International Olympic Committee for exogenous testosterone or precursor administration. In addition, supplementation at this dosage and duration was non-toxic to the liver and renal functions. However, future studies with a higher dosage and longer supplementation period is still warranted to ensure that ElJ does not pose any doping issue or compromise the safety of the individuals consuming them.
ACKNOWLEDGEMENTS
We wish to extend our sincere gratitude to all the subjects who have participated in this study. We also want to express our appreciation to Mdm. Jamaayah bt. Meor Osman, Mdm. Norlida bt Azalan and Mdm. Hafizah bt. Hamzah for their technical assistance in the Sports Science Laboratory, Universiti Sains Malaysia, Kelantan, Malaysia throughout this study. Special thanks to Prof. Aishah bt. Abdul Latiff and Normaliza bt.Hj. Abdul Manaf from the Doping Control Center, Universiti Sains Malaysia, Penang for the analysis of testosterone: Epitestosterone ratio. We would like to thank Biotropics Malaysia Berhad, Kuala Lumpur, Malaysia, for financial support of this clinical trial. Annie George is an employee of this company.
Footnotes
Source of Support: Biotropics Malaysia Berhad
Conflict of Interest: None declared.
REFERENCES
- 1.Kuo PC, Shi LS, Damu AG, Su CR, Huang CH, Ke CH, et al. Cytotoxic and antimalarial beta-carboline alkaloids from the roots of Eurycoma longifolia. J Nat Prod. 2003;66:1324–7. doi: 10.1021/np030277n. [DOI] [PubMed] [Google Scholar]
- 2.Goreja WG. Vol. 2. New York: Amazing Herb Press; 2004. Tongkat Ali: The Tree that Cures a Hundred Diseases. [Google Scholar]
- 3.Mohd Effendy N, Mohamed N, Muhammad N, Naina Mohamad I, Shuid AN. Eurycoma longifolia: Medicinal plant in the prevention and treatment of male osteoporosis due to androgen deficiency. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/125761. 125761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaganath JB, Ng LT. Kuala Lumpur: Vinpress Sdn. Bhd. and Malaysian Agricultural Research and Development Institute; 2000. Herbs: The Green Pharmacy of Malaysia. [Google Scholar]
- 5.Sahelian R. New York: Square One Publishers; 2004. Tongkat Ali: Exotic Asian Aphrodisiac in Natural Sex Boosters: Supplements that Enhance Stamina, Sensation and Sexuality for Men and Women. [Google Scholar]
- 6.Darise M, Kohda H, Mizutani K, Tanaka O. Eurycomanone and eurycomanol, quassinoids from the roots of Eurycoma longifolia. Phytochemistry. 1982;21:2091–3. [Google Scholar]
- 7.Morita H, Kishi E, Takeya K, Itokawa H, Litaka Y. Squalene derivatives from Eurycoma longifolia. Phytochemistry. 1993;34:765–71. [Google Scholar]
- 8.Choo CY, Chan KL. High performance liquid chromatography analysis of canthinone alkaloids from Eurycoma longifolia. Planta Med. 2002;68:382–4. doi: 10.1055/s-2002-26745. [DOI] [PubMed] [Google Scholar]
- 9.Kuo PC, Damu AG, Lee KH, Wu TS. Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Bioorg Med Chem. 2004;12:537–44. doi: 10.1016/j.bmc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Osman A, Jordan B, Lessard PA, Muhammad N, Haron MR, Riffin NM, et al. Genetic diversity of Eurycoma longifolia inferred from single nucleotide polymorphisms. Plant Physiol. 2003;131:1294–301. doi: 10.1104/pp.012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ang HH, Sim MK. Eurycoma longifolia increases sexual motivation in sexually naive male rats. Arch Pharm Res. 1998;21:779–81. doi: 10.1007/BF02976776. [DOI] [PubMed] [Google Scholar]
- 12.Ang HH, Ngai TH, Tan TH. Effects of Eurycoma longifolia Jack on sexual qualities in middle aged male rats. Phytomedicine. 2003;10:590–3. doi: 10.1078/094471103322331881. [DOI] [PubMed] [Google Scholar]
- 13.Ang HH, Lee KL, Kiyoshi M. Sexual arousal in sexually sluggish old male rats after oral administration of Eurycoma longifolia Jack. J Basic Clin Physiol Pharmacol. 2004;15:303–9. doi: 10.1515/jbcpp.2004.15.3-4.303. [DOI] [PubMed] [Google Scholar]
- 14.Chan KL, O’Neill MJ, Phillipson JD, Warhurst DC. Plants as sources of antimalarial drugs. Part 3. Eurycoma longifolia Planta Med. 1986;2:105–7. doi: 10.1055/s-2007-969091. [DOI] [PubMed] [Google Scholar]
- 15.Kardono LB, Angerhofer CK, Tsauri S, Padmawinata K, Pezzuto JM, Kinghorn AD. Cytotoxic and antimalarial constituents of the roots of Eurycoma longifolia. J Nat Prod. 1991;54:1360–7. doi: 10.1021/np50077a020. [DOI] [PubMed] [Google Scholar]
- 16.Ang HH, Chan KL, Mak JW. In vitro antimalarial activity of quassinoids from Eurycoma longifolia against Malaysian chloroquine-resistant Plasmodium falciparum isolates. Planta Med. 1995;61:177–8. doi: 10.1055/s-2006-958042. [DOI] [PubMed] [Google Scholar]
- 17.Farouk AE, Benafri A. Antibacterial activity of Eurycoma longifolia Jack. A Malaysian medicinal plant. Saudi Med J. 2007;28:1422–4. [PubMed] [Google Scholar]
- 18.Tee TT, Cheah YH, Hawariah LP. F16, a fraction from Eurycoma longifolia jack extract, induces apoptosis via a caspase-9-independent manner in MCF-7 cells. Anticancer Res. 2007;27:3425–30. [PubMed] [Google Scholar]
- 19.Ang HH, Cheang HS. Studies on the anxiolytic activity of Eurycoma longifolia Jack roots in mice. Jpn J Pharmacol. 1999;79:497–500. doi: 10.1254/jjp.79.497. [DOI] [PubMed] [Google Scholar]
- 20.Tada H, Yasuda F, Otani K, Doteuchi M, Ishihara Y, Shiro M. New antiulcer quassinoids from Eurycoma longifolia. Eur J Med Chem. 1991;26:345–9. [Google Scholar]
- 21.Tambi MI. Standardised water soluble extract of Eurycoma longifolia (LJ100) on men's health. Int J Androl. 2005;28(Suppl 1):25–44. doi: 10.1111/j.1439-0272.2011.01168.x. [DOI] [PubMed] [Google Scholar]
- 22.Shuid AN, Abu Bakar MF, Abdul Shukor TA, Muhammad N, Mohamed N, Soelaiman IN. The anti-osteoporotic effect of Eurycoma Longifolia in aged orchidectomised rat model. Aging Male. 2011;14:150–4. doi: 10.3109/13685538.2010.511327. [DOI] [PubMed] [Google Scholar]
- 23.Tambi MI. Las Vegas, USA: Proceedings of the International Trade Show and Conference 2003; 2003. Oct 1-3, Water soluble extract of Eurycoma longifolia in enhancing testosterone in males. [Google Scholar]
- 24.Tambi MI, Imran MK, Henkel RR. Standardised water-soluble extract of Eurycoma longifolia, Tongkat ali, as testosterone booster for managing men with late-onset hypogonadism? Andrologia. 2012;44(Suppl 1):226–30. doi: 10.1111/j.1439-0272.2011.01168.x. [DOI] [PubMed] [Google Scholar]
- 25.Aguilera R, Chapman TE, Starcevic B, Hatton CK, Catlin DH. Performance characteristics of a carbon isotope ratio method for detecting doping with testosterone based on urine diols: Controls and athletes with elevated testosterone/epitestosterone ratios. Clin Chem. 2001;47:292–300. [PubMed] [Google Scholar]
- 26.Ooi FK, Singh R, Sirisinghe RG, Ang B, Jamalullail S. Effects of a herbal ergogenic drink on cycling performance in young cyclists. Malays J Nutr. 2001;7:33–40. [PubMed] [Google Scholar]
- 27.Kiew OF, Singh R, Sirisinghe RG, Suen AB, Jamalullail SM. Effects of a herbal drink on cycling endurance performance. Malays J Med Sci. 2003;10:78–85. [PMC free article] [PubMed] [Google Scholar]
- 28.Muhamad AS, Keong CC, Kiew OF, Abdullah MR, Lam CT. Effects of Eurycoma longifolia Jack supplementation on recreational athletes endurance running capacity and physiological responses in the heat. Int J Appl Sports Sci. 2010;22:1–19. [Google Scholar]
- 29.Hamzah S, Yusof A. The ergogenic effects of Eurycoma longifolia Jack: A pilot study. Br J Sports Med. 2003;37:465–6. [Google Scholar]


