Summary
In Cleome species (Cleomaceae) having two different forms and evolutionary origins of Kranz anatomy there is convergence in the structural and biochemical expression of C4 traits during leaf ontogeny.
Key words: C4 anatomy, C4 development, Cleome angustifolia, Cleome gynandra, C4 photosynthesis, C4 plants, immunolocalization, ultrastructure.
Abstract
In family Cleomaceae there are NAD-malic enzyme-type C4 species having different forms of leaf anatomy. Leaves of Cleome angustifolia have Glossocardioid-type anatomy with a single complex Kranz unit which surrounds all the veins, while C. gynandra has Atriplicoid anatomy with multiple Kranz units, each surrounding an individual vein. Biochemical and ultrastructural differentiation of mesophyll (M) and bundle sheath (BS) cells were studied along a developmental gradient, from the leaf base (youngest) to the tip (mature). Initially, there is cell-specific expression of certain photosynthetic enzymes, which subsequently increase along with structural differentiation. At the base of the leaf, following division of ground tissue to form M and BS cells which are structurally similar, there is selective localization of Rubisco and glycine decarboxylase to BS cells. Thus, a biochemical C3 default stage, with Rubisco expression in both cell types, does not occur. Additionally, phosphoenolpyruvate carboxylase (PEPC) is selectively expressed in M cells near the base. Surprisingly, in both species, an additional layer of spongy M cells on the abaxial side of the leaf has the same differentiation with PEPC, even though it is not in contact with BS cells. During development along the longitudinal gradient there is structural differentiation of the cells, chloroplasts, and mitochondria, resulting in complete formation of Kranz anatomy. In both species, development of the C4 system occurs similarly, irrespective of having very different types of Kranz anatomy, different ontogenetic origins of BS and M, and independent evolutionary origins of C4 photosynthesis.
Introduction
In most C4 plants, the dual-cell system (so-called Kranz anatomy) operates to concentrate CO2 around ribulose 1,5-bisphosphate carboxylase-oxygenase (Rubisco). Primary fixation of CO2 into C4 acids occurs in the outer layer of mesophyll (M) cells using phosphoenolpyruvate carboxylase (PEPC), while decarboxylation of C4 acids and refixation of CO2 by Rubisco in the C3 pathway occur in the inner layer of bundle sheath (BS) cells (Hatch, 1987). This CO2-concentrating mechanism restricts the competing ribulose bisphosphate (RuBP) oxygenase reaction and synthesis of glycolate, and the loss of CO2 by photorespiration. Glycine decarboxylase (GDC), which is essential for photorespiratory CO2 release in the glycolate pathway, is confined exclusively to BS mitochondria (Edwards and Walker, 1983; Edwards and Voznesenskaya, 2011; Sage et al., 2012).
C4 species are classified according to their biochemical subtype based on the predominant type of C4 decarboxylase, NADP-malic enzyme (NADP-ME), NAD-malic enzyme (NAD-ME), or phosphoenolpyruvate carboxykinase, used to release and concentrate CO2 around Rubisco (Hatch, 1987). The two main types of C4 cycles in eudicots function either through NADP-ME, which is located in the chloroplasts, or through NAD-ME, which is located in the mitochondria of BS cells. There are structural differences in grana development between M and BS chloroplasts in NADP-ME- versus NAD-ME-type C4 species associated with differences in photochemistry. For C4 to function, NADPH is required to reduce 3-phosphoglycerate, the product of CO2 fixation by Rubisco, to triose-phosphates. In NADP-ME-type C4 species, the primary shuttle of malate from M cells to BS chloroplasts provides both CO2 and NADPH, which reduces the requirement for photosystem II (PSII) activity, and the associated development of grana, in BS chloroplasts. In NAD-ME-type species, BS chloroplasts have increased grana development and PSII activity, since the primary shuttle of aspartate from the M to BS cells delivers CO2, but not reductive power (Edwards and Walker, 1983; Edwards and Voznesenskaya, 2011).
C4 photosynthesis is considered to have evolved independently >60 times (Sage et al., 2011), resulting in much structural diversity in the forms of C4 which evolved from C3 ancestors (Dengler and Nelson, 1999; Peter and Katinas, 2003; Muhaidat et al., 2007; Edwards and Voznesenskaya, 2011). Among the forms identified, 16 develop Kranz around individual veins, while eight develop a compound Kranz unit near the periphery of the leaf, which surrounds all veins (Edwards and Voznesenskaya, 2011). The diversity in forms occurs by special positioning of the M and BS layers in relation to the distribution of veins and other tissues, by the occurrence of different types of tissues in the leaf (e.g. mestome sheath in monocots, hypodermal, or water storage cells in dicots), by the way organelles (chloroplast and mitochondria) are arranged in BS cells and their differentiation between M and BS cells, and by structural diversity in developing gas diffusion barriers to support the CO2-concentrating mechanism (Edwards and Voznesenskaya, 2011).
The ontogeny of the complex C4 system requires a highly coordinated selective expression of many genes during development, concurrent with establishment of Kranz anatomy. Genes controlling development of M and BS specialization for function of the C4 system, and the degree of redundancy which has occurred during the multiple times C4 has evolved is unknown. Among monocot and eudicot C4 species which are being used as genetic models, concerted efforts are needed to identify regulatory factors along leaf developmental gradients which control the expression of specific C4 traits (Covshoff et al., 2013).
There have been a number of studies on structural and biochemical development of forms of C4 having Kranz anatomy around individual veins. This includes extensive analysis of leaf development in Zea mays which has classical NADP-ME-type anatomy (Sheen and Bogorad, 1985; Langdale et al., 1988b; Dengler and Nelson, 1999; Sheen, 1999). In the interest of identifying transcription factors controlling expression of specific traits which are required for C4 function, there have been recent studies on the developmental gradient along the leaf of maize, including analyses of transcriptome, proteome, and structural changes (see Li et al., 2010; Majeran et al., 2010; Covshoff et al., 2013; Wang et al., 2013). Developmental studies have also been conducted on some other forms of Kranz anatomy in grasses (Dengler et al., 1985, 1986, 1996; Wakayama et al., 2003) and on different structural forms of Kranz in family Cyperaceae (Soros and Dengler, 2001). Among eudicots, significant developmental studies have been conducted on two NAD-ME-type species having Atriplicoid-type anatomy, namely Atriplex rosea (Liu and Dengler, 1994; Dengler et al., 1995; Dengler and Nelson, 1999) and Amaranthus hypochondriacus (Ramsperger et al., 1996; Patel and Berry, 2008). There has been less attention to development of types of Kranz anatomy which surround all veins in the leaves. There are reports on NADP-ME Salsoloid type with Salsola richteri (Voznesenskaya et al., 2003b), NAD-ME Salsinoid type with Suaeda taxifolia, and NAD-ME Schoberioid type with Suaeda eltonica (Koteyeva et al., 2011a ).
These studies on different forms of C4 show a progression in structural and biochemical differentiation during development, with dimorphic M and BS cells originating from monomorphic cells. Characterization of the development of Kranz anatomy includes identification of the origins of M and BS cells during leaf initiation, which is different depending on Kranz anatomical type or plant lineages (Dengler et al., 1985; Dengler and Nelson, 1999; Soros and Dengler, 2001; Koteyeva et al., 2011a ). This characterization includes structural differentiation of M and BS chloroplasts and their function in photochemistry to provide energy cooperatively for CO2 assimilation (Brown, 1975; Dengler et al., 1985; Langdale et al., 1988a , b; Dengler and Nelson, 1999; Sheen, 1999; von Caemmerer and Furbank, 2003; Patel and Berry, 2008; Li et al., 2010; Majeran et al., 2010; Edwards and Voznesenskaya, 2011; Koteyeva et al., 2011a ; Wang et al., 2013). It also consists of analysis of the differences among forms of C4 in the development of barriers to diffusion of CO2 from sites of decarboxylation in BS cells, which enables CO2 to be concentrated around Rubisco (e.g. BS cell wall thickness, location of the C4 acid decarboxylase, and the position of organelles) (von Caemmerer and Furbank, 2003). Determining when selective expression of enzymes in M and BS cells for C4 function occurs during development among different forms of C4 is also important (Dengler et al., 1985; Soros and Dengler, 2001; Koteyeva et al., 2011a ; Langdale, 2012). Analyses show that there are differences in the sequence and the timing of biochemical and structural changes, with respect to establishment of the vascular system, and whether expression of traits is regulated by environmental (light) or developmental cues (Langdale et al., 1988a ; Wang et al., 1992; Dengler et al., 1995; Ramsperger et al., 1996; Dengler and Nelson, 1999; Sheen, 1999; Voznesenskaya et al., 2003a ; Wakayama et al., 2003; Koteyeva et al., 2011a ; Langdale, 2012).
There is recent interest in the occurrence of C4 photosynthesis in Cleomaceae, one of 19 families in which C4 species have been found (Sage et al., 2011). The genus Cleome sensu lato consists of >200 species. Most species have the C3 type of photosynthesis; but, it has been shown that three species, Cleome angustifolia, C. gynandra, and C. oxalidea, have NAD-ME-type C4 photosynthesis in leaves and cotyledons (Marshall et al., 2007; Voznesenskaya et al., 2007; Feodorova et al., 2010; Koteyeva et al., 2011b). Two of them, C. gynandra and C. oxalidea, have flat leaves with an Atriplicoid type of anatomy consisting of multiple Kranz units around individual veins, while C. angustifolia with Glossocardioid-type anatomy, has a single compound Kranz unit with a double layer of concentric chlorenchyma surrounding all the veins and water storage cells (Koteyeva et al., 2011b). They belong to separate lineages in the genus and they have different distribution, pantropical for C. gynandra, Australian for C. oxalidea, and African for C. angustifolia (Feodorova et al., 2010). Cleome gynandra was originally proposed as a model NAD-ME eudicot C4 species for identifying factors controlling development and function of C4 photosynthesis (Brown et al., 2005). The attractive features of this species are that it can be transformed, grown under a short life cycle, has C3 and C3–C4 intermediate Cleome relatives for comparative studies, and, phylogenetically, among C4 genera it has the closest position to the well-established model species Arabidopsis thaliana (a C3 dicot) (Feodorova et al., 2010; Covshoff et al., 2013). Progress towards development of C. gynandra has been made, including identifying transcripts associated with C4 biochemistry and metabolite transporters (Kajala et al., 2012), in comparative RNaseq profiling (Bräutigam et al., 2011), and in the ability to transform it (Newell et al., 2010).
The purpose of the current study was to examine development of C4 in two NAD-ME-type C4 Cleome species, C. gynandra and C. angustifolia, which have major differences in leaf morphology, vascular pattern, and forms of Kranz anatomy. This included analysis of M and BS cell formation from leaf primordia, and structural and biochemical transitions during leaf ontogeny, to determine whether there is convergence in development and expression of C4 traits.
Materials and methods
Plant material
Seeds of Cleome angustifolia Forssk. (from herbarium material kindly provided by Drs A. Oskolskii and O. Maurin) and C. gynandra L. (kindly provided by Dr A.S. Raghavendra) were stored at 3–5 ºC prior to use and were germinated on moist paper in Petri dishes at room temperature and a photosynthetic photon flux density ~20 μmol quanta m–2 s–1. The seedlings were then transplanted to 15cm diameter pots with commercial potting soil and grown in the greenhouse during the mid-winter/spring months, with an approximate 26 ºC day/18 ºC night temperatures. Maximum midday photosynthetic photon flux density was 500 μmol quanta m–2 s–1 on clear days. Plants were fertilized once a week with Peter’s Professional (20:20:20; Scotts Miracle-Gro Co., Marysville, OH, USA). For microscopy and biochemical analyses, leaves of different lengths were taken from plants of ~3–6 weeks old. Voucher specimens are available at the Marion Ownbey Herbarium, Washington State University under # WS 375818 for C. angustifolia and WS 369765 for C. gynandra.
Light and electron microscopy
Study of the structural basis of leaf development was carried out on expanding leaves with lengths of leaflets from 0.5mm to 7mm. Vegetative shoot tips were used to study the earliest events of leaf initiation and development. Three samples of each leaflet length (using the terminal leaflet in the palmate leaf) were harvested from three independent plants of each species (a total of nine samples from each species for each leaf length). Vegetative shoot apices with several of the youngest leaf primordia, and leaves of intermediate size (6–7mm in length), were sectioned longitudinally. Additionally, cross-sections were made in 0.5mm steps from the tip to base of 3–5mm long leaves.
Sample preparation for light microscopy (LM) and transmission electron microscopy (TEM) was carried out following Koteyeva et al. (2011a ). An Olympus BH-2 (Olympus Optical Co. Ltd) light microscope equipped with an LM Digital Camera and Software (Jenoptik ProgRes Camera, C12plus, Jena, Germany) was used for observation and collection of images at the LM level. A Hitachi H-600 (Hitachi Scientific Instruments, Tokyo, Japan) and FEI Tecnai G2 (Field Emission Instruments Company, Hillsboro, OR, USA) equipped with Eagle FP 5271/82 4K HR200KV digital camera transmission electron microscopes were used for TEM studies.
The cell size, length of the chloroplasts, and the thickness of cell walls were measured for the adaxial M and BS on micrographs from leaf longitudinal and cross-sections at different distances from the leaf base using an image analysis program (UTHSCSA, Image Tool for Windows, version 3.00). For both types of cells, the measurements of cell wall thickness were made on cell walls facing intercellular airspaces between M and BS. From images of the cell walls, plasmodesmata between M and BS cells (which were radially but not longitudinally oriented in the wall sections) were counted. The plasmodesmata frequency was referred to as the number of plasmodesmata per 1 μm of cell–cell contact interface length.
Images of the shoot apices with the youngest leaf primordia and surface of young leaves for analysis of stomata were captured under the low vacuum mode with an FEI Scanning Electron Microscope Quanta 200F (Feild Emission Instruments Company) without additional treatments.
To observe the leaf vascular pattern, leaves of different ages, from youngest primordia to fully expanded leaves, were cleared in 70% ethanol (v/v) until chlorophyll was removed, bleached with 5% (w/v) NaOH overnight, and then rinsed three times in water. At least five leaves of different ages were studied from two to three different plants. The leaves were mounted in water on slides and examined under a UV light [with a 4′,6-diamidino-2-phenylindole (DAPI) filter] on a Fluorescence Microscope Leica DMFSA (Leica Microsystems Wetzlar GmbH, Germany) using autofluorescence of lignified tracheary elements of the xylem.
In situ immunolocalization
Sample preparation and immunolocalization by LM and TEM were carried out on longitudinal sections of leaves, 6–7mm long, following the procedure in Koteyeva et al. (2011a ). Antibodies used were anti-spinach Rubisco [large subunit (LSU)] IgG (courtesy of B. McFadden) commercially available anti-maize PEPC IgG (Chemicon, Temecula, CA, USA), and the P-subunit of GDC, all raised in rabbits. The density of labelling was determined by counting the gold particles on digital electron micrographs using the UTHSCSA image analysis program and calculating the number per unit area (μm2). Standard errors were determined.
Western blot analysis
For the study of accumulation of the main photosynthetic enzymes during development, individual leaflets of different ages, with lengths of 1–3, 3–6, 6–10, 10–15mm, and 30–35mm (totally expanded mature leaflet), were used. Soluble proteins were extracted from leaves and prepared for SDS–PAGE following Koteyeva et al. (2011b). Protein concentration was determined with an RCDC protein quantification kit (Bio-Rad), which tolerates detergents and reducing agents. A 10 μg aliquot of soluble protein was applied per lane, with separation by 12% (w/v) SDS–PAGE, and transferred to a nitrocellulose membrane for analysis of the several photosynthetic enzymes and GDC. Primary antibodies used were anti-Amaranthus hypochondriacus NAD-ME IgG against the 65kDa α-subunit (Long and Berry, 1996) (dilution 1:5000), anti-Zea mays PEPC IgG (1:100 000), anti-Zea mays pyruvate,Pi dikinase (PPDK) IgG (courtesy of T. Sugiyama) (1:5000), anti-Pisum sativum L. GDC P-subunit (courtesy of Dr D. Oliver) (1:10 000), and anti-Spinacia oleracea Rubisco LSU IgG (courtesy of B. McFadden) (1:10 000). Goat anti-rabbit IgG–alkaline phosphatase-conjugated secondary antibodies (Sigma) were used at a dilution of 1:10 000 for detection. Bound antibodies were visualized by developing the blots with 20mM nitroblue tetrazolium and 75mM 5-bromo-4-chloro-3-indolyl phosphate in detection buffer (100mM TRIS-HCl, pH 9.5, 100mM NaCl, and 5mM MgCl2). Two separate blots from two separate extractions were made for each enzyme. The intensities of bands in western blots were quantified with an image analysis program (ImageJ 1.37, NIH, USA) and expressed relative to the level in the fully expanded leaves.
Mass spectrometric measurements of Γ, R d, and leaf carbon isotope composition
A membrane inlet mass spectrometer (DELTA V Plus; Thermo Scientific) connected to a closed leaf chamber via a membrane inlet was used to measure rates of CO2 exchange and the CO2 compensation point (Γ) in C. gynandra young and mature leaves, as described previously (Maxwell et al., 1998; Ruuska et al., 2000). Cuttings from mature leaves ~1.5cm2 or 5–7 young (~7mm) leaves of C. gynandra harvested from several branches were placed in the chamber and, once CO2 and O2 concentrations were balanced with ambient concentrations, the leaf chamber was sealed and after 5min sitting in the dark was submitted to an irradiance of 1000 μmol m–2 s–1. The temperature was controlled at 25 °C using a water bath circulating around the chamber. Net CO2 assimilation was followed in the sealed chamber by measuring the changes in 12CO2 concentration over time until reaching Γ; that is, when the amount of CO2 assimilated by photosynthesis was balanced with the amount of CO2 released by respiration and photorespiration. The rate of dark respiration (R d) was calculated from the rate of CO2 released after a minimum of 5min in the dark. The system was zeroed before and after each measurement by flushing the chamber with a mixture of nitrogen and oxygen.
Leaf carbon isotope composition was determined in C. gynandra from sections taken at the base, middle, and tip of young 0.7cm leaves, and from mature leaves (n=3–6). As previously described (Voznesenskaya et al., 2013), samples (1–2mg) were placed in a tin capsule and combusted in a Eurovector elemental analyser; the resulting N2 and CO2 gases were separated by gas chromatography and admitted into the inlet of a Micromass, Isoprime isotope ratio mass spectrometer (IRMS) for determination of 13C/12C ratios (R). δ13C values were determined where 1000×Rsample/Rstandard)–1, using PDB (Pee Dee Belemnite) as the standard.
Statistical analysis
Where indicated, standard errors were determined, and analysis of variance (ANOVA) was performed with Statistica 7.0 software (StatSoft, Inc.). Tukey’s HSD (honest significant difference) tests were used to analyse differences in cell and chloroplast sizes, cell wall thickness, plasmodesmata frequency, amounts of gold particles, intensities of bands in western blots, and Г and R d values at different stages of leaf development. All analyses were performed at the 95% significance level.
Results
Early events in primary leaf morphogenesis
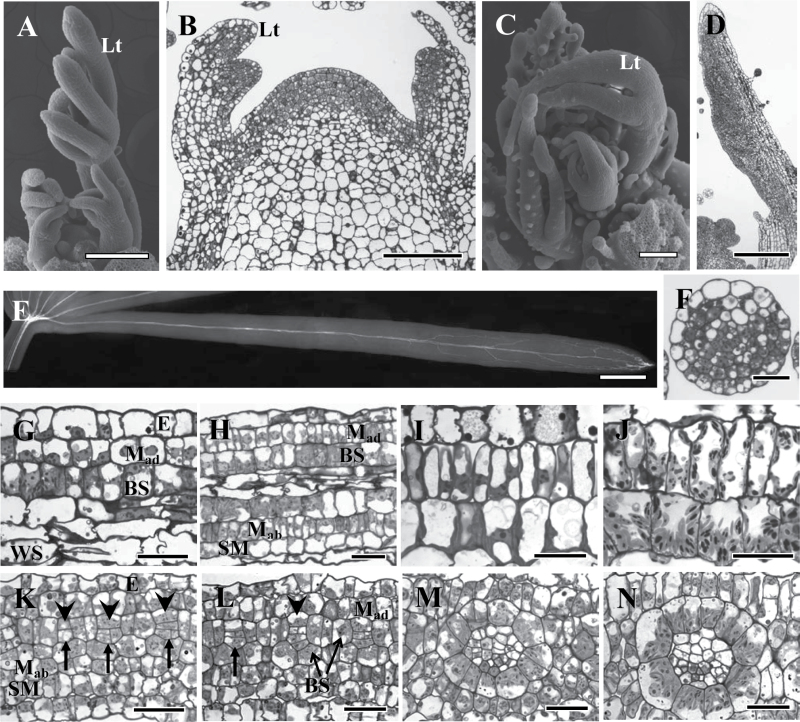
Cleome angustifolia and C. gynandra have palmate compound leaves with up to five flat, broad leaflets in C. gynandra and 5–8 narrow leaflets in C. angustifolia. Figure 1 shows the morphology and anatomy of a vegetative shoot apical meristem of C. angustifolia and C. gynandra at the stage of active organogenesis, together with the early stages of morphogenesis of compound leaves (Fig. 1A–D). Leaf primordia in both species are initiated alternately. When the primordium reaches ~100 μm, the proliferation of marginal and submarginal initials appears at the distal adaxial flank (Fig. 1B), giving rise to the terminal followed by the lateral primordial leaflets (Fig. 1A, C). Leaflets of C. angustifolia grow by meristem initials at the base generating files of cells with restricted marginal growth resulting in narrow leaflets. Laminar growth in the leaflets of C. gynandra is initiated when they reach 0.4–0.5mm long, forming a flat blade (Fig. 1D).
Fig. 1.
Vegetative shoot tip structure and early stages of leaf development in two Cleome species, C. angustifolia (A, B, E–J) and C. gynandra (C, D, K–N). (A, C) Scanning electron microscopy of shoot tips. (B, D) Light microscopy of longitudinal sections of the shoot apical meristem showing initiation and development of the leaflet. (E) Cleared young leaflet of C. angustifolia under UV light showing the basipetal direction of lateral vein development. (F) Cross-section of the young (0.5mm) C. angustifolia leaf. (G–J) Longitudinal sections showing the formation of cell lineages from the base of young leaves (G) to the tip (J), with the direction of maturation from left to right in C. angustifolia. The adaxial double-layered chlorenchyma (M and BS) differentiation is shown at Stages 1 (G), 2 (H, note the abaxial SM delimited), 3 (I), and 4 (J). (K–N) Procambial strand initiation and Kranz anatomy development around the veins from the base of young leaves (K) to the tip (N), with the direction of maturation from left to right in C. gynandra. (K) Procambium initials are indicated by arrows; arrowheads show the first adaxial BS cell progenitor; note the additional abaxial SM layer formed. (L) BS progenitors derived from second and third ground meristem layers are indicated. Different stages of M and BS differentiation are shown for C. gynandra: Stage 1 (L), 2 (M), and 3 (N). E, epidermis; BS, bundle sheath; Lt, leaflet; Mad, adaxial mesophyll; Mab, abaxial mesophyll; SM, spongy mesophyll; WS, water storage. Scale bars=200 μm for A, C, D; 100 μm for B; 0.5mm for E; 20 μm for F–N.
In both species, the processes of tissue differentiation in terminal and lateral leaflets are similar; but they differ in growth dynamics, resulting in different sizes (Fig. 1A, C). Procambial strands of the future major veins are initiated acropetally (developing from the base towards the tip) in leaflets. From analysis of cleared young leaves of different ages, the visualization of lignified elements of xylem confirms an acropetal pattern of differentiation of major veins, and shows a basipetal pattern of peripheral/minor vasculature development. In C. angustifolia, the lateral veins develop from the tip (Fig. 1E). In general, C. gynandra also has a basipetal pattern of secondary vascular system development from the tip of the leaf and the leaf margins to the base; however, veins of different orders have different time courses of initiation, with later development of the higher order veins (not shown).
Leaflet development in compound leaves does not differ from that in simple leaves, as shown earlier for different species (Esau, 1965). The leaflet of Cleome species will be referred to later as ‘leaf’ for simplicity in presentation.
Ontogenetic origin of mesophyll and bundle sheath cells
The origin of M and BS cells was observed in cross- and longitudinal sections at the base of young leaves (leaf length of 0.5–5mm for C. angustifolia and 3–7mm for C. gynandra). In C. angustifolia, M and BS cell precursors originate during the initiation of leaf primordium from the ground meristem, with subsequent division of abaxial M progenitors which give rise to the M layer adjacent to BS cells and an additional layer of abaxial chlorenchyma [precursors of the spongy mesophyll (SM) cell layer]. The middle layers of ground meristem generate water storage tissue and procambial strands. Young leaf primordia (up to 0.5mm) consist of 6–7 layers of ground meristem and the forming central procambial strand (excluding epidermis, Fig. 1F). During the subsequent leaf blade development, the M and BS cells divide only anticlinally, and the developing leaf consists of 7–8 cell layers between epidermises depending on the number of progenitors of water storage cells (Fig. 1H).
In C. gynandra, the youngest leaves (up to 3mm long) and the base of young leaves (7mm long) consist of four layers of ground meristem between the adaxial and abaxial epidermis (not shown), with the abaxial subepidermal cells undergoing periclinal divisions very early in development and forming an additional abaxial layer which will later become SM (Fig. 1K, L). Periclinal division of the ground meristem cells in the second layer leads to initiation of the procambial strand from the abaxial derivative (Fig. 1K, arrows show the different stages of procambial strand formation). The adaxial derivative is the progenitor of the upper BS cell (or 2–4 upper BS cells in the case of subsequent anticlinal divisions; Fig. 1K, arrowheads show the upper BS progenitors). All other BS cells originate from the second and third layers of ground meristem which surround the forming vascular tissues (Fig. 1L). The M is differentiated from the first, second, third, or fourth layer of the ground meristem cells depending on the position of M cells around the ring (on the cross-section) of BS cells.
Development of Kranz anatomy
The formation of Kranz anatomy was studied at the LM and TEM levels using longitudinal sections of intermediate size young leaves. Clear gradual progression in distinct developmental events can be observed along the leaf from the base to the tip. In both species, the meristematic activity is evident near the base of the leaf, followed by BS cell expansion with M cell intensive divisions, resulting in 2–3M cells per BS cell as viewed on the leaf section. In both species the additional abaxial layer of SM cells has not undergone anticlinal divisions, in contrast to their sister abaxial M (Mab) cells which are adjacent to the BS (Fig. 1H, L). After transition of M cells to expansion, the structural differentiation of M and BS cells begins, and close to the leaf tip complete Kranz anatomy is established. Following the initial formation of M and BS cells from progenitor cells, four stages of chlorenchyma development were characterized in the progression towards the formation of the Kranz syndrome.
Stages of chlorenchyma development in C. angustifolia
Development was visualized using files of cells which were continuous from the base to the tip of the 6mm long leaf. In Stage 1 at the meristematic zone of the base of the leaf (0–1mm from the base), M and BS cells are similar in shape, size, and structure (Fig. 1G; Table 1). They can be identified by their position, from the adaxial side of the leaf subepidermal layer for M, and BS for the next layer, with an additional subepidermal layer of SM cells on the abaxial side. At this time M and BS cells undergo anticlinal divisions.
Table 1.
Cell size (length, L, and width, W), thickness of cell walls, size of chloroplasts for mesophyll (M) and bundle sheath (BS) cells, and the number of plasmodesmata in the cell wall between M and BS cells
| Stage of development | Cell size (μm) | Thickness of individual cell wall towards the IAS (μm) | No. of plasmodesmata per μm M/BS cell wall | Chloroplast length (μm) | |||||
|---|---|---|---|---|---|---|---|---|---|
| M | BS | M | BS | M | BS | ||||
| L | W | L | W | ||||||
| Cleome angustifolia | |||||||||
| 1 | 12.5±0.5 a | 9.1±0.5 b | 12.5±0.3 a | 9.7±0.7 a | ND | ND | ND | 1.5±0.07 a | 1.6±0.06 a |
| 2 | 15.4±0.2 b | 5.4±0.3 a | 16.4±0.4 b | 11.1±0.6 a | 0.12±0.009 a | 0.23±0.01 a | 0.53±0.12 a | 1.5±0.08 a | 2.3±0.1 a |
| 3 | 20.3±0.2 c | 4.9±0.2 a | 19.6±0.5 b | 13.1±0.4 b | 0.12±0.02 a | 0.27±0.01 b | 1.25±0.16 b | 2.3±0.08 b | 3.0±0.08 b |
| 4 | 35.6±1.0 d | 8.5±0.4 b | 27.1±0.7 c | 15.6±1.1 c | 0.14±0.006 a | 0.28±0.01 b | 1.1±0.14 b | 5.6±0.2 c | 4.8±0.2 c |
| Cleome gynandra | |||||||||
| 1 | 10.3±0.3 a | 6.6±0.3 a | 9.7±0.3 a | 10.8±0.6 a | ND | ND | ND | 2.5±0.1 a | 2.4±0.1 a |
| 2 | 16.0±0.6 b | 6.7±0.3 a | 13.6±0.5 b | 15.3±0.9 b | 0.08±0.01 a | 0.21±0.02 a | 1.11±0.09 a | 3.5±0.2 a | 3.8±0.2 b |
| 3 | 19.1±0.6 c | 6.4±0.2 a | 15.7±0.5 b | 15.1±1.0 b | 0.10±0.01 a | 0.30±0.02 b | 0.88±0.17 a | 4.6±0.1 b | 5.1±0.2 c |
| 4 | 21.5±0.6 d | 6.5±0.2 a | 18.8±0.6 c | 17.9±0.8 b | 0.10±0.006 a | 0.43±0.02 c | 0.81±0.09 a | 5.6±0.2 c | 7.1±0.2 d |
The average number of partial cell profiles examined was 20.
Different letters indicate significant differences between developmental stages in a column for each species separately, at P≤0.05.
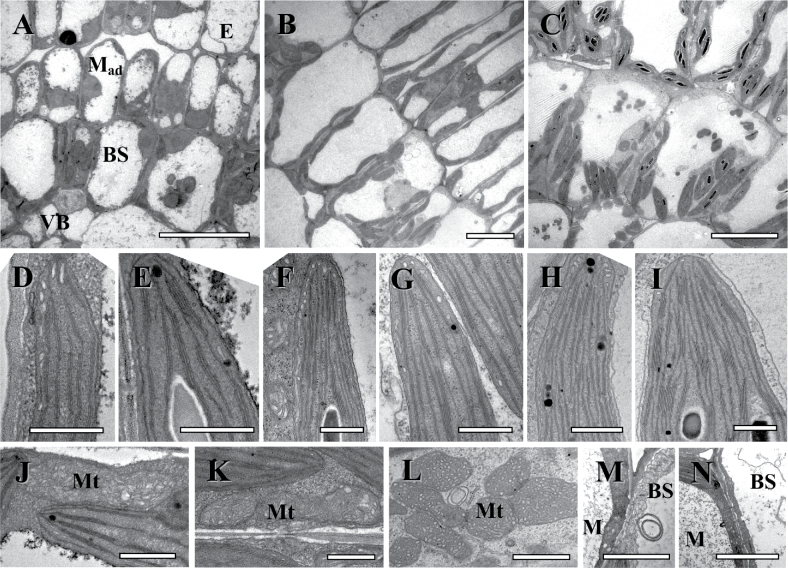
Following the completion of division of M cells, the expansion of M and BS cells is evident (~2–3mm from the base) (Table 1), and this was designated as Stage 2. At this time M and BS cells have a central vacuole, and chloroplasts are distributed around the cell periphery. In M cells, the nucleus often has a central position (Fig. 2A). Chloroplasts have a relatively well-developed thylakoid system with numerous small grana which are structurally similar in both M and BS cells (Fig. 2D, E). Mitochondria in the chlorenchyma cells are few in number, small, and they have crescent-like cristae (Fig. 2J). The cells of all tissues are tightly packed; minor intercellular air spaces (IAS) exist between M and epidermal cells (Fig. 2A). Xylem and phloem elements are not differentiated in veins.
Fig. 2.
Electron microscopy of Cleome angustifolia longitudinal sections showing three structural stages along the leaf from the base to the tip with the undifferentiated cells at the base, developmental Stage 2 (A, D, E, J), through Stage 3 (B, F, G, K, M), and nearly mature cell structure at the tip, Stage 4 (C, H, I, L, N). (A–C) Micrographs show the development of leaf structure and organelle distribution in BS and Mad cells. (D, F, G) Thylakoid system in M chloroplasts. (E, G, I) Thylakoid system in BS chloroplasts. (J–L) Progression in BS mitochondria ultrastructure specialization and size increase. Note the specific cristae in mitochondria in mature BS cells. (M, N) Plasmodesmata between M and BS cells and BS cell wall thickening. E, epidermis; BS, bundle sheath; M, mesophyll; Mad, adaxial mesophyll; Mt, mitochondria; VB, vascular bundle; WS, water storage tissue. Scale bars=10 μm for A–C; 0.5 μm for D–K; 1 μm for L; 2 μm for M, N.
Stage 3 (~3–5.5mm from the base) of chlorenchyma development is characterized by structural differentiation of M and BS. M cells have developed a palisade shape with a well-developed central vacuole and organelles distributed around the cell periphery (Figs 1I, 2B). The characteristic centripetal positioning of organelles in BS cells is established (Fig. 1I, Fig. 2B). BS and M chloroplasts are structurally similar; but, compared with Stage 2, they have become enlarged (Table 1) and have a more developed thylakoid system (Fig. 2F, G). BS mitochondria are co-localized with chloroplasts, and they are also slightly enlarged (Fig. 2K). Intercellular air spaces throughout the M cell layer are more prominent (Figs 1I, 2B). The numbers of plasmodesmata connecting M and BS cells have increased (Fig. 2M; Table 1). At this stage, there are the first signs of vascular tissue differentiation in the minor veins. In most vascular bundles, 1–2 tracheary elements are well developed, while the sieve elements of the phloem are still not completely differentiated as they contain a cytoplasmic layer with organelles delimited by the tonoplast. Mature xylem vessels can be identified by the prominent secondary wall thickening and the lack of cytoplasm. Mature sieve tubes contain only a peripheral layer of cytosol without nuclei, ribosomes, and tonoplasts, with rare occurrence of mitochondria, plastids, and smooth endoplasmic reticulum (not shown).
In Stage 4, observed in the leaf tip (~5.5–6mm from the base), differentiation of chloroplasts and mitochondria is completed and there is full formation of Glossocardioid-type Kranz anatomy. Chloroplasts of BS cells have numerous grana with 5–15 thylakoids in stacks, while M chloroplasts have poorly developed grana usually with 2–3 thylakoids in stacks (Fig. 2H, I). BS mitochondria are larger and more numerous compared with Stage 3, with cristae showing a distinct tubular structure (Fig. 2L). BS organelles maintain their characteristic positioning in the cell. Also, multiple plasmodesmata connect BS cells with M, as was noted in Stage 3 (Fig. 2N; Table 1). Between Stages 2 and 4, there is not a significant difference in the thickness of the BS cell wall towards the IAS; however, the BS cell walls are about twice as thick as those in M cells (Table 1).
Stages of chlorenchyma development in C. gynandra
Compared with C. angustifolia, in C. gynandra the basal meristematic zone is extended, with delimitation of BS cells occurring after 1mm. Development of Kranz anatomy was followed in cross-sections of veins of the highest orders (4 and 5, minor veins) along the longitudinal section from the base, where cell proliferation occurs, to the tip where BS and M cells are differentiated. While in general the veins differentiate basipetally, the differentiation of neighbouring veins alternates according to their order and timing of initiation, with corresponding changes in development of BS cells. On the other hand, M cells have a clear longitudinal developmental gradient from the base to the tip of the leaf, which is independent of the level of vein and BS differentiation.
At the earliest stages near the base of the leaf, the BS cells are identified by their position adjacent to developing vein tissues, and subsequently by their size and roundish shape (Fig. 1K–N). In Stage 1 (~1–3mm from the base), anticlinal and radial cell divisions occur in M and BS cells; the two types of cells have nearly the same appearance, with the BS cells being a little wider (Fig. 1L; Table 1). Vascular bundles are not differentiated and they appear as multiplying procambium cells (Fig. 1L).
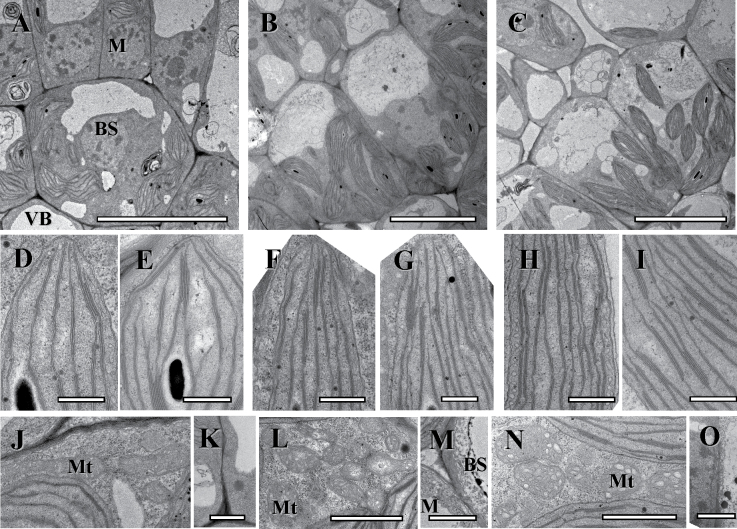
Recognition of Stage 2 in C. gynandra is associated structurally with transition of M cells from division to expansion (~4–5.5mm from the base). BS cells of the highest order veins are characterized by the vacuoles which are asymmetrically forming at the centrifugal pole (Figs 1M, 3A) which ultimately results in the shift of organelles to the centripetal position in Stage 3. As in C. angustifolia, at this stage of development BS and M chloroplasts are enlarged compared with Stage 1 (Table 1) and they have a similar thylakoid system with numerous small grana (Fig. 3D, E). The mitochondria are small with crescent-like cristae (Fig. 3J for BS cell). Most leaf tissues are tightly packed. The BS cell wall is about twice as thick as the M cell wall, and there are multiple plasmodesmata connecting BS and M cells (Table 1). In veins, there is the beginning of differentiation of xylem and phloem elements.
Fig. 3.
Electron microscopy of Cleome gynandra longitudinal sections showing three structural stages along the leaf from the base to the tip, with the undifferentiated cells at the base, Stage 2 (A, D, E, J, K), through Stage 3 (B, F, G, L, M), and nearly mature cell structure at the tip, Stage 4 (C, H, I, N, O). (A–C) Micrographs show the development of leaf structure and organelle distribution in BS cells. (D, F, H) Thylakoid system in M chloroplasts. (E, G, I) Thylakoid system in BS chloroplasts. (J, L, N) Progression in BS mitochondria ultrastructure specialization and size increase. Note the specific cristae in mitochondria in mature BS cells. (K, M, O) Plasmodesmata between M and BS cells and BS cell wall thickening. BS, bundle sheath; Mad, adaxial mesophyll; Mt, mitochondria; VB, vascular bundle. Scale bars=10 μm for A–C; 0.5 μm for D–I; 1 μm for J–O.
At Stage 3 of development (~5.5–6.5mm from the base), M and BS cell structural specialization becomes clearer, with well-developed central vacuoles in the M cells and distinctive centripetal organelle positioning in BS cells (Figs 1N, 3B). Chloroplasts of both cell types have increased in size (Table 1) and they have a similar level of grana development (Fig. 3F, G). Bundle sheath mitochondria have increased in number and are localized towards the inner cell wall (Fig. 3L). At this stage, the BS cell walls have continued to thicken and there is no change in plasmodesmata number per unit of cell wall length between the M and BS (Fig. 3M; Table 1). Also, differentiation of 2–3 xylem tracheary elements and first phloem sieve elements has occurred at this stage of development.
In Stage 4, at the leaf tip (~6.5–7mm from the base), the Atriplicoid type of Kranz anatomy is fully developed. The size of M and BS cells and chloroplasts increased (Table 1). The ultrastructural dimorphism of the chloroplast and mitochondria has formed. Chloroplasts of M cells usually have 2–3 thylakoids in grana stacks, while BS chloroplasts have numerous grana with 4–10 thylakoids in stacks (Fig. 2H, I). BS cells have abundant, large mitochondria with distinct tubular cristae (Fig. 3N). The BS cell wall facing the IAS is four times thicker than in M; the numbers of plasmodesmata between the M and BS are similar to those in Stage 3 (Fig. 3O; Table 1).
Stomatal initiation from epidermal cells occurred along the longitudinal leaf gradient, with a clear progression in the portion of fully differentiated stomata from the base to mid-region to the tip which was similar for the two species (8, 26, and 72%, respectively, in C. angustifolia and 5, 33, and 69%, respectively in C. gynandra) (Supplementary Fig. S1 available at JXB online). Supplementary Fig. S1 shows images of the abaxial surface of the leaves (results were similar for the adaxial surface, not shown).
Immunolabelling along the developmental gradient
The pattern of PEPC, Rubisco LSU, and GDC expression was studied on longitudinal sections of intermediate size leaves (~6–7mm) using the reflected/scanning mode of confocal microscopy. The more precise immunogold technique by TEM was used to analyse the base, mid-region, and tip of the same leaf to evaluate the density of labelling in cellular compartments.
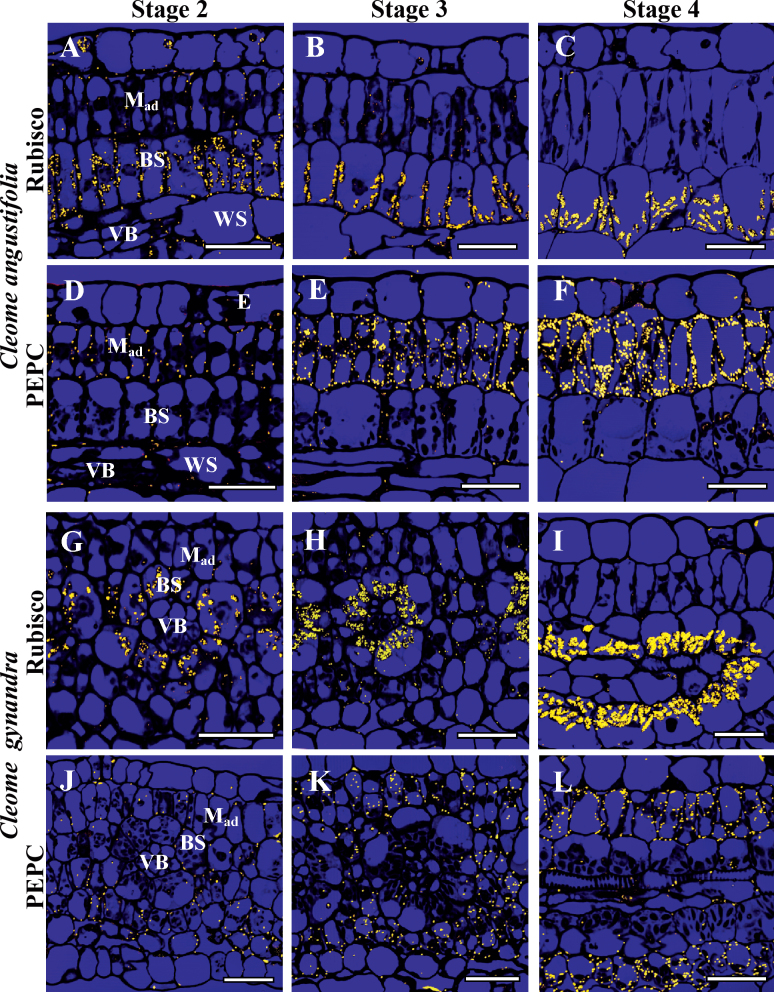
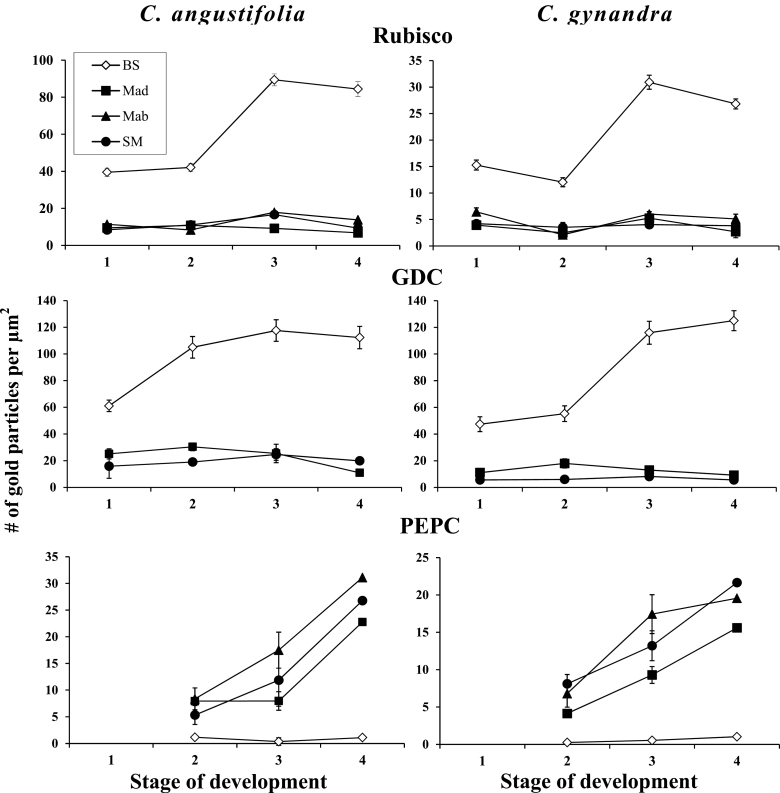
In both Cleome species, Rubisco is strongly associated with BS chloroplasts from Stage 1 when M and BS cells become distinguishable (Figs 4A, C, 5). Rubisco is suppressed not only in chloroplasts in M cells adjacent to BS (Mad and Mab), but also in the SM cells which are not in contact with the BS cells (Figs 4G, H, 5). Rubisco accumulation per BS chloroplast area reaches a maximum level in Stage 3 (Figs 4B, H, 5), and maintains a high level in Stage 4 at the tip of leaves (Figs 4C, I, 5).
Fig. 4.
In situ immunolocalization of Rubisco LSU (A–C, G–I) and PEPC (D–F, J–L) in leaves of Cleome angustifolia (A–F) and C. gynandra (G–L) at three developmental stages. Reflected/transmitted confocal imaging of longitudinal leaf sections shows Rubisco LSU selectively localized to BS cells beginning from developmental Stage 2 (A, G); note labelling in the few chloroplasts in the epidermis and water storage cells (A). The weak labelling for PEPC is M specific from the earliest stages (D, J), with increased labelling beginning from Stage 3 (E, K). At Stage 4, high labelling for Rubisco and PEPC is partitioned to BS and M, respectively. BS, bundle sheath; E, epidermis; Mad, adaxial mesophyll; VB, vascular bundle, WS, water storage. Scale bars=20 μm for A–L.
Fig. 5.
Graphical representation showing the density of immunolabelling for Rubisco LSU (top panels) in BS versus M chloroplasts, GDC (middle panels) in BS versus M mitochondria, and PEPC (bottom panel) in BS versus M cytosol at four stages of leaf development for Cleome angustifolia and C. gynandra. Also, the comparison was made between three types of M cells: Mad, adaxial mesophyll; Mab, abaxial mesophyll cells which are in contact with the BS cells; and SM (spongy mesophyll), an additional M layer on the abaxial side of the leaf which does not have contact with BS. In both graphs, the y-axis represents the number of gold particles per μm2 of chloroplast, mitochondria, or cytosol area, and the x-axis represents the developmental stages. For each cell type and stage of development, 10–15 cell areas were used for counting. BS, bundle sheath; M, mesophyll.
In both species there is selective labelling of GDC in BS mitochondria in Stage 1. In C. angustifolia GDC expression increases and reaches a maximum at Stage 2 of development, which is well before BS cell differentiation and mitochondrial structural specialization. In C. gynandra a steady increase of GDC was shown in parallel with an increase in BS Rubisco levels (Fig. 5) and cell structural maturation.
PEPC was not detected by immunolocalization in Stage 1 (not shown). In Stage 2, at the confocal microscopy level, there is very low M-specific labelling for PEPC in both Cleome species (Fig. 4D, J). The TEM level shows a low and comparable amount of PEPC selectively localized in Mad cells and in both Mab and SM cells (Fig. 5). Both methods show steady accumulation of PEPC preferentially in M cells, with levels reaching a maximum at the tip of the leaf (Stage 4) with little or no difference in particle density between Mab cells adjacent to BS and the SM layers (Figs 4E, F, K, L, 5). Compared with the Mad cells, the expression of PEPC begins earlier in the Mab and SM cells, and usually the density of labelling is higher in these cells at all stages of leaf development (Fig. 5). For C. gynandra, comparative counting of the number of particles in the M cells located around the neighbouring veins of different order (with a different level of BS differentiation) shows no difference (not shown).
Western blot analysis
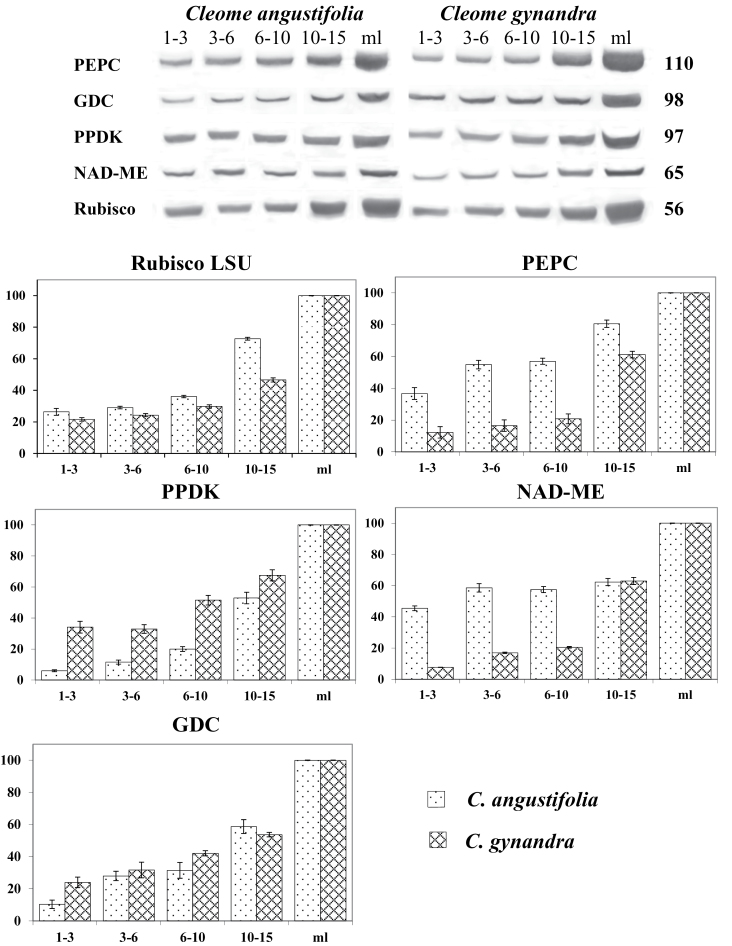
Western blots were performed on extractions of total soluble proteins from individual leaves (young leaves of different lengths, 1–3, 3–6, 6–10, and 10–15mm, and mature leaves, 30–35mm) (Fig. 6). Proteins for the C3 pathway (Rubisco), C4 cycle (PEPC, PPDK, and NAD-ME), and photorespiratory pathway (GDC) were analysed and represented quantitatively (on a soluble protein basis). The youngest leaves (1–3mm) of both Cleome species contain 22–26% of Rubisco (LSU) compared with the mature stage, with a slow increase in leaves up to 6–10mm long and a large increase thereafter. The youngest leaves (1–3mm) of C. gynandra contain a low amount of C4 enzymes; only 12% of PEPC, 6% of PPDK, and 8% of NAD-ME compared with mature leaves on a soluble protein basis; there was a slow rise in levels in leaves up to 10mm long, and a large increase thereafter. In contrast, the youngest leaves of C. angustifolia have a high amount of C4 enzymes which on average is 40% of that in the mature stage, with increasing levels as leaf age progressed. The level of GDC shows a steady increase from the youngest up to mature leaves, with higher expression in C. gynandra than in C. angustifolia (Fig. 6).
Fig. 6.
Western blot analysis showing accumulation of C4 enzymes, GDC, and Rubisco LSU during leaf development in two Cleome species. Total soluble proteins were extracted from individual leaves of different lengths of C. angustifolia and C. gynandra. Blots were probed with antibodies raised against PEPC, GDC, PPDK, NAD-ME, and Rubisco LSU. Top: representative western blots showing detection of each protein. Numbers on the right indicate molecular mass in kilodaltons. Bottom: quantitative representation of western blot data. 100% on the y-axis refers to the level achieved in mature leaves. The x-axis represents the length of the leaves.
CO2 compensation point, dark respiration, and carbon isotope composition in C. gynandra
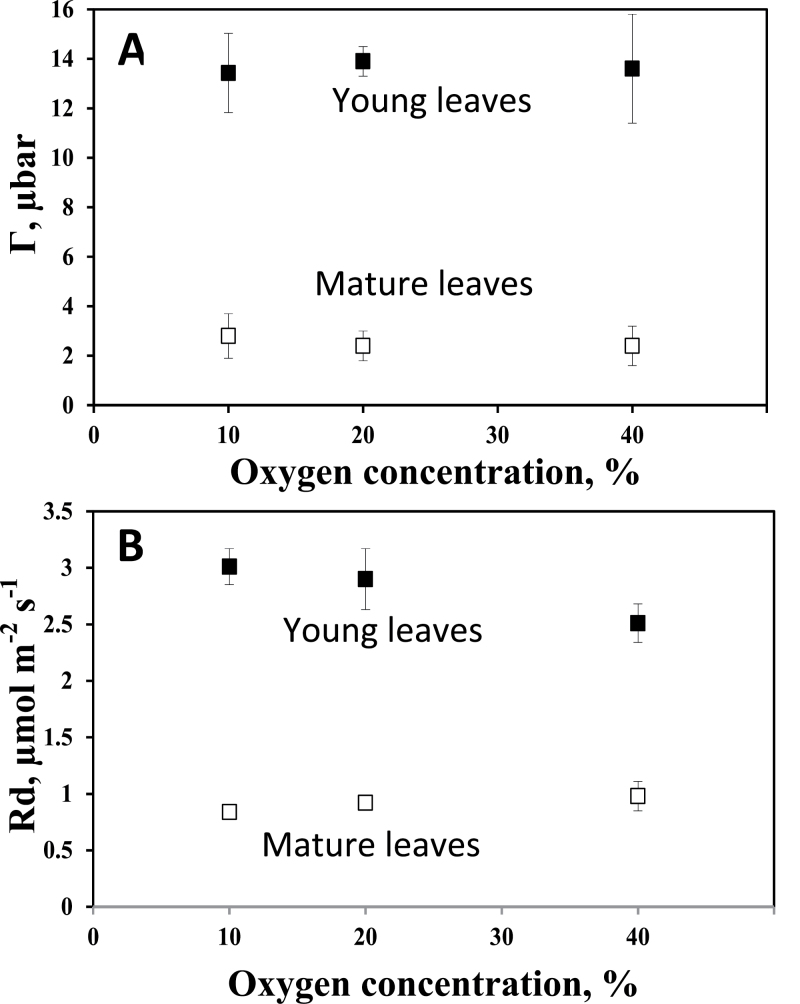
Г and R d were measured at 10, 20, and 40% O2 in young (~7mm) and mature leaves of C. gynandra (Fig. 7). Г was insensitive to increases in O2 in both; however, the mean value of Г across all O2 levels was higher in the young (13.7±1.7 μbar) than in mature leaves (2.6±0.8 μbar). Rates of R d were higher in young than in mature leaves, with mean values across O2 levels of 2.91±0.33 in young versus 0.91±0.08 μmol m–2 s–1 in mature leaves. Excised leaves of C. angustifolia were unstable in the leaf chamber, so it was not possible to obtain steady-state readings with the mass spectrometer. Values of carbon isotope composition (δ13C) measured from leaf sections of C. gynandra at the base, tip, and middle of young leaves were –14.1 (±0.03), –14.1 (±0.02), and –14.7 (±0.08), respectively, compared with –14.6 (±0.12) in mature leaves.
Fig. 7.
The effect of oxygen on the CO2 compensation point (Γ; A) and dark respiration (R d; B) in young and mature leaves of Cleome gynandra. Number of replications n=5–8 for young leaves and n=3–6 for mature leaves. One-way analysis of variance shows that varying O2 had no significant effect on Γ or R d in young or mature leaves.
Discussion
Origin of M and BS cells in C4 Cleome
Cleome angustifolia and C. gynandra have compound leaves with a similar pattern of initiation, but with distinct differences in the patterns of minor veins and Kranz anatomy spatial development. In C. angustifolia, which has a form of Kranz (called Glossocardioid) that consists of a single complex unit, M and BS cells originate from subepidermal layers of ground meristem during leaflet initiation. The pattern of origins of M and BS cells is very close to that in S. taxifolia which has Schoberioid-type anatomy with a similar positioning of two chlorenchyma layers at the leaf periphery (Koteyeva et al., 2011a ). In C. gynandra, which has Atriplicoid-type anatomy with Kranz units around individual veins, the BS cells towards the adaxial side are sister to procambium cells arising from the adaxial derivative of the first periclinal division of the ground meristem cell (the abaxial derivative gives rise to the procambium initial). The rest of the BS cells ontogenetically originate from the ground meristem cells of the second, third, and fourth layers adjacent to the vein. M cells are also derived from the ground meristem having different ontogeny, namely subepidermal and central cells, which is similar to that of A. rosea with Atriplicoid-type anatomy (Liu and Dengler, 1994). In both C. angustifolia and C. gynandra it is difficult to reveal the progenitor cells of the shoot apical meristem due to initiation of leaflets from the primary primordium which is the future petiole. As shown in previous studies, the ontogenetic origin of M and BS cells has no effect on the structural, biochemical, and functional peculiarities of C4 tissues which shows a clear convergent evolution of a highly coordinated functioning of the two-cell system (Dengler et al., 1985, 1986, 1996; Koteyeva et al., 2011a ).
Longitudinal patterns of development in C4 Cleome
The form of Kranz in leaves of C. angustifolia allows all stages of the developmental progression to be studied along one file of chlorenchyma cells from the base to the tip of an intermediate size leaf. A similar basipetal pattern of development along a single lineage of cells has also been observed in two other forms having a single compound Kranz unit (Schoberioid and Salsinoid type in family Chenopodiaceae, Koteyeva et al., 2011a ), and in several C4 monocots with parallel venation (Langdale et al., 1988a ; Soros and Dengler, 2001; Wakayama et al., 2003; Li et al., 2010; Majeran et al., 2010; Nelson, 2011).
In contrast to C. angustifolia, development of Kranz anatomy in leaves of C. gynandra is closely associated with vein differentiation. Having in general a longitudinal basipetal pattern, the initiation of minor veins is sequential in time. Veins at different stages of differentiation in C. gynandra, which are alternate in the longitudinal section, can be easily recognized by size, anatomy, chloroplast ultrastructure, and accumulation of Rubisco in the BS cells. The pattern of vascular development in C. gynandra, and associated progression in specialization of BS cells, resembles that described for C4 A. rosea (Dengler et al., 1995), C4 A. hypochondriacus (Wang et al., 1993), and C4 Flaveria trinervia (McKown and Dengler, 2009).
Stages of C4 development along the leaf
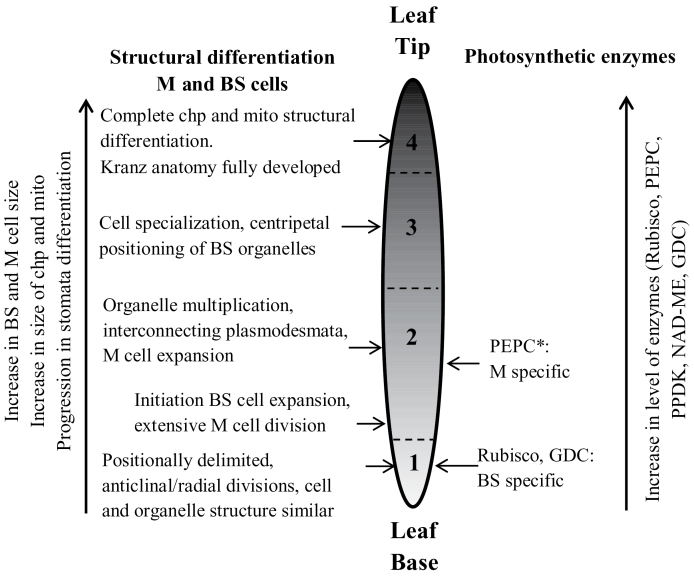
Irrespective of having vastly different Kranz anatomical types, C. angustifolia and C. gynandra have a similar sequence of events during C4 leaf development. However, the lengths of the zones along the leaves where transitions occur are different between the two species, which may be related to differences in anatomy or growth rates. Four notable stages in C4 development were recognized from base to tip of young leaves of both species based on anatomical and ultrastructural features (illustrated in Fig. 8).
Fig. 8.
Illustration of major changes during development of C4 photosynthesis in Cleome angustifolia and C. gynandra from the basal region to the tip of young leaves. The numbers refer to four stages of chlorenchyma development; see text for the corresponding length of the zones for each species. The horizontal arrows point to specific changes occurring in each of the four stages. The vertical arrows indicate continual changes which occur along the longitudinal gradient. BS, bundle sheath, chp, chloroplasts; GDC, glycine decarboxylase; M, mesophyll; mito, mitochondria. *PEPC was selectively localized to M cells in stage 2 (there was insufficient labelling to determine its localization by immunolocalization in stage 1).
Structural development
Stage 1, the beginning of Kranz, is distinguished by M and BS cells which are positionally delimited from meristem precursors, performing anticlinal/radial divisions and having similar shape and structure with ultrastructurally uniform organelles. In Stage 2 there is expansion of M and BS cells, vacuoles are developing, and chloroplasts and mitochondria are replicating and enlarging. The plasmodesmata frequency between M and BS cells is also high at this time. In Stage 3, structural specialization of M and BS begins. The M cells develop a palisade-like shape with a large central vacuole and organelles around the cell periphery. Organelles in BS cells become localized in a centripetal position towards the inner cell wall characteristic of mature Kranz anatomy in C4 Cleome species. The chloroplasts, and the mitochondria, between M and BS cells are structurally similar, indicating an immature stage of development. In Stage 4, full Kranz anatomy has developed, with structurally specialized dimorphic chloroplasts and mitochondria in M and BS cells.
There were some structural parameters which show a continuous change along the leaf gradient. In both species there was an increase in the sizes of M and BS cells, chloroplasts, and mitochondria, and in stomatal development and density (illustrated on the left side of Fig. 8). There was an increase in the length of the M cells with no change in width, while there was an ~2-fold increase in both the length and width of the BS cells. During development there is continual enlargement of M and BS chloroplasts, with an increase in length by ~3-fold in both species. The density of plasmodesmata at the interphase between M and BS cells increases or remains constant (see Table 1) despite the increase in size of M and BS cells (and contact between the two) due to the formation of secondary plasmodesmata during development. This indicates establishment of symplastic connections between M and BS from the earliest stages of development which enables intercellular movement of metabolites for C4 photosynthesis. In both species, BS cell wall thickening, which starts soon after the BS cell become positionally delimited, is considered to contribute to diffusive resistance and limit CO2 leakage from the BS during C4 photosynthesis (von Caemmerer and Furbank, 2003).
Biochemical development
In both species there is a very early cell-specific C4-like pattern of enzyme accumulation. BS cells complete divisions, and initiate cell expansion with multiplication of chloroplasts and mitochondria earlier than M cells which continue to have active anticlinal divisions up to Stage 2. However, biochemical specialization in M and BS begins simultaneously from the meristematic zone in Stage 1. Rubisco and GDC are selectively expressed in chloroplasts and mitochondria, respectively, in BS cells, and at the same time they are suppressed in chloroplasts and mitochondria of the M cells, characteristic of the C4 system. So, while organelles, chloroplasts and mitochondria, do not become ultrastructurally differentiated in BS and M until full maturation of Kranz, biochemical specialization of organelles in the two chlorenchyma cells is occurring already in the meristematic stages right after the layers become positionally delimited. This shows that Rubisco and GDC expression in these two Cleome species is not directly associated with structural differentiation of M and BS cells.
Early cell-specific Rubisco expression during leaf development has also been observed in several other C4 species, including Arundinella hirta (Wakayama et al., 2003), A. rosea (Dengler et al., 1995), S. eltonica, and S. taxifolia (Koteyeva et al., 2011a ). In contrast, a C3 default state with Rubisco appearing initially in both M and BS chloroplasts has been observed in other C4 species, with selective expression of Rubisco in BS depending on light or developmental cues in maize (Sheen and Bogorad, 1985; Langdale et al., 1988b; Sheen, 1999), in A. hypochondriacus (Patel and Berry, 2008), in three structural forms of C4 in family Cyperaceae (Soros and Dengler, 2001), and in Salsola richteri (Voznesenskaya et al., 2003b). Clearly these differences in transitions to C4 suggest evolution of alternative cues for initiation of C4 development; although there may be convergence in factors (transcriptional or transacting) controlling synthesis of components of the C4 system.
PEPC levels were too low to detect in Stage 1. From its earliest detection in Stage 2, it was M specific and the level increased in parallel with M differentiation. In a number of other developmental studies, PEPC gene expression also appears to be initially M specific and increased depending on M differentiation (Langdale et al., 1988a ; Dengler et al., 1995; Stockhaus et al., 1997; Soros and Dengler, 2001; Wakayama et al., 2003; Koteyeva et al., 2011a ).
The results in both C4 Cleome species indicate that intercellular compartmentation for C4 photosynthesis is established early without a C3 default state, that enzyme levels to support the C4 system continue to rise during the four stages of leaf development, and that structural positioning of organelles and differentiation of chloroplasts and mitochondria occur later (Fig. 8). This suggests that development of C4 biochemistry may be a driver for organelle differentiation, namely for M and BS chloroplasts to provide the balance in ATP and NADPH from photochemistry to support the NAD-ME-type C4 cycle (see Edwards and Voznesenskaya, 2011), and for mitochondria to provide the capacity for transport and metabolism to support decarboxylation via NAD-ME. The same pattern of development in these two C4 Cleome species which have very different types of Kranz anatomy and independent evolutionary lineages is consistent with the concept that genes to support the C4 cycle were already primed for recruitment and selective expression in M and BS cells (Brown et al., 2011; Kajala et al., 2012).
A functional analysis of young versus mature leaves of C. gynandra was made by analysing the effect of O2 on Г to determine if the response is indicative of C4, intermediate, or C3 photosynthesis. C4 plants have very low Г (1–5 μbar) while C3 plants have high values of Г (~40–50 μbar at 25 ○C and 20% O2). Additionally, Г in C4 plants has little or no sensitivity to O2, while values in C3 plants approximately double with a 2-fold increases in O2. C3–C4 species have intermediate Г values, which increase with the level of O2 (Krenzer et al., 1975; Brooks and Farquhar, 1985; von Caemmerer, 1989, 2000; Ku et al., 1991; Vogan et al., 2007; Voznesenskaya et al., 2007). Mature leaves of C. gynandra show a clear C4-type response, with values of Г of ~2.5 μbar with insensitivity to O2. In young leaves, the lack of sensitivity of Г to O2, between 10% and 40%, is characteristic of C4 plants. The higher Г values in young leaves (~14 μbar) are probably due to the higher R d and lower V cmax for Rubisco (see western blots), due to the dependence of Г on the R d/V cmax ratio (von Caemmerer, 2000), in addition to its dependence on Rubisco kinetic properties and the level of photorespiration. In plant leaves, R d saturates at relatively low levels of O2 (~2–3% O2), whereas the reaction of O2 with RuBP oxygenase, in competition with CO2, results in an increase in Г in response to higher levels of O2. Thus, in the absence of R d, extrapolated plots of Г versus O2 would intercept at zero, whereas contribution from R d results in the intercept on the y-axis (Brooks and Farquhar, 1985). Thus, the results suggest that young leaves of C. gynandra are functionally C4. A factor which may minimize the occurrence of photorespiration and maintain a lower Г in young leaves is the selective localization of photosynthetic enzymes to M and BS cells for function of C4 early in development. Also, the selective localization of GDC to the BS indicates the presence of conditions favourable for refixation of photorespired CO2 around Rubisco from the very early stages of development. As leaves develop, there is an increase in the level of GDC in the two Cleome species; this may be associated with multiplication and increase in size of BS mitochondria (where GDC is localized), which was observed during development along a longitudinal gradient in young leaves (Stage 3). There is the possibility that sensitivity of photosynthesis to O2 occurs at the base of young leaves, but its contribution to carbon assimilation is expected to be very low (due to low Rubisco, and undeveloped stomata).
Expression of C4 enzymes in M cells which do not have contact with the BS
Both Cleome species have an additional layer of chlorenchyma cells on the abaxial side of leaves (SM) which is ontogenetically related to the abaxial M layer which is adjacent to the BS. They are not associated with BS, and structurally resemble M cells with well-developed chloroplasts. It was shown by confocal immunohistochemistry and TEM immunogold labelling that their cytosol contains PEPC at a similar level to that of adaxial and abaxial M cells which are in contact with BS. The expression of Rubisco and GDC in these distal M cells is suppressed, and structural and biochemical differentiation of these cells occurs in parallel with the M cells in contact with BS.
There are not many cases where C4 plants have more than two M cells between neighbouring BS cells; analysis of 119 C4 grasses (Hattersley and Watson, 1975) showed that no M cell is separated from the nearest BS cell by more than one other M cell (the maximum cell distant count). Based on studies with maize, it has long been considered that M cells differentiate to form a C4-type cell only when they are in contact with BS; and that M cells distant from BS are deprived of PEPC expression and contain Rubisco in chloroplasts (Langdale et al., 1988b). In maize husk having >20 additional cells between BS cells, the structural and biochemical specialization for C4 was shown only in M cells which are in contact with BS cells, which supports this hypothesis (Langdale et al., 1988b; Pengelly et al., 2011). Also, in A. rosea there is an extra layer of abaxial and adaxial cells which are not in contact with the BS cells; Dengler et al. (1995) found that PEPC expression was restricted to the M cells adjacent to BS cells along the whole maturation gradient. However, the authors noted that those cells contain few organelles and are non-photosynthetic (hypodermal cells, probably functioning in water storage). Thus, the results with the two C4 Cleome species, which clearly shows formation of C4-type M cells which are not adjacent to the BS, is an interesting variation on regulation of C4 development.
Signalling for tissue-specific development of BS and M cells
Veins in C4 plants have been considered to play an organizing role in the differentiation of Kranz anatomy as a source of the positional signal which controls the coordinated development of at least two cells in a radius around individual veins, as in C. gynandra, generating the pattern of Vein–BS–M–M–BS–Vein units (Langdale et al., 1988a , b; Nelson and Langdale, 1992; Langdale, 2012) and candidate genes which may be associated with differentiation of Kranz anatomy have been proposed (Wang et al., 2013). This is a logical hypothesis for the leaf structures where the anatomy consists of multiple simple Kranz units around individual veins which occur in many C4 lineages coupled with increased leaf venation during evolution from C3 to C4 species (McKown and Dengler, 2007; Griffiths et al., 2013). However, the pattern of development of Kranz anatomy in the Cleome species in the current study is not compatible with this hypothesis. In C. gynandra, which has Atriplicoid-type anatomy, the structural development of M specialization is not correlated with BS cell development. Rather BS cell development is correlated with stages of differentiation of alternate veins, but not the M cells which have only a basipetal gradient of maturation. Also, the hypothesis of signalling from vascular tissue to control differentiation of the M cells adjacent to BS cells is not consistent with the same expression pattern of C4 in the cells of the abaxial SM layer not adjacent to BS. These two abaxial M layers represent sister cells which originated from the division of the abaxial subepidermal ground meristem; but, their segregation occurs before, or simultaneously with, the start of procambial strand initiation. One could imagine that the ‘M’ signal may come to the progenitor cell before its division, which could explain the existence of PEPC in both M layers; but, in this case, the signal cannot come from the undifferentiated vein. Also, the selective expression of PEPC in the M cells occurs later, during Stage 2, while the selective expression of Rubisco and GDC occurs during Stage 1, which suggests temporal differences in signalling.
With respect to C. angustifolia, recently it was shown that evolution of C4 species having a single compound Kranz unit in C4 Salsola occurs not with increasing vein density, but by increased succulence and development of a layer of BS cells around veins and water storage tissue (Voznesenskaya et al., 2013). For differentiation of tissue for this type of anatomy, veins could not be the centre for a positional signal since they are not evenly distributed relative to the position of Kranz anatomy, and their contact with BS is not essential (Koteyeva et al., 2011a ). The spatial positioning of M and BS cells relative to each other, unlike the positioning of Kranz around individual veins, is essential to the mechanism controlling their differentiation.
Concluding remarks
The results indicate that two C4 Cleome species, which have different Kranz anatomy and evolved independently in family Cleomaceae, have a similar developmental pathway for C4 during structural and biochemical maturation. The ontogenetic programme for this development involves control of cell-specific patterns of mitosis (rates and plane) and cell expansion, division of chloroplasts and mitochondria, structural differentiation and spatial distribution, cell wall modification, and plasmodesmata formation, with all of these characteristics occurring in close coordination with vascular system differentiation. The early partitioning of Rubisco, GDC, and PEPC expression between M and BS in both species shows that factors controlling their cell specificity must be activated as soon as the tissue become delimited. Further regulation occurs during development, with increased levels of expression of photosynthetic enzymes, and structural differentiation of M and BS cells and organelles. The results provide insight into stages to consider for transcriptome and proteomic studies to search for candidate genes which control structural and biochemical transitions in C4 development.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Scanning electron microscopy of the abaxial surfaces of the young leaves of Cleome angustifolia (6mm) and C. gynandra (7mm) showing the level of stomata differentiation in the tip, middle, and base.
Acknowledgements
This material is based upon work supported by the National Science Foundation under funds MCB #1146928, by the Russian Foundation of Basic Research, grant 11-04-01457 and by the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences, Photosynthetic Systems through DE-FG02_09ER16062. We thank C. Cody for plant growth management, and the Franceschi Microscopy and Imaging Center of Washington State University for use of its facilities and staff assistance.
Glossary
Abbreviations:
- BS
bundle sheath
- GDC
glycine decarboxylase
- M
mesophyll
- Mab
abaxial mesophyll
- Mad
adaxial mesophyll
- NAD-ME
NAD-malic enzyme
- PEPC
phosphoenolpyruvate carboxylase
- Rd
dark type respiration
- Rubisco
ribulose 1,5-bisphosphate carboxylase-oxygenase
- SM
spongy mesophyll
- Γ
CO2 compensation point.
References
- Bräutigam A, Kajala K, Wullenweber J, et al. 2011. An mRNA blueprint for C4 photosynthesis derived from comparative transcriptomics of closely related C3 and C4 species. Plant Physiology 155, 142–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A, Farquhar GD. 1985. Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165, 397–406 [DOI] [PubMed] [Google Scholar]
- Brown NJ, Newell CA, Stanley S, Chen JE, Perrin AJ, Kajala K, Hibberd JM. 2011. Independent and parallel recruitment of preexisting mechanisms underlying C4 photosynthesis. Science 331, 1436–1439 [DOI] [PubMed] [Google Scholar]
- Brown WV. 1975. Variations in anatomy, associations, and origin of Kranz tissue. American Journal of Botany 62, 395–402 [Google Scholar]
- Covshoff S, Knerova J, Burgess SJ, Kumpers BMC. 2013. Getting the most out of natural variation in C4 photosynthesis. Photosynthesis Research 119, 157–167 [DOI] [PubMed] [Google Scholar]
- Dengler NG, Dengler RE, Donnelly PM, Filosa MF. 1995. Expression of the C4 pattern of photosynthetic enzyme accumulation during leaf development in Atriplex rosea (Chenopodiaceae). American Journal of Botany 82, 318–327 [Google Scholar]
- Dengler NG, Dengler RE, Hattersley PW. 1985. Differing ontogenetic origins of PCR (Kranz) sheaths in leaf blades of C4 grasses (Poaceae). American Journal of Botany 72, 284–302 [Google Scholar]
- Dengler NG, Dengler RE, Hattersley PW. 1986. Comparative bundle sheath and mesophyll differentiation in the leaves of the C4 grasses Panicum effusum and P. bulbosum . American Journal of Botany 73, 1431–1442 [Google Scholar]
- Dengler NG, Donnelly PM, Dengler RE. 1996. Differentiation of bundle sheath, mesophyll, and distinctive cells in the C4 grass Arundinella hirta (Poaceae). American Journal of Botany 83, 1391–1405 [Google Scholar]
- Dengler NG, Nelson T. 1999. Leaf structure and development in C4 plants. In: Sage RF, Monson RK, eds. C4 plant biology. Physiological ecology series. San Diego: Academic Press, 133–172 [Google Scholar]
- Edwards GE, Voznesenskaya EV. 2011. C4 photosynthesis: Kranz forms and single-cell C4 in terrestrial plants. In: Raghavendra AS, Sage RF, eds. C4 photosynthesis and related CO2 concentrating mechanisms. Dordrecht, The Netherlands: Springer, 29–61 [Google Scholar]
- Edwards GE, Walker DA. 1983. C3, C4: mechanisms, and cellular and environmental regulation, of photosynthesis. Oxford: Blackwell Scientific publications; [DOI] [PubMed] [Google Scholar]
- Esau K. 1965. Plant anatomy. New York: Wiley Interscience [Google Scholar]
- Feodorova TA, Voznesenskaya EV, Edwards GE, Roalson EH. 2010. Biogeographic patterns of diversification and the origins of C4 in Cleome (Cleomaceae). Systematic Botany 35, 811–826 [Google Scholar]
- Griffiths H, Weller G, Toy LFM, Dennis RJ. 2013. You’re so vein: bundle sheath physiology, phylogeny and evolution in C3 and C4 plants. Plant, Cell and Environment 36, 249–261 [DOI] [PubMed] [Google Scholar]
- Hatch MD. 1987. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta 895, 81–106 [Google Scholar]
- Hattersley PW, Watson L. 1975. Anatomical parameters for predicting photosynthetic pathways of grass leaves: ‘the maximum lateral count’ and ‘the maximum cells distant count’. Phytomorphology 25, 325–333 [Google Scholar]
- Kajala K, Brown NJ, Williams BP, Borrill P, Taylor LE, Hibberd JM. 2012. Multiple Arabidopsis genes primed for recruitment into C4 photosynthesis. The Plant Journal 69, 47–56 [DOI] [PubMed] [Google Scholar]
- Koteyeva NK, Voznesenskaya EV, Berry JO, Chuong SDX, Franceschi VR, Edwards GE. 2011a. Development of structural and biochemical characteristics of C4 photosynthesis in two types of Kranz anatomy in genus Suaeda (family Chenopodiaceae). Journal of Experimental Botany 62, 3197–3212 [DOI] [PubMed] [Google Scholar]
- Koteyeva NK, Voznesenskaya EV, Roalson EH, Edwards GE. 2011b. Diversity in forms of C4 in the genus Cleome (Cleomaceae). Annals of Botany 107, 269–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenzer EG, Moss DN, Crookston RK. 1975. Carbon dioxide compensation points of flowering plants. Plant Physiology 56, 194–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku MS, Wu J, Dai Z, Scott RA, Chu C, Edwards GE. 1991. Photosynthetic and photorespiratory characteristics of Flaveria species. Plant Physiology 96, 518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdale JA. 2012. C4 cycles: past, present, and future research on C4 photosynthesis. The Plant Cell 23, 3879–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdale JA, Rothermel BA, Nelson T. 1988a. Cellular pattern of photosynthetic gene expression in developing maize leaves. Genes and Development 2, 106–115 [DOI] [PubMed] [Google Scholar]
- Langdale JA, Zelitch I, Miller E, Nelson T. 1988b. Cell position and light influence C4 versus C3 patterns of photosynthetic gene expression in maize. EMBO Journal 7, 3643–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Ponnala L, Gandotra N, et al. 2010. The developmental dynamics of the maize leaf transcriptome. Nature Genetics 42, 1060–1067 [DOI] [PubMed] [Google Scholar]
- Liu Y, Dengler NG. 1994. Bundle sheath and mesophyll cell differentiation in the C4 dicotyledon Atriplex rosea: quantitative ultrastructure. Canadian Journal of Botany 72, 644–657 [Google Scholar]
- Long JJ, Berry JO. 1996. Tissue-specific and light-mediated expression of the C4 photosynthetic NAD-dependent malic enzyme of amaranth mitochondria. Plant Physiology 112, 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeran W, Friso G, Ponnala L, et al. 2010. Structural and metabolic transitions of C4 leaf development and differentiation defined by microscopy and quantitative proteomics. The Plant Cell 22, 3509–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DM, Muhaidat R, Brown NJ, Liu Z, Stanley S, Griffiths H, Sage RF, Hibberd JM. 2007. Cleome, a genus closely related to Arabidopsis, contains species spanning a developmental progression from C3 to C4 photosynthesis. The Plant Journal 51, 886–896 [DOI] [PubMed] [Google Scholar]
- Maxwell K, Badger MR, Osmond CB. 1998. A comparison of CO2 and O2 exchange patterns and the relationship with chlorophyll fluorescence during photosynthesis in C3 and CAM plants. Functional Plant Biology 25, 45–52 [Google Scholar]
- McKown AD, Dengler NG. 2007. Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae). American Journal of Botany 94, 382–399 [DOI] [PubMed] [Google Scholar]
- McKown AD, Dengler NG. 2009. Shifts in leaf vein density through accelerated vein formation in C4 Flaveria (Asteraceae). Annals of Botany 104, 1085–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhaidat R, Sage RF, Dengler NG. 2007. Diversity of Kranz anatomy and biochemistry in C4 eudicots. American Journal of Botany 94, 362–381 [DOI] [PubMed] [Google Scholar]
- Nelson T. 2011. The grass leaf developmental gradient as a platform for a systems understanding of the anatomical specialization of C4 leaves. Journal of Experimental Botany 62, 3039–3048 [DOI] [PubMed] [Google Scholar]
- Nelson T, Langdale JA. 1992. Developmental genetics of C4 photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 43, 25–47 [Google Scholar]
- Newell CA, Brown NJ, Liu Z, Pflug A, Gowik U, Westhoff P, Hibberd JM. 2010. Agrobacterium tumefaciens-mediated transformation of Cleome gynandra L., a C4 dicotyledon that is closely related to Arabidopsis thaliana . Journal of Experimental Botany 61, 1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Berry JO. 2008. Rubisco gene expression in C4 plants. Journal of Experimental Botany 59, 1625–1634 [DOI] [PubMed] [Google Scholar]
- Pengelly JJL, Kwasny S, Bala S, Evans JR, Voznesenskaya EV, Koteyeva NK, Edwards GE, Furbank RT, von Caemmerer S. 2011. Functional analysis of corn husk photosynthesis. Plant Physiology 156, 503–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter G, Katinas L. 2003. A new type of Kranz anatomy in Asteraceae. Australian Journal of Botany 51, 217–226 [Google Scholar]
- Ramsperger VC, Summers RG, Berry JO. 1996. Photosynthetic gene expression in meristems and during initial leaf development in a C4 dicotyledonous plant. Plant Physiology 111, 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska SA, Badger MR, Andrews TJ, von Caemmerer S. 2000. Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: little evidence for significant Mehler reaction. Journal of Experimental Botany 51, 357–368 [DOI] [PubMed] [Google Scholar]
- Sage RF, Christin P-A, Edwards EJ. 2011. The C4 plant lineages of planet Earth. Journal of Experimental Botany 62, 3155–3169 [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47 [DOI] [PubMed] [Google Scholar]
- Sheen J-Y, Bogorad L. 1985. Differential expression of the ribulose bisphosphate carboxylase large subunit gene in bundle sheath and mesophyll cells of developing maize leaves is influenced by light. Plant Physiology 79, 1072–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. 1999. C4 gene expression. Annual Review of Plant Physiology and Plant Molecular Biology 50, 187–217 [DOI] [PubMed] [Google Scholar]
- Soros CL, Dengler NG. 2001. Ontogenetic derivation and cell differentiation in photosynthetic tissues of C3 and C4 Cyperaceae. American Journal of Botany 88, 992–1005 [PubMed] [Google Scholar]
- Stockhaus J, Schlue U, Koczor M, Chitty JA, Taylor WC, Westhoff P. 1997. The promoter of the gene encoding the C4 form of phosphoenolpyruvate carboxylase directs mesophyll-specific expression in transgenic C4 Flaveria spp. The Plant Cell 9, 479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogan PJ, Frohlich MW, Sage RF. 2007. The functional significance of C3–C4 intermediate traits in Heliotropium L. (Boraginaceae): gas exchange perspectives. Plant, Cell and Environment 30, 1337–1345 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. 1989. A model of photosynthetic CO2 assimilation and carbon-isotope discrimination in leaves of certain C3–C4 intermediates. Planta 178, 463–474 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. 2000. Biochemical models of leaf photosynthesis. Collingwood, Australia: CSIRO Publishing [Google Scholar]
- von Caemmerer S, Furbank RT. 2003. The C4 pathway: an efficient CO2 pump. Photosynthesis Research 77, 191–207 [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Edwards GE, Kiirats O, Artyusheva EG, Franceschi VR. 2003a. Development of biochemical specialization and organelle partitioning in the single celled C4 system in leaves of Borszczowia aralocaspica (Chenopodiaceae). American Journal of Botany 90, 1669–1680 [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Artyusheva EG, Black CC, Jr, Pyankov VI, Edwards GE. 2003b. Development of the C4 photosynthetic apparatus in cotyledons and leaves of Salsola richteri (Chenopodiaceae). International Journal of Plant Sciences 164, 471–487 [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Akhani H, Roalson EH, Edwards GE. 2013. Structural and physiological analyses in Salsoleae (Chenopodiaceae) indicate multiple transitions among C3, intermediate and C4 photosynthesis. Journal of Experimental Botany 64, 3583–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voznesenskaya E, Koteyeva NK, Chuong SDX, Ivanova AN, Barroca J, Craven L, Edwards GE. 2007. Physiological, anatomical and biochemical characterization of the type of photosynthesis in Cleome species (Cleomaceae). Functional Plant Biology 34, 247–267 [DOI] [PubMed] [Google Scholar]
- Wakayama M, Ueno O, Ohnishi J. 2003. Photosynthetic enzyme accumulation during leaf development of Arundinella hirta, a C4 grass having Kranz cells not associated with vascular tissues. Plant and Cell Physiology 44, 1330–1340 [DOI] [PubMed] [Google Scholar]
- Wang J-L, Klessig DF, Berry JO. 1992. Regulation of C4 gene expression in developing amaranth leaves. The Plant Cell 4, 173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-L, Turgeon R, Carr JP, Berry JO. 1993. Carbon sink-to-source transition is coordinated with establishment of cell-specific gene expression in a C4 plant. The Plant Cell 5, 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Kelly S, Fouracre JP, Langdale JA. 2013. Genome-wide transcript analysis of early maize leaf development reveals gene cohorts associated with the differentiation of C4 Kranz anatomy. The Plant Journal 75, 656–670 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.