Summary
Phylogenetic analysis of independent gains of C4 photosynthesis in Suaedoideae shows differences compared with other C4 lineages in amino acids of phosphoenolpyruvate carboxylase under positive selection and their position relative to functional residues.
Key words: Bienertia, C4 photosynthesis, PAML, phosphoenolpyruvate carboxylase, positive selection analysis, Suaeda aralocaspica, Suaedoideae.
Abstract
In subfamily Suaedoideae, four independent gains of C4 photosynthesis are proposed, which includes two parallel origins of Kranz anatomy (sections Salsina and Schoberia) and two independent origins of single-cell C4 anatomy (Bienertia and Suaeda aralocaspica). Additional phylogenetic support for this hypothesis was generated from sequence data of the C-terminal portion of the phosphoenolpyruvate carboxylase (PEPC) gene used in C4 photosynthesis (ppc-1) in combination with previous sequence data. ppc-1 sequence was generated for 20 species in Suaedoideae and two outgroup Salsola species that included all types of C4 anatomies as well as two types of C3 anatomies. A branch-site test for positively selected codons was performed using the software package PAML. From labelling of the four branches where C4 is hypothesized to have developed (foreground branches), residue 733 (maize numbering) was identified to be under positive selection with a posterior probability >0.99 and residue 868 at the >0.95 interval using Bayes empirical Bayes (BEB). When labelling all the branches within C4 clades, the branch-site test identified 13 codons to be under selection with a posterior probability >0.95 by BEB; this is discussed considering current information on functional residues. The signature C4 substitution of an alanine for a serine at position 780 in the C-terminal end (which is considered a major determinant of affinity for PEP) was only found in four of the C4 species sampled, while eight of the C4 species and all the C3 species have an alanine residue; indicating that this substitution is not a requirement for C4 function.
Introduction
Phosphoenolpyruvate carboxylase (PEPC) (EC 4.1.1.31) plays an important biochemical role in higher plants by converting bicarbonate (HCO3 –) and phosphoenolpyruvate (PEP), in the presence of Mg2+ or Mn2+, into the four-carbon acid oxaloacetate (OAA) and Pi (O’Leary, 1982; Chollet et al., 1996; Izui et al., 2004). OAA is readily reduced into the more stable product malate or transaminated to aspartate. Plants that have high levels of PEPC protein in their leaves to generate a pool of aspartate or malate as intermediate products of photosynthesis are known as C4 or CAM (Crassulacean acid metabolism) species, as opposed to C3 species that use PEPC primarily in an anaplerotic role. C4 and CAM plants subsequently de-carboxylate the pool of C4 acids, distally or temporally, respectively, to increase the concentration of CO2 around Rubisco. Thus, all plant genomes encode several paralogues of PEPC, with only one orthologue being used in the C4 pathway (Hermans and Westhoff, 1990; Lepiniec et al., 1994; Chollet et al., 1996; Svensson et al., 2003; Gowik et al., 2006; Rao et al., 2006; Christin et al., 2007, 2011; Besnard et al., 2009). The function of non-C4 PEPC paralogues has been summarized recently (O’Leary et al., 2011); they have a role in many plant functions, such as the regulation of stomatal movement, seed development, seed germination, root excretion for abiotic stress acclimation, energy production, carbon storage, nitrogen fixation, and an anaplerotic role in the citric acid cycle. Given the physiological importance of PEPC for C4 photosynthesis, numerous studies have shown that PEPC used for C4 biochemistry has distinct kinetic differences in comparison with orthologous genes (Ting and Osmond, 1973; Blasing et al., 2000, 2002; Gowik et al., 2006; Lara et al., 2006; Jacobs et al., 2008; Rao et al., 2008). The exact amino acid residues that are responsible for the various observed kinetic difference is still being resolved in order to explain further how PEPC kinetics impact the flux of CO2 assimilation through the C4 pathway under a wide range of changing conditions.
PEPC in vascular plants is functional as a homodimer of dimers composed of subunits with a molecular mass of 95–116kDa, and is allosterically regulated by the metabolic context of the enzyme (Kai et al., 2003). At physiological pH, PEPC is activated by it substrate Mg-PEP (Tovar-Mendez et al., 1998), phosphorylated sugars such as glucose 6-phosphate (G6P) (Coombs et al., 1973; Wong and Davies, 1973), and neutral amino acids such as glycine, alanine, and serine (Nishikido and Takanashi, 1973; Bandarian et al., 1992; Tovar-Mendez et al., 2000). Dicarboxylic acids such as the downstream products malate and aspartate negatively inhibit PEPC activity (Huber and Edwards, 1975). Enzyme activity can also be modified by phosphorylation on a conserved N-terminal serine residue, which causes a decrease in affinity for dicarboxylic acids and an increase in affinity for PEP (Jiao and Chollet, 1988; Tovar-Mendez et al., 1998). A protein crystal structure for PEPC from Escherichia coli, Zea mays (C4), Flaveria pringlei (C3), and F. trinervia (C4) has been resolved to help facilitate understanding of the relationship of amino acid substitutions to PEPC kinetics (Kai et al., 1999; Matsumura et al., 2002; Paulus et al., 2013). Identifying amino acids in the PEPC protein that are under the most selective pressure after being recruited for use in C4 biochemistry will further elucidate what changes can potentially enhance C4 photosynthesis, potential metabolic limitations, and the regulatory network underlying plant adaptation.
The appearance of C4 biochemistry has occurred at least 62 independent times in angiosperms (36 lineages in eudicots, six in the sedges, and 18 in the grasses), making it one of the most common convergent processes studied to date (Sage et al., 2011). Among dicot families, the Chenopodiaceae has the largest number of C4 species with the greatest diversity in leaf anatomy, with Kranz anatomy and single-cell C4 species as well as C3 species (Kadereit et al., 2003; Edwards and Voznesenskaya, 2011). All of the Chenopodiaceae C4 genera except Atriplex are in the Salicorniodeae/Suaedoideae/Salsoloideae/Camphorosmoideae (Kadereit et al., 2003; Kadereit and Freitag, 2011). The three different types of C3 leaf anatomy within the genus Suaeda are characterized according to their sections: Brezia, Vera, and Schanginia (Schütze et al., 2003). There are hypothesized to be four independent origins of C4 photosynthesis within the Suaedoideae, including two separate origins of distinctive Kranz C4 anatomies, in Suaeda sections Salsina sensu lato (s.l.) and Schoberia, and two independent origins of unique single-cell C4 anatomy, in Bienertia and in Suaeda aralocaspica (Kapralov et al., 2006).
Nomenclature for PEPC in higher plants is varied throughout the literature, an artifact that is attributable to the numerous independent characterizations of PEPC genes, independent gene and genome duplication events that occurred after species divergence, as well as non-standardized nomenclature. In the grasses, there are four PEPC genes that have been predominantly characterized, with the PEPC gene that is most often recruited for use in C4 photosynthesis being named initially ppc-C 4 and subsequently ppc-B2 (Christin et al., 2007). In the sedges, there are five PEPC genes that have been predominantly characterized, with the gene being recruited for use in C4 being labelled ppc-1 (Besnard et al., 2009). In Arabidopsis there are four PEPC genes that were arbitrarily numbered 1–4 (Sanchez and Cejudo, 2003). One of the earliest dicot C4 PEPC genes analysed was in Flaveria (Asteraceae), where three genes were identified and labelled A, B, and C, with the ppc-A gene being identified as the gene recruited for use in the C4 photosyntehic pathway (Hermans and Westhoff, 1990; Engelmann et al., 2003). Alphabetical nomenclature for PEPC genes was subsequently used in Alternthera (Amaranthaceae) where the three characterized PEPC genes were phylogenetically sister to the Flaveria PEPC genes (Gowik et al., 2006). More recent phylogenetic analysis shows that there are two paralogous eudicot PEPC genes, with the one most often being recruited for use in the C4 photosynthetic pathway being labelled ppc-1, with the exception of Flaveria where the apparent loss of the ppc-1 gene required the recruitment of a twice duplicated ppc-2 gene (Christin et al., 2011). Here the nomenclature of Christin et al. (2011) is followed, since this is to date the most detailed phylogenetic study of eudicot PEPC genes, even though only two of the 3–5 eudicot PEPC genes are presented.
Genes from closely related species tend to have a high amino acid homology, and in the case of PEPC, ~10% (or ~100 residues) are invariant across all PEPC genes (Nakamura et al., 1995). Orthologous protein variation is the result of changes to the nucleotide sequence that alter the codon and resulting amino acid. Changes to the coding sequence of a gene are often classified as being either a non-synonmous (dN) substitution that alters the resulting amino acid, or a synonymous (dS) substitution that changes the codon but does not change the amino acid. The direction of selective pressure at each amino acid residue is determined by comparing the rates of dN and dS substitutions across orthologous proteins, usually expressed as the ratio of dN to dS (ω=dN/dS). Equal rates of both types of substitutions (ω=1) suggest neutral evolution or low selective pressure for a specific amino acid at that residue. A low number of dN substitutions relative to the number of dS substitutions (ω<1) indicates purifying selection pressure against changes to the amino acid present. A high number of dN substitutions relative to dS substitutions (ω>1) suggests that the new amino acid present offers some fitness advantage probably associated with adaptive change (Yang and Nielsen, 2002; Huelsenbeck and Dyer, 2004). At the molecular level, there are functional constraints across the protein, so the type of selective pressure at each residue is generally purifying. In most proteins, a functional difference is often the result of positive selection at only a few sites (Yang et al., 2005; Christin et al., 2008; Kapralov et al., 2012). Phylogenetic analysis in the grasses showed that ppc-B2 was recruited eight independent times for use in the C4 pathway (Christin et al., 2007). During this switch to C4, 21 amino acids evolved under positive selection and converged to similar or identical amino acids, some of which have also been recorded in non-grass C4 species (Blasing et al., 2000; Gowik et al., 2006; Christin et al., 2007, 2011; Besnard et al., 2009). Such convergence at some sites, such as serine at position 780, appears to reflect the need for specific amino acid residues for C4 function, whereas at other sites there appears to be a requirement for loss of the C3-associated amino acid. These substitutions are thought to optimize PEPC for C4 photosynthetic function.
The specific aims of this study are to: (i) find additional phylogenetic support for the four origins of C4 photosynthesis in Suaedoideae by using PEPC sequence data; (ii) identify which PEPC paralogous gene is being recruited for use in C4 photosynthesis in comparison with other previously characterized PEPC genes; (iii) identify any amino acids under positive selection in the PEPC gene used in C4 photosynthesis; and (iv) determine the spatial location of positively selected amino acids in relation to catalytic and allosteric regulatory amino acids.
Materials and methods
Plant material
All plants used in this study were started from seed and were grown in controlled environmental chambers (Econair GC-16; Bio Chambers). Seedlings were started under lower light [100 photosynthetic photon flux density (PPFD; μmol quanta m–2 s–1)] and temperature conditions with a day/night temperature of 25/22 °C and a photoperiod of 14/10h, and then moved to high light and temperature conditions (1000 PPFD, with a day/night temperature of 35/25 °C and a photoperiod of 14/10h) once plants were well established. A few leaves from 2- to 6-month-old plants were used for DNA extraction.
DNA sequencing
The PEPC gene ppc-1 was sequenced for two Bienertia species and 18 Suaeda species, with two Salsola species sequenced as outgroups for the selection analyses. The PEPC gene ppc-2 was sequenced for 12 Suaeda species. DNA was extracted from 250mg of plant material using the CTAB (cetyltrimethylammonium bromide) method following the protocol of Doyle and Doyle (1987). Primers were developed to similar regions previously analysed for positive selection in order to amplify exons 8–10 (Supplementary Table S1 available at JXB online). Initial PCR conditions were 2min at 95 °C, followed by 35 cycles of: 30 s at 95 °C, a 30 s 52 °C annealing step, and a 3min extension at 72 °C. The PCR product was visualized and purified using a PCR clean-up kit according to the manufacturer’s protocol (Qiagen, USA). Purified PCR product was cloned into pGEM T-easy vector using the manufacturer’s protocol (Promega, USA). Single colonies were grown overnight and plasmid DNA was purified using alkaline lysis with SDS (Sambrook and Russell, 2001). Plasmid inserts were PCR amplified using GOTaq (Promega, USA), and Sp6 and T7 primers, and were visualized on a gel. Prior to sequencing, the PCR product was mixed with 2.5U of antarctic phosphatase and 4U of exo-sap nuclease in antarctic phosphatase buffer (New England BioSciences, USA) to degrade primers and nucleotides, and subsequently diluted 1:10. Sequencing reactions were performed using the Big Dye terminator master mix v3.1 (Applied BioSciences, USA), using sequence-specific internal primers along with Sp6 and T7 (Supplementary Table S1). Sequencing was carried out by Operon Sequences (USA) and at Washington State University genomics core. Sequence data were assembled using Sequencher software (USA). Nucleotide sequences were translated, aligned, and visualized using Se-Al and MacVector (USA). All sequences were deposited in GenBank (Supplementary Table S2).
Phylogenetic analyses
Three DNA sequence data matrices were analysed in this study: (i) the previously published matrix of the nuclear ribosomal internal transcribed spacer (ITS), along with the chloroplast intergenic spacers of atpB-rbcL, and psbB-psbH from Kapralov et al. (2006) (Supplementary Table S3 at JXB online), combined with the third nucleotide from each codon and introns for ppc-1 from the subsample of the species sampled; (ii) ppc-1 and ppc-2 coding sequence from Suaedoideae sequenced here and other eudicot sequences from GenBank; and (ii) ppc-1 third position sites and introns of the suaedoids sequenced in this study with two ppc-1 Salsola samples used as the outgroup. Alignments of PEPC genes and their introns were conducted with MUSCLE (Edgar, 2004), and visually inspected using Se-Al. Maximum likelihood (ML) analyses were performed using the GTRGAMMA model in RAxML version 7.2.8 (Stamatakis, 2006; Silvestro and Michalak, 2012) with 1000 bootstraps using multithreading on four cores.
Positive selection analysis
Positive selection analysis used the ML tree from the ppc-1 suaedoid data set analysis described above, consisting of eight C3 species, nine C4 Kranz species, and three single-cell C4 species, of which Bienertia sinuspersici had two ppc-1 accessions. Two Salsola species (one C3 and one C3–C4 intermediate) were used as outgroups. To test for positive selection at particular sites of the ppc-1 gene, the codeml program in the PAML v4.4 package was used to perform likelihood ratio tests (LRTs) to identify the best model for codon change while concurrently identifying dN amino acid changes that are under positive selection (Yang, 2007). Each model adds additional parameters to try and fit the data better by assuming similar ω values either across all of the phylogeny (site test) or on pre-specified C4 branches only (branch-site test). A comparison of LRT scores shows which model fits the data the best. Details of each model are as follows. Model M0 allows for a single ω value across the whole phylogenetic tree at all sites. Subsequent models allow ω to vary at different sites. Model M1a (nearly neutral) allows for two rates of ω to vary between 0 and 1, while Model M2a (positive selection) is the same as Model M1a but allows for an additional rate of ω to be >1. Model M8a assumes a discrete beta distribution for ω, which is constrained between 0 and 1 including a class with ω=1, similar to Model M8 which allow the same distribution as M8a but has an extra class under positive selection with ω>1. Branch-site tests, using pre-specified branches where changes associated with C4 photosynthesis are hypothesized to have occurred (foreground branches), were made with the null Model A1. This allows ω ratios to vary among sites and among lineages, and it also provides two additional classes of codons with ω=1 along pre-specified foreground branches, while restricting ω to be ≤1, on background branches. The alternative Model A allows ω to vary between 0 and 1, be equal to 1 for all branches, and also has two additional classes of codons under positive selection with ω>1 along pre-specified foreground branches while restricting ω to either 0–1 or ω=1 on background branches. C4 lineages were marked as foreground branches.
For all LRTs, the null model is a simplified version of the selection model, with fewer parameters, and is thus expected to provide a poorer fit to the data (lower maximum likelihood). The null models (M1a, M8a, and A1) do not allow codons with ω>1, whereas the selection models (M2a, M8, and A) are alternative models that allow for codons with ω>1. The significance of the LRTs was calculated assuming that twice the difference in the log of maximum likelihood between the two models was distributed as a χ2 distribution with the degrees of freedom (df) given by the difference in the number of parameters in the two types of models (Yang and Nielsen, 2002; Yang and Swanson, 2002). For the M1a–M2a comparison df=2, and for M8a–M8 and A1–A comparisons df=1. Each LRT was run at least twice using different initial omega values to test for suboptimal local peaks. To identify amino acid sites potentially under positive selection, the parameter estimates from M2a, M8, and A models were used to calculate the posterior probabilities that an amino acid belongs to a class with ω>1, using the Bayes empirical Bayes (BEB) approaches implemented in PAML (Yang et al., 2005).
Structural analysis of PEPC
The recently published PEPC (ppc-2) protein structure from the C4 species F. trinervia (Asteraceae) (Paulus et al., 2013) was obtained from the RCSB Protein data bank (www.rcsb.org, 3ZGB). Throughout this paper, the numbering of PEPC residues is based on the maize ppc-B2 sequence CAA33317 (Besnard et al., 2003) for easy comparison with previous studies. The properties, locations, and spatial relationships of individual amino acids in the PEPC structure were analysed using CUPSAT and PyMOL (Schrödinger; Parthiban et al., 2006). Figures of the atomic structures and distances measurements were made with PyMOL, and formatted using Adobe Photoshop CS5 (USA).
Results
Phylogenetic analyses
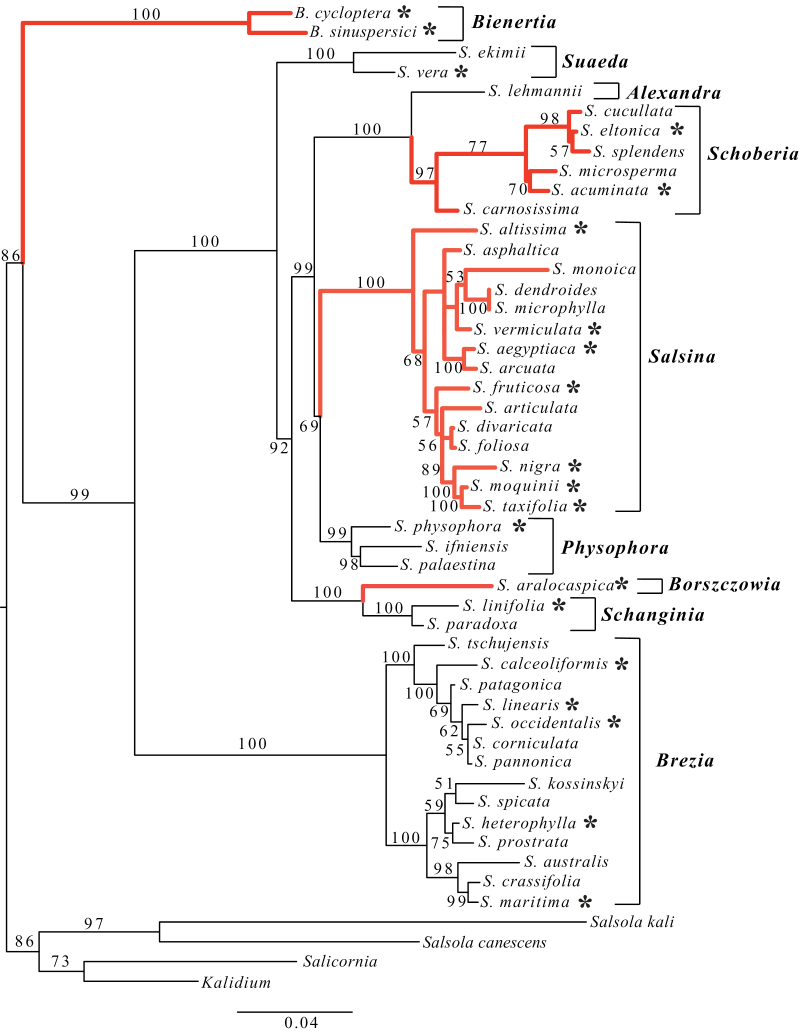
Analysis of third position sites and introns of ppc-1, in combination with previous ITS, atpB-rbcL, and the psbB-H sequence data, provided additional support for relationships in Suaedoideae showing four independent origins of C4. The ML phylogenetic tree (Fig. 1) for 46 Suaedoideae species and four outgroup species is in agreement with previously obtained phylogenies for this subfamily (Schütze et al., 2003; Kapralov et al., 2006). Previous phylogenetic analyses of relationships within Suaeda provided a strongly supported phylogenetic hypothesis of relationships in the clade, with the exception of two branches: the branch grouping Schoberia+Alexandra to Physophora and the branch grouping Schanginia+Borszczowia to Suaeda both had bootstrap support <50% (Kapralov et al., 2006). With the addition of ppc-1 data to the previously analyzed data sets, a similar but generally much more strongly supported phylogeny suggests a grade of Suaeda, Schanginia+Borszczowia, Schoberia+Alexandra, Physophora, and Salsina clades (Fig. 1). Among the clades, the only branch grouping major clades that does not have >90% bootstrap support is the Physophora+Salsina branch (bootstrap support=69%). Despite this, these results continue to support strongly four independent gains of C4 photosynthesis within the Suaedoideae, including two parallel origins of distinctive Kranz C4 anatomy in Suaeda sections Salsina s.l. and Schoberia, and two independent origins of unique single-cell C4 anatomy in Bienertia and Suaeda aralocaspica.
Fig. 1.
Suaedoideae phylogeny using ITS, atp-rbcL, psbB-psbH, and ppc1 third position plus intron sequence. Number above branches refer to bootstrap percentages. Clades leading to C4 photosynthesis are highlighted in red. Taxa used for positive selection analysis are indicated with an asterisk. Abbreviations: B., Bienertia; S., Suaeda. Bracketed names refer to section names within Suaedoideae.
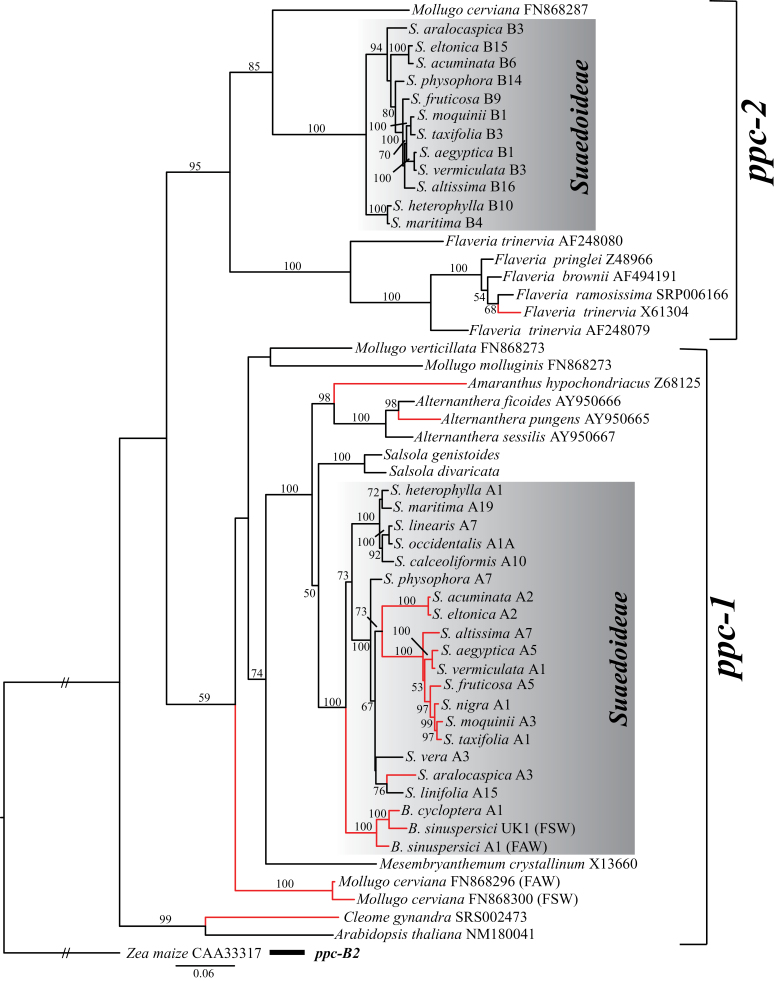
The phylogenetic relationships of eudicot PEPC genes, using exons 8, 9, and 10, rooted on the monocot ppc-B2 maize gene, shows that the ppc-1 gene that was recruited for use in C4 photosynthesis in Suaedoideae is the same orthologous gene that has been previously shown to be recruited for use in C4 photosynthesis in other C4 eudicot species (Fig. 2). The exception to this parallel recruitment of the same PEPC gene is found in the Asteraceae where the paralogous ppc-2 gene is recruited for use in C4 photosynthesis. There is strong bootstrap support for all Suaedoideae ppc-1 genes being sister to closely related Amaranthaceae ppc-1 genes.
Fig. 2.
Eudicot PEPC exon 8, 9, and 10 phylogeny rooted on the monocot ppc-B2 maize gene. Accessions numbers are indicated after species names for sequences retrieved from GenBank. Paralogous PEPC genes (ppc-1 and ppc-2) are delimited on the right. Eudicot families are indicated on the right. Branches leading to C4 clades are in red. Numbers above branches are the bootstrap percentages.
Positive selection analyses of the PEPC coding sequence
The sequenced region of ppc-1 and ppc-2 includes exons 8, 9, and 10 and accounts for 511 of the 973 (53%) amino acids present in the PEPC protein. Comparison of the ppc-1 gene across the 22 species showed that 372 of these 511 (73%) amino acids are conserved. The same sequenced region for the ppc-2 gene, for 12 of the 22 sampled species, showed that 449 of the 506 (88%) amino acids are conserved.
A phylogenetic tree, generated from intron and third position sites, was used to obtain a supported tree that is minimally affected by selective pressures (Supplementary Fig. S1 at JXB online). The tree had 23 tips, which included 22 sampled species and an additional tip for the variation found at residue 780 in the B. sinuspersici ppc-1 gene (denoted FAW for an alanine residue, or FSW for a serine residue present at position 780). Identification of codons under positive selection was performed using the software package PAML, which provided a likelihood score for each model, that was subsequently used to test for significance between the null and selection model. LRT showed that the site models assuming positive selection (M2a and M8) did not fit the data better than models without positive selection (M1a and M8a), with a P-value of 0.5273 and 0.0192, respectively (Table 1).
Table 1.
Comparison of modelled amino acid model change in the Suaedoideae ppc-1 gene, to identify sites under positive selection
| Model with positive selectiona | Null modela | LRTb | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Model | Log-likelihood | Parametersc | Positively selected sitesd | Model | Log-likelihood | Parametersc | 2L | |
| Analysis for positively selected sites common for C3 and C4 clades | ||||||||
| M2a | –6741.7 | κ=2.94, p0=0.82, ω0=0.07, ps=0.003, ωS=3.24 | None | M1a | –6742.34 | κ=2.92, p0=0.82, ω0=0.07 | 1.28 | 0.5273 |
| M8 | –6740.28 | κ=2.89, p0=0.98, p=0.24, q=1.0, ωS=1.93 | None | M8a | –6743.02 | κ=2.86, p0=0.96, p=0.19, q=0.7 | 5.48 | 0.0192 |
| Analysis for positive selection along branches leading to C4 clades | ||||||||
| A | –6729.78 | κ=2.95, p0=0.81, ω0=0.08, ps=0.04, ωS=4.2 | 733, 868 | A1 | –6737.24 | κ=2.89, p0=0.77, ω0=0.06 | 14.9 | 0.0001 |
| Analysis for positive selection along branches leading to Kranz C4 clades | ||||||||
| A | –6732.25 | κ=2.94, p0=0.77,ω0=0.06, ps=0.06, ωS=3.64 | 485, 519, 735 | A1 | –6735.67 | κ=2.91, p0=0.71, ω0=0.06 | 6.8 | 0.0091 |
| Analysis for positive selection along branches leading to single-cell C4 clades | ||||||||
| A | –6742.34 | κ=2.92, p0=0.82, ω0=0.07, ps=0.00, ωS=NA | None | A1 | –6742.34 | κ=2.92, p0=0.82, ω0=0.07 | 0 | 1 |
| Analysis for positive selection along all C4 branches | ||||||||
| A | –6702.46 | κ=2.91, p0=0.74, ω0=0.05, ps=0.18, ωS=1.5 | 480, 513, 627, 662, 695, 707, 733, 744, 794, 863, 868, 880, 931 | A1 | –6705.34 | κ=2.86, p0=0.69, ω0=0.04 | 5.08 | 0.0242 |
a M1a (nearly neutral), M2a (positive selection), M8a (beta and ω=1), and M8 (beta and ω) are PAML site models; A1 and A are PAML branch-site models.
b LRT is the likelihood ratio test; 2L is twice the difference of model log-likelihoods.
c κ is the transition/transversion rate ratio; ω is the dN/dS ratio; ωs is the dN/dS ratio in a class under putative positive selection; p0 and ps are the proportion of codons with ω<1 and ω>1, respectively; p and q are parameters of beta distribution in the range (0, 1).
d Sites listed are those at which positive selection is detected at the significance level of >95%, or >99% in bold italics.
To test if codon selection occurs specifically in C4 clades, the branch site Model A, which allows for ω>1 along foreground branches (branches where C4-specific changes were hypothesized to occur), was compared with the null Model A1, which only allows for ω≤1 along foreground and background branches. This model comparison was made by labelling foreground branches in four different ways: (i) only those foreground branches leading to C4 clades; (ii) only those foreground branches leading to Kranz C4 clades; (iii) only those branches leading to single-cell C4 clades; or (iv) by labelling all branches within C4 clades. Comparing the selection Model A with the null Model A1 showed that labelling of just the branches leading to C4 clades was the most significant, with a P-value <0.0001 (Table 1). Labelling just the branches leading to Kranz C4 clades, as well as all branches within all C4 clades, both produced significant results; P-value 0.0091 and 0.0242, respectively (Table 1). There was no variation in the likelihood score for the selection model compared with the null model when labelling just foreground branches leading to single-cell C4 clades (Table 1). There was some variation in sites identified under selection depending on how the foreground branches were labelled.
Sites under positive selection
There were no codons identified as being under positive selection with a posterior probability >0.95 by BEB in the M2A or M8 model (Table 1). There were two codons (position 733 and 868) that were shown to be under positive selection with a posterior probability >0.95 by BEB when only branches leading to C4 clades were labelled as foreground branches, with position 733 being the only residue identified to have a posterior probability >0.99 by BEB in Model A (Table 1). Both sites had four alternative amino acids present in this data set, an amino acid present mostly in C3 species, and one of three amino acids present in C4 species (Table 2). Only 733 had a substitution present in all the C4 species sampled from the C3 amino acid, while 868 had a substitution in all C4 species sampled except the two Bienertia species. Model A identified three codons (485, 519, and 735) that were shown to be under positive selection with a posterior probability >0.95 by BEB, when only branches leading to Kranz C4 clades were labelled as foreground branches (Table 1). Substitutions at codons 485 and 735 had two possible amino acids present in the data set, and all species in the Salsina clade had a substitution to the C4 amino acid (Table 2; Supplementary Table S4 at JXB online). Additionally, codon 519 also had two amino acids present in the data set, and a substitution to the C4 amino acid was only present in the two Schloberia sampled species (Table 2; Supplementary Table S4). There were no codons identified as being under positive selection that was specific to branches leading to single-cell C4 clades (Table 1). By labelling all C4 branches as foreground branches, 13 codons were identified as being under positive selection (480, 513, 627, 662, 695, 707, 733, 744, 794, 863, 868, 880, and 931) with a posterior probability >0.95 by BEB (Table 1). Two of these residues (733 and 868) were identified as being on branches leading to C4 clades.
Table 2.
Characteristics of amino acid replacements under positive selection in the C4 lineages of Suaedoideae
| AA no.a | AA change C3→C4 | Type of changeb | ΔHc | ΔPd | ΔVe | SAf (%) | ΔGg (kJ mol–1) | No. of C3/ no. of C4 h | No. of transitions for C4 AA | No. of transversions for C4 AA | Location of residue |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 480 | D→E | D→D | 0.0 | –0.7 | 27.3 | 92.7 | S (0.15) | 0/11 | – | 1 | α-Helix 18 |
| 485i | K→A | R→A | 5.7 | –3.2 | –80.0 | 40.3 | DS (–1.12) | 0/7 | 1 | 1 | α-Helix 19 |
| 513 | D→A | D→A | 5.3 | –4.9 | –22.5 | 18.7 | DS (–1.17) | 0/9 | – | 1 | α-Helix 20 |
| 519j | H→K | R→R | –0.7 | 0.9 | 15.4 | 46.7 | S (2.04) | 0/2 | – | 2 | α-Helix 20 |
| 627 | V→I | A→A | 0.3 | –0.7 | 26.7 | 10.3 | S (1.4) | 0/9 | 1 | – | α-Helix 25 |
| 662 | D→E | D→D | 0.0 | –0.7 | 27.3 | 45.1 | DS (–1.11) | 0/3 | – | 1 | Loop |
| 695 | T→V | P→A | 4.9 | –2.7 | 23.9 | 0.7 | DS (–4.39) | 0/1 | 2 | – | α-Helix 28 |
| T→I | P→A | 5.2 | –3.4 | 50.6 | 0.7 | DS (–0.97) | 0/1 | 1 | α-Helix 28 | ||
| 707 | I→M | A→A | –2.6 | 0.5 | –3.8 | 47.6 | DS (–0.64) | 0/1 | 1 | – | Loop |
| I→L | A→A | –0.7 | –0.3 | 0.0 | 47.6 | DS (–1.55) | 0/2 | – | 1 | Loop | |
| I→S | A→P | –5.3 | 4.0 | –77.7 | 47.6 | S (0.38) | 0/1 | 1 | – | Loop | |
| I→T | A→P | –5.2 | 3.4 | –50.6 | 47.6 | DS (–0.3) | 0/1 | 1 | – | Loop | |
| 733 | F→M | F→A | –0.9 | 0.5 | –27.0 | 39.9 | DS (–3.73) | 0/2 | 1 | 1 | Loop |
| F→L | F→A | 1.0 | –0.3 | –23.2 | 39.9 | DS (–3.14) | 0/8 | – | 1 | Loop | |
| F→R | F→R | –7.3 | 5.3 | –16.5 | 39.9 | DS (–2.42) | 0/2 | 1 | 1 | Loop | |
| 735i | E→N | D→P | 0.0 | –0.7 | –24.3 | 48.7 | DS (–0.76) | 0/7 | 1 | 1 | Loop |
| 744 | L→C | A→P | –1.3 | 0.6 | –58.2 | 29.5 | DS (–2.63) | 0/2 | – | 2 | α-Helix 30 |
| L→R | A→R | –8.3 | 5.6 | 6.7 | 29.5 | DS (–4.14) | 0/2 | – | 1 | α-Helix 30 | |
| 780 | A→S | A→P | –2.6 | 1.1 | 0.4 | 0.0 | DS (–3.1) | 0/4 | – | 1 | α-Helix 32 |
| 794 | F→I | F→A | 1.7 | 0.0 | –23.2 | 0.0 | DS (–2.03) | 0/6 | – | 1 | α-Helix 34 |
| F→M | F→A | –0.9 | 0.5 | –27.0 | 0.0 | DS (–2.51) | 0/2 | 1 | 1 | α-Helix 34 | |
| F→L | F→A | 1.0 | –0.3 | –23.2 | 0.0 | DS (–0.87) | 0/1 | – | 1 | α-Helix 34 | |
| 863 | S→K | A→R | 0.6 | 2.1 | 79.6 | 15.7 | DS (–0.22) | 0/1 | 1 | 1 | α-Helix 38 |
| S→D | A→D | –2.7 | 3.8 | 22.1 | 15.7 | S (0.07) | 0/1 | 2 | – | α-Helix 38 | |
| S→N | A→P | –2.7 | 2.4 | 5.1 | 15.7 | S (2.2) | 0/9 | 1 | – | α-Helix 38 | |
| S→T | P→P | 0.1 | –0.6 | 27.1 | DS (–0.43) | 0/1 | – | 1 | α-Helix 38 | ||
| 868 | K→R | R→R | –0.6 | –0.8 | 4.8 | 16.5 | DS (–0.58) | 0/2 | 1 | – | α-Helix 38 |
| K→Q | R→P | 0.4 | –0.8 | –24.8 | 16.5 | DS (–0.07) | 0/1 | – | 1 | α-Helix 38 | |
| K→L | R→A | 7.7 | –3.4 | –1.9 | 16.5 | DS (–0.88) | 0/7 | – | 2 | α-Helix 38 | |
| 880 | D→N | D→P | 0.0 | –1.4 | 3.0 | 46.5 | DS (–1.49) | 0/5 | 1 | – | Loop |
| D→E | D→D | 0.0 | –0.7 | 27.3 | 46.5 | DS (–0.22) | 0/1 | – | 1 | Loop | |
| D→Y | D→A | 2.2 | –6.8 | 82.5 | 46.5 | DS (–3.79) | 0/1 | – | 1 | Loop | |
| 931 | M→I | A→A | 2.6 | –0.5 | 3.8 | – | – | 0/2 | 1 | – | α-Helix 39 |
a Amino acid (AA) numbering is based on the maize sequence after Hudspeth and Grula (1989).
b Side chain type changes: A, non-polar aliphatic; P, polar uncharged; D, polar negatively charged; R, polar positively charged.
c Hydropathicity difference (Kyte and Doolittle, 1982).
d Polarity difference (Grantham, 1974).
e van der Waals volume difference (Zamyatin, 1972).
f Solvent accessibility calculated using the Flaveria trinervia ppc-2 structure (pdb file 3ZGE) by CUPSAT (Parthiban et al. 2006).
g Overall stability of the protein predicted using the F. trinervia ppc-2 structure (pdb file 3ZGE) by CUPSAT (Parthiban et al., 2006): DS, destabilizing; S, stabilizing.
h Number of C3 or C4 Suaedoideae species that have the indicated amino acid substitution (amino acid on right side of arrow).
i Specific for Salsina Kranz anatomy.
j Specific for Schoberia Kranz anatomy.
The amount of substitution at a particular residue for a C4 amino acid from a C3 amino acid varies quite considerably across the data set. Residues which have a low frequency of substitutions are found at positions 695 and 931 where only the two Bienertia species have a difference in the amino acid present, while at positions 662 and 744 only three or four Kranz C4 species, respectively, have a different amino acid present (Table 2; Supplementary Table S4 at JXB online). Residues that have a high frequency of substitutions, but not in all species, are found at positions 480, 513, 627, 794, and 863 (Table 2; Supplementary Table S4). Additionally, sites under selection show differences in the degree of parallelisms. For example, residues at positions 480, 485, 513, 627, and 735 all changed to an identical amino acid along different ppc-1 lineages, while residues at positions 707, 733, 794, 863, 868, and 880 changed to different amino acids (Table 2). This suggests that the C4 characteristics might be conferred by a change to a specific amino acid or by the absence of a particular amino acid.
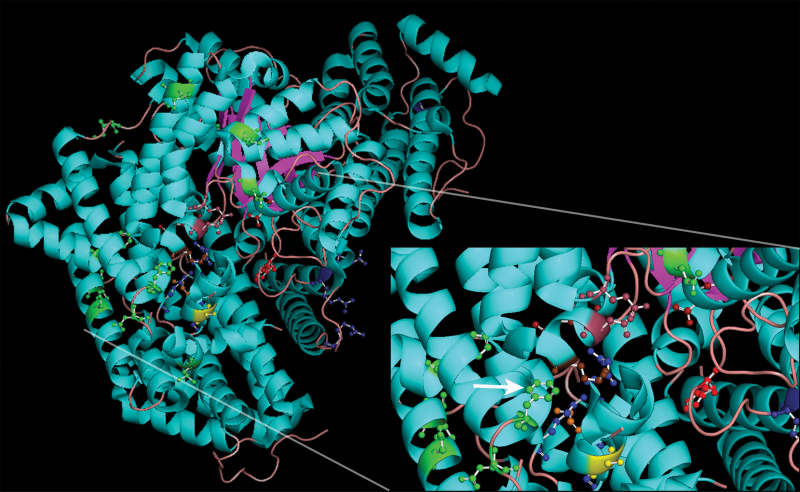
Spatial analysis of the sites identified to be under positive selection indicates that most identified residues are found around the enzyme reaction site/hydrophobic pocket (Fig. 3). Residues close to the G6P-binding pocket were not analysed in this study since they are present in exons 2, 3, and 4. Some residues (794, 863, 868, and 880) under positive selection are in the vicinity of the allosteric site for aspartate and malate regulation (Fig. 3). Two residues (480 and 485) identified to be under positive selection are far from the reaction site and are most probably involved in the dimer–dimer interaction. Interestingly, no sites involved in the β-barrel structure were identified to be under selection, meaning all sites were located in an α-helix or a connecting loop (Table 2).
Fig. 3.
Cartoon representation of the C4 PEPC enzyme structure of Flaveria trinervia (ppc-2 gene) (Paulus et al., 2013), showing the location of functional residues in comparison with sites that were identified to be under positive selection in Suaedoideae. Green residues are sites under positive selection, biochemically essential residues are in a shade of red (histone 177 is bright red, Mg2+-binding sites are ruby, PEP- and HCO3 –-binding sites are dark red, Lys606 is brown), allosteric regulatory sites are in a shade of blue (deep-blue residues are glucose 6-phosphate-binding sites, light blue are malate/aspartate-binding sites), Gly890 is orange, and Ser780 is yellow (maize numbering). Residue 733 is indicated with a white arrow. The proposed residue function is adapted from Kai et al. (2003).
Discussion
Recruitment of the ppc-1 gene in four gains of C4 photosynthesis in Suaedoideae
Extensive phylogenetic analyses on the relationships of higher plant PEPC genes has been previously carried out in families Cyperaceae, Poaceae, and Molluginaceae to determine which PEPC gene is recruited for use in C4 biochemistry (Christin et al., 2007, 2011; Besnard et al., 2009; Christin and Besnard, 2009). In the monocots, the same ppc-B2 orthologue is recruited for each independent gain of C4 photosynthesis, except for Stipagrostis where the ppc-B2 orthologue is lost and ppc-aL1b is instead used for C4 photosynthesis (Christin and Besnard, 2009). In the core eudicots, there are two primary PEPC gene lineages that have been studied to date: ppc-1 and ppc-2 (Fig. 2) (Christin et al., 2011). Results from this study show that C4 Suaedoideae species (Chenopodiaceae) all recruit the orthologous ppc-1 gene for C4 use (Fig. 2), as has been found in Amaranthaceae and Molluginaceae (Gowik et al., 2006; Christin et al., 2011). This differs from Flaveria (Asteraceae) where the paralogous ppc-2 gene was recruited (Hermans and Westhoff, 1992; Westhoff and Gowik, 2004).
Selection for PEPC amino acid residues in C4 species
In Suaedoideae, there are four independent ppc-1 lineages that have a number of non-synonomyous substitutions (Table 1; Fig. 2). This is analogous to previous positive selection analyses on PEPC that showed there are important adaptive changes to the PEPC sequences in C4 species (Christin et al., 2007, 2012; Besnard et al., 2009). What is divergent about these changes are the residues at which the changes have occurred. Of the 15 amino acids identified to be under positive selection in this analysis (Table 1), only residue 733 was previously identified to be under selection in both the grasses and sedges, while residues 794 and 863 were identified to be under selection in the grasses (Christin et al., 2007; Besnard et al., 2009). This variation in what amino acids are under selection may be attributed to the recruitment of different PEPC paralogues with different starting amino acid sequences. However, when looking at paralogous PEPC genes that were recruited for use in C4 photosynthesis in the grasses, parallel changes for eight of the 21 codons previously shown to be under positive selection in C4 grasses (ppc-B2 gene) also had the same amino acid substitution on the paralogous ppc-aL1b branch of the C4 Stipagrostis (positions 517, 531, 572, 579, 625, 665, 733, and 780) (Christin and Besnard, 2009). The positive selection of ppc-1 amino acid residues in C4 Suaedoideae species suggests that there is some fitness advantage associated with these changes. The lack of parallel substitutions between monocots and Suaedoideae might also be due to structural and ecological differences associated with optimization for C4 function. For C4 function there needs to be spatial separation between the site of atmospheric CO2 capture and the site of decarboxylation in the C4 cycle, which provides resistance to leakage and allows CO2 to be concentrated and assimilated by Rubisco. There are major differences in anatomy and structural differences in how this is achieved among the four independent origins of C4 in Suaedoideae (and in comparison with monocots), namely in the arrangement of the cytoplasmic compartments of the two types of single-cell C4 species, and in the position of organelles in bundle sheath cells between the two Kranz lineages (Schütze et al., 2003; Edwards and Voznesenskaya, 2011). These are succulent halophytes living in semi-arid deserts where high temperature, limited water, and saline soils could all contribute to CO2 limitations on photosynthesis where C4 would be beneficial. The evolution of C4 photosynthesis in Chenopodiaceae was promoted by adaptation of species to dry and/or saline habitats (Kadereit et al., 2012). Further kinetic analyses of PEPC in C4 lineages are needed to characterize differences that may be linked to optimization for C4 function. Thus the exact PEPC amino acid modifications that are necessary for optimized C4 biochemical kinetics seem to vary across deeply divergent C4 origins.
With respect to PEPC function, there have been several residues which, when mutated individually, are clearly shown to be essential for enzyme catalytic function (reviewed in Kai et al., 2003). However, substitution for amino acids along the PEPC sequence that either has no effect, or actually improves enzyme kinetics for function in C4, is hard to determine experimentally. Thus, it is difficult to know if all the observed changes in both Suaedoideae C4 ppc-1 genes and previously analysed PEPC genes in the grasses and sedges are absolutely necessary, or act in a synergistic way, to improve the enzyme function for C4 biochemistry. Closely related species that use the same PEPC gene for C4 photosynthesis probably have an optimal molecular path for amino acid changes that is different from that of distantly related species that recruited a different PEPC gene (Besnard et al., 2009). With all of the family-specific PEPC adaptive changes that alter PEPC kinetics for use in C4 photosynthesis, residue 733 is the only codon that underwent a similar change in the sedges, grasses, and Suaedoideae (Supplementary Table S4 at JXB online). There seems to be no requirement for a specific residue at this location, as four different amino acid substitutions are observed in C4 species (Table 2; Supplementary Table S4). All of the substitutions at position 733 are from the bulky C3 phenylalanine to the less bulky amino acids methionine, leucine, or arginine in Suaedoideae, or, comparatively, a valine substitution in the grasses and sedges. Substitution at 733 in Suaedoideae was observed in all C4 species sampled, while none of the C3 species had this substitution. This substitution is also observed in some C4 species such as Amaranthus hypochondriacus, Cleome gynandra, and some grasses and sedges, but not all C4 species (Supplementary Table S4). Single amino acid substitutions of PEPC have been recently shown to have dramatic effects on enzyme kinetics (Paulus et al., 2013). While no analysis has specifically analysed the effect of a substitution at residue 733, it is in close proximity (>4 Å) to a lysine residue that is conserved across higher plant PEPC genes (606 maize numbering/600 Flaveria numbering) (Supplementary Fig. S2 at JXB online). Lysine (606/600) is proposed to be involved in substrate binding through mutation analysis that showed that when Lys606 is mutated to an arginine or threonine, the K m for HCO3 – increased up to 9-fold, but there was a minimal effect on the overall maximal velocity (V max) (Gao and Woo, 1995). The exact function of Lys606 is not known since the residue is not required for enzyme activity, but when mutated becomes less active at physiological pH and is more inhibited by malate (Gao and Woo, 1995). Closer analysis of the phenylalanine substitution at 733 shows that every substitution is to a smaller amino acid (Table 2), that increases the space between Lys606 and changes the solvent-exposed surface (Supplementary Fig. S2). If Lys606 is involved in HCO3 – or, to a lesser extent, PEP binding, as has been proposed (Kai et al., 2003), then by making it more accessible by substituting out phenylalanine, the K m for HCO3 –, and possibly for PEP may increase. Substitution at 733 could thus be beneficial to C4 plants, as this could increase the rate of HCO3 – utilization and potentially C4 acid generation, which indicates that detailed kinetic analyses are needed.
Two amino acid positions have been described to be beneficial for C4 function by increasing the efficiency of PEPC by substitutions at position 890 and 780 (Blasing et al., 2000; Paulus et al., 2013). Studies on PEPC in Flaveria show that substitution of arginine with glycine at position 890 reduces the affinity for malate and aspartate which are inhibitors of PEPC. Studies also show that substitution of alanine by a serine at position 780 lowers the affinity (raises the K m) for PEP, meaning it would take higher levels of PEP to saturate the enzyme, which allows for higher concentrations of PEP to accumulate (Blasing et al., 2000). Neither one of these sites was identified to be under positive selection in Suaedoideae (Table 1). Both Schoberia Kranz species sampled along with the two Bienertia species had a serine at position 780 (Supplementary Table S4 at JXB online). While none of the C4 species sampled had a glycine at residue 890, all the Salsinia Kranz C4 species as well as three C3 species had a methionine at this position (Supplementary Table S4). Conversely, there was positive selection for residues 880, 868, and 863, in order of proximity to 890, respectively (Fig. 3). These residues are close to the proposed site of inhibition by C4 acids aspartate and malate, with a substitution at residues 868 and 880 being present in the majority but not all C4 species (Supplementary Table S4). This lack of parallel amino acid conversion in Suaedoideae C4 species indicates either that these substitutions are not necessary for effective function of C4 due to compensating adjustments in C4 biochemistry, or growth conditions, or that alternative amino acid substitutions can fulfil these functions. These results also suggest that C4 may be able to function effectively with minimal changes in orthologous PEPC genes.
The results from variations in the branch-site test (Table 1), labelling all the Suaedoideae C4 branches or just the branches where C4 is thought to evolve, suggests there may be an order of selection or stronger selective pressures on residues at position 733 and 868 as noted above. At residue 733, all 12 C4 species had a substitution for something other than phenylalanine. However, as noted above, there is not strong selection for serine at position 780 (lacking in eight C4 species). This raises the question of how selective pressures on separate PEPC gene lineages may change if one mutation can affect the selective pressure on subsequent mutations in the same functional area of the enzyme. For example, if residue 733 mutates from the bulky phenylalanine to a less bulky amino acid (potentially increasing the affinity for HCO3 –), before a substitution at 780 to serine (which may decrease the affinity for PEP), does this lower the selective pressure for a subsequent substitution at position 780? Reciprocally, would a substitution at residue 780 to serine lower the selective pressure for fixation of a mutation at residue 733, possibly explaining what is observed in F. trinervia, Alternanthera pungens, and Mollugo cerviana where a substitution at residue 733 is absent but there is a serine at residue 780? Thus, kinetic analysis for the different PEPC forms to determine how positive selection at specific codons affects the kinetic properties and response to allosteric effectors compared with the C3 orthologues is needed.
The substitution of serine for alanine at position 780 has been observed in almost every C4 species analysed to date (Blasing et al., 2000; Christin et al., 2007, 2011; Besnard et al., 2009; Christin and Besnard, 2009). This serine substitution at position 780 has also been observed in the paralogous ppc-B1 gene for the C4 species Centropodia forskaliiis (Christin et al., 2007), although its not clear if this gene is being co-expressed with the ppc-B2 gene or why there would be selection for this residue. However, Hydrilla verticulata (inducible aquatic single-cell C4 system) is an example of a PEPC gene that participates in C4 biochemistry but lacks the C4 signature serine—having the characteristic C3 alanine. Surprisingly, in vitro kinetic analysis showed that the serine was not essential for C4-like kinetics, and in fact substitution of serine for alanine in HVPEPC4 (the product of the PEPC gene that is proposed to function in the C4 cycle) was detrimental in terms of reduced V max and K cat values, although the same (serine for alanine) substitution in the anaplerotic form, HVPEPC3, altered the kinetics to become more C4 like (Rao et al., 2008).
The results of the current study showing that only four out of 12 C4 species sampled (Table 2; Supplementary Table S4 at JXB online) have a serine at position 780, indicating that species can perform C4 photosynthesis with an alanine at position 780. Bienertia sinuspersici is the second C4 plant after Mollugo cerviana (fragilis group) that has been identified to have what appears to be a recent gene duplication of the ppc-1 gene, with selection acting on only one of the two gene copies (Christin et al., 2011). Whether both are expressed is not known, but if selection occurs on only one of the two paralogues it may shed light on regulation of C4 gene expression. Furthermore, Mollugo cerviana (cerviana group) populations that lacked a substitution for a serine residue at position 780 in the ppc-1 gene were suggested not to be fully optimized for C4 biochemistry, although its carbon isotope composition is typical of c4 plants. Physiological analyses of species from the four C4 clades in Suaedoideae, which have diversity in the form of PEPC at position 780, indicate that they are functionally C4 (Smith et al., 2009; King et al., 2012). This indicates that the C4-ness of a species cannot be determined by Ser780 alone.
Finding C4 species without a Ser780 would not be the first time that experimental evidence for PEPC caused a re-evaluation of how enzyme kinetics are modified and the context of in vivo regulation. For example, PEPC has a conserved N-terminal serine that is subject to reversible phosphorylation in response to light and has been determined to reduce the inhibitory effects of malate in vitro (Tsuchida et al., 2001). When this residue is not phosphorylated in vivo, there are no observable effects on CO2 assimilation rates in transgenic F. bidentis, raising the question that if phosphorylation is not essential for efficient C4 photosynthesis how biochemically is it related (Furumoto et al., 2007). PEPCs from both single-cell C4 types and S. eltonica were shown to undergo phosphorylation in the light, at this conserved N-terminal serine analogous to other C4 systems, while the C3 plant S. linifolia did not (Lara et al., 2006). This suggests there is the potential for tolerating accumulation of high levels of malate during photosynthesis in these C4 plants, but further analysis is needed to understand whether this is biochemically necessary and the context to in vivo levels of metabolites. This is analogous to the question of what effect substitution at position 780 in PEPC has on kinetics in vitro, and whether this can be observed to be beneficial under certain conditions in vivo.
Conclusion
To date there are only a few reports on the recruitment of and modifications to PEPC along lineages that evolved C4 in the eudicots. In Suaedoideae, the ppc-1 gene is used in C4 photosynthesis as observed in C4 eudicot families Amaranthaceae and Molluginaceae, which is analogous to the predominant recruitment of the same PEPC orthologue in C4 grasses and sedges. Unlike in the monocots, there is less evidence for the necessity for a high number of positively selected PEPC residues in Suaedoideae. Further analysis is needed to determine if the observed amino acid differences in Suaedoideae are more or less common across the Chenopodiaceae, their effect on PEPC kinetic properties, and ultimately how these changes are beneficial for C4 photosynthesis.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Suaedoideae phylogeny, using only the ppc1 third position plus intron sequence, that was used for positive selection analysis.
Figure S2. Cartoon representation of the C4-PEPC enzyme structure of Flaveria trinervia (ppc-2 gene) (Paulus et al., 2013), showing the spatial effect of a substitution at residue 733 (maize numbering) in relation to residue 606.
Table S1. Name, sequence of primer, and which species the primer was used for in sequencing Suaedoideae ppc-1 and ppc-2 genes.
Table S2. List of species origin, voucher, and ppc sequence accession numbers generated in this study.
Table S3. Chenopodioideae species list used in phylogenetic analyses with marker accession numbers.
Table S4. Comparison of ppc-1 exon 8, 9, and 10 amino acids, that were identified to be under positive selection in Suaedoideae, across Eudicot families.
Acknowledgements
This material is based upon work supported by the National Science Foundation under funds MCB #1146928, including support from the Office of International Science and Engineering (OISE) to GEE. Thanks to Maxim Kapralov for help with running and discussing PAML, and to Richard Sharpe for discussion on PEPC genes. Travel support to the C4/CAM workshop in Illinois was provided by a Betty Higginbotham grant through the School of Biological Sciences at WSU. This manuscript is in partial fulfilment of a doctoral thesis at Washington State University.
Glossary
Abbreviations:
- dN
non-synonmous substitution rate
- dS
synonymous substitution rate
- G6P
glucose 6-phosphate
- LRT
likelihood ratio test
- ML
maximum likelihood
- OAA
oxaloacetate
- PEP
phosphoenolpyruvate
- PEPC
phosphoenolpyruvate carboxylase
- ω
the non-synonymous/synonymous substitution rate ratio.
References
- Bandarian V, Poehner WJ, Grover SD. 1992. Metabolite activation of crassulacean acid metabolism and C4 phosphoenolpyruvate carboxylase. Plant Physiology 100, 1411–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard G, Muasya AM, Russier F, Roalson EH, Salamin N, Christin P-A. 2009. Phylogenomics of C4 photosynthesis in sedges (Cyperaceae): multiple appearances and genetic convergence. Molecular Biology and Evolution 26, 1909–1919 [DOI] [PubMed] [Google Scholar]
- Besnard G, Pincon G, D’Hont A, Hoarau J-Y, Cadet F, Offmann B. 2003. Characterisation of the phosphoenolpyruvate carboxylase gene family in sugarcane (Saccharum spp.). Theoretical and Applied Genetics 107, 470–478 [DOI] [PubMed] [Google Scholar]
- Blasing OE, Ernst K, Streubel M, Westhoff P, Svensson P. 2002. The non-photosynthetic phosphoenolpyruvate carboxylases of the C4 dicot Flaveria trinervia—implications for the evolution of C4 photosynthesis. Planta 215, 448–456 [DOI] [PubMed] [Google Scholar]
- Blasing OE, Westhoff P, Svensson P. 2000. Evolution of C4 phosphoenolpyruvate carboxylase in Flaveria, a conserved serine residue in the carboxyl-terminal part of the enzyme is a major determinant for C4-specific characteristics. Journal of Biological Chemistry 275, 27917–27923 [DOI] [PubMed] [Google Scholar]
- Chollet R, Vidal J, O’Leary MH. 1996. Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants Annual Review of Plant Physiology and Plant Molecular Biology 47, 273–298 [DOI] [PubMed] [Google Scholar]
- Christin P-A, Besnard G. 2009. Two independent C4 origins in Aristidoideae (Poaceae) revealed by the recruitment of distinct phosphoenolpyruvate carboxylase genes. American Journal of Botany 96, 2234–2239 [DOI] [PubMed] [Google Scholar]
- Christin P-A, Edwards EJ, Besnard G, Boxall SF, Gregory R, Kellogg EA, Hartwell J, Osborne CP. 2012. Adaptive evolution of C4 photosynthesis through recurrent lateral gene transfer. Current Biology 22, 445–449 [DOI] [PubMed] [Google Scholar]
- Christin P-A, Sage TL, Edwards EJ, Ogburn RM, Khoshravesh R, Sage RF. 2011. Complex evolutionary transitions and the significance of C3–C4 intermediate forms of photosynthesis in Molluginaceae. Evolution 65, 643–660 [DOI] [PubMed] [Google Scholar]
- Christin P-A, Salamin N, Muasya AM, Roalson EH, Russier F, Besnard G. 2008. Evolutionary switch and genetic convergence on rbcL following the evolution of C4 photosynthesis. Molecular Biology and Evolution 25, 2361–2368 [DOI] [PubMed] [Google Scholar]
- Christin P-A, Salamin N, Savolainen V, Duvall MR, Besnard G. 2007. C4 photosynthesis evolved in grasses via parallel adaptive genetic changes. Current Biology 17, 1241–1247 [DOI] [PubMed] [Google Scholar]
- Coombs J, Baldry CW, Bucke C. 1973. The C-4 pathway in Pennisetum purpureum: the allosteric nature of PEP carboxylase. Planta 110, 95–107 [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19, 11–15 [Google Scholar]
- Edwards GE, Voznesenskaya EV. 2011. C4 photosynthesis: Kranz forms and single-cell C4 in terrestrial plants. In: Raghavendra AS, Sage RF, eds. C4 photosynthesis and related CO2 concentrating mechanisms. Berlin: Springer Science, 29–61 [Google Scholar]
- Engelmann S, Blasing OE, Gowik U, Svensson P, Westhoff P. 2003. Molecular evolution of C4 phosphoenolpyruvate carboxylase in the genus Flaveria—a gradual increase from C3 to C4 characteristics. Planta 217, 717–725 [DOI] [PubMed] [Google Scholar]
- Furumoto T, Izui K, Quinn V, Furbank RT, von Caemmerer S. 2007. Phosphorylation of phosphoenolpyruvate carboxylase is not essential for high photosyntheitc rates in the C4 species Flaveria bidentis. Plant Physiology 144, 1936–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Woo KC. 1995. Site-directed mutagenesis of Lys600 in phosphoenolpyruvate carboxylase of Flaveria trinervia: its roles in catalytic and regulatory functions. FEBS Letters 375, 95–98 [DOI] [PubMed] [Google Scholar]
- Gowik U, Engelmann S, Blasing OE, Raghavendra AS, Westhoff P. 2006. Evolution of C4 phosphoenolpyruvate carboxylase in the genus Alternanthera: gene families and the enzymatic characterisitcs of the C4 isozyme and its orthologues in C3 and C3/C4 Alternantheras . Planta 223, 359–368 [DOI] [PubMed] [Google Scholar]
- Hermans J, Westhoff P. 1990. Analysis of expression and evolutionary relationships of phosphoenolpyruvate carboxylase genes in Flaveria trinervia (C4) and F. pringlei (C3). Molecular and General Genetics 224, 459–468 [DOI] [PubMed] [Google Scholar]
- Hermans J, Westhoff P. 1992. Homologous genes for the C4 isoform of phosphoenolpyruvate carboxylase in a C3 and a C4 Flaveria species. Molecular and General Genetics 234, 275–284 [DOI] [PubMed] [Google Scholar]
- Huber SC, Edwards GE. 1975. Inhibition of phosphoenolpyruvate carboxylase from C4 plants by malate and aspartate. Canadian Journal of Botany 53, 1925–1933 [Google Scholar]
- Huelsenbeck JP, Dyer KA. 2004. Bayesian estimation of positively selected sites. Journal of Molecular Evolution 58, 661–672 [DOI] [PubMed] [Google Scholar]
- Izui K, Matsumura H, Furumoto T, Kai Y. 2004. Phosphoenolpyruvate carboxylase: a new era of structural biology. Annual Review of Plant Biology 55, 69–80 [DOI] [PubMed] [Google Scholar]
- Jacobs B, Engelmann S, Westhoff P, Gowik U. 2008. Evolution of C4 phosphoenolpyruvate carboxylase in Flaveria: determinants for high tolerance towards the inhibitor l -malate. Plant, Cell and Environment 31, 793–803 [DOI] [PubMed] [Google Scholar]
- Jiao J-A, Chollet R. 1988. Light/dark regulation of maize leaf phosphoenolpyruvate carboxylase by in vivo phosphorylation. Archives of Biochemistry and Biophysics 261, 409–417 [DOI] [PubMed] [Google Scholar]
- Kadereit G, Ackerly D, Pirie MD. 2012. A broader model for C4 photosynthesis evolution in plants inferred from the goosefoot family (Chenopodiaceae s.s.). Proceedings of the Royal Society B: Biological Sciences 279, 3304–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadereit G, Borsch T, Weising K, Freitag H. 2003. Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C4 photosynthesis International Journal of Plant Sciences 164, 959–986 [Google Scholar]
- Kadereit G, Freitag H. 2011. Molecular phylogeny of Camphorosmeae (Camphorosmoidease, Chenopodiaceae): implications for biogeography, evolution of C4-photosynthesis and taxonomy. Taxon 60, 51–78 [Google Scholar]
- Kai Y, Matsumura H, Inoue T, Terada K, Nagara Y, Yoshinaga T, Kihara A, Tsumura K, Izui K. 1999. Three-dimensional strucutre of phosphoenolpyruvate carboxylase: a proposed mechanism for allosteric inhibition. Proceedings of the National Academy of Sciences, USA 96, 823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai Y, Matsumura H, Izui K. 2003. Phosphoenolpyruvate carboxylase: three-dimensional structure and molecular mechanisms. Archives of Biochemistry and Biophysics 414, 170–179 [DOI] [PubMed] [Google Scholar]
- Kapralov MV, Akhani H, Voznesenskaya EV, Edwards G, Franceschi V, Roalson EH. 2006. Phylogenetic relationships in the Salicornioideae/Suaedoideae/Salsoloideae s.l. (Chenopodiaceae) clade and a clarification of the phylogenetic position of Bienertia and Alexandra using multiple DNA sequence datasets. Systematic Botany 31, 571–585 [Google Scholar]
- Kapralov MV, Smith AC, Filatov DA. 2012. Rubisco evolution in C4 eudicots: an analysis of Amaranthaceae sensu lato . PLoS One 7, e52974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JL, Edwards GE, Cousins AB. 2012. The efficiency of the CO2-concentrating mechanism during single-cell C4 photosynthesis. Plant, Cell and Environment 35, 513–523 [DOI] [PubMed] [Google Scholar]
- Lara MV, Chuong SDX, Akhani H, Andreo CS, Edwards GE. 2006. Species having C4 single-cell-type photosynthesis in the Chenopodiaceae family evolved a photosynthetic phosphoenolpyruvate carboxylase like that of kranz-type C4 species. Plant Physiology 142, 673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepiniec L, Vidal J, Chollet R, Gadal P, Cretin C. 1994. Phosphoenolpyruvate carboxylase: structure, regulation and evolution. Plant Science 99, 111–124 [Google Scholar]
- Matsumura H, Xie Y, Shirakata S, Inoue T, Yoshinaga T, Ueno Y, Izui K, Kai Y. 2002. Crystal structures of C4 from maize and quaternary complex of E. coli phosphoenolpyruvate carboxylases. Structure 10, 1721–1730 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yoshioka I, Takahashi M, Toh H, Izui K. 1995. Cloning and sequence analysis of the gene for phosphoenolpyruvate carboxylase from an extreme thermophile, Thermus sp. Journal of Biochemistry 118, 319–324 [DOI] [PubMed] [Google Scholar]
- Nishikido T, Takanashi H. 1973. Glycine activation of PEP carboxylase from monocotyledoneous C4 plants. Biochemical and Biophysical Research Communications 53, 126–133 [DOI] [PubMed] [Google Scholar]
- O’Leary B, Park J, Plaxton WC. 2011. The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochemical Journal 436, 15–34 [DOI] [PubMed] [Google Scholar]
- O’Leary MH. 1982. Phosphoenolpyruvate carboxylase: an enzymologist’s view. Annual Review of Plant Physiology 33, 297–315 [Google Scholar]
- Parthiban V, Gromiha MM, Schomburg D. 2006. CUPSAT: prediction of protein stability upon point mutations. Nucleic Acids Research 34, W239–W242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus JK, Schlieper D, Groth G. 2013. Greater efficiency of photosynthetic carbon fixation due to single amino-acid substitution. Nature Communications 4, 1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SK, Fukayama H, Reiskind JB, Miyao M, Bowes G. 2006. Identification of C4 responsive genes in the facultative C4 plant Hydrilla verticillata . Photosynthesis Research 88, 173–183 [DOI] [PubMed] [Google Scholar]
- Rao SK, Reiskind JB, Bowes G. 2008. Kinetic analyses of recombinant isoforms of phosphoenolpyruvate carboxylase from Hydrilla verticillata leaves and the impact of substituting a C4-signature serine. Plant Science 174, 475–483 [Google Scholar]
- Sage RF, Christin P-A, Edwards EJ. 2011. The C4 plant lineages of planet Earth. Journal of Experimental Botany 62, 3155–3169 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Sanchez R, Cejudo FJ. 2003. Identification and expression analysis of a gene encoding a bacterial-type phosphoenolpyruvate carboxylase from Arabidopsis and rice. Plant Physiology 132, 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger. The PyMOL Molecular Graphics System, Version 1.5.0.4.
- Schütze P, Freitag H, Weising K. 2003. An integrated molecular and morphological study of the subfamily Suaedoideae Ulbr. (Chenopodiaceae). Plant Systematics and Evolution 239, 257–286 [Google Scholar]
- Silvestro D, Michalak I. 2012. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity and Evolution 12, 335–337 [Google Scholar]
- Smith ME, Koteyeva NK, Voznesenskaya EV, Okita TW, Edwards GE. 2009. Photosynthetic features of non-Kranz type C4 versus Kranz type C4 and C3 species in subfamily Suaedoideae (Chenopodiaceae). Functional Plant Biology 36, 770–782 [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 [DOI] [PubMed] [Google Scholar]
- Svensson P, Blasing OE, Westhoff P. 2003. Evolution of C4 phosphoenolpyruvate carboxylase. Archives of Biochemistry and Biophysics 414, 180–188 [DOI] [PubMed] [Google Scholar]
- Ting IP, Osmond CB. 1973. Photosynthetic phosphoenolpyruvate carboxylases. Plant Physiology 51, 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Mendez A, Mujica-Jimenez C, Munoz-Clares R. 2000. Physiological implications of the kinetics of maize leaf phosphoenolpyruvate carboxylase. Plant Physiology 123, 149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Mendez A, Rodriguez-Sotres R, Lopez-Valentin DM, Munoz-Clares RA. 1998. Re-examination of the roles of PEP and Mg2+ in the reaction catalysed by the phosphorylated and non-phosphorylated forms of phosphoenolpyruvate carboxylase from leaves of Zea mays: effects of the activators glucose 6-phosphate and glycine. Biochemical Journal 332, 633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida Y, Furumoto T, Izumida A, Hata S, Izui K. 2001. Phosphoenolpyruvate carboxylase kinase involved in C4 photosynthesis in Flaveria trinervia: cDNA cloning and characterization. FEBS Letters 507, 318–322 [DOI] [PubMed] [Google Scholar]
- Westhoff P, Gowik U. 2004. Evolution of C4 phosphoenolpyruvate carboxylase. Genes and proteins: a case study with the genus Flaveria . Annals of Botany 93, 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KF, Davies DD. 1973. Regulation of phosphoenolpyruvate carboxylase of Zea mays by metabolites. Biochemical Journal 131, 451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution 24, 1586–1591 [DOI] [PubMed] [Google Scholar]
- Yang Z, Nielsen R. 2002. Codon-substitution models for detecting molecular adaptation at individual sites along specific lineages. Molecular Biology and Evolution 19, 908–917 [DOI] [PubMed] [Google Scholar]
- Yang Z, Swanson WJ. 2002. Codon-substitution models to detect adaptive evolution that account for heterogeneous selctive pressures among site classes. Molecular Biology and Evolution 19, 49–57 [DOI] [PubMed] [Google Scholar]
- Yang Z, Wong WSW, Nielsen R. 2005. Bayes empirical Bayes inference of amino acid site under positive selection. Molecular Biology and Evolution 22, 1107–1118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.