Summary
In maize, salinity reduced photosynthesis by decreasing stomatal conductance. Shade downregulated PEPC more than Rubisco, leading to decreased bundle-sheath CO2 leakiness. The treatments elicited plasticity in NADP-ME/PEP-CK activity ratio.
Key words: C4 photosynthesis, carbon isotope discrimination, leakiness, NADP-ME, PEP-CK, PEPC, Rubisco.
Abstract
C4 photosynthesis involves a close collaboration of the C3 and C4 metabolic cycles across the mesophyll and bundle-sheath cells. This study investigated the coordination of C4 photosynthesis in maize plants subjected to two salinity (50 and 100mM NaCl) treatments and one shade (20% of full sunlight) treatment. Photosynthetic efficiency was probed by combining leaf gas-exchange measurements with carbon isotope discrimination and assaying the key carboxylases [ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and phosphoenolpyruvate carboxylase (PEPC)] and decarboxylases [nicotinamide adenine dinucleotide phosphate malic enzyme (NADP-ME) and phosphoenolpyruvate carboxykinase (PEP-CK)] operating in maize leaves. Generally, salinity inhibited plant growth and photosynthesis to a lesser extent than shade. Salinity reduced photosynthesis primarily by reducing stomatal conductance and secondarily by equally reducing Rubisco and PEPC activities; the decarboxylases were inhibited more than the carboxylases. Salinity increased photosynthetic carbon isotope discrimination (Δp) and reduced leaf dry-matter carbon isotope composition (13δ) due to changes in p i/p a (intercellular to ambient CO2 partial pressure), while CO2 leakiness out of the bundle sheath (ϕ) was similar to that in control plants. Acclimation to shade was underpinned by a greater downregulation of PEPC relative to Rubisco activity, and a lesser inhibition of NADP-ME (primary decarboxylase) relative to PEP-CK (secondary decarboxylase). Shade reduced Δp and ɸ without significantly affecting leaf 13δ or p i/p a relative to control plants. Accordingly, shade perturbed the balance between the C3 and C4 cycles during photosynthesis in maize, and demonstrated the flexible partitioning of C4 acid decarboxylation activity between NADP-ME and PEP-CK in response to the environment. This study highlights the need to improve our understanding of the links between leaf 13δ and photosynthetic Δp, and the role of the secondary decarboxylase PEP-CK in NADP-ME plants such as maize.
Introduction
C4 photosynthesis evolved as a spatial and biochemical adaptation to remedy the inefficiency of C3 photosynthesis under conditions of high temperature, low CO2, and water stress, all of which exacerbate photorespiration (Ludwig, 2013). The propensity of photorespiration is determined by the extent of oxygenation carried out by ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) (Andrews et al., 1973; Cleland et al., 1998). For C3 plants, the current O2 concentration in the atmosphere (210 mmol mol–1) severely inhibits the carboxylation of ribulose-1,5-bisphosphate by Rubisco. Together, these environmental conditions are hypothesized to be the drivers for the independent evolution of plants operating a CO2 concentration mechanism (CCM; Sage et al., 2012). The distinguishing features of the CCM in most C4 plants include the operation of two metabolic cycles (C3 and C4) across two photosynthetic cell types, mesophyll cells (MCs) and bundle-sheath cells (BSCs), which compartmentalize the initial carboxylation and decarboxylation reactions (Hatch, 1987; Langdale, 2011). The primary step of HCO3 – fixation to phosphoenolpyruvate is catalysed by phosphoenolpyruvate carboxylase (PEPC) to produce oxaloacetate, which is subsequently converted into C4 acids within the MCs (Jenkins et al., 1987). These organic acids then diffuse into neighbouring BSCs where decarboxylation of C4 acids releases CO2. A high CO2 concentration within the semi-gas-tight BSCs suppresses photorespiration and enhances the capacity for CO2 fixation by Rubisco. C4 photosynthesis has three biochemical subtypes depending on the C4 decarboxylase enzyme: nicotinamide adenine dinucleotide phosphate malic enzyme (NADP-ME), NAD malic enzyme, and phosphoenolpyruvate carboxykinase (PEP-CK) (Gutierrez et al., 1974; Kanai and Edwards, 1999). C4 plants have been classified into one of the three subtypes based on the dominant C4 acid decarboxylation enzyme. Specialized leaf anatomy, biochemistry, and physiology are associated with each of the C4 subtypes (Ghannoum et al., 2005, 2011).
Nevertheless, there is evidence emerging that PEP-CK activity is more widespread among the biochemical subtypes, suggesting that a degree of flexibility within the C4 cycle may exist depending on species or environmental conditions (Leegood and Walker, 2003; Furbank, 2011). In maize, an NADP-ME C4 grass, 25% of the oxaloacetate produced is cycled through an alternative pathway involving the aspartate aminotransferase shuttle and the subsequent decarboxylation of oxaloacetate within the cytosol of BSCs catalysed by PEP-CK (Wingler et al., 1999; Furbank, 2011). This has been shown to exist for maize and other C4 grasses (Gutierrez et al., 1974). Therefore, the presence of alternative decarboxylase pathways within maize provides the possibility for flexibility in the use of the decarboxylation pathways of the CCM under certain growth conditions (Leegood and Walker, 2003).
For C4 plants, there is an additional energetic cost associated with the operation and overcycling of the CCM. Minimally, an extra two ATP molecules per CO2 fixed are required for the regeneration of PEP from pyruvate. During C4 photosynthesis, the C4 cycle operates faster than the C3 (Calvin) cycle in order to raise the BSC CO2 concentration and saturate the carboxylation reaction of Rubisco. Inevitably, a fraction of this CO2 is not fixed by Rubisco and ultimately leaks back from the BSCs to the MCs. This fraction is termed leakiness (ɸ) and entails additional energy costs associated with the overcycling of the C4 cycle (Farquhar, 1983; Furbank et al., 1990). Consequently, the efficiency of C4 photosynthesis requires the tight regulation of CO2 supply with Rubisco activity within the BSCs in order to minimize leakiness and associated energy costs. This is often the case, given that leakiness varies within a narrow range and averages about 20% for a wide range of C4 plants and environments (Henderson et al., 1992; von Caemmerer et al., 1997a ; Cousins et al., 2008). Bundle-sheath leakiness can be estimated by concurrently measuring leaf gas exchange with carbon isotope discrimination (Evans et al., 1986). A number of studies have examined the effects of short-term and long-term changes in environmental parameters, such as light, water stress, and salinity, yielding mixed results. A few studies have estimated leakiness from measurements of dry-matter carbon isotope, and found that leakiness was impacted by light, salinity, or water stress (Buchmann et al., 1996; Saliendra et al., 1996; Fravolinil et al., 2002). When leakiness was estimated from carbon isotope discrimination measured during gas exchange, small changes in leakiness have been reported in some studies but not others in response to short- or long-term changes in the environment (Bowman et al., 1989; Kubasek et al., 2007). In particular, Bowman et al. (1989) found that leakiness changed diurnally in salt-stressed Zea mays and Andropogon glomeratus, two C4, NADP-ME grasses, while Kubasek et al. (2007) reported that leakiness increased with low light and low temperature. Lowering light intensity during gas-exchange measurements had no effect on bundle-sheath leakiness in a number of C4 plants (Henderson et al., 1992), and leakiness was unchanged under long-term exposure to low light (Bellasio and Griffiths, 2013a ). Ubierna et al. (2013) found that the increase in leakiness commonly reported at low light (Henderson et al., 1992) was only marginally present when using the full model for carbon isotope discrimination in C4 leaves (Farquhar and Cernusak, 2012). Leakiness depends on a number of anatomical (e.g. CO2 diffusion path length, chloroplast position in the BSC, BSC wall conductance) and biochemical (e.g. activities of the carboxylases and decarboxylases during C4 photosynthesis) factors (Henderson et al., 1992; von Caemmerer and Furbank, 2003). In contrast to manipulations using transgenic C4 plants (von Caemmerer et al., 1997b ; Cousins et al., 2006; Pengelly et al., 2012), few studies have investigated the effects of environmental variables on leakiness together with possible underlying biochemical mechanisms.
Consequently, the current study was aimed at investigating the efficiency of C4 photosynthesis in maize exposed to long-term shade and salinity, by combining measurements of leakiness with assays of the two carboxylases and decarboxylases known to operate in maize leaves. A second aim of this study was to probe the plasticity of the C4 acid decarboxylases in response to these environmental variables. Salinity and shade were chosen because they impact on photosynthesis through contrasting effects on leaf CO2 diffusion and fixation. Mild to moderate salinity inhibits root water uptake, thus indirectly reducing the plant water status, as detected by increased leaf water potential and reduced stomatal conductance, both of which reduce photosynthesis (Munns and Tester, 2008; Omoto et al., 2012; Shabala and Munns, 2012). Low light reduces photosynthesis mainly by reducing activity and activation of photosynthetic enzymes (Edwards et al., 1985).
Materials and methods
Plant culture
Maize seeds (Sweet Corn, Kelvedon Glory 5713) were germinated in 5 l pots (shaded plants were raised in 2 l pots) containing standard potting mix in a sunlit glasshouse during summer (December–March 2012). Nutrients were supplied through the addition of Osmocote and periodic watering with soluble Aquasol supplemented with magnesium sulfate. Maize plants destined for the salinity treatments were initially watered with tap water. Once seedlings were well established (2 weeks after germination), NaCl was added at increasing concentrations to the watering solution over a period of 2 weeks until the endpoint concentrations of 50 and 100mM NaCl were reached. To minimize NaCl accumulation, pots were flushed with water once a week, and then irrigated with the desired NaCl concentration. Plants destined for shading were germinated as above in full sunlight and then placed under a shade cloth, which limited light to 20% of the ambient sunlight. At midday, the photosynthetic active radiation of full sunlight ranged between 1000 and 1800 µmol m–2 s–1 when measured at pot level during the experiment. Air temperature inside the glasshouse compartment was regulated by a temperature-control system, and day/night temperatures averaged 26/18 °C. Relative humidity was monitored and ranged between 60 and 80% during the day. There were five pots per treatments. Plants were harvested 12 weeks after germination.
Measuring leaf gas exchange
Leaf gas-exchange measurements were carried out 1–2 weeks before harvest using a portable open photosynthesis system (LI-6400XT; LI-COR, Lincoln, USA). Measurements of light-saturated photosynthetic rate (A sat) and stomatal conductance (g s) were taken between 10:00 and 14:00 at ambient CO2 (400 µl l–1), a leaf temperature of 26 °C, and a photosynthetic photon flux density of 1800 µmol m–2 s–1. Each leaf was allowed to reach a steady state of CO2 uptake in the LI-6400XT leaf chamber before measurements were taken.
Photosynthetic responses to intercellular CO2 concentration (A/C i curves) were measured at 10 CO2 steps using similar conditions as described above. The A/C i curves were fitted using a C4 photosynthesis model (von Caemmerer, 2000) to estimate maximal PEPC (in vivo V pmax) and Rubisco (in vivo V cmax) activities. V cmax and V pmax were varied simultaneously until the best fit with the gas-exchange data was obtained.
Photosynthetic carbon isotope discrimination
Bundle-sheath leakiness was determined by measuring real-time 13CO2/12CO2 carbon isotope discrimination using a gas exchange system (LI-6400XT: LI-COR) attached to a tunable diode-laser (model TGA100; Campbell Scientific, Logan, UT, USA), under similar conditions to the spot gas exchange measurements. Photosynthetic discrimination against 13C (Δp) was calculated using the following equations (Evans et al., 1986):
| (1) |
| (2) |
where δ e, δ o, C e, and C o are the δ13C (δ) and CO2 mol fraction (C) of the air entering (e) and leaving (o) the leaf chamber and were measured with the tunable diode-laser. In this study, ξ ranged between 5 and 11. Leakiness (ɸ) was calculated using the model of Farquhar (1983) as modified by Pengelly et al. (2010, 2012). The formulae used are described briefly below.
| (3) |
where the term t, which represents the ternary effect, is defined as by Farquhar and Cernusak, (2012):
| (4) |
where E is the transpiration rate and g t ac the total conductance to CO2 diffusion including boundary layer and stomatal conductance (von Caemmerer and Farquhar, 1981). The symbol a′ denotes the combined fractionation factor through the leaf boundary layer and through stomata:
| (5) |
where C a, C i, and C ls are the ambient, intercellular, and leaf surface CO2 partial pressures, a b (2.9‰) is the fractionation occurring through diffusion in the boundary layer, a (4.4‰) is the fractionation due to diffusion in air (Evans et al., 1986), s (1.8‰) is the fractionation during leakage of CO2 out of the bundle sheath, and a i is the fractionation factor associated with the dissolution of CO2 and diffusion through water. Here, we assume that s=a i.
| (6) |
and
| (7) |
where b 3 is the fractionation by Rubisco (30‰), b 4 is the combined fractionation of the conversion of CO2 to HCO3 − and PEP carboxylation (–5.74‰ at 25 °C), f is the fraction associated with photosrespiration, and V o and V c are the rates of oxygenation and carboxylation, respectively. The fractionation factor e associated with respiration was calculated from the difference between δ13C in the CO2 cylinder (–40.5‰) used during experiments and that in the atmosphere under growth conditions (–8‰; Tazoe et al., 2008). A and R d denote the CO2 assimilation rate and day respiration, respectively; R d was assumed to equal dark respiration. We assumed a mesophyll conductance (g m)=1mol m−2 s−1 bar−1 for these calculations. In this study, leaf gas exchange was measured at high light, and hence V o=0 ( =0) (Pengelly et al., 2010, 2012; Ubierna et al., 2013).
Rubisco content and soluble protein determination
Following gas-exchange measurements, replicate leaf discs (0.74cm2) were rapidly frozen in liquid nitrogen and then stored at –80 °C until analysed. Each leaf disc was extracted in 1ml of ice-cold extraction buffer [50mM EPPS/NaOH (pH 8.0), 5mM dithiothreitol, 20mM NaHCO3, 20mM MgCl2, 1mM EDTA, 4% (v/v) Protease Inhibitor Cocktail (Sigma), and 1% (w/v) polyvinyl polypyrrolidone] using a 2ml Potter–Elvehjem glass homogenizer kept on ice. Subsamples were taken from the total extract for SDS-PAGE analysis (see below) of total leaf protein. The remaining extract was centrifuged at 16, 100g for 1min and the supernatant used for Rubisco and soluble protein assays. Rubisco content was estimated by the irreversible binding of [14C]carboxyarabinitol bisphosphate (CABP) to the fully carbamylated enzyme (Sharwood et al., 2008). Extractable soluble proteins were measured using a Coomassie Plus kit (Pierce).
Activity of carboxylase and decarboxylase enzymes
Activity of Rubisco in maize extracts was determined by multiplying the number of Rubisco active sites determined using the [14C]CABP binding assay by the Rubisco in vitro k cat (5.5 s–1) determined using a 14CO2 fixation assay (Sharwood et al., 2008). The activity of the PEPC and NADP-ME enzymes were determined spectrophotometrically as described previously (Ashton et al., 1990; Pengelly et al., 2012).
The activity of PEP-CK in maize extracts was measured in the carboxylase direction using the method outlined by Walker et al. (2002). For each assay, a separate leaf disc was homogenized in extraction buffer containing 50mM HEPES (pH 7.2), 5mM dithiothreitol, 1% polyvinyl polypyrrolidone, 2mM EDTA, 2mM MnCl2, and 0.05% Triton X-100. MgCl2 was not added to the extraction or assay buffer to remove the possibility of interference from other enzymes. PEP-CK activity was measured in assay buffer [100mM HEPES (pH 7.0), 4% mercaptoethanol (w/v), 100mM KCl, 90mM NaHCO3, 1mM ADP, 2mM MnCl2, 0.14mM NADH, and malate dehydrogenase (6U)] after the addition of PEP to 5mM. The final concentration of 4mM MnCl2 has been shown to be sufficient for PEP-CK activity (Chen et al., 2002; Walker et al., 2002).
SDS-PAGE and immunoblot analysis of Rubisco and CCM proteins
Subsamples of total protein fractions were mixed with 0.25 vols of 4× LDS buffer (Invitrogen) containing 100mM dithiothreitol and placed on ice until analysed within 2h. For confirmatory visualization, protein samples were separated by SDS-PAGE in TGX Any kD (BioRad) pre-cast polyacrylamide gels buffered with 1× Tris/glycine SDS buffer (BioRad) at 200V using a Mini-Protean apparatus at 4 °C. Proteins were visualized by staining with Bio-Safe Coomassie G-250 (BioRad) and imaged using a VersaDoc imaging system (BioRad).
For immunoblot analyses of total leaf protein, samples were separated by SDS-PAGE as outlined above and then transferred at 4 °C to nitrocellulose membranes (0.45 µm; BioRad) using a Xcell Surelock western transfer module (Invitrogen) buffered with 1× transfer buffer [20×: 25mM Bicine, 25mM Bis/Tris, 1mM EDTA, 20% (v/v) methanol]. After 1h transfer at 30V, the membrane was placed in blocking solution [3% (w/v) skimmed milk powder in TBS, 50mM Tris/HCl (pH 8), 150mM NaCl)] for 1h at room temperature with gentle agitation.
Primary antisera raised in rabbit against tobacco Rubisco (prepared by S. M. Whitney, Australian National University, Canberra, Australia) was diluted 1:4000 in TBS before incubation at 1h with membranes at room temperature with gentle agitation. Antisera raised against PEPC was obtained from AgriSera and diluted 1:2000 with TBS. For NADP-ME and PEP-CK, synthetic peptides based on monocot amino acid sequences for each protein were synthesized by GL Biochem and antisera were raised against each peptide in rabbits. The reactive antisera were the antigen purified for use in immunoblot analysis (GL Biochem). The NADP-ME and PEP-CK antisera were diluted in TBS at 1:1000 and 1:500, respectively.
All primary antisera were incubated with membranes at room temperature for 1h with gentle agitation before washing three times with TBS. Secondary goat anti-rabbit antiserum conjugated to horseradish peroxidase (Perkin Elmer) was diluted 1:3000 in TBS and incubated with the membranes for 1h at room temperature followed by three washes with TBS. Immunoreactive peptides were detected using an Immun-Star WesternC kit (BioRad) and imaged using VersaDoc.
Plant biomass, leaf water potential, and nitrogen and carbon isotope composition
Before harvest, leaf water potential (ΨL) was measured on a cut, matching gas-exchange leaf using a Scholander-style pressure chamber (PMS Instrument Company, Corvallis, OR, USA). At harvest, leaves were sampled and their area determined using a leaf area meter (LI-3100A; LI-COR) and roots were washed free of soil. Plant tissues were oven dried at 80 °C for 48h, weighed, and ground to a homogenous powder in a ball mill (MM-400; Retsch).
Leaf N content was determined on the ground tissue using a CN analyser (LECO TruSpec; LECO Corp., MI, USA). For carbon isotope composition (13δ), ground leaf samples were combusted in a Carlo Erba elemental analyser (Model 1108) and the CO2 was analysed by mass spectrometry. Isotopic composition (δ) was calculated as [(R sample – R standard)/R standard]×1000, where R sample and R standard are the 13C/12C ratios of the sample and standard (Pee Dee Belemnite), respectively.
Statistical analysis
Statistical significance tests were conducted using one-way analysis of variance computed in a general linear model. Treatment means were ranked using a post-hoc Tukey test.
Results
Plant growth and leaf nitrogen
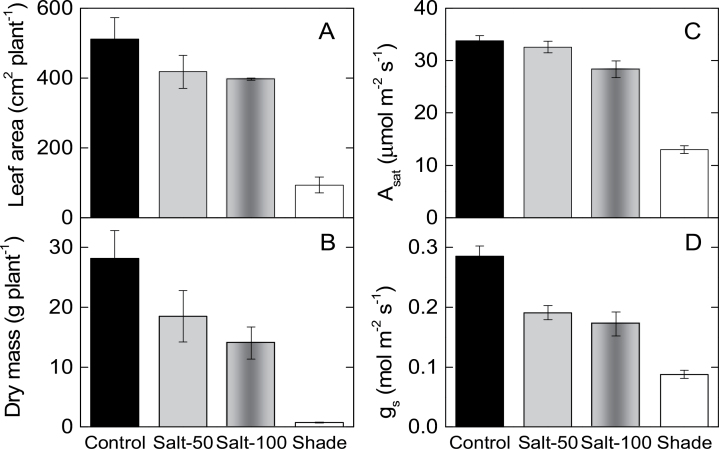
Plant leaf area was reduced by 18 and 22% for plants exposed to 50 and 100mM NaCl, respectively, whereas plant biomass was decreased by 34 and 50% for the same treatments when compared with the control (Fig. 1A, B, Table 1). Leaf mass per area was not significantly affected by salinity (Table 1).
Fig. 1.
Growth of maize plants exposed to salinity and shade. Total leaf area (A), plant dry mass (B), light-saturated photosynthetic rates in ambient air, A sat (C), and stomatal conductance, g s (D), of maize plants grown in full sunlight and irrigated with water (control), 50mM NaCl (Salt-50), or 100mM NaCl (Salt-100), or grown in 20% sunlight (shade).
Table 1.
Summary of plant growth, leaf chemistry, leaf gas exchange and photosynthetic enzyme activity determined for maize plants grown in full sunlight and irrigated with normal water (control), 50mM NaCl (Salt-50) or 100mM NaCl (Salt-100)
Shade plants were grown in 20% sunlight and irrigated with normal water.Values are treatment means of three replicates±standard error. Statistical significance tests were conducted using one-way analysis of variance computed in a general linear model. Treatment means were ranked using a post-hoc Tukey test, and values followed by the same letter are not significantly different at the 5% level (P<0.05). ND, not determined
| Parameter | Control | Shade | Salt-50 | Salt-100 | Model P value |
|---|---|---|---|---|---|
| Plant and leaf traits | |||||
| Total leaf area (cm2 plant–1) | 512±61b | 94±23a | 418±47b | 397±4b | 0.0012 |
| Plant dry mass (g plant–1) | 28.2±4.6c | 0.75±0.05a | 18.5±4.3bc | 14.0±2.7b | 0.0031 |
| Leaf mass per area (g m–2) | 71±5b | 26±2a | 69±6b | 59±15b | 0.0343 |
| Leaf water potential, ΨL (MPa) | 0.32±0.07a | ND | 0.53±0.10a | 1.08±0.09b | 0.0000 |
| Leaf N content (mg g–1) | 36±2b | 38±0b | 33±2ab | 29±1a | 0.0054 |
| Leaf N content (mmol m–2) | 184±10a | 95±25a | 132±24a | 124±28a | 0.1027 |
| Leaf C isotope composition, 13δ (‰) | –14.68±0.24a | –14.38±0.19a | –15.43±0.05b | –15.85±0.07b | 0.0006 |
| Leaf gas exchange | |||||
| Photosynthesis, A sat (µmol m–2 s–1) | 33.7±1.0c | 13.0±0.7a | 32.6±1.1c | 28.3±1.6b | 0.0000 |
| Stomatal conductance, g s (mol m–2 s–1) | 0.285±0.017c | 0.088±0.007a | 0.191±0.012b | 0.172±0.020b | 0.0000 |
| in vivo V cmax (µmol m–2 s–1) | 40±10b | 19±6a | 40±1b | 33±1b | 0.0125 |
| in vivo V pmax (µmol m–2 s–1) | 104±6b | 45±5a | 94±1b | 94±1b | 0.0012 |
| V pmax/V cmax | 2.6±0.2a | 2.5±0.4a | 2.3±0.1a | 2.8±0.1a | 0.4682 |
| Photosynthetic Δp (‰) | 3.66±0.13b | 2.46±0.36a | 3.90±0.04b | 4.23±0.11b | 0.0040 |
| Leakiness, ɸ | 0.26±0.02ab | 0.13±0.04a | 0.24±0.02ab | 0.31±0.01b | 0.0125 |
| Photosynthetic enzymes | |||||
| Rubisco content (g m–2) | 0.31±0.04b | 0.14±0.03a | 0.23±0.01ab | 0.24±0.03ab | 0.0141 |
| Soluble proteins (g m–2) | 4.2±0.2b | 2.5±0.2a | 3.2±0.2ab | 3.9±0.4b | 0.0057 |
| Rubisco activity (µmol m–2 s–1) | 27.5±1.4c | 11.5±2.0a | 18.1±0.8b | 19.5±2.1b | 0.0034 |
| PEPC activity (µmol m–2 s–1) | 107±7d | 21±4a | 52±4b | 72±4c | 0.0000 |
| PEPC/Rubisco | 3.9±0.10c | 1.8±0.08a | 2.9±0.13b | 3.3±0.15b | 0.0000 |
| NADP-ME activity (µmol m–2 s–1) | 53±8b | 32±5a | 18±0.1a | 19±3a | 0.0073 |
| PEP-CK activity (µmol m–2 s–1) | 12.4±1.6c | 3.0±0.4a | 7.6±0.7b | 8.2±1.2b | 0.0021 |
The impact of 80% shading (shaded plants received 20% of ambient sunlight) on the maize plants was profound. Leaf area and total plant biomass were reduced to 18 and 3% of that of the control plants, respectively, while leaf mass per area was reduced to 37% of that of the control plants (Fig. 1A, B, Table 1).
Leaf N content per unit mass decreased in the high-salt-treated plants only relative to the control. When expressed on an areas basis, leaf N concentration tended to be lower in the shaded plants relative to the control (Table 1).
Leaf photosynthesis
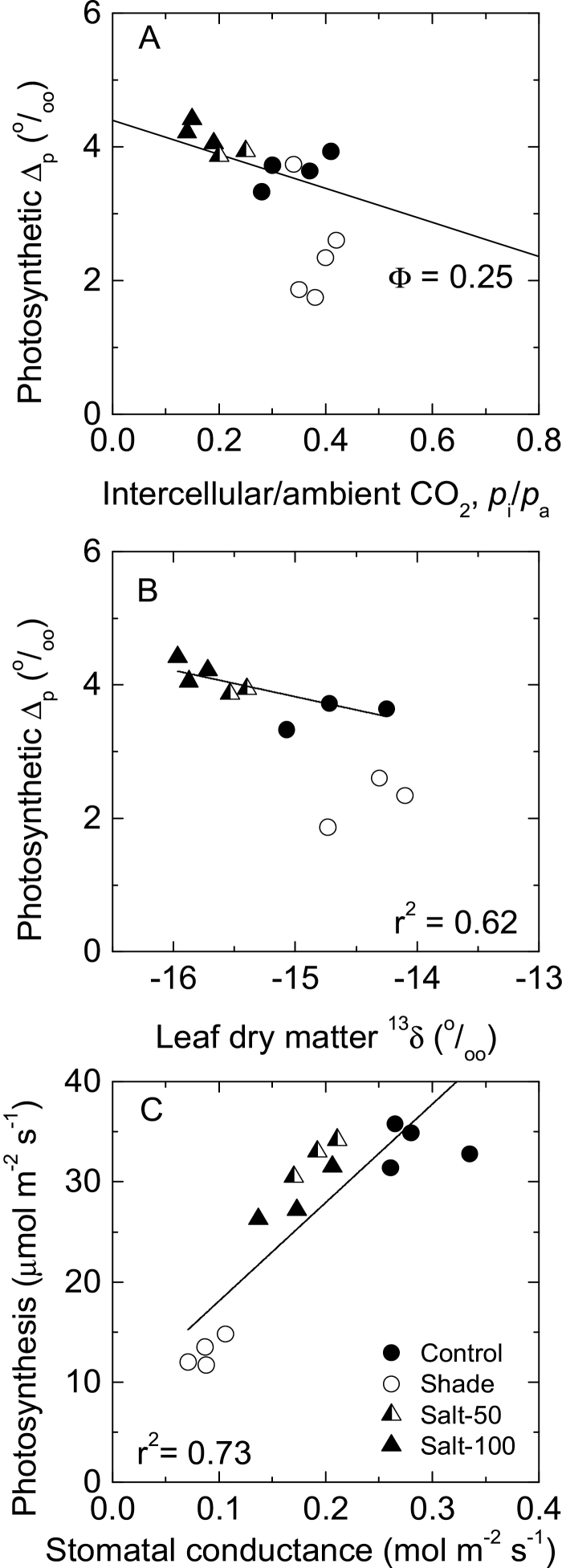
Leaf water potential (ΨL) decreased in plants exposed to moderate (100mM NaCl) but not mild (50mM NaCl) levels of soil salinity (Table 1). Consequently, photosynthetic rates measured at ambient CO2 (A sat) decreased in maize plants exposed to the higher salinity treatment only (Fig. 1C, Table 1), whereas g s decreased in plants exposed to both salinity levels (Fig. 1D, Table 1). Plants exposed to shade underwent larger decreases in photosynthesis and g s (Fig. 1C, D, Table 1). A common linear relationship related A sat to g s (r 2=0.73) in all the maize plants regardless of the treatment (Fig. 2C).
Fig. 2.
Leaf gas exchange and carbon isotope discrimination in maize plants exposed to salinity and shade. Photosynthetic carbon isotope discrimination, Δp measured during the gas exchange of maize leaves as a function of intercellular to ambient CO2 ratio (A) or leaf dry matter carbon isotope composition, 13δ (B). Photosynthetic rates as a function of stomatal conductance are also shown (C). In (A), the solid line is the solution for the C4 discrimination model (Farquhar, 1983) using a leakiness (ɸ) value of 0.25. Leaf gas exchange was measured at high light (1800 µmol m–2 s–1), ambient CO2 (400 µl l–1) and 26 °C. In (B), the solid line is the linear regression of all data points excluding the shade treatment. In (C), the solid line is the linear regression of all data points. Maize plants were grown in full sunlight and irrigated with water (control, filled circle), 50mM NaCl (Salt-50, half-filled triangle), or 100mM NaCl (Salt-100, filled triangle), or grown in 20% sunlight (shade, open circle).
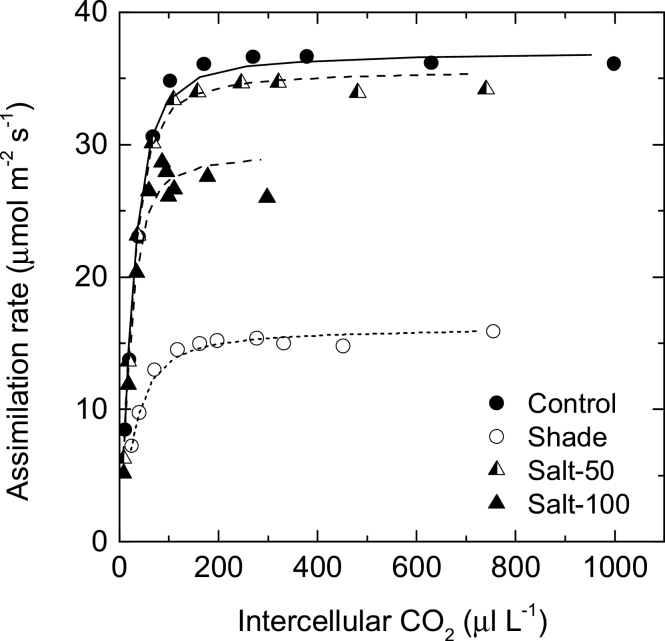
A/C i curves were fitted using the C4 photosynthesis model (von Caemmerer, 2000) to estimate in vivo V cmax and V pmax. Both parameters decreased in the shaded plants relative to the control, while there was a small but non-significant reduction in V cmax in the higher salinity treatment. The ratio V pmax/V cmax (2.3–2.8) was similar for all the maize plants, regardless of the treatment (Fig. 3, Table 1).
Fig. 3.
A/C i response curves for leaves of maize exposed to salinity and shade. Responses of assimilation rates to intercellular CO2 (A/C i curves) measured at a light intensity of 1800 µmol m–2 s–1 and a leaf temperature of 26 °C. Data points are the average of two replicates. Lines are the mathematical fits using the C4 photosynthesis model (von Caemmerer, 2000). Maize plants were grown in full sunlight and irrigated with water (control, filled circle), 50mM NaCl (Salt-50, half-filled triangle), or 100mM NaCl (Salt-100, filled triangle), or grown in 20% sunlight (shade, open circle).
Photosynthetic and dry-matter carbon isotope discrimination
Concurrent measurements of 13CO2/12CO2 discrimination and leaf gas exchange showed that photosynthetic discrimination (Δp) varied linearly with p i/p a for plants in the control and salinity treatments, yielding a common bundle-sheath ɸ value of 0.25 according to the carbon discrimination model for C4 plants (Farquhar, 1983). Thus, salinity changed p i/p a without affecting ɸ. In contrast, shaded plants had lower Δp, p i/p a, and ɸ relative to both control and salt-stressed plants (Fig. 2A).
Leaf dry-matter carbon isotope composition (13δ) decreased (more negative) significantly in the salt-treated plants only, while shade plants had similar leaf 13δ to the control plants (Table 1). Photosynthetic Δp and leaf dry-matter 13δ changed proportionately for the control and salt-treated plants (Fig. 2B). In contrast, the shade plants fell outside the common relationship because their photosynthetic Δp decreased but not their leaf 13δ relative to the control plants (Fig. 2B).
Activity of photosynthetic enzymes
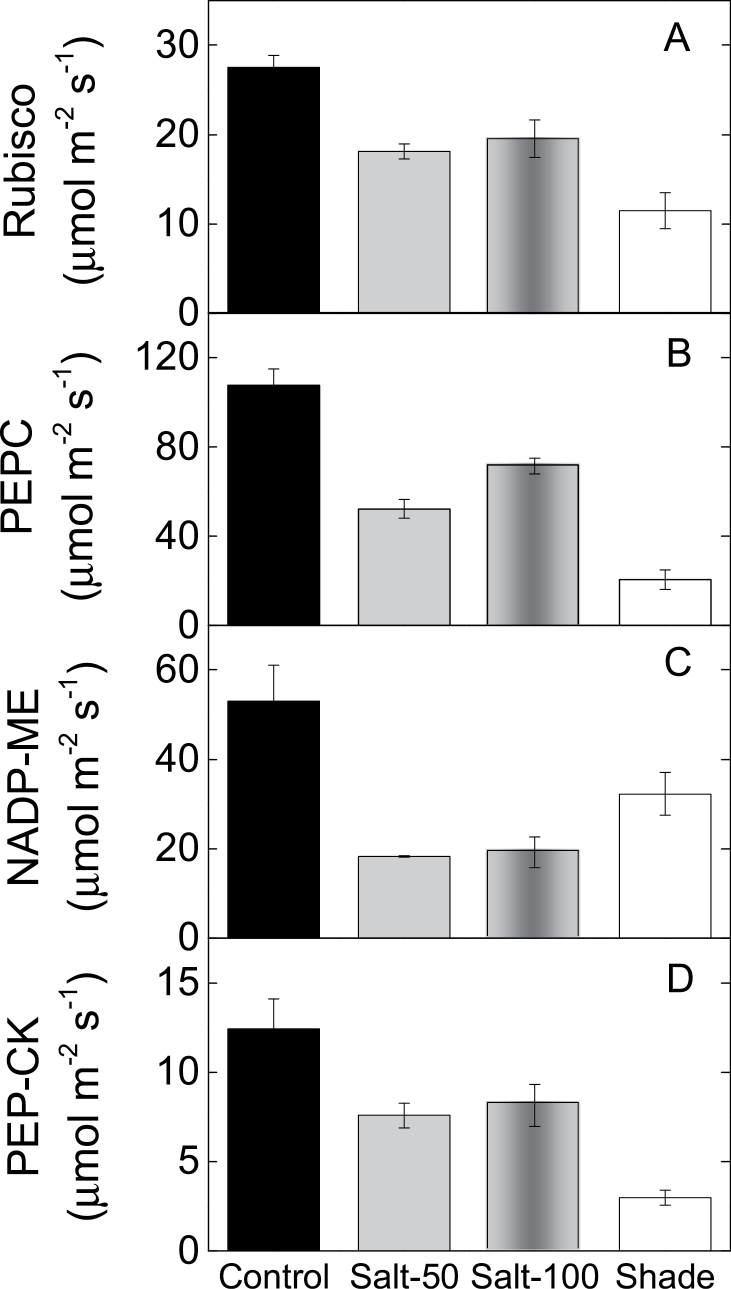
Leaf Rubisco content and Rubisco activity calculated from k cat and the irreversible binding of the transition state analogue [14C]CABP decreased by 25 and 50% in the salt-treated and shaded plants, respectively. As expected for C4 leaves, Rubisco activity was equivalent to A sat for the control and shade leaves; this was not the case for the salt-treated leaves (Fig. 4A, Table 1). Leaf soluble proteins changed together with Rubisco such that Rubisco constituted a constant fraction of soluble proteins under all treatments (Table 1). PEPC activity measured in leaf extracts was reduced by 80% in the shaded plants and by 30–50% in the salt-treated plants relative to the control treatment (Fig. 4B, Table 1). Generally, changes in Rubisco and PEPC activities were reflected by the immunoblots probed with antibodies raised against each of the carboxylase enzymes (Fig. 5). Shading reduced PEPC activity to a greater extent than Rubisco activity, and consequently halved the PEPC/Rubisco activity ratio relative to the control treatment. The PEPC/Rubisco ratio was not significantly affected by salinity in the maize plants (Table 1). It is worth noting that in vivo and in vitro estimates of Rubisco (V cmax) and PEPC (V pmax) activities did not closely correlate in this study. Reconciling these parameters requires more detailed parameterization of C4 photosynthesis model (von Caemmerer, 2000).
Fig. 4.
Activity of carboxylases and decarboxylases in maize plants exposed to salinity and shade. Activity of Rubisco (A), PEPC (B), NADP-ME (C), and PEP-CK (D) for maize plants grown in full sunlight and irrigated with water (control), 50mM NaCl (Salt-50), or 100mM NaCl (Salt-100), or grown in 20% sunlight (shade).
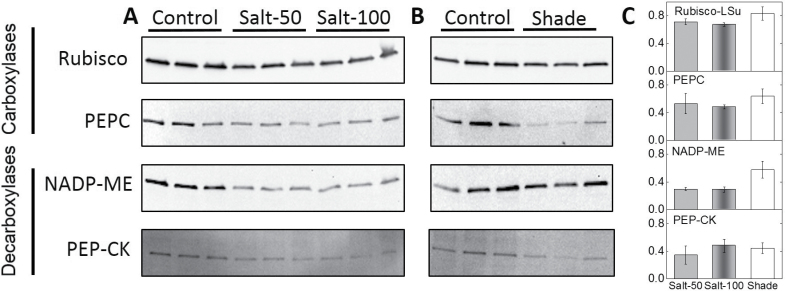
Fig. 5.
Immunoblots of carboxylases and decarboxylases in maize plants exposed to salinity and shade. Immunoblots of total leaf proteins probed with antisera raised against the four photosynthetic enzymes Rubisco large subunit, PEPC, NADP-ME, and PEP-CK, as described in Materials and methods. The analysis was undertaken separately for the salinity-treated (A) and shade-treated (B) maize plants. Changes in immunoblot densitometry were calculated relative to the control treatment (C).
The activity of the primary decarboxylase NADP-ME and its relative content determined by immunoblot analysis showed 35% reductions across both salinity treatments. NADP-ME activity declined by 60% in the shaded relative to the control plants (Figs 4C and 5, Table 1). Activity and protein expression of the secondary decarboxylase, PEP-CK, was detected in the leaf extracts of all maize plants (Figs 4D and 5). In the control treatment, PEP-CK accounted for 20% of the total C4 acid decarboxylation activity measured in maize leaves. This proportion increased to 30% in salt-treated plants and declined to 10% in the shaded plants (Table 1). In absolute terms, PEP-CK activity decreased by 40% in salt-treated plants and by 75% in shaded plants (Figs 4D and 5, Table 1).
Discussion
Contrasting impacts of salinity and shade on maize
The main objective of this study was to investigate the regulation of C4 photosynthesis subjected to environmental manipulations that are known to have contrasting impacts on the processes of CO2 assimilation and diffusion. One of the main acclimation responses to shade is the downregulation of leaf photosynthetic capacity (Boardman, 1977). In contrast, mild to moderate salinity will primarily reduce stomatal conductance by negatively impacting on soil, and hence on leaf water potential. Compared with water stress, salinity has the added advantage of providing a steady stress while avoiding the complications associated with controlling soil water supply (Neumann et al., 1988; Chaves et al., 2009). To this end, both treatments used in this study achieved their goals. While shade markedly reduced plant growth and photosynthetic capacity, salinity reduced stomatal conductance with small effects on photosynthetic rates of the maize plants. Salinity inhibited plant growth to a lesser extent than shade (Table 1).
The evolution of a CCM in higher plants represents a key step to improving photosynthesis under environmental conditions favouring photorespiration by circumventing the inefficiency of Rubisco. The efficient operation of C4 photosynthesis requires close coordination between the C4 and C3 cycles, which is achieved through the distinct cellular compartmentalization of the initial and final carboxylases PEPC (in MCs) and Rubisco (in BSCs), respectively, as well as the localization of the decarboxylases (NADP-ME and PEP-CK for maize) within the BSC. In addition, the maintenance of a high PEPC/Rubisco activity ratio is critical for the build-up of CO2 within the BSCs. Importantly, regulating the balance between Rubisco, PEPC, NADP-ME, and other enzymes of the C3 and C4 cycles allows the dynamic regulation of C4 efficiency that other features such as BSC wall conductance or CO2 diffusion path length cannot offer in the short to medium term (Hatch, 1987; von Caemmerer and Furbank, 2003). Perturbation of the PEPC/Rubisco ratio by genetically suppressing PEPC results in C4 plants unable to grow effectively in air (Dever et al., 1997; Cousins et al., 2007). Leakiness of CO2 from the BSCs as determined from measurements of 13C/12C carbon isotope discrimination represents a key surrogate indicator of the coordination between the C3 and C4 cycles (Farquhar, 1983). Combining measurements of leakiness with activities of the key enzymes in the C3 and C4 cycles can elucidate the regulation and efficiency of C4 photosynthesis under different environments (Evans et al., 1986; Henderson et al., 1992). Below, we demonstrate that shade, but not salinity, can perturb CCM efficiency as evidenced by changed leakiness.
Mild to moderate salinity impacts on carbon isotope discrimination through stomatal conductance without affecting leakiness
In maize, mild salinity (50mM NaCl) reduced leaf g s but not A sat, while moderate salinity (100mM NaCl) reduced both g s and A sat. Hence, reduced photosynthetic rates were largely caused by increased resistance to CO2 diffusion under both salinity treatments, and this was born out in the lower p i/p a ratio and more negative dry-matter 13δ observed in the leaves of salt-treated maize plants (Fig. 2). Reduced stomatal conductance and leaf 13δ in response to salinity is commonly reported in C3 (Seemann and Critchley, 1985; Brugnoli and Lauteri, 1991) and C4 (Bowman et al., 1989; Meinzer et al., 1994; Meinzer and Zhu, 1999) plants. In maize, reduced photosynthetic rates, especially at the highest salinity treatment was also caused by the lower Rubisco and PEPC activities. This reduction was observed in the spectrophotometric assays and the immunodetection of the expressed proteins. Reduced expression of Rubisco under salinity was part of a general reduction in soluble proteins and leaf N. Leaf N is known to decline under salinity due to Cl– interference with nitrate uptake by roots (Munns and Termaat, 1986).
The activity of both carboxylases declined to the same extent under salinity conditions such that the PEPC/Rubisco ratio was indistinguishable from that of the control leaves. This may explain why leakiness was unaffected by salinity in maize leaves despite the changes in photosynthetic Δp and leaf 13δ, which were caused by reduced p i/p a (Fig. 2). In line with these results, when the C4 shrub Atriplex lentiformis was exposed for 4 weeks to salinity levels equivalent to those used in the current study, photosynthesis and stomatal conductance decreased, while the PEPC/Rubisco ratio remained unchanged until the salinity increased above 120mM. The same study also reported that leakiness, estimated from leaf 13δ rather than from photosynthetic Δp, correlated positively with the PEPC/Rubisco ratio (Meinzer and Zhu, 1999). Similarly to Atriplex, salinity reduced photosynthesis and increased p i/p a and ɸ values in sugarcane genotypes. Changes in ɸ derived from leaf 13δ were also related to the PEPC/Rubisco ratio in sugarcane (Meinzer et al., 1994).
The discrepancy between the studies using Atriplex and sugarcane with the current study using maize may be related to a number of factors, the main ones being the salinity level and the basis for leakiness calculation. Meinzer and Zhu (1999) found that mild salinity mainly affected g s and had little impacts on ɸ (a similar scenario to the current maize study), and that ɸ and the PEPC/Rubisco ratio were affected at high salinity, indicating profound damage of the photosynthetic apparatus by the accumulating salt, unlike the treatments used in the current maize study. In addition, the difference between leaf 13δ and photosynthetic Δp have not been reconciled yet for C4 plants. Post-photosynthetic fractionation of 13C/12C may be important in C4 leaves, thus representing a source of uncertainty in leakiness calculations based on leaf 13δ (Henderson et al., 1992).
In maize, both salinity treatments reduced the activity of the primary (NADP-ME) and secondary (PEP-CK) decarboxylases. These observations, together with reduced PEPC activity, suggest that the CCM was down regulated in response to salinity. Results obtained with enzyme activity and immunoblot analysis indicated that the decarboxylases were inhibited more than the carboxylases under salinity. Evidence from transgenic Flaveria plants with reduced amounts of NADP-ME have indicated that this decarboxylase is in excess, as photosynthesis was not impacted until activity was reduced to less than 40% of that of wild type (Pengelly et al., 2012). In summary, salinity treatments reduced photosynthesis primarily by reducing g s and secondarily by reducing Rubisco and PEPC activities. The balance between the C3 and C4 cycles was unaffected, as indicated by a similar leakiness between the salt-treated and control maize plants.
Shade profoundly reduces photosynthetic capacity and leakiness, thus perturbing the coordination between the C3 and C4 cycles
The shade treatment used in this study had profound impacts on the growth and photosynthesis of the maize plants (Table 1). In particular, shade reduced the photosynthetic capacity measured in terms of in vivo V cmax and V pmax estimated from the A/C i curves and in terms of enzyme activity of the carboxylases and decarboxylases. In contrast to salinity, shade had two significantly distinct effects on leaf photosynthesis. Firstly, decreased photosynthetic capacity was mediated by a general downregulation of the activity and protein expression of all measured photosynthetic enzymes. Secondly, the PEPC/Rubisco ratio, photosynthetic Δp, and its derived leakiness decreased relative to those of the control plants, while leaf 13δ was not significantly affected (Fig. 2).
The responses of C4 photosynthesis to low light vary depending on whether the condition is transient or a short-term acclimation. Under low light (<200 µmol quanta m–2 s–1), ɸ may increase, possibly as a result of decreased Rubisco activation or increased Rubisco oxygenation due to the low BSC CO2 concentration. These factors decrease CO2 fixation by Rubisco more than by PEPC, thus maintaining a higher supply of CO2 to the BSCs than Rubisco can fix (Henderson et al., 1992; Kromdijk et al., 2008, 2010; Tazoe et al., 2008). However, ɸ in maize leaves was unaffected under conditions of short-term acclimation to low light (Bellasio and Griffiths, 2013a , b).
In contrast to these studies, leakiness decreased in our study as a result of reduced Δp with little impact on p i/p a, suggesting two main conclusions. Firstly, reduced leakiness in our maize study was accompanied by a reduced PEPC/Rubisco ratio, highlighting the role of this ratio in particular, and the balance between the activity of the C3 and C4 cycle enzymes in general, for optimizing the efficiency of C4 photosynthesis. Our results in maize make it clear that acclimation to low light reduced PEPC activity and protein expression to a greater extent than those of Rubisco. High light dependence of PEPC gene expression is well documented in C4 plants (Chollet et al., 1996). Secondly, leaf 13δ and photosynthetic Δp in our maize study did not change together under low light, mainly because the former decreased while the latter was only marginally and not significantly affected by shade (Fig. 2). This is in contrast to a large survey of C4 grasses, showing that leaf 13δ decreased under shade conditions (Buchmann et al., 1996). On the one hand, our results highlight the problems of using leaf 13δ as a proxy for photosynthetic Δp, especially when inferring leakiness and C4 regulation. On the other hand, our results point to a stronger dependence of leaf 13δ on the diffusive components (salinity effects) within the Δp equation as opposed to the metabolic factors for which light can have complex effects (Farquhar, 1983; Henderson et al., 1992; von Caemmerer et al., 1997a ; Ubierna et al., 2011). Solving the link between leaf 13δ and photosynthetic Δp remains a key challenge for elucidating the underpinnings of carbon isotope discrimination in C4 leaves.
In another contrast with the salinity treatments, shade reduced the activity of the primary decarboxylase NADP-ME less, while strongly suppressing the activity of the secondary decarboxylase PEP-CK. Taken together, these results constitute rare evidence for decarboxylase flexibility in response to environmental conditions, with salinity and shade having opposite effects on the ratio of PEP-CK to NADP-ME activity in maize. It is unlikely that the observed changes in NADP-ME and PEP-CK were due to anaplerotic activities due to their low contribution relative to that of the photosynthetic isoforms (Drincovich et al., 2001). The differential engagement of the decarboxylation pathways enables C4 plants to acclimate to varying conditions of light (Furbank, 2011). For example, it has been shown that the flexible operation of NADP-ME and PEP-CK decarboxylases in maize allows the bundle sheath to regulate NADPH supply under variable light conditions (Bellasio and Griffiths, 2013b ). In the current study, we demonstrated the differential engagement of the primary and secondary decarboxylases under long-term acclimation to low light through the significant reductions of PEP-CK activity and protein content (Figs 4 and 5).
In summary, we demonstrated that long-term acclimation to low light in maize causes a reduction in BSC leakiness. This reduction was underpinned by a greater downregulation of PEPC activity and content relative to those of Rubisco, and by a flexible partitioning of C4 acid decarboxylation activity between NADP-ME and PEP-CK.
Acknowledgements
We thank Hilary Stuart-Williams from the Australian National University for assistance with the analysis of leaf dry-matter carbon isotope composition and N content. We thank Anthony Newton for help with plant culture. This research was partially funded by the Hawkesbury Institute for the Environment at University of Western Sydney through the award of a research fellowship to RES. This research was also supported by a Discovery Project awarded to OG by the Australian Research Council (DP120101603).
Glossary
Abbreviations:
- BSC
bundle-sheath cell
- CABP
carboxyarabinitol bisphosphate
- CCM
CO2 concentration mechanism
- MC
mesophyll cell
- NADP-ME
nicotinamide adenine dinucleotide phosphate malic enzyme
- PEPC
phosphoenolpyruvate carboxylase
- PEP-CK
phosphoenolpyruvate carboxykinase
- Rubisco
ribulose-1,5-bisphosphate carboxylase/oxygenase.
References
- Andrews TJ, Lorimer GH, Tolbert NE. 1973. Ribulose diphosphate oxygenase. I. Synthesis of phosphoglycolate by fraction-1 protein of leaves. Biochemistry 12, 11–18 [DOI] [PubMed] [Google Scholar]
- Ashton AR, Burnell JN, Furbank RT, Jenkins CLD, Hatch MD. 1990. The enzymes in C4 photosynthesis. In: Lea PJ, ed. Enzymes of primary metabolism. London: Academic Press, 39–72 [Google Scholar]
- Bellasio C, Griffiths H. 2013. a Acclimation to low light by C4 maize: implications for bundle sheath leakiness. Plant Cell, & Environment, 10.1111/pce.12194. [DOI] [PubMed] [Google Scholar]
- Bellasio C, Griffiths H. 2013. b The operation of two decarboxylases (NADP-ME and PEP-CK), transamination and partitioning of C4 metabolic processes between mesophylll and bundle sheath cells allows light capture to be balanced for the maize C4 pathway. Plant Physiology http://dx.doi.org/10.1104/pp.113.228221 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman NK. 1977. Comparative photosynthesis of sun and shade plants. Annual Review of Plant Physiology and Plant Molecular Biology 28, 355–377 [Google Scholar]
- Bowman WD, Hubick KT, von caemmerer S, Farquhar GD. 1989. Short-term changes in leaf carbon isotope discrimination in salt-stressed and water-stressed C4 grasses. Plant Physiology 90, 162–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnoli E, Lauteri M. 1991. Effects of salinity on stomatal conductance, photosynthetic capacity, and carbon isotope discrimination of salt-tolerant (Gossypium hirsutum L) and salt-sensitive (Phaseolus vulgaris L) C3 non-halophytes. Plant Physiology 95, 628–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann N, Brooks JR, Rapp KD, Ehleringer JR. 1996. Carbon isotope composition of C4 grasses is influenced by light and water supply. Plant,Cell & Environment 19, 392–402 [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C. 2009. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany 103, 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Walker RP, Acheson RM, Leegood RC. 2002. Phosphoenolpyruvate carboxykinase assayed at physiological concentrations of metal ions has a high affinity for CO2 . Plant Physiology 128, 160–164 [PMC free article] [PubMed] [Google Scholar]
- Chollet R, Vidal J, O’Leary MH. 1996. Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47, 273–298 [DOI] [PubMed] [Google Scholar]
- Cleland WW, Andrews TJ, Gutteridge S, Hartman FC, Lorimer GH. 1998. Mechanism of Rubisco: the carbamate as general base. Chemical Reviews 98, 549–562 [DOI] [PubMed] [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S. 2006. Carbonic anhydrase and its influence on carbon isotope discrimination during C4 photosynthesis. Insights from antisense RNA in Flaveria bidentis . Plant Physiology 141, 232–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S. 2008. C4 photosynthetic isotope exchange in NAD-ME- and NADP-ME-type grasses. Journal of Experimental Botany 59, 1695–1703 [DOI] [PubMed] [Google Scholar]
- Cousins AB, Baroli I, Badger MR, Ivakov A, Lea PJ, Leegood RC, von Caemmerer S. 2007. The role of phosphoenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance. Plant Physiology 145, 1006–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever LV, Bailey KJ, Leegood RC, Lea PJ. 1997. Control of photosynthesis in Amaranthus edulis mutants with reduced amounts of PEP carboxylase. Functional Plant Biology 24, 469–476 [Google Scholar]
- Drincovich MaF, Casati P, Andreo CS. 2001. NADP-malic enzyme from plants: a ubiquitous enzyme involved in different metabolic pathways. FEBS Letters 490, 1–6 [DOI] [PubMed] [Google Scholar]
- Edwards GE, Nakamoto H, Burnell JN, Hatch MD. 1985. Pyruvate, Pi dikinase and NADP-malate dehydrogenase in C4 photosynthesis: properties and mechanism of light/dark regulation. Annual Review of Plant Physiology 36, 255–286 [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. 1986. Carbon isotope discrimination measured concurrently with gas-exchange to investigate CO2 diffusion in leaves of higher-plants. Australian Journal of Plant Physiology 13, 281–292 [Google Scholar]
- Farquhar GD. 1983. On the nature of carbon isotope discrimination in C4 species. Australian Journal of Plant Physiology 10, 205–226 [Google Scholar]
- Farquhar GD, Cernusak LA. 2012. Ternary effects on the gas exchange of isotopologues of carbon dioxide. Plant, Cell & Environment 35, 1221–1231 [DOI] [PubMed] [Google Scholar]
- Fravolinil A, Williams DG, Thompson TL. 2002. Carbon isotope discrimination and bundle sheath leakiness in three C4 subtypes grown under variable nitrogen, water and atmospheric CO2 supply. Journal of Experimental Botany 53, 2261–2269 [DOI] [PubMed] [Google Scholar]
- Furbank RT. 2011. Evolution of the C4 photosynthetic mechanism: are there really three C4 acid decarboxylation types? Journal of Experimental Botany 62, 3103–3108 [DOI] [PubMed] [Google Scholar]
- Furbank RT, Jenkins CLD, Hatch MD. 1990. C4 photosynthesis—quantum requirement, C4 acid overcycling and Q-cycle involvement. Australian Journal of Plant Physiology 17, 1–7 [Google Scholar]
- Ghannoum O, Evans JR, Caemmerer S. 2011. Nitrogen and water use efficiency of C4 plants. In: Raghavendra AS, Sage RF, eds. C4 photosynthesis and related CO2 concentrating mechanisms, vol. 32 The Netherlands: Springer, 129–146 [Google Scholar]
- Ghannoum O, Evans JR, Wah Soon C, Andrews TJ, Conroy JP, Susanne von C. 2005. Faster Rubisco is the key to superior nitrogen use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiology 137, 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M, Gracen VE, Edwards GE. 1974. Biochemical and cytological relationships in C4 plants. Planta 119, 279–300 [DOI] [PubMed] [Google Scholar]
- Hatch MD. 1987. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochemica et Biophysica Acta 895, 81–106 [Google Scholar]
- Henderson S, Caemmerer S, Farquhar G. 1992. Short-term measurements of carbon isotope discrimination in several C4 species. Functional Plant Biology 19, 263–285 [Google Scholar]
- Jenkins CL, Burnell JN, Hatch MD. 1987. Form of inorganic carbon involved as a product and as an inhibitor of C4 acid decarboxylases operating in C4 photosynthesis. Plant Physiology 85, 952–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Edwards GE. 1999. The biochemistry of C4 photosynthesis. In: Rowan FS, Russell KM, eds. C4plant biology. San Diego: Academic Press, 49–87 [Google Scholar]
- Kromdijk J, Griffiths H, Schepers HE. 2010. Can the progressive increase of C4 bundle sheath leakiness at low PFD be explained by incomplete suppression of photorespiration? Plant, Cell & Environment 33, 1935–1948 [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Schepers HE, Albanito F, Fitton N, Carroll F, Jones MB, Finnan J, Lanigan GJ, Griffiths H. 2008. Bundle sheath leakiness and light limitation during C4 leaf and canopy CO2 uptake. Plant Physiology 148, 2144–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasek J, Setlik J, Dwyer S, Santrucek J. 2007. Light and growth temperature alter carbon isotope discrimination and estimated bundle sheath leakiness in C4 grasses and dicots. Photosynthesis Research 91, 47–58 [DOI] [PubMed] [Google Scholar]
- Langdale JA. 2011. C4 cycles: past, present, and future research on C4 photosynthesis. Plant Cell 23, 3879–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood RC, Walker RP. 2003. Regulation and roles of phosphoenolpyruvate carboxykinase in plants. Archives Biochemistry and Biophysics 414, 204–210 [DOI] [PubMed] [Google Scholar]
- Ludwig M. 2013. Evolution of the C4 photosynthetic pathway: events at the cellular and molecular levels. Photosynthesis Research 117, 147–161 [DOI] [PubMed] [Google Scholar]
- Meinzer FC, Plaut Z, Saliendra NZ. 1994. Carbon isotope discrimination, gas exchange, and growth of sugarcane cultivars under salinity. Plant Physiology 104, 521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer FC, Zhu J. 1999. Efficiency of C4 photosynthesis in Atriplex lentiformis under salinity stress. Functional Plant Biology 26, 79–86 [Google Scholar]
- Munns R, Termaat A. 1986. Whole-plant responses to salinity. Australian Journal of Plant Physiology 13, 143–160 [Google Scholar]
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681 [DOI] [PubMed] [Google Scholar]
- Neumann PM, Van Volkenburgh E, Cleland RE. 1988. Salinity stress inhibits bean leaf expansion by reducing turgor, not wall extensibility. Plant Physiology 88, 233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto E, Taniguchi M, Miyake H. 2012. Adaptation responses in C4 photosynthesis of maize under salinity. Journal of Plant Physiology 169, 469–477 [DOI] [PubMed] [Google Scholar]
- Pengelly JJ, Sirault XR, Tazoe Y, Evans JR, Furbank RT, von Caemmerer S. 2010. Growth of the C4 dicot Flaveria bidentis: photosynthetic acclimation to low light through shifts in leaf anatomy and biochemistry. Journal of Experimental Botany 61, 4109–4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengelly JJ, Tan J, Furbank RT, von Caemmerer S. 2012. Antisense reduction of NADP-malic enzyme in Flaveria bidentis reduces flow of CO2 through the C4 cycle. Plant Physiology 160, 1070–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47 [DOI] [PubMed] [Google Scholar]
- Saliendra NZ, Meinzer FC, Perry M, Thom M. 1996. Associations between partitioning of carboxylase activity and bundle sheath leakiness to CO2, carbon isotope discrimination, photosynthesis, and growth in sugarcane. Journal of Experimental Botany 47, 907–914 [Google Scholar]
- Seemann J, Critchley C. 1985. Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta 164, 151–162 [DOI] [PubMed] [Google Scholar]
- Shabala S, Munns R. 2012. Salinity stress: physiological constraints and adaptive mechanisms. In: Plant Stress Physiology. Wallingford, UK: CAB International, 59–93 [Google Scholar]
- Sharwood RE, von Caemmerer S, Maliga P, Whitney SM. 2008. The catalytic properties of hybrid Rubisco comprising tobacco small and sunflower large subunits mirror the kinetically equivalent source Rubiscos and can support tobacco growth. Plant Physiology 146, 83–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe Y, Hanba YT, Furumoto T, Noguchi K, Terashima I. 2008. Relationships between quantum yield for CO2 assimilation, activity of key enzymes and CO2 leakiness in Amaranthus cruentus, a C4 dicot, grown in high or low light. Plant and Cell Physiology 49, 19–29 [DOI] [PubMed] [Google Scholar]
- Ubierna N, Sun W, Cousins AB. 2011. The efficiency of C4 photosynthesis under low light conditions: assumptions and calculations with CO2 isotope discrimination. Journal of Experimental Botany 62, 3119–3134 [DOI] [PubMed] [Google Scholar]
- Ubierna N, Sun W, Kramer DM, Cousins AB. 2013. The efficiency of C4 photosynthesis under low light conditions in Zea mays, Miscanthus x giganteus and Flaveria bidentis . Plant, Cell & Environment 36, 365–381 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. 2000. Biochemical models of leaf photosynthesis. Melbourne: CSIRO Publishing [Google Scholar]
- von Caemmerer S, Farquhar GD. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Furbank RT. 2003. The C4 pathway: an efficient CO2 pump. Photosynthesis Research 77, 191–207 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Ludwig M, Millgate A, Farquhar GD, Price GD, Badger M, Furbank RT. 1997. a Carbon isotope discrimination during C4 photosynthesis: insights from transgenic plants. Australian Journal of Plant Physiology 24, 487–494 [Google Scholar]
- von Caemmerer S, Millgate A, Farquhar GD, Furbank RT. 1997. b Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase by antisense RNA in the C4 plant Flaveria bidentis leads to reduced assimilation rates and increased carbon isotope discrimination. Plant Physiology 113, 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RP, Chen ZH, Acheson RM, Leegood RC. 2002. Effects of phosphorylation on phosphoenolpyruvate carboxykinase from the C4 plant Guinea grass. Plant Physiology 128, 165–172 [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Walker RP, Chen ZH, Leegood RC. 1999. Phosphoenolpyruvate carboxykinase is involved in the decarboxylation of aspartate in the bundle sheath of maize. Plant Physiology 120, 539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]