Summary
At glacial CO2, NAD-ME grasses have higher photosynthetic water use efficiency than NADP-ME and PCK counterparts. Photosynthetic carboxylases rather than decarboxylases modulate the response of C4 photosynthesis to glacial CO2
Key words: C3, C3–C4, and C4 photosynthesis; glacial CO2; NAD-ME; NADP-ME; PEPC; PEP-CK; Rubisco; water and nitrogen use efficiency.
Abstract
Most physiology comparisons of C3 and C4 plants are made under current or elevated concentrations of atmospheric CO2 which do not reflect the low CO2 environment under which C4 photosynthesis has evolved. Accordingly, photosynthetic nitrogen (PNUE) and water (PWUE) use efficiency, and the activity of the photosynthetic carboxylases [Rubisco and phosphoenolpyruvate carboxylase (PEPC)] and decarboxylases [NADP-malic enzyme (NADP-ME) and phosphoenolpyruvate carboxykinase (PEP-CK)] were compared in eight C4 grasses with NAD-ME, PCK, and NADP-ME subtypes, one C3 grass, and one C3–C4 grass grown under ambient (400 μl l–1) and glacial (180 μl l–1) CO2. Glacial CO2 caused a smaller reduction of photosynthesis and a greater increase of stomatal conductance in C4 relative to C3 and C3–C4 species. Panicum bisulcatum (C3) acclimated to glacial [CO2] by doubling Rubisco activity, while Rubisco was unchanged in Panicum milioides (C3–C4), possibly due to its high leaf N and Rubisco contents. Glacial CO2 up-regulated Rubisco and PEPC activities in concert for several C4 grasses, while NADP-ME and PEP-CK activities were unchanged, reflecting the high control exerted by the carboxylases relative to the decarboxylases on the efficiency of C4 metabolism. Despite having larger stomatal conductance at glacial CO2, C4 species maintained greater PWUE and PNUE relative to C3–C4 and C3 species due to higher photosynthetic rates. Relative to other C4 subtypes, NAD-ME and PEP-CK grasses had the highest PWUE and PNUE, respectively; relative to C3, the C3–C4 grass had higher PWUE and similar PNUE at glacial CO2. Biomass accumulation was reduced by glacial CO2 in the C3 grass relative to the C3–C4 grass, while biomass was less reduced in NAD-ME grasses compared with NADP-ME and PCK grasses. Under glacial CO2, high resource use efficiency offers a key evolutionary advantage for the transition from C3 to C4 photosynthesis in water- and nutrient-limited environments.
Introduction
The decline in atmospheric CO2 concentration ([CO2]) in the late Oligocene (30 million years ago) is considered to be the primary driver for the evolution of the C4 photosynthetic pathway (Christin et al., 2008; Ehleringer et al., 1997; Sage et al., 2012). Geological fluctuations in atmospheric [CO2] have shaped the Earth’s vegetation, yet relatively little is known about the responses of C4 plants to the low [CO2] levels that dominated during their evolution, and that are close to the atmospheric [CO2] of the recent glaciation (Pagani et al., 2005). Low [CO2] promotes high rates of photorespiration and reduces the carboxylation efficiency of C3 photosynthesis. The key feature of C4 photosynthesis is the operation of a CO2-concentrating mechanism (CCM) which suppresses photorespiration by raising [CO2] around Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase). During C4 photosynthesis, phosphoenolpyruvate carboxylase (PEPC) catalyses the initial carboxylation of CO2 into organic C4 acids in the mesophyll. Decarboxylation of C4 acids in the bundle sheath releases CO2 for refixation by Rubisco (Hatch, 1987). The C4 photosynthetic pathway is classified into three biochemical subtypes based on the primary C4 decarboxylase enzyme. These enzymes are NADP-malic enzyme (NADP-ME), NAD-malic enzyme (NAD-ME), and phosphoenolpyruvate carboxykinase (PEP-CK, also known as PCK) (Gutierrez et al., 1974; Kanai and Edwards, 1999). There are strong anatomical and biochemical variations associated with these biochemical subtypes (Prendergast et al., 1987; Dengler et al., 1994; Edwards and Voznesenskaya, 2011).
The operation of a CCM enhances the efficiency of C4 relative to C3 photosynthesis (Osmond, 1982). In particular, C4 species attain higher photosynthetic water use efficiency (PWUE) because lower stomatal conductance (g s) and intercellular [CO2] (C i) are needed to saturate Rubisco carboxylation. C4 plants achieve higher photosynthetic nitrogen use efficiency (PNUE) due to their lower leaf N requirement as a result of a higher Rubisco catalytic turnover rate (k cat) (Long, 1999; Taylor et al., 2010; Ghannoum et al., 2011). Variations in resource use efficiency also occur among the C4 subtypes (Ghannoum et al., 2011). For example, NADP-ME grasses tend to have lower leaf N content than their NAD-ME counterparts (Bowman, 1991; Knapp and Medina, 1999; Taub and Lerdau, 2000), as a result of faster Rubisco k cat in NADP-ME species (Ghannoum et al., 2005). Furthermore, Ghannoum et al. (2002) showed that under water stress, NAD-ME grasses increased their whole-plant WUE to a greater extent than NADP-ME counterparts. These aforementioned studies were undertaken under current ambient [CO2] which does not reflect the low CO2 environment under which C4 grasses have evolved. Hence, the main aim of the current study was to investigate whether previously reported physiological differences among the C4 subtypes at ambient [CO2] are similarly observed at glacial [CO2].
Growth at low [CO2] reduces growth and photosynthesis of C3 plants. C3 plants respond to low [CO2] by increasing g s to improve CO2 supply and by up-regulating photosynthetic enzymes to improve CO2 capture (Polley et al., 1992; Dippery et al., 1995; Tissue et al., 1995; Gesch et al., 2000; Anderson et al., 2001). The occurrence of a CCM in C4 leaves makes the C4 pathway less limited by CO2 supply and, hence, less likely to respond and acclimate to growth at low [CO2] relative to C3 photosynthesis (Hatch, 1987; Gerhart and Ward, 2010). Nevertheless, increased leaf N content and g s have been observed under low [CO2] in some C4 species (Anderson et al., 2001; Maherali et al., 2002). To the authors’ knowledge there are no published studies comparing the impact of low [CO2] on the photosynthetic gas exchange or biochemistry of C4 grasses with different biochemical subtypes. The current study aims at addressing this knowledge gap.
A hypothezised intermediate stage during C4 evolution, known as C3–C4 intermediate, restricts the activity of glycine decarboxylase to the bundle sheath (Sage et al., 2012), thus improving Rubisco efficiency by facilitating the recapture of photorespired CO2 (Monson and Moore, 1989; Monson and Rawsthorne, 2000). The operation of a photorespiratory pump in C3–C4 photosynthesis is expected to elicit a response to [CO2] that is intermediate between C3 and C4 photosynthesis (Monson and Rawsthorne, 2000; Sage et al., 2012). Under low [CO2], C3–C4 plants have been reported to maintain greater photosynthetic rates, PWUE, and PNUE relative to C3 species (Ku and Edwards, 1978; Bolton and Brown, 1980; Ku et al., 1991; Monson and Rawsthorne, 2000; Vogan et al., 2007; Pinto et al., 2011; Vogan and Sage, 2012). The current study seeks to determine how C3–C4 species perform relative to the various C4 subtypes at low [CO2].
Comparing the sensitivity to glacial [CO2] of the different pathways of photosynthesis and subtypes of C4 photosynthesis among closely related grass species may provide critical insight into the physiology of C4 plants under conditions that led to their evolution. Consequently, this study compared the photosynthetic physiology (PWUE and PNUE) and biochemistry (activity of the photosynthetic carboxylase and decarboxylase enzymes) in C4 grasses with different biochemical subtypes grown under ambient (400 μl l–1) or glacial (180 μl l–1) [CO2]. Closely related C3 and C3–C4 grass species were included for comparison.
Materials and methods
Plant culture
Two matched growth chambers (1.8 m3 each; BioChambers, Winnipeg, Manitoba, Canada) were used in this study. The chambers were maintained at either glacial (180 μl l–1) or ambient (400 μl l–1) [CO2]. Low [CO2] was achieved by passing incoming air over a CO2 absorbent (Grace SodaSorb, WR Grace and Co.-Conn., Chicago, USA) and controlled by CO2 gas analysers (LI-820, LI-COR, Lincoln, NE, USA). The average growth conditions during the experiment are shown in Table 1.
Table 1.
Average growth conditions in the glacial and ambient CO2 growth chambers during the experimental period
Light intensity was measured at the pot level. The photoperiod was 12h. Values are averages (± standard deviation) over the growing period.
| Glacial CO2 | Ambient CO2 | |||
|---|---|---|---|---|
| Day | Night | Day | Night | |
| Light (μmol m–2 s–1) | 900±2 | 900±3 | ||
| [CO2] (μl l–1) | 181±4 | 182±2 | 400±2 | 400±2 |
| Temperature (°C) | 27±1 | 17±1 | 27±1 | 17±1 |
| Relative humidity (%) | 70±1 | 70±1 | 70±1 | 70±1 |
Locally collected soil (Ghannoum et al., 2010) was air-dried, coarsely sieved, and added (3.7kg) to 3.5 l cylindrical pots, which were watered to 100% capacity, then transferred to the two growth chambers. Seeds for the grass species used in this study (Table 2) were obtained from the Australian Plant Genetic Resources Information System (ACT, Australia) and Queensland Agricultural Seeds Pty. Ltd (Toowoomba, Australia). Seeds were sown in trays containing a common germination mix. Three to four weeks after germination, three seedlings were transplanted into each of the soil-filled pots. Within a week of transplanting, one healthy seedling was left in the pot while the other seedlings were removed; there were four pots per species and CO2 treatment. Two environmentally controlled growth chambers were used to generate the CO2 treatments. In order to minimize the impact of having a single growth chamber per CO2 treatment, pots and CO2 treatments were switched between chambers on two occasions. In addition, pots were randomly rotated within each chamber on a weekly basis throughout the experiment. Plants were watered daily and a commercial fertilizer (General Purpose, Thrive Professional, Yates, Australia) was applied weekly (0.2g N l–1).
Table 2.
List of grass species used in the current study
| Species | Photosynthetic type |
|---|---|
| Panicum bisulcatum Thunb. | C3 |
| Panicum milioides Nees | C3–C4 |
| Astrebla lappacea (Lindl.) Domin. | C4, NAD-ME |
| Panicum coloratum L. | C4, NAD-ME |
| Heteropogon contortus (L) P. Beauv. Ex Roem. & Schult. | C4, PCK |
| Panicum monticola Hook. F. | C4, PCK |
| Panicum maximum Jacq. | C4, PCK |
| Chloris gayana Kunth. | C4, PCK |
| Zea mays L. | C4, NADP-ME |
| Echinochloa frumentaceae L. | C4, NADP-ME |
Leaf gas exchange measurements
Gas exchange measurements were made using a portable open gas exchange system (LI-6400XT, LI-COR). At 7–8 weeks after transplanting, gas exchange measurements were made at a photosynthetic photon flux density of 1800 μmol m–2 s–1 between 10:00h and 14:00h on attached, last fully expanded leaves (LFELs) of the main stems. Spot measurements of the light-saturated photosynthetic rate (A sat) and g s were made at target growth [CO2] (180 μl l–1 or 400 μl l–1) and leaf temperature of 27 ºC. Leaf-to-air vapour pressure deficit ranged between 1.7 kPa and 2.4 kPa during the measurements. Before each measurement, the leaf was allowed to stabilize for 10–20min until it reached a steady state of CO2 uptake.
The responses of CO2 assimilation rates (A) to step increases of C i were measured under conditions similar to spot measurements by raising the cuvette [CO2] in 10 steps between 50 μl l–1 and 1500 μl l–1. There were 3–4 replicates per treatment. The A–C i curves were fitted using the C4 photosynthesis model (von Caemmerer, 2000) to estimate maximal PEPC (in vivo V pmax) and Rubisco (in vivo V cmax) activities. The biochemical model of C3 photosynthesis was used to estimate V cmax (apparent, maximal RuBP-carboxylation limited rate) for the C3 grass (Farquhar et al., 1980), using Rubisco catalytic parameters obtained for Panicum bisulcatum (RE Sharwood, O Ghannoum, and SM Whitney, unpublished).
Growth and nitrogen analyses
Plants were harvested 12–13 weeks after transplanting. At harvest, the area of the LFELs and total leaf area were measured using a leaf area meter (LI-3100A, LI-COR). Shoots were separated into stems and leaves. Roots were washed free of soil. Plant materials were oven-dried at 80 ºC for 48h before dry mass was measured. Leaf mass per area (LMA, g m–2) was calculated as total leaf dry mass/total leaf area. For each treatment, three dried LFELs of each species were milled to a fine powder. Tissue N was determined on the ground samples using a CHN analyser (LECO TruSpec, LECO Corporation, MI, USA).
Activity of Rubisco, PEPC, NADP-ME, and PEP-CK
Following gas exchange measurements made at growth [CO2], replicate leaf discs (1–2cm2) were cut under high light and rapidly frozen in liquid nitrogen then stored at –80 °C for biochemical analysis. Each leaf disc was extracted in 1ml of ice-cold extraction buffer [50mM EPPS-NaOH pH 8.0, 5mM dithiothreitol (DTT), 15mM NaHCO3, 20mM MgCl2, 2mM EDTA, 4% (v/v) protease inhibitor cocktail (Sigma), and 1% (w/v) polyvinylpolypyrrolidone (PVPP)] using a 2ml Potter–Elvehjem glass homogenizer kept on ice. Subsamples (75 μl) were taken from the total extract for SDS–PAGE analysis of total leaf protein. The remaining extract was centrifuged at 16 100 g for 1min and the supernatant used for enzyme activity, Rubisco content, and soluble protein assays. Rubisco content was estimated by the irreversible binding of [14C]carboxyarabinitol bisphosphate (CABP) to the fully carbamylated enzyme (Ruuska et al., 1998). Rubisco activity (in vitro V cmax) was estimated by multiplying the concentration of active sites determined using the [14C]CABP assay by the in vitro turnover rate (k cat at 25 °C) of the various C4 grasses (Supplementary Table S1 available at JXB online). Activities of PEPC and NADP-ME enzymes were determined at 25 °C using a UV-VIS spectrophotometer (model 8453, Agilent Technologies Australia, Mulgrave, Victoria) as previously described by Pengelly et al. (2012) and Ashton et al. (1990). Soluble proteins were measured using the Pierce Coomassie Plus (Bradford) protein assay kit (Thermo Scientific, Rockford, IL, USA).
PEP-CK activity was assayed at 25 °C in the carboxylase direction (Walker et al., 2002). Each leaf disc was extracted in 1ml of ice-cold extraction buffer [50mM HEPES pH 7.2, 5mM DTT, 2mM EDTA, 2mM MnCl2, 0.05% Triton, 4% (v/v) protease inhibitor cocktail (Sigma), and 1% (w/v) PVPP] using a 2ml Potter–Elvehjem glass homogenizer kept on ice. The extract was centrifuged at 16 100 g for 1min and the supernatant was used. PEP-CK activity was measured in assay buffer containing 100mM HEPES, pH 7.0, 4% mercaptoethanol (w/v), 100mM KCl, 90mM NaHCO3, 1mM ADP, 2mM MnCl2, 0.14mM NADH, and malate dehydrogenase after the addition of phosphoenolpyruvate (PEP) to 5mM. It was not possible to assay reliably for NAD-ME activity in this study.
Immunoblot analysis
To confirm the presence or absence of assayed enzyme activities, especially the decarboxylases in the C4 species and PEPC in C3 and C3–C4 species, immunoblot analysis of the proteins in question was carried out. Subsamples of total leaf extracts (used for enzyme assays) were mixed with 0.25vol of 4× LDS buffer (Invitrogen) containing 100mM DTT, snap-frozen in liquid nitrogen, and stored at –20 °C until analysed. Protein samples were separated by SDS–PAGE at 200V using TGX Any kD (Bio-Rad Laboratories, Hercules, CA, USA) pre-cast polyacrylamide gels in the Mini-Protean apparatus buffered with TRIS-glycine SDS buffer (Bio-Rad). Separated proteins were transferred at 4 °C to nitrocellulose membranes (0.45 μm; Bio-Rad) using the Xcell Surelock western transfer module (Invitrogen) buffered with 1× Transfer buffer [20×; 25mM Bicine, 25mM Bis-Tris, 1mM EDTA, 20% (v/v) methanol]. After 1h of transfer at 30V, the membrane was placed in blocking solution [3% (w/v) skim milk powder in TRIS-buffered saline (TBS); 50mM TRIS-HCl pH 8, 150mM NaCl] for 1h at room temperature with gentle agitation.
For immunoblot analysis, primary antisera raised in rabbit against tobacco Rubisco (prepared by SM Whitney) were diluted 1:4000 in TBS before incubation at 1h with membranes at room temperature with gentle agitation. Antiserum raised against PEPC (Cat. AS09 458) was obtained from Agrisera (Agrisera AB, Vännäs, Sweden) and diluted 1:2000 with TBS. For immunoblot analysis of NADP-ME and PEP-CK, synthetic peptides based on monocot amino acid sequences for each were synthesized by GL Biochem [GL Biochem (Shanghai) Ltd., Shanghai, China] and antiserum was raised to each peptide in rabbits. The reactive antisera were antigen purified and used for immunoblots (GL Biochem). The NADP-ME (Product ID A-003198) and PEP-CK (Product ID A-003200) antisera were diluted in TBS 1:1000 and 1:500, respectively. All primary antisera were incubated with membranes at room temperature for 1h with gentle agitation before washing three times with TBS. Secondary goat anti-rabbit antisera conjugated to horseradish peroxidase (HRP; Cat. NEF 812001EA, Perkin Elmer) were diluted 1:3000 in TBS and incubated with the membranes for 1h at room temperature followed by three washes with TBS. Immunoreactive peptides were detected using the Immun-Star Western C kit (Cat. 170–5070, Bio-Rad) and imaged using the VersaDoc imaging system (Bio-Rad).
Statistical and data analysis
PWUE was calculated as A sat (μmol m–2 s–1)/g s (mol m–2 s–1). PNUE was calculated as A sat (μmol m–2 s–1)/leaf [N]area (mmol m–2). The proportion of leaf N invested in Rubisco (Rubisco-N) was calculated by assuming that Rubisco contained 16% N on a mass basis (Evans and Seemann, 1989).
There were four replicates per treatment for growth, gas exchange, and enzyme assay measurements. There were three replicate measurements for the leaf N analysis and the A–C i curves. The relationship between the various response variables and the main effects (species, photosynthetic type, and CO2 treatment) and their interactions were fitted using a linear model in R (V. 3.0.2; R Foundation for statistical computing, Vienna, Austria). Analysis of variance (ANOVA; summarized in Table 2) was conducted for each fitted model. Multiple comparisons (shown in Table 4 and Supplementary Table S1 at JXB online) of species means were made using the Tukey test.
Table 4.
Summary of leaf N, soluble protein, and Rubisco contents
Ten grass species were grown at glacial (180 μl l–1) or ambient (400 μl l–1) [CO2]. Values are means (n=3–4) ±SE. Lower case letters indicate the ranking of species within each row using a multiple comparison, Tukey test. Values followed by the same letter are not significantly different at the 5% level.
| Parameter | [CO2] (μl L–1) | C3 | C3–C4 | C4, NAD-ME | C4, PCK | C4, NADP-ME | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P. bisulcatum | P. milioides | A. lappacea | P. coloratum | H. contortus | P. monticola | P. maximum | C. gayana | Z. mays | E. frumentaceae | ||
| LMA (g m–2) | 180 | 23±9 a | 21±8 a | 36±13 a | 46±16 a | 46±22 a | 48±2 a | 40±14 a | 16±6 a | 140±11 b | |
| 400 | 61±10 a,b,c,d | 25±3 a | 58±3 b,c,d | 51±5 a,b,c,d | 76±5 d | 57±5 c,d | 56±4 a,b,c,d | 27±8 a,b | 62±3 c,d | ||
| Leaf [N]area (mmol m–2) | 180 | 118±32 a,b | 132±25 a,b | 150±16 a,b | 251±40 b | 131±40 a,b | 141±8 a,b | 192±28 a,b | 74±4 a | 492±50 c | 145±11 a,b |
| 400 | 98±18 a | 89±15 a | 154±10 a,b | 170±27 a,b | 144±17 a,b | 190±3 b | 97±11 a | 91±26 a | 151±2 a,b | 122±17 a,b | |
| Rubisco sites (nmol m–2) | 180 | 21±6.0 b | 19±6.0 b | 13±0.5 a,b | 15±1.0 a,b | 7±0.4 a | 4±0.5 a | 6±0.3 a | 5±0.4 a | 7±0.6 a | 4±0.6 a |
| 400 | 9±0.6 a,b | 20±5.0 c | 11±0.4 b | 9±0.6 b | 4±0.1 a | 5±0.3 a,b | 5±0.5 a | 6±0.6 a,b | 6±0.4 a,b | 4±0.4 a | |
| Soluble proteins (g m–2) | 180 | 4.4±0.5 a,b,c | 4.6±0.2 a,b,c | 6.2±0.6 c | 6.6±1.0 c | 3.9±0.3 a,b,c | 3.2±0.3 a,b | 2.8±0.5 a | 3.4±0.2 a,b | 5.3±0.5 b,c | 4.2±0.1 a,b,c |
| 400 | 2.5±0.2 a | 4.0±0.3 a–d | 5.0±0.5 d | 4.8±0.3 c,d | 2.7±0.2 a,b | 3.0±0.2 a,b | 3.0±0.3 a,b,c | 3.2±0.3 a,b | 4.1±0.2 b,c,d | 3.6±0.1 a–d | |
| Rubisco/Soluble protein | 180 | 0.32±0.04 | 0.29±0.05 b,c | 0.14±0.03 a,b,c | 0.17±0.04 a,b,c | 0.14±0.03 a,b,c | 0.10±0.04 a,b | 0.30±0.04c | 0.10±0.04a | 0.09±0.04a | 0.04±0.05 a |
| 400 | 0.25±0.02 b,c | 0.34±0.02 c | 0.15±0.01 a | 0.16±0.02 a,b | 0.09±0.01 a | 0.10±0.02 a | 0.12±0.02 a | 0.14±0.02 a | 0.09±0.01 a | 0.08±0.02 a | |
| Rubisco-N (% leaf N) | 180 | 9.8±2 a,b | 14.0±2 b | 5.0±1 a,b | 4.2±2 a | 4.8±2 a,b | 2.7±1 a | 2.5±2 a | 5.6±2 a,b | 2.9±2 a | 2.1±2 a |
| 400 | 5.5±2 a | 26.3±2 b | 5.6±2 a | 5.4±2 a | 2.2±2 a | 2.3±2 a | 4.0±2 a | 6.6±2 a | 3.3±2 a | 2.5±2 a | |
Results
Photosynthetic rates and WUE
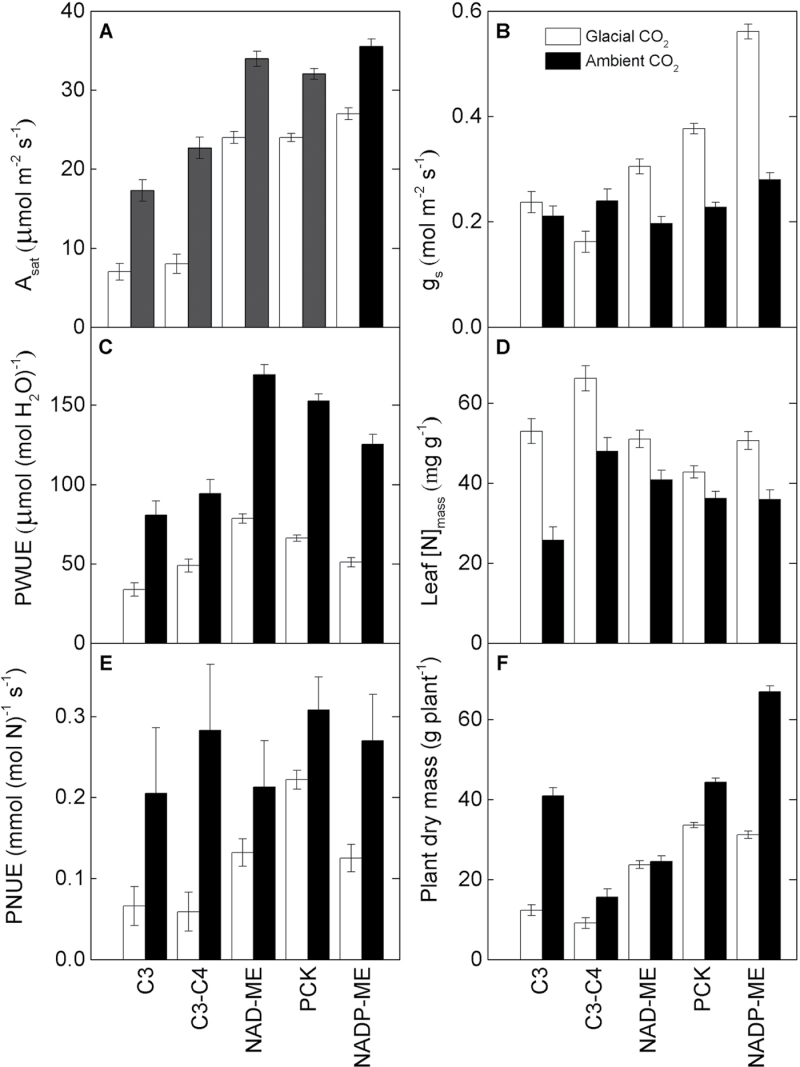
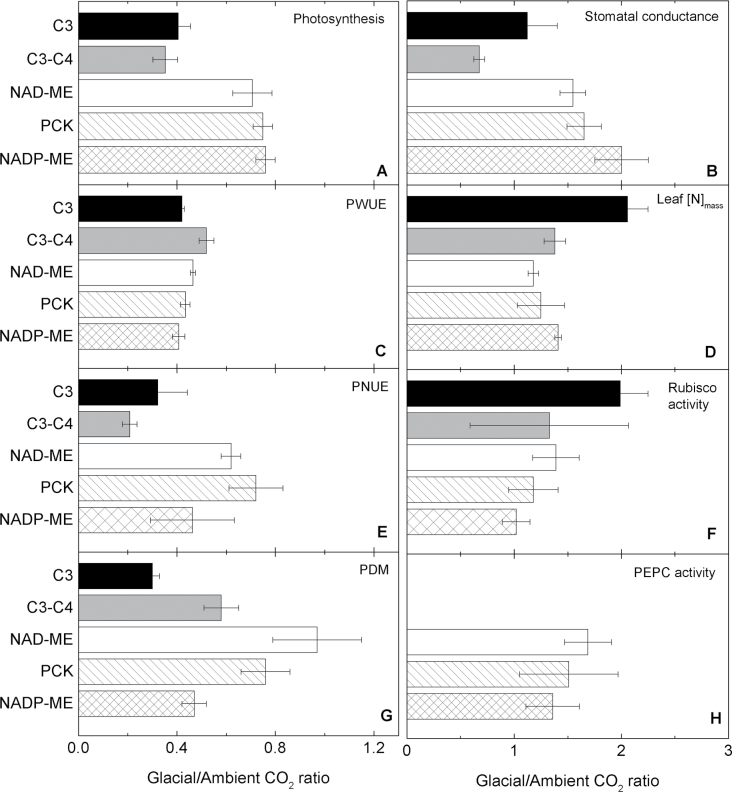
Under both [CO2] treatments, photosynthetic rates measured at high light and growth [CO2] (A sat) were higher in the C4 species relative to the C3–C4 and C3 species. Amongst the C4 species, variation in A sat was unrelated to their subtype. Relative to ambient [CO2], glacial [CO2] decreased A sat to a greater extent in the C3–C4 (65%) and C3 (60%) species relative to the C4 species (26%) (Figs 1A, 2A; Table 3; Supplementary Table S1 at JXB online).
Fig. 1.
Gas exchange and growth parameters. Light-saturated photosynthesis, A sat (A), stomatal conductance, g s (B), photosynthetic water use efficiency, PWUE (C), leaf N per unit dry mass, [N]mass (D), photosynthetic nitrogen use efficiency, PNUE (E), and plant dry mass, PDM (F) of 10 grass species belonging to C3, C3–C4, and C4 (NAD-ME, PCK, NADP-ME) photosynthetic types grown at glacial (180 μl l–1, open columns) or ambient (400 μl L–1, filled columns) [CO2]. Values are means ±SE of species within each photosynthetic type.
Fig. 2.
CO2 sensitivity of photosynthetic and growth parameters. Glacial to ambient CO2 ratios of light-saturated photosynthesis, A sat (A), stomatal conductance, g s (B), photosynthetic water use efficiency, PWUE (C), leaf N per unit dry mass, [N]mass (D), photosynthetic nitrogen use efficiency, PNUE (E), Rubisco activity (F), plant dry mass, PDM (G), and PEPC activity (H). Original data are shown in Supplementary Table S1 at JXB online.
Table 3.
Statistical summary
Summary of statistical analysis using three-way ANOVA for the effects of [CO2], species, and the photosynthetic type on various parameters collected for 10 grass species grown at glacial (180 μl l–1) and ambient (400 μl l–1) [CO2].
| Parameter | Main effects (P) | Interactions (P) | |||
|---|---|---|---|---|---|
| Species | Type | [CO2] | [CO2]×species | [CO2]×type | |
| Photosynthesis, Asat (μmol m–2 s–1) | 0.000 | 0.000 | 0.000 | 0.016 | 0.000 |
| Conductance, gs (mol m–2 s–1) | 0.000 | 0.000 | 0.000 | 0.110 | 0.000 |
| Intercellular [CO2], Ci (μl l–1) | 0.000 | 0.000 | 0.000 | 0.028 | 0.005 |
| PWUE [μmol (mol H2O)–1] | 0.000 | 0.000 | 0.000 | 0.694 | 0.083 |
| LMA (g m–2) | 0.000 | 0.028 | 0.000 | 0.692 | 0.378 |
| Leaf [N]mass (mg g–1) | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| Leaf [N]area (mmol m–2) | 0.000 | 0.000 | 0.036 | 0.039 | 0.516 |
| PNUE [mmol (mol N)–1 s–1] | 0.000 | 0.000 | 0.000 | 0.460 | 0.001 |
| Plant dry mass, PDM (g per plant) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Soluble protein (g m–2) | 0.004 | 0.000 | 0.000 | 0.710 | 0.044 |
| Rubisco sites (nmol m–2) | 0.594 | 0.000 | 0.000 | 0.158 | 0.000 |
| Rubisco/soluble protein | 0.001 | 0.000 | 0.448 | 0.009 | 0.463 |
| Rubisco-N (% leaf N) | 0.006 | 0.000 | 0.407 | 0.230 | 0.000 |
| Rubisco activity (μmol m–2 s–1) | 0.000 | 0.000 | 0.000 | 0.004 | 0.000 |
| PEPC activity (μmol m–2 s–1) | 0.000 | 0.000 | 0.000 | 0.000 | 0.140 |
| NADP-ME activity (μmol m–2 s–1) | 0.000 | 0.000 | 0.340 | 0.048 | 0.461 |
| PEP-CK activity (μmol m–2 s–1) | 0.000 | 0.000 | 0.133 | 0.319 | 0.960 |
| PEPC/Rubisco | 0.000 | 0.000 | 0.003 | 0.068 | 0.579 |
| In vivo V cmax (μmol m–2 s–1) | 0.000 | 0.010 | 0.000 | 0.004 | 0.000 |
| In vivo V pmax (μmol m–2 s–1) | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 |
| In vivo V p/Vc | 0.000 | 0.010 | 0.000 | 0.000 | 0.001 |
At ambient [CO2], variation in g s was unrelated to the photosynthetic type or subtype of the grasses. At glacial [CO2], the C4 species had higher g s relative to the C3 and C3–C4 counterparts. Glacial [CO2] increased g s to a greater extent in the C4 relative to the C3 (1.1-fold) and C3–C4 (1.3-fold) species, with NADP-ME (1.5-fold) grasses showing the greatest increase in g s relative to the other C4 species (1.35-fold) (Figs 1B, 2B; Table 3; Supplementary Table S1 at JXB online).
At ambient [CO2], PWUE was higher in the C4 relative to the two C3–C4 and C3 species. At glacial [CO2], PWUE was highest in NAD-ME and PCK species, intermediate in NADP-ME and C3–C4, and lowest in C3 species. Amongst the C4 species, the two NAD-ME grasses had higher PWUE relative to their PCK and NADP-ME counterparts. Glacial [CO2] decreased PWUE in all species by an average of 55% (Figs 1C, 2C; Table 3; Supplementary Table S1 at JXB online).
Leaf N use efficiency and plant dry mass
Under both [CO2] treatments, leaf [N]mass was highest in P. milioides (C3–C4) and lowest in Heteropogon contortus (PCK). Glacial [CO2] enhanced leaf [N]mass in all grasses except for Panicum monticola and Chloris gayana (PCK). The largest enhancement was observed in the C3 (51%) and NADP-ME (29%) species (Figs 1D, 2D; Table 3; Supplementary Table S1 at JXB online).
At ambient [CO2], PNUE varied 3-fold amongst the species in a manner unrelated to their photosynthetic type. Glacial [CO2] reduced PNUE to a lesser extent in the C4 (30%) relative to the C3 (58%) and C3–C4 (79%) species. At glacial [CO2], PNUE was highest in C4 plants (PCK >NADP-ME and NAD-ME) and lowest in C3 and C3–C4 plants (Figs 1E, 2E; Table 3; Supplementary Table S1 at JXB online).
At ambient [CO2], plant dry mass (PDM) was lower in the C3–C4 and NAD-ME species relative to the C3 and other C4 species. At glacial [CO2], the C4 species accumulated more biomass than their C3 and C3–C4 counterparts, which had similar PDM. Glacial [CO2] reduced PDM to a greater extent in the C3 (70%) and C3–C4 (42%) species relative to the C4 (25%) species. Amongst the C4 species, PDM was least and most inhibited by glacial [CO2] in the NAD-ME and NADP-ME grasses, respectively (Figs 1F, 2H; Table 3; Supplementary Table S1 at JXB online).
Rubisco and soluble protein content
Under both [CO2] treatments, leaf Rubisco content was higher in Panicum milioides (C3–C4) relative to the other species, and in the two NAD-ME species relative to the other C4 grasses. At ambient [CO2], P. bisulcatum (C3) and NAD-ME grasses had similar Rubisco contents. Glacial [CO2] increased Rubisco content in P. bisulcatum (2.3-fold) and in three (Astrebla lappacea, Panicum coloratum, and H. contortus; 1.2- to 1.7-fold) of the eight C4 species (Tables 3, 4).
The ratio of Rubisco to soluble proteins and the proportion of leaf N invested in Rubisco (Rubisco-N) were higher in the C3 and C3–C4 species relative to the C4 species. Amongst the C4 species, the NADP-ME grasses tended to have the lowest leaf N or soluble protein investment in Rubisco. Glacial [CO2] increased Rubisco-N in the C3 species, reduced it in the C3–C4 species, and had little effect in the C4 species (Tables 3, 4).
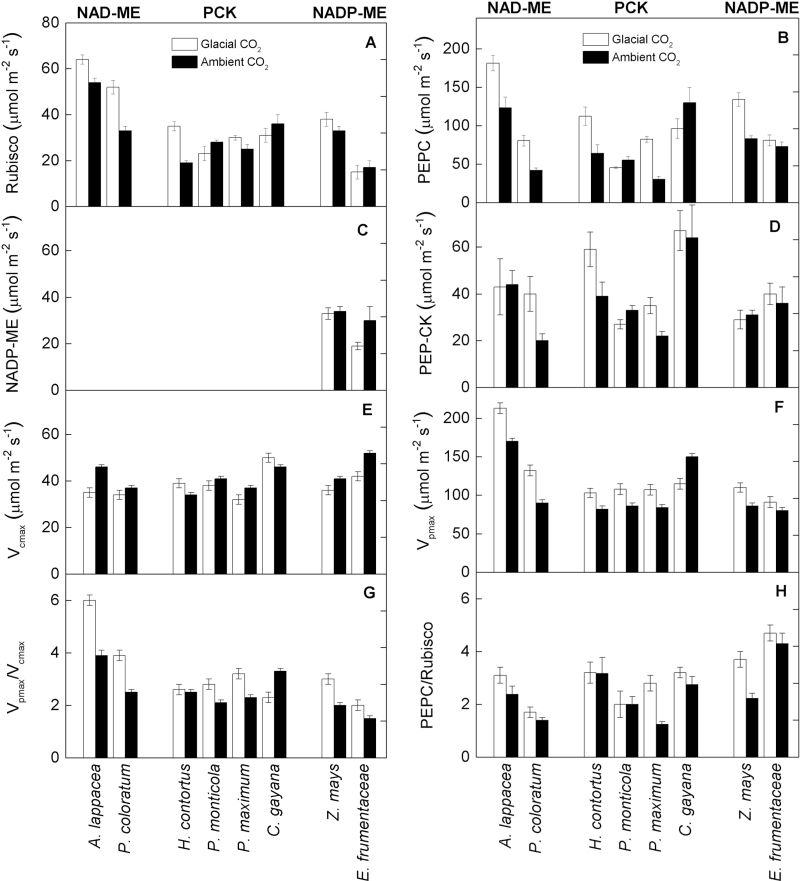
The C3, C3–C4, and NAD-ME species had similar Rubisco activities, which were higher relative to the PCK and NADP-ME species. Glacial [CO2] significantly up-regulated Rubisco activity in the C3 and NAD-ME grasses only (Figs 2F, 3A, Table 3; Supplementary Table S1 at JXB online).
Fig. 3.
Activity of photosynthetic enzymes. Activities of Rubisco (A), PEPC (B), NADP-ME (C), PEP-CK (D), in vivo V cmax (E), in vivo V pmax (F), V pmax/V cmax ratio (G), and PEPC/Rubisco activity ratio (H) of eight C4 grass species (NAD-ME, PCK, NADP-ME) grown at glacial (180 μl l–1, open columns) or ambient (400 μl l–1, filled columns) [CO2]. Values are means (n=3–4) ±SE.
Activity of C4 cycle enzymes in C4 grasses
At ambient [CO2], PEPC activity was highest in A. lappacea (NAD-ME) and C. gayana (PCK), and lowest in P. maximum (PCK). At glacial [CO2], PEPC activity was highest in A. lappacea and lowest in P. monticola (PCK). Glacial [CO2] stimulated PEPC activity in five out of the eight C4 species (Figs 2H, 3B; Table 3; Supplementary Table S1 at JXB online). Variations in the ratio of PEPC to Rubisco activity reflected changes in PEPC activity (Fig. 3H; Table 3; Supplementary Table S1).
In this study, only the activities of the decarboxylases NADP-ME and PEP-CK were measured. Significant activity of NADP-ME was measured in the two NADP-ME species, while marginal NADP-ME activity was detected in the two NAD-ME species and in one of the PCK species (Fig. 3C). In contrast, PEP-CK activity was ubiquitous among the C4 species used, with C. gayana showing the highest PEP-CK activity. Overall, growth [CO2] had no significant effect on the activity of either decarboxylase (Fig. 3C–D, Table 3; Supplementary Table S1 at JXB online).
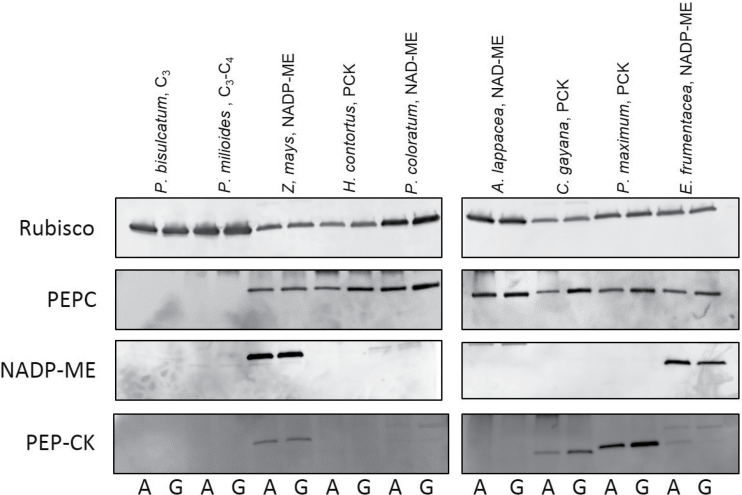
The detectability of the activity of both carboxylases and decarboxylases was corroborated by immunodetection of the corresponding protein (Fig. 6). PEPC activity and protein were lacking from the C3 and C3–C4 species and present in all C4 grasses. NADP-ME activity and protein were found in two C4 species only. PEP-CK activity was measured in all C4 grasses, and the protein was readily detected in six grasses, with A. lappacea and H. contortus exhibiting weak immunoreaction with the available antibody, possibly due to divergent amino acid sequences of PEP-CK in these two species (Fig. 6).
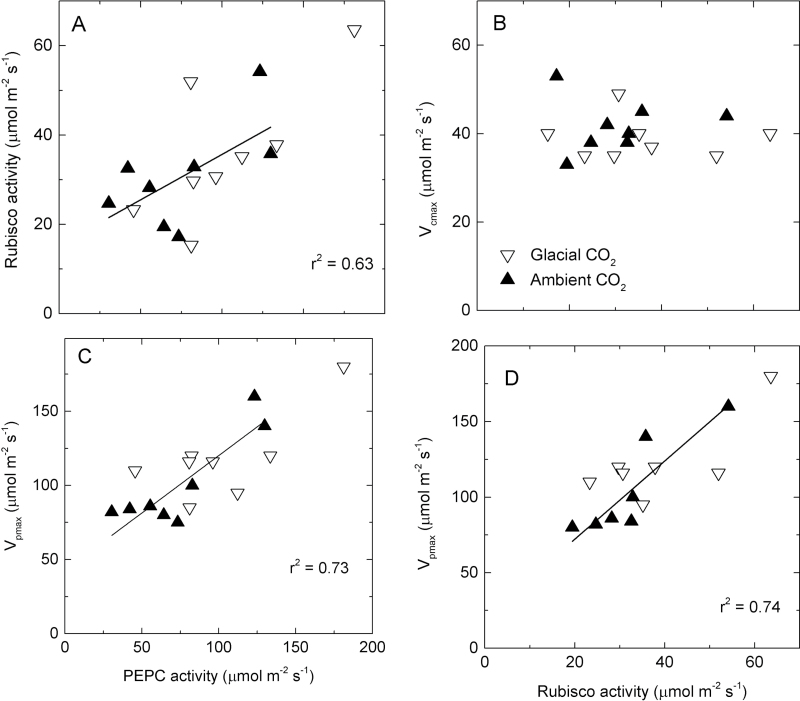
Fig. 6.
Relationships between the in vitro and in vivo estimates of Rubisco and PEPC activities in eight C4 grass species. Values are means for each species grown at glacial (180 μl l–1, inverted open triangles) or ambient (400 μl l–1, filled triangles) [CO2]. Solid lines represent linear regressions of all data points. Original data are shown in Supplementary Table S1 at JXB online.
In vivo estimates of maximal Rubisco (V cmax) and PEPC activity (V pmax) in C4 grasses
In vivo estimates of V cmax and V pmax were calculated using the C4 photosynthesis model (von Caemmerer, 2000) from A–C i curves measured at high light and 27 °C (Fig. 5). The variation of gas exchange-derived V cmax between the C4 species was unrelated to their biochemical subtype. In contrast to its effect on in vitro V cmax (Rubisco activity), glacial [CO2] reduced gas exchange V cmax in two out of the eight C4 species (Fig. 3E; Table 3; Supplementary Table S1 at JXB online). Consequently, in vivo and in vitro estimates of V cmax were unrelated among the C4 grasses (Fig. 6B). In contrast, PEPC activity was positively correlated with that of Rubisco across the C4 species and [CO2] treatments (Fig. 6A).
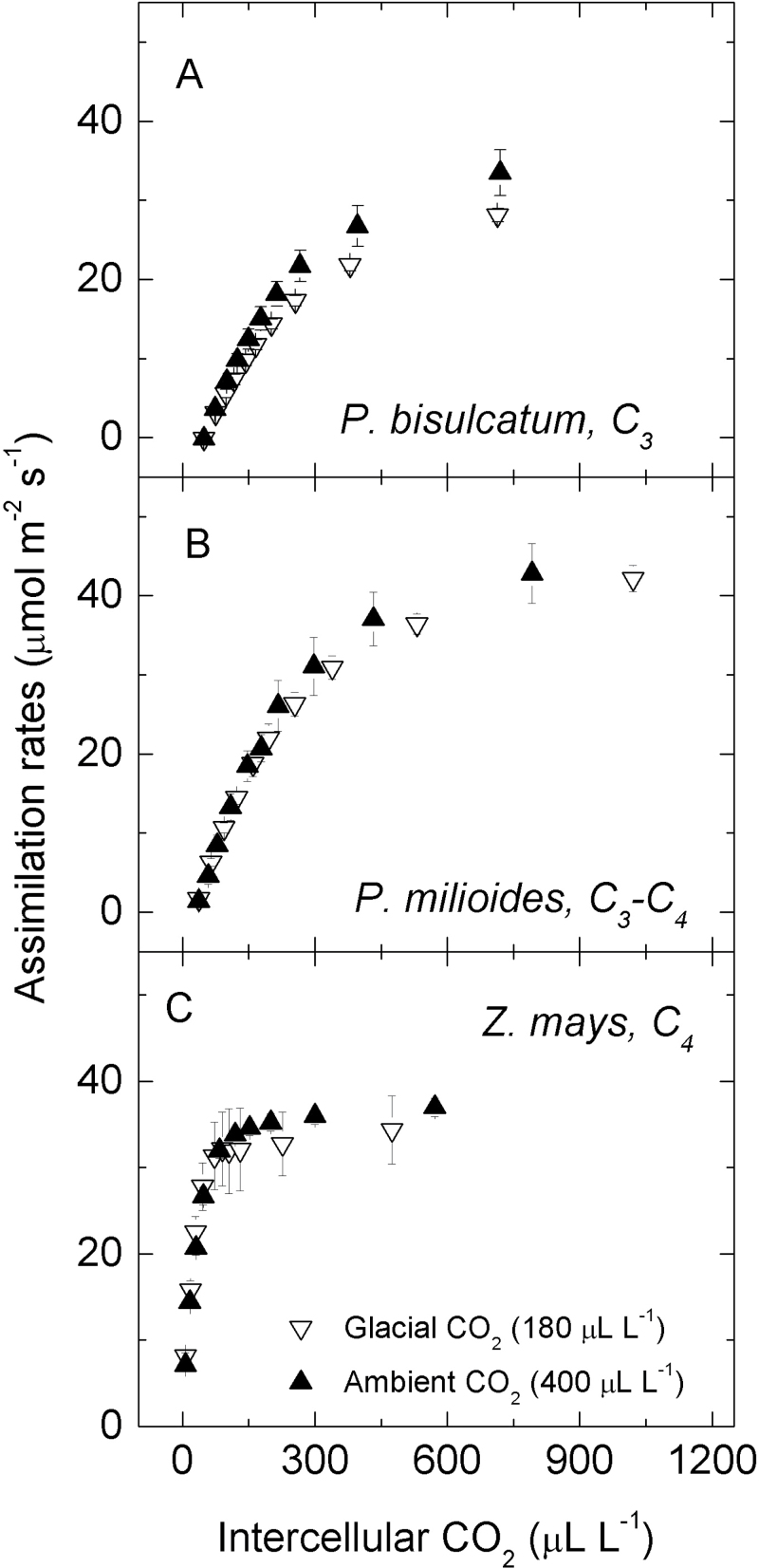
Fig. 5.
Responses of CO2 assimilation rate to increasing intercellular [CO2]. Examples of A–C i curves measured in C3, C3–C4, and C4 species grown at glacial (180 μl l–1, inverted open triangles) or ambient (400 μl l–1, filled triangles) [CO2]. Values represent the means ±SE of three replicates.
On average, NAD-ME species tended to have higher V pmax and V pmax/V cmax relative to the other C4 grasses, especially at glacial [CO2]. Glacial [CO2] increased V pmax and the V pmax/V cmax ratio in all C4 species, except for C. gayana, by an average of 25% and 19%, respectively (Fig. 3F, G; Table 3; Supplementary Table S1 at JXB online). Within the C4 species, V pmax showed significant positive correlations with in vitro PEPC and Rubisco activities (Fig. 6C, D).
Discussion
Photosynthetic efficiency under glacial CO2: C3, C3–C4, and C4 pathways
In accordance with theoretical understanding, the current study revealed that photosynthetic rates (A sat) were most responsive to decreased [CO2] from ambient to glacial levels in C3 followed by C3–C4 and then C4 species. In addition, the C4 grasses had higher photosynthesis under ambient and glacial [CO2] relative to their C3 and C3–C4 counterparts (Figs 1A, 2A). Similar responses were observed for other C3, C3–C4, and C4 species exposed to 180 μl CO2 l–1 and 380 μl CO2 l–1 (Ward et al., 1999; Cunniff et al., 2010; Pinto et al., 2011; Vogan and Sage, 2012).
Stomatal conductance was greater at glacial [CO2] compared with ambient [CO2] in all species, but in particular was higher in C4 species relative to the C3 and C3–C4 species (Figs 1B, 2B). Huxman and Monson (2003) found that g s was more sensitive to changing C i in C4 relative to C3 and C3–C4 Flaveria species. Recently, Vogan and Sage (2011) presented evidence of changed C i sensitivity for g s in Flaveria species during their evolutionary transition from C3 to C4 photosynthesis. In contrast, Morison and Gifford (1983) observed little difference in stomatal sensitivity to short-term changes of [CO2] or vapour pressure deficit between two C3 and two C4 grasses. Growth at low [CO2] may cause acclimation of the stomatal response that is not necessarily captured during short-term gas exchange measurements. However, a number of studies found no evidence of differential stomatal acclimation between C3 and C4 plants (Cunniff et al., 2010; Vogan and Sage, 2012). Hence, there does not seem to be a consensus regarding the relative stomatal sensitivity to short- or long-term changes in [CO2] between C3 and C4 plants, which remains an area worthy of further investigation.
Despite having larger g s at glacial [CO2], C4 species maintained greater PWUE than C3–C4 and C3 species as a result of higher photosynthetic rates in C4 plants (Fig. 1). Improved PWUE is one of the most consistently reported advantages of C4 species (Long, 1999; Taylor et al., 2010). Higher PWUE in the C3–C4 species relative to the C3 species under both growth [CO2] confirmed that the photorespiratory pump of the intermediate pathway confers greater water use efficiency relative to the C3 pathway (Pinto et al., 2011; Vogan and Sage, 2011), thereby achieving PWUE similar to the C4, NADP-ME pathway under glacial [CO2] (Fig. 1C).
Fig. 4.
Immunoblot analyses of photosynthetic enzymes. Examples of immunoblot analysis for the photosynthetic proteins Rubisco (A), PEPC (B), NADP-ME (C), and PEP-CK (D) extracted from leaves of selected grass species grown at glacial (180 μl l–1, G) or ambient (400 μl l–1, A) [CO2].
Higher PNUE in C4 relative to C3 plants under ambient [CO2] is well established (Brown, 1978; Long, 1999; Taylor et al., 2010). In this study, these differences were maintained under glacial [CO2] as a result of higher photosynthetic rates and lower leaf [N] in the C4 relative to the C3 and C3–C4 species (Fig. 1). The C3–C4 species had no PNUE advantage over the C3 species, mainly due to the higher leaf [N] and Rubisco-N of the intermediate species (Table 4). In contrast, intermediate Flaveria species maintained higher photosynthesis and PNUE relative to C3 congeners at ambient and glacial [CO2] (Vogan and Sage, 2012).
Growth of P. bisulcatum (C3) at glacial [CO2] increased Rubisco activity and g s to improve photosynthetic capacity and CO2 supply, respectively (Tissue et al., 1995; Gesch et al., 2000; Anderson et al., 2001). These commonly reported responses represent significant N and water costs for C3 plants at glacial [CO2], thus reducing their PWUE and PNUE. The additional resource requirements at low [CO2] may have contributed to the more pronounced reduction in plant biomass in C3 relative to C4 plants observed in this study (Fig. 2F) as in others (Ward et al., 1999; Cunniff et al., 2010; Ripley et al., 2013). Consequently, low WUE and NUE of C3 photosynthesis at low [CO2] may have favoured the evolution of C4 phototosynthesis.
Photosynthetic efficiency under glacial CO2: the C4 subtypes
Results obtained in this study at glacial [CO2] largely confirmed previously reported differences in photosynthetic efficiency among the C4 subtypes at ambient [CO2], and revealed a number of insights into the physiology of C4 subtypes, as discussed below.
First, there were no subtype differences in photosynthetic rates or their sensitivity to decreased growth [CO2]. These results constitute new evidence that there are no discernible differences in the efficiency of the CCM operating in the three C4 subtypes, despite their diverse leaf biochemistry and anatomy. This conclusion is supported by the findings that CO2 leakiness out of the bundle sheath (a surrogate measure of CCM efficiency) is similar among C4 grasses with different subtypes (Henderson et al., 1992; Cousins et al., 2008).
Secondly, NAD-ME species had lower g s and higher PWUE relative to NADP-ME and PCK counterparts at glacial [CO2]. Moreover, g s was less affected by glacial [CO2] in NAD-ME than in NADP-ME and PCK grasses (Fig. 2). Previous studies demonstrated that photosynthetic activity was less sensitive to water deficit, and leaf traits were better suited for arid habitats in an NAD-ME relative to an NADP-ME and a PCK grass (Carmo-Silva et al., 2007, 2009). In another study, Ghannoum et al. (2002) showed that NAD-ME grasses increased their whole-plant WUE to a greater extent than their NADP-ME counterparts under water stress. Taken together, these findings are consistent with the observation that grasses with the NAD-ME subtype predominate in more arid regions relative to the other two C4 subtypes (Hattersley, 1992; Taub, 2000).
Thirdly, NADP-ME grasses showed the greatest increase of leaf [N]mass, which may be linked to their stomatal response in that the correlation between N uptake (proxy leaf [N]) and mass flow of soil water through the transpiration stream (proxy g s) is commonly reported in plants grown under different atmospheric [CO2] (Conroy and Hocking, 1993; McDonald et al., 2002; Sherwin et al., 2013).
Fourthly, NAD-ME grasses showed the lowest biomass reduction in response to decreased growth [CO2] relative to the PCK and NADP-ME species. NAD-ME grasses also had lower plant biomass relative to the other C4 species at both growth [CO2]. Studies conducted at elevated [CO2] have shown that growth response to high [CO2] decreases with decreasing growth potential (Poorter, 1993; Ziska and Bunce, 1997). Extrapolating these findings to low [CO2] suggests that the lower growth response to glacial [CO2] in NAD-ME plants may be related to their smaller biomass accumulation relative to the other, larger C4 species.
Photosynthetic enzymes under glacial CO2
Generally, growth at low [CO2] leads to increased photosynthetic capacity, g s, and leaf [N] in C3 plants (Dippery et al., 1995; Ward et al., 1999; Anderson et al., 2001; Cunniff et al., 2010; Gerhart and Ward, 2010; Ripley et al., 2013). Accordingly, P. bisulcatum (C3) exhibited increased leaf proteins, including Rubisco at glacial [CO2] (Fig. 2; Table 4). Panicum milioides (C3–C4) did not up-regulate Rubisco content at glacial [CO2], possibly due to the high leaf [N] and Rubisco-N in this species; a consequence of the high N costs of operating two Calvin cycles in the mesophyll and bundle sheath cells (Monson, 1989; Monson and Rawsthorne, 2000).
The operation of Rubisco under elevated [CO2] in the bundle sheath, the multiplicity of metabolic cycles and cells involved in C4 photosynthesis, and the complexity of its regulation thwart the task of predicting how C4 photosynthesis will acclimate to growth at low [CO2]. Measurements of photosynthetic rates under growth [CO2] (A sat) indicated that photosynthesis in the C4 grasses was CO2 limited at glacial [CO2], albeit to a lesser extent than C3 and C3–C4 counterparts (Fig. 2A). This may explain the significant up-regulation of the two carboxylases, Rubisco and PEPC, which was observed in a number of the C4 grasses (Figs 3–6). Generally, the activities of Rubisco and PEPC changed in concert, a reflection of the fine balance operating between these two enzymes which modulate the pace of the C3 and C4 cycles during C4 photosynthesis, respectively (von Caemmerer and Furbank, 2003). There is strong evidence showing that CO2 delivery into the bundle sheath and fixation in the mesophyll are tightly regulated, as indicated by the constancy of leakiness (a measure of CO2 fixed by PEPC but not Rubisco, subsequently leaking back from the bundle sheath) under a wide range of environmental conditions (Henderson et al., 1992; Cousins et al., 2008). Nevertheless, the PEPC/Rubisco ratio increased at glacial [CO2] in two C4 species (Fig. 3H). Increasing PEPC/Rubisco via transgenic transformation in Flaveria bidentis led to increased leakiness, an indication of reduced efficiency of the C4 mechanism (von Caemmerer et al., 1997b). In the current study, V pmax and PEPC activity were linearly correlated, while V cmax and Rubisco activity showed no correlation (Fig. 5). Reconciling the in vivo and in vitro estimates of Rubisco and PEPC activity will require greater knowledge about bundle sheath cell wall conductance and [CO2] than is currently available (von Caemmerer et al., 1997a; von Caemmerer and Furbank, 2003).
The activities of the two measured decarboxylases were not affected by growth [CO2], possibly reflecting the low control that decarboxylases exert on the photosynthetic flux. Pengelly et al. (2012) reported that NADP-ME activity in transgenic F. bidentis can be halved without affecting photosynthetic rates or growth. Accordingly, the rate of the decarboxylases measured at ambient [CO2] may be sufficient under glacial [CO2], where Rubisco and PEPC activities were up-regulated in a number of C4 species. Although PEPC and NADP-ME have significant effects on the efficiency of the C4 pathway as evidenced by changes in leakiness, Rubisco retains a high control of metabolic flux in C4 leaves (Furbank et al., 1997; von Caemmerer et al., 1997b; Pengelly et al., 2012).
It is worth noting that PEP-CK activity and, to a lesser extent, PEP-CK protein were ubiquitously detected in the C4 species used in this study. Significant PEP-CK activity in C4 grasses and eudicots of the NADP-ME and NAD-ME subtypes has been previously reported (Walker et al., 1997; Wingler et al., 1999; Carmo-Silva et al., 2008; Muhaidat and McKown, 2013). These findings challenge the classical view of the C4 subtypes, where a single decarboxylase dominates (Hatch, 1987; Furbank, 2011). Recent studies have postulated a role for PEP-CK as a second decarboxylase in maize that serves to match ATP and NADPH demand in bundle sheath and mesophyll cells under different light environments (Bellasio and Griffiths, 2013). The full physiological significance of PEP-CK in a wider range of C4 grasses and environments is yet to be elucidated.
Conclusions
Various photosynthetic responses, including increased leaf Rubisco, nitrogen, and g s, were observed in response to growth at glacial [CO2]. Nevertheless, the operation of a CCM ensured that PWUE and PNUE remained higher in C4 species relative to C3 and C3–C4 species, while the photorespiration pump ensured higher PWUE in the C3–C4 relative to the C3 species. Greater resource use efficiency promotes cheaper biomass construction costs, and hence reduces productivity losses at low [CO2]. Accordingly, high resource use efficiency may have constituted a key evolutionary advantage for the transition from C3 to C4 photosynthesis under low [CO2] (Cerling et al., 1998; Sage, 2004). Results obtained in this study support the notion that Rubisco and PEPC, rather than the decarboxylases, modulate the response to glacial [CO2] for C4 grasses with different biochemical subtypes.
Supplementary data
Supplementary data are available at JXB online
Table S1. Summary of leaf gas exchange, resource use efficiency, and activity of photosynthetic enzymes.
Acknowledgements
We thank Balasaheb Sonawane for assistance with biochemical analysis. This research was partially funded by the Hawkesbury Institute for the Environment at UWS through the award of a PhD scholarship to HP and a research fellowship to RES. This research was also supported by a Discovery Project awarded to OG from the Australian Research Council (DP120101603).
References
- Anderson LJ, Maherali H, Johnson HB, Polley HW, Jackson RB. 2001. Gas exchange and photosynthetic acclimation over subambient to elevated CO2 in a C3–C4 grassland. Global Change Biology 7, 693–707 [Google Scholar]
- Ashton AR, Burnell JN, Furbank RT, Jenkins CLD, Hatch MD. 1990. The enzymes in C4 photosynthesis. In: Lea PJ, ed. Enzymes of primary metabolism. London: Academic Press, 39–72 [Google Scholar]
- Bellasio C, Griffiths H. 2013. The operation of two decarboxylases (NADPME and PEPCK), transamination and partitioning of C4 metabolic processes between mesophylll and bundle sheath cells allows light capture to be balanced for the maize C4 pathway. Plant Physiology (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JK, Brown RH. 1980. Photosynthesis of grass species differing in carbon dioxide fixation pathways.V. Response of Panicum maximum, Panicum milioides, and tall fescue (Festuca arundinacea) to nitrogen nutrition. Plant Physiology 66, 97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman WD. 1991. Effect of nitrogen nutrition on photosynthesis and growth in C4 Panicum species. Plant, Cell and Environment 14, 295–301 [Google Scholar]
- Brown RH. 1978. Difference in N use efficiency in C3 and C4 plants and its implications in adaptation and evolution. Crop Science 18, 93–98 [Google Scholar]
- Carmo-Silva AE, Bernardes da Silva A, Keys AJ, Parry MA, Arrabaca MC. 2008. The activities of PEP carboxylase and the C4 acid decarboxylases are little changed by drought stress in three C4 grasses of different subtypes. Photosynthesis Research 97, 223–233 [DOI] [PubMed] [Google Scholar]
- Carmo-Silva AE, Francisco A, Powers SJ, Keys AJ, Ascensao L, Parry MA, Arrabaca MC. 2009. Grasses of different C4 subtypes reveal leaf traits related to drought tolerance in their natural habitats: changes in structure, water potential, and amino acid content. American Journal of Botany 96, 1222–1235 [DOI] [PubMed] [Google Scholar]
- Carmo-Silva AE, Soares AS, Marques da Silva J, Bernardes da Silva A, Keys AJ, Arrabaça MC. 2007. Photosynthetic responses of three C4 grasses of different metabolic subtypes to water deficit. Functional Plant Biology 34, 204–213 [DOI] [PubMed] [Google Scholar]
- Cerling TE, Ehleringer JR, Harris J. 1998. Carbon dioxide starvation, the development of C4 ecosystems, and mammalian evolution. Philosophical Transactions of the Royal Society B: Biological Sciences 353, 159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Besnard G, Samaritani E, Duvall MR, Hodkinson TR, Savolainen V, Salamin N. 2008. Oligocene CO2 decline promoted C4 photosynthesis in grasses. Current Biology 18, 37–43 [DOI] [PubMed] [Google Scholar]
- Conroy J, Hocking P. 1993. Nitrogen nutrition of C3 plants at elevated atmospheric CO2 concentrations. Physiologia Plantarum 89, 570–576 [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S. 2008. C4 photosynthetic isotope exchange in NAD-ME- and NADP-ME-type grasses. Journal of Experimental Botany 59, 1695–1703 [DOI] [PubMed] [Google Scholar]
- Cunniff J, Charles M, Jones G, Osborne CP. 2010. Was low atmospheric CO2 a limiting factor in the origin of agriculture? Environmental Archaeology 15, 113–123 [Google Scholar]
- Dengler NG, Dengler RE, Donnelly PM, Hattersley PW. 1994. Quantitative leaf anatomy of C3 and C4 grasses (Poaceae) – bundle sheath and mesophyll surface-area relationships. Annals of Botany 73, 241–255 [Google Scholar]
- Dippery J, Tissue D, Thomas R, Strain B. 1995. Effects of low and elevated CO2 on C3 and C4 annuals. Oecologia 101, 13–20 [DOI] [PubMed] [Google Scholar]
- Edwards G, Voznesenskaya E. 2011. C4 photosynthesis: Kranz forms and single-cell C4 in terrestrial plants. In: Raghavendra AS, Sage RF, eds. C4 photosynthesis and related CO2 concentrating mechanisms. Dordrecht: Springer, 29–61 [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR. 1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112, 285–299 [DOI] [PubMed] [Google Scholar]
- Evans JR, Seemann JR. 1989. The allocation of nitrogen in the photosynthetic apparatus: costs, consequences and control. In: Briggs WR, ed. Photosynthesis. New York: Alan R Liss, Inc., 183–205 [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 [DOI] [PubMed] [Google Scholar]
- Furbank RT. 2011. Evolution of the C4 photosynthetic mechanism: are there really three C4 acid decarboxylation types? Journal of Experimental Botany 62, 3103–3108 [DOI] [PubMed] [Google Scholar]
- Furbank RT, Chitty JA, Jenkins CLD, Taylor WC, Trevanion SJ, von Caemmerer S, Ashton AR. 1997. Genetic manipulation of key photosynthetic enzymes in the C4 plant Flaveria bidentis . Functional Plant Biology 24, 477–485 [Google Scholar]
- Gerhart LM, Ward JK. 2010. Plant responses to low [CO2] of the past. New Phytologist 188, 674–695 [DOI] [PubMed] [Google Scholar]
- Gesch RW, Vu JCV, Boote KJ, Hartwell Allen L, Jr, Bowes G. 2000. Subambient growth CO2 leads to increased Rubisco small subunit gene expression in developing rice leaves. Journal of Plant Physiology 157, 235–238 [Google Scholar]
- Ghannoum O, Caemmerer Sv, Conroy JP. 2002. The effect of drought on plant water use efficiency of nine NAD-ME and nine NADP-ME Australian C4 grasses. Functional Plant Biology 29, 1337–1348 [DOI] [PubMed] [Google Scholar]
- Ghannoum O, Evans JR, Caemmerer S. 2011. Nitrogen and water use efficiency of C4 plants. In: Raghavendra AS, Sage RF, eds. C4 photosynthesis and related CO2 concentrating mechanisms. Dordreccht: Springer, 129–146 [Google Scholar]
- Ghannoum O, Evans JR, Wah Soon C, Andrews TJ, Conroy JP, von Caemmerer S. 2005. Faster Rubisco is the key to superior nitrogen use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiology 137, 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, Phillips NG, Conroy JP, Smith RA, Attard RD, Woodfield R, Logan BA, Lewis JD, Tissue DT. 2010. Exposure to preindustrial, current and future atmospheric CO2 and temperature differentially affects growth and photosynthesis in Eucalyptus. Global Change Biology 16, 303–319 [Google Scholar]
- Gill RA, Polley HW, Johnson HB, Anderson LJ, Maherali H, Jackson RB. 2002. Nonlinear grassland responses to past and future atmospheric CO2 . Nature 417, 279–282 [DOI] [PubMed] [Google Scholar]
- Gutierrez M, Gracen VE, Edwards GE. 1974. Biochemical and cytological relationships in C4 plants. Planta 119, 279–300 [DOI] [PubMed] [Google Scholar]
- Hatch MD. 1987. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta 895, 81–106 [Google Scholar]
- Hattersley P. 1992. C4 photosynthetic pathway variation in grasses (Poaceae): its significance for arid and semi-arid lands. In: Chapman GP, ed. Desertified grasslands—their biology and management. London: Academic Press, 181–212 [Google Scholar]
- Henderson S, Caemmerer S, Farquhar G. 1992. Short-term measurements of carbon isotope discrimination in several C4 species. Functional Plant Biology 19, 263–285 [Google Scholar]
- Huxman TE, Monson RK. 2003. Stomatal responses of C3, C3–C4 and C4 Flaveria species to light and intercellular CO2 concentration: implications for the evolution of stomatal behaviour. Plant, Cell and Environment 26, 313–322 [Google Scholar]
- Kanai R, Edwards GE. 1999. The biochemistry of C4 photosynthesis. In: Rowan FS, Russell KM, eds. C4 plant biology. San Diego: Academic Press, 49–87 [Google Scholar]
- Knapp AK, Medina E. 1999. Success of C4 photosynthesis in the field. In: Sage R, Monson RK, eds. C4 plant biology. San Diego: Academic Press, 251–283 [Google Scholar]
- Ku MSB, Wu J, Dai Z, Scott RA, Chu C, Edwards GE. 1991. Photosynthetic and photorespiratory characteristics of Flaveria species. Plant Physiology 96, 518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku SB, Edwards GE. 1978. Photosynthetic efficiency of Panicum hians and Panicum milioides in relation to C3 and C4 plants. Plant and Cell Physiology 19, 665–675 [Google Scholar]
- Long SP. 1999. Environmental responses. In: Rowan FS, Russell KM, eds. C4 plant biology. San Diego: Academic Press, 215–249 [Google Scholar]
- Maherali H, Reid C, Polley H, Johnson H, Jackson R. 2002. Stomatal acclimation over a subambient to elevated CO2 gradient in a C3/C4 grassland. Plant, Cell and Environment 25, 557–566 [Google Scholar]
- McDonald EP, Erickson JE, Kruger EL. 2002. Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Functional Plant Biology 29, 1115–1120 [DOI] [PubMed] [Google Scholar]
- Monson R. 1989. The relative contributions of reduced photorespiration, and improved water- and nitrogen-use efficiencies, to the advantages of C3–C4 intermediate photosynthesis in Flaveria . Oecologia 80, 215–221 [DOI] [PubMed] [Google Scholar]
- Monson R, Moore BD. 1989. On the significance of C3–C4 intermediate photosynthesis to the evolution of C4 photosynthesis. Plant, Cell and Environment 12, 689–699 [Google Scholar]
- Monson R, Rawsthorne S. 2000. CO2 assimilation in C3–C4 intermediate plants. In: Leegood RC, Sharkey TD, von Caemmerer S, eds. Photosynthesis: physiology, metabolism. Dordrecht, The Netherlands: Kluwer, 533–550 [Google Scholar]
- Morison JI, Gifford RM. 1983. Stomatal sensitivity to carbon dioxide and humidity a comparison of two C3 and two C4 grass species. Plant Physiology 71, 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhaidat R, McKown AD. 2013. Significant involvement of PEP-CK in carbon assimilation of C4 eudicots. Annals of Botany 111, 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond B. 1982. Functional significance of different pathways of CO2 fixation in photosynthesis. In: Lange OL, Nobel PS, Osmond B, Ziegler H, eds. Physiological plant ecology II. Encylopedia of plant physiology New Series. Berlin: Springer-Verlag, 479–549 [Google Scholar]
- Pagani M, Zachos JC, Freeman KH, Tipple B, Bohaty S. 2005. Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science 309, 600–603 [DOI] [PubMed] [Google Scholar]
- Pengelly JJ, Tan J, Furbank RT, von Caemmerer S. 2012. Antisense reduction of NADP-malic enzyme in Flaveria bidentis reduces flow of CO2 through the C4 cycle. Plant Physiology 160, 1070–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto H, Tissue DT, Ghannoum O. 2011. Panicum milioides (C3–C4) does not have improved water or nitrogen economies relative to C3 and C4 congeners exposed to industrial-age climate change. Journal of Experimental Botany 62, 3223–3234 [DOI] [PubMed] [Google Scholar]
- Polley H, Johnson H, Mayeux H. 1992. Carbon dioxide and water fluxes of C3 annuals and C3 and C4 perennials at subambient CO2 concentrations. Functional Ecology 6, 693–703 [Google Scholar]
- Poorter H. 1993. Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio 104–105, 77–97 [Google Scholar]
- Prendergast H, Hattersley P, Stone N. 1987. New structural/biochemical associations in leaf blades of C4 grasses (Poaceae). Functional Plant Biology 14, 403–420 [Google Scholar]
- Ripley BS, Cunniff J, Osborne CP. 2013. Photosynthetic acclimation and resource use by the C3 and C4 subspecies of Alloteropsis semialata in low CO2 atmospheres. Global Change Biology 19, 900–910 [DOI] [PubMed] [Google Scholar]
- Ruuska S, Andrews TJ, Badger MR, Hudson GS, Laisk A, Price GD, von Caemmerer S. 1998. The interplay between limiting processes in C3 photosynthesis studied by rapid-response gas exchange using transgenic tobacco impaired in photosynthesis. Australian Journal of Plant Physiology 25, 859–870 [Google Scholar]
- Sage RF. 2004. The evolution of C4 photosynthesis. New Phytologist 161, 341–370 [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47 [DOI] [PubMed] [Google Scholar]
- Sherwin GL, George L, Kannangara K, Tissue DT, Ghannoum O. 2013. Impact of industrial-age climate change on the relationship between water uptake and tissue nitrogen in eucalypt seedlings. Functional Plant Biology 40, 201–212 [DOI] [PubMed] [Google Scholar]
- Taub DR. 2000. Climate and the U.S. distribution of C4 grass subfamilies and decarboxylation variants of C4 photosynthesis. American Journal of Botany 87, 1211–1215 [PubMed] [Google Scholar]
- Taub DR, Lerdau MT. 2000. Relationship between leaf nitrogen and photosynthetic rate for three NAD-ME and three NADP-ME C4 grasses. American Journal of Botany 87, 412–417 [PubMed] [Google Scholar]
- Taylor SH, Hulme SP, Rees M, Ripley BS, Ian Woodward F, Osborne CP. 2010. Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening experiment. New Phytologist 185, 780–791 [DOI] [PubMed] [Google Scholar]
- Tissue D, Griffin K, Thomas R, Strain B. 1995. Effects of low and elevated CO2 on C3 and C4 annuals. Oecologia 101, 21–28 [DOI] [PubMed] [Google Scholar]
- Vogan PJ, Frohlich MW, Sage RF. 2007. The functional significance of C3–C4 intermediate traits in Heliotropium L. (Boraginaceae): gas exchange perspectives. Plant, Cell and Environment 30, 1337–1345 [DOI] [PubMed] [Google Scholar]
- Vogan PJ, Sage RF. 2011. Water-use efficiency and nitrogen-use efficiency of C3–C4 intermediate species of Flaveria Juss. (Asteraceae). Plant, Cell and Environment 34, 1415–1430 [DOI] [PubMed] [Google Scholar]
- Vogan P, Sage R. 2012. Effects of low atmospheric CO2 and elevated temperature during growth on the gas exchange responses of C3, C3–C4 intermediate, and C4 species from three evolutionary lineages of C4 photosynthesis. Oecologia 169, 341–352 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. 2000. Biochemical models of leaf photosynthesis. Melbourne: CSIRO Publishing [Google Scholar]
- von Caemmerer S, Furbank RT. 2003. The C4 pathway: an efficient CO2 pump. Photosynthesis Research 77, 191–207 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Ludwig M, Millgate A, Farquhar GD, Price GD, Badger M, Furbank RT. 1997a. Carbon isotope discrimination during C4 photosynthesis: insights from transgenic plants. Australian Journal of Plant Physiology 24, 487–494 [Google Scholar]
- von Caemmerer S, Millgate A, Farquhar GD, Furbank RT. 1997b. Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase by antisense RNA in the C4 plant Flaveria bidentis leads to reduced assimilation rates and increased carbon isotope discrimination. Plant Physiology 113, 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RP, Acheson RM, Técsi LI, Leegood RC. 1997. Phosphoenolpyruvate carboxykinase in C4 plants: its role and regulation. Functional Plant Biology 24, 459–468 [Google Scholar]
- Walker RP, Chen ZH, Acheson RM, Leegood RC. 2002. Effects of phosphorylation on phosphoenolpyruvate carboxykinase from the C4 plant Guinea grass. Plant Physiology 128, 165–172 [PMC free article] [PubMed] [Google Scholar]
- Ward J, Tissue DT, Thomas RB, Strain B. 1999. Comparative responses of model C3 and C4 plants to drought in low and elevated CO2 . Global Change Biology 5, 857–867 [Google Scholar]
- Wingler A, Walker RP, Chen ZH, Leegood RC. 1999. Phosphoenolpyruvate carboxykinase is involved in the decarboxylation of aspartate in the bundle sheath of maize. Plant Physiology 120, 539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska L, Bunce J. 1997. Influence of increasing carbon dioxide concentration on the photosynthetic and growth stimulation of selected C4 crops and weeds. Photosynthesis Research 54, 199–208 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.