Summary
Besides the acknowledged roles of red light, blue light is a key determinant for synchronizing the metabolic and physiological components of CAM over the day/night cycle.
Key words: CAM, carbohydrate, gas exchange, light quality, PEPC, PEPCK, titratable acidity.
Abstract
Temporal compartmentation of carboxylation processes is a defining feature of crassulacean acid metabolism and involves circadian control of key metabolic and transport steps that regulate the supply and demand for carbon over a 24h cycle. Recent insights on the molecular workings of the circadian clock and its connection with environmental inputs raise new questions on the importance of light quality and, by analogy, certain photoreceptors for synchronizing the metabolic components of CAM. The present work tested the hypothesis that optimal coupling of stomatal conductance, net CO2 uptake, and the reciprocal turnover of carbohydrates and organic acids over the diel CAM cycle requires both blue and red light input signals. Contrasting monochromatic wavelengths of blue, green, and red light (i.e. 475, 530, 630nm) with low fluence rates (10 μmol m–2 s–1) were administered for 16 hours each diel cycle for a total treatment time of 48 hours to the obligate CAM bromeliad, Aechmea ‘Maya’. Of the light treatments imposed, low-fluence blue light was a key determinant in regulating stomatal responses, organic acid mobilization from the vacuole, and daytime decarboxylation. However, the reciprocal relationship between starch and organic acid turnover that is typical for CAM was uncoupled under low-fluence blue light. Under low-fluence red or green light, the diel turnover of storage carbohydrates was orchestrated in line with the requirements of CAM, but a consistent delay in acid consumption at dawn compared with plants under white or low-fluence blue light was noted. Consistent with the acknowledged influences of both red and blue light as input signals for the circadian clock, the data stress the importance of both red and blue-light signalling pathways for synchronizing the metabolic and physiological components of CAM over the day/night cycle.
Introduction
Crassulacean acid metabolism (CAM) is a photosynthetic specialization, which permits the net uptake of CO2 at night and thereby improves the water-use efficiency (WUE) of carbon assimilation in up to 7% of terrestrial higher plants (Crayn et al., 2004). CAM plants show a remarkable metabolic plasticity for modulating nocturnal and diurnal CO2 uptake and have been identified as competitive biomass accumulators in comparison with many C3 and C4 crops (Borland et al., 2009, 2011). The diel cycle of CAM photosynthesis is commonly divided into four phases that integrate patterns of net CO2 uptake and changes in the abundance of key metabolites that define carbon supply and demand over the 24h cycle (Osmond, 1978). The enzyme phosphoenolpyruvate carboxylase (PEPC) is activated at night (Phase I) with the resultant uptake of CO2 leading to nocturnal accumulation of malic acid and a concomitant depletion in storage carbohydrates (i.e. soluble sugars or starch), which deliver the phosphoenolpyruvate (PEP) building blocks to sustain malate formation. During the day, PEPC is deactivated and malate decarboxylation mediated by phosphoenolpyruvate carboxykinase (PEPCK) or (NAD)P malic enzymes, depending on the species, releases CO2 within the leaf chlorenchyma cells. Consequently ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) fixes the CO2 released from decarboxylation (Phase III) behind closed stomata. These major CAM phases are punctuated by Phase II at the start of the day and Phase IV at the end of the day (Borland and Taybi, 2004). All four phases of the CAM cycle show plasticity in terms of magnitude and duration, which seems to be critical for optimizing carbon gain and water use under different environmental situations (Ceusters et al., 2008a, 2009a, 2009b, 2010, 2011; Dodd et al., 2003).
Light intensity (photosynthetic photon flux density, PPFD) is a critical factor for determining the magnitude and duration of each of the four phases of CAM, illustrating a cardinal role for the light reactions of photosynthesis in achieving metabolic synchronization. PPFD during Phase III determines the rate of organic acid mobilization from the vacuole (Barrow and Cockburn, 1982; Thomas et al., 1987), which seems to be linked to the rates of electron transport and Rubisco mediated net CO2 uptake in vivo (Lüttge, 2004). Daytime integrated PPFD also influences the magnitude of Phase I dark CO2 uptake by determining the abundance of carbohydrate generated via the Calvin cycle and gluconeogenesis which is subsequently required for the nocturnal provision of PEP (Nobel and Hartsock, 1983). In addition, high PPFD can elicit the induction of CAM in some facultative species that include Guzmania monostachia and Clusia minor (Maxwell et al., 1994, 1995; Grams and Thiel 2002). The operation of CAM is also influenced by light quality, which implies cardinal roles for certain photoreceptors in synchronizing metabolism over the diel phases. Phytochrome was shown to play a central role in the persistence of the CAM-defining circadian rhythms of CO2 fixation under red light as first demonstrated in Bryophyllum fedtschenkoi by Harris and Wilkins (1976, 1978a, 1978b). In addition, phytochrome has also been implicated in the short-photoperiod induction of CAM in Kalanchoe blossfeldiana (Brulfert et al., 1982, 1988; Taybi et al., 2002). The role of blue light receptors in the operation and synchronization of the metabolic components of CAM is less clear. Previously, it was suggested that a UV-A blue light receptor mediates the high PPFD induced switch from C3 to CAM in Clusia minor (Grams and Thiel, 2002). However, no detectable effect of blue light on the persistence, phase, or period of the rhythm of CO2 metabolism in Bryophyllum leaves was noted by Wilkins (1992), implying a minor role for the blue/ultra violet A (UV-A) absorbing cryptochromes in the synchronization of CAM phases. The apparent blue-light insensitivity of CAM stomata reported for Portulacaria afra and Mesembryanthemum crystallinum has been proposed as a central component for ensuring daytime closure of stomata during phase III of CAM (Lee and Assmann, 1992, Tallman et al. 1997). Taken together, these observations might imply a possible C3 to CAM divergence in light signalling pathways mediated by blue and red light photoreceptors. Recent insights into the molecular components of the CAM circadian clock, which seem to be comparable to those of the C3 clock of Arabidopsis thaliana (Boxall et al., 2005), raise new questions about the influence of light quality on orchestrating the diel cycle of CAM. Both phytochromes and cryptochromes constitute the main photoreceptors that mediate light input into the circadian clock (Hotta et al., 2007). With CAM relying on a strict temporal compartmentation of metabolic processes (Borland et al., 1999; Hartwell, 2005), it might be hypothesized that optimal coupling of stomatal conductance, net CO2 uptake, and the reciprocal turnover of carbohydrates and organic acids over the diel cycle requires both blue and red light input signals.
The aim of the present work was to examine the influence of light quality on synchronizing the phases of CAM. The obligate CAM bromeliad, Aechmea ‘Maya’, was exposed to different monochromatic wavelengths of light for periods of 16 hours over two complete diel cycles. By providing the different monochromatic light treatments at low fluence rates of 10 μmol m–2 s–1 using LED illumination, the intention was to minimize direct involvement of photosynthetic processes in driving CAM and thereby highlight the involvement of different photoreceptors and associated signalling pathways in metabolic synchronization. As natural daylight is composed of nearly equal amounts of red and blue light, similar quantum fluence rates were applied for these wavelengths of light. Leaf gas exchange patterns, diel gene expression analyses, protein abundance and activities of key enzymes such as phosphoenolpyruvate carboxylase (PEPC) and phosphoenolpyruvate carboxykinase (PEPCK), titratable acidities, and storage carbohydrate turnover (i.e. starch and sucrose) were monitored to examine metabolic synchronization.
Materials and methods
Plant material and experimental sampling
Aechmea ‘Maya’ is a spineless cultivar resulting from a cross between A. tessmannii and A. fasciata. These Aechmea species are CAM bromeliads and belong to the subfamily of the Bromelioideae (Benzing, 2000; De Proft et al., 2007). Previous studies have indicated that A. ‘Maya’ is an obligate CAM plant (Ceusters et al., 2008b, 2009c, 2010). Plants were cultivated in a growth room with a daytime temperature of 20±2 °C and a minimum nocturnal temperature of 18±2 °C and PPFD of 100 μmol m–2 s–1 between 06.00 and 22.00h. Watering was performed once weekly with a conventional nutrient solution of 1 mS cm–1 (Londers et al., 2009).
To investigate the influences of light quality on the phases of CAM, different LED (Roithner LaserTechnik, Vienna, Austria) configurations were used; i.e. blue (475±25nm), green (530±25nm) and red (630±25nm) light. At the onset of the day (06.00h) separate batches of twelve months old vegetative Aechmea ‘Maya’ plants (n=5) were placed under each configuration with a low fluence rate of 10±2 μmol m–2 s–1 and a photoperiod from 06.00–22.00h for 2 complete diel cycles. In addition, a control batch of plants was exposed to continuous darkness for 48h and a further batch of plants was exposed to white light (100 μmol m–2 s–1, photoperiod from 06.00–22.00h). Previous experiments indicated that white light of 15 μmol m–2 s–1 was insufficient to sustain metabolic activity on the short term (Ceusters et al., 2011) and therefore 100 μmol m–2 s–1 was used instead of 10 μmol m–2 s–1. Leaf samples (n=5 plants) were taken from the upper one-third of young fully expanded leaves during the second light treatment cycle of 24h starting from 06.00h every 4h. Samples were immediately frozen in liquid nitrogen and stored at –80 °C except for the determination of enzyme activities, which were performed on fresh leaf samples. Additionally, net leaf gas exchange was also monitored for three replicate plants of each treatment.
Gas exchange measurements
Net CO2 exchange was measured on the youngest fully expanded leaves, using a LCi Portable Photosynthesis System (ADC BioScientific Ltd., UK). The top part of the leaf was enclosed in a broad leaf chamber (6.25cm2) and the incoming air was passed through a 25 l bottle to buffer short-term fluctuations in the CO2 concentration. Gas exchange data were collected over a 24h period with measurements obtained at 15min intervals (n=3 plants). Integrated net CO2 uptake was determined for specific periods during the 24h time course by calculating specified areas under the CO2 exchange curves (Griffiths et al., 1986).
Chemical analyses of metabolites
Titratable acidity was determined by titration of methanol extracts against 1 N NaOH to a neutral endpoint, as indicated by phenolphthalein.
Soluble sugars (glucose, fructose, and sucrose) were extracted, subjected to enzymatic treatment (Enzytec, Scil Diagnostics GmbH, Germany) and analysed at 340nm using a spectrophotometer (DU-65, Beckman, Fullerton, USA). Starch content was determined as glucose equivalents following digestion with amyloglucosidase (Enzytec, Scil Diagnostics GmbH, Germany). The analyses were conducted as earlier described by Ceusters et al. (2008a).
Western blotting
Approximately 250mg of powdered tissue was mixed with 250 μl of a buffer containing 100mol m–3 Tris, pH 8.3 at 4 °C, 10mol m–3 NaCl, 5mol m–3 ethylenediamine tetraacetic acid (EDTA), 10mol m–3 dithiotreitol (DTT) plus 4 μg leupeptin, 4 μg E-64, 2mol m–3 phenylmethanesulfonyl fluoride (PMSF) and 20 μl of a plant protease inhibitor cocktail (all protease inhibitors from Sigma, UK). The extract was centrifuged at 4 °C for 10min at 12,000 g and the supernatant was mixed thoroughly with glycerol (10% final volume). Protein contents were determined as described by Bradford (1976). Exactly 15 μg protein was resolved on 12% polyacrylamide gels and blotted onto polyvinyldene fluoride membranes (Sigma, UK). PEPC abundance was determined using antibodies raised against the purified enzyme from Panicum miliaceum (gift from Prof H. Bohnert) and PEPCK with antibodies against the purified enzyme from Panicum maximum (gift from RP Walker). Anti-IgG alkaline phosphatase conjugate was used as the secondary antibody. Detection was achieved using the enhanced chemi-luminescence (ECL) system (Amersham, Buckinghamshire, UK) following manufacturer’s instructions. For all blots, duplicate gels were stained with Coomassie Blue to confirm equal loading of the samples and protein integrity.
Enzyme activities
The extraction and assay of PEPC was based on the method described by Borland and Griffiths (1997). Exactly 0.6g leaf material was homogenized in 3ml extraction buffer at 4 °C containing 200mM Tris-HCl (pH 8.0), 2mM EDTA, 1mM DTT, 2% (w/v) PEG 20 000, 1mM benzamidine, and 10mM malate with 60mg sodium bicarbonate. The homogenate was filtered through three layers of muslin and centrifuged for 2min at 13000 g. The extract was then desalted in a column of Sephadex G-25, equilibrated with 100mM Tris-HCl (pH 7.5 at 4 °C), 1mM DDT, 1mM benzamidine, and 5 % (w/v) glycerol. The maximal activity of PEPC was assayed in a reaction mix containing, in 1 ml: 50mM Tris-HCl (pH 8.0), 5mM MgCl2, 0.2mM NADH, 10mM NaHCO3, and 2.5mM PEP. The reaction was initiated by the addition of 50 μl of extract and change in absorbance at 340nm was measured for 4min at 25 °C.
The extraction and assay of PEPCK was adapted from the methods described by Cooper et al. (1968) and Walker and Leegood (1995). Exactly 0.3g leaf tissue was homogenised at 4 °C with 100mg PVPP in 3ml extraction buffer containing 50mM Hepes-NaOH (pH 7.7), 1mM EDTA, 5mM Mg-acetate, 1% (w/v) bovine serum albumin, 5mM DTT, and 1mM PMSF. The homogenate was centrifuged for 1min at 13 000rpm at 4 °C and 75 μl of the supernatant was used immediately in a reaction mix containing, in 1 ml: 40mM Mes-KOH (pH 6.7), 4.2mM glutathione reduced, 0.35mM NADH, 1mM MnCl2, 50mM KHCO3, 2mM ADP, 2mM PEP, and 6 units malate dehydrogenase (Sigma Aldrich). To determine the maximal activity of PEPCK the change in absorbance at 340nm was measured for 4min at 25 °C. Similar reactions without ADP or PEP were carried out to eliminate potential background reactions catalysed by PEPC or pyruvate kinase plus lactate dehydrogenase (Hatch, 1973).
RNA extraction and semi-quantitative RT-PCR
Total RNA was purified from 250mg of powdered leaf tissue using Tri-Reagent (Helena Biosciences, UK) as described by Taybi et al., (2000). Exactly 5 μl of RNA extract was treated with DNase I (Life Technologies, Paisley UK), to prevent amplification of genomic DNA. RT-PCR was conducted as a single-tube reaction and cDNA synthesis was promoted using the reverse primer. Each 25 μl reaction contained 10x PCR reaction buffer, 10mol m–3 DTT, 2.5mol m–3 MgCl2, 0.25mol m–3 dNTPs (Bioline Scientific, London, UK), 400nM each of forward and reverse primers, 12U RNase Out (Life Technologies, UK), 0.5U Taq DNA polymerase (Bioline Scientific), 30U Superscript II reverse transcriptase (Life Technologies) and 100ng total RNA. Annealing temperature was 50 °C and 25 and 30 cycles were used for amplification of ppc and pepck respectively. The following primers were used: PPC F (5’ GTG GGA CTG TGG GGA GA 3’), PPC R (5’ CTT GTC TGT GTC CAC GCA 3’), PEPCK F (5’ CAC GCC TGA AGA GCT AG 3’), PEPCK R (5’ CAG GGC ATC TTT GCG TTA 3’). Ubiquitin served as internal control as well as control for equal RNA inputs and RT-PCR conditions. To confirm specificity of RT-PCR, the PCR products obtained from using the ppc and pepck primers were sequenced and confirmed against publicly available sequences for the two genes using blast. The partial sequences obtained for ppc and pepck from A. ‘maya’ were deposited on the National Center for Biotechnology Information (NCBI; KC007431 for ppc and KC007432 for pepck).
Data analysis
Where appropriate, data were analysed using the statistical software package SAS Enterprise Guide 4.0. Before carrying out statistical tests, normality of the data was checked by means of the Kolmogorov-Smirnoff statistic (p>0.05). Means are compared either by two sample t-test or Tukey’s studentized range test (α=0.05).
Results
Leaf gas exchange
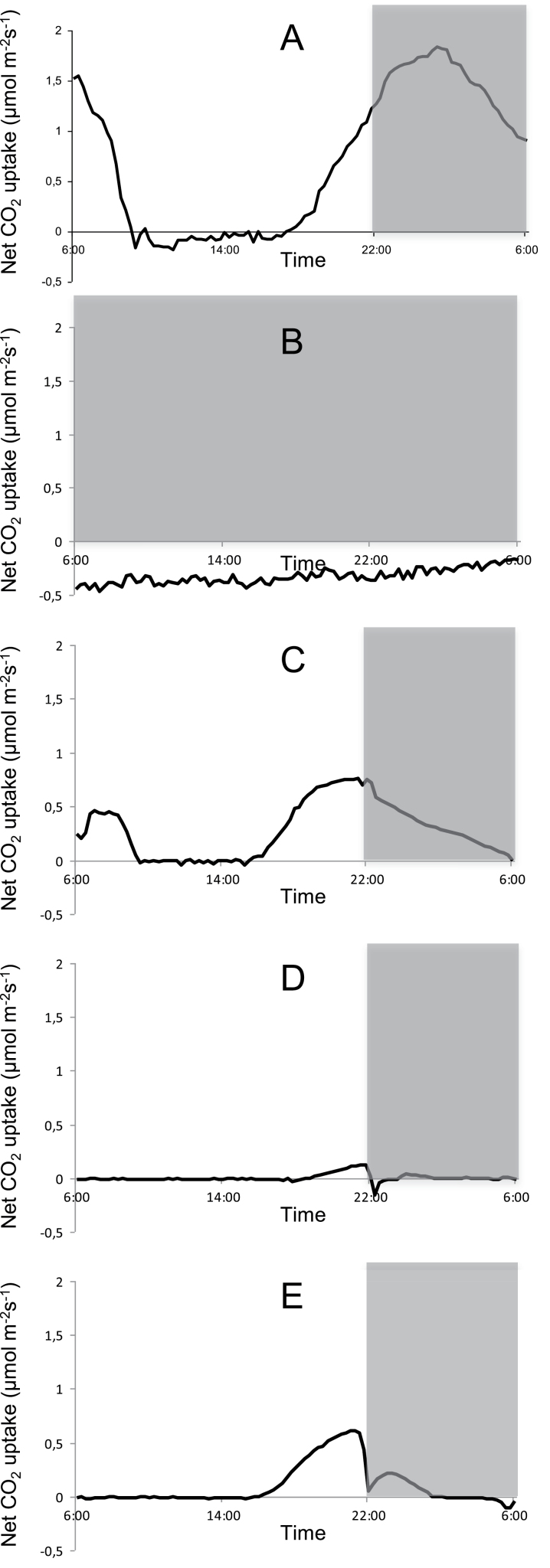
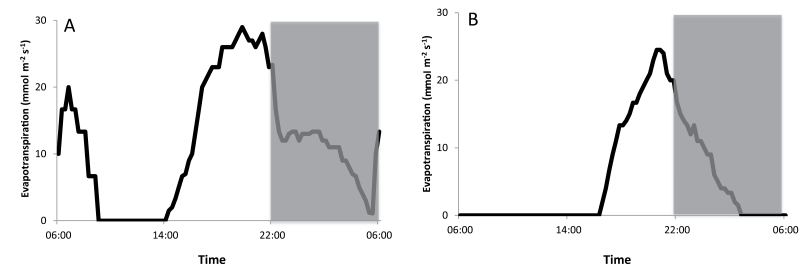
For each of the investigated wavelengths together with continuous dark and white light/dark conditions, diel gas exchange patterns were monitored and integrated net CO2 uptake was calculated for each of the different CAM phases (Fig. 1, Table 1). In contrast to the white light/dark control where a complete 24h CAM cycle occurred with a total diel uptake of 61.1±9.4 mmol CO2 m–2, under continuous darkness sustained respiration of CO2 resulted in a net loss of 28.4±4.9 mmol CO2 m–2 over 24h. Under low fluence rates of blue light (10 μmol m–2 s–1) all four phases of CAM were expressed and approximately 33% of CO2 was captured over 24h in comparison with the white light/dark control treatment. Afternoon net CO2 uptake (Phase IV) was unaffected (relative to that under white light; P>0.05) but night-time net CO2 uptake was significantly curtailed compared with that noted under the usual day/night conditions (P<0.05). Under low-fluence red light, 24h net CO2 uptake represented ~16.5% of that measured in the white light control. About 90% of total net CO2 uptake under red light occurred during the afternoon (Phase IV) with no Phase II and a restricted Phase I. The abolishment of an early day stomatal opening for red illuminated plants, although clearly present for blue illuminated ones, was further confirmed by leaf evapotranspiration data for blue and red illumination (Fig. 2). Under low-fluence green light a small but significant (P<0.05) amount of net CO2 uptake (1.1±0.5 mmol CO2 m–2) during a shortened Phase IV was noted but no net respiration was recorded over the diel cycle in contrast to that observed under continuous darkness (Fig. 1, Table 1).
Fig. 1.
Net 24h CO2 uptake (μmol m–2 s–1) for young fully developed leaves of Aechmea ‘Maya’ under control light-dark cycle (A, white light, 100 μmol m–2 s–1), continuous dark (B), and different monochromatic light-dark cycles (10 µmol m–2s–1; C=blue 475nm; D=green 530nm; E=red 630nm). The dark period is indicated in grey. Gas exchange curves are representative of three replicate runs with SE<15%.
Table 1.
Integrated net CO2 uptake for each of the four CAM Phases (mmol CO2 m–2 Phase–1) by young fully developed leaves of Aechmea ‘Maya’ under control light-dark cycle (LD, white light, 100 μmol m–2 s–1), continuous dark (DD) and different monochromatic light-dark cycles (10 μmol m–2s–1)
Data are means±SE (n=3) and significant net CO2 uptake/loss (P<0.05) is indicated by an asterisk
| Phase II | Phase III | Phase IV | Phase I | Total 24 h | |
|---|---|---|---|---|---|
| LD | 11.1±2.5* | –2.0±1.8 | 9.9±3.2* | 42.8±5.4* | 61.1±9.4* |
| DD | N/A | N/A | N/A | N/A | –28.4±4.9* |
| Blue | 3.9±0.5* | 0.1±0.8 | 9.2±1.7* | 9.9±1.9* | 23.1±3.4* |
| Green | –0.1±0.2 | –0.2±0.2 | 1.1±0.5* | 0.1±0.2 | 0.8±0.5* |
| Red | –0.1±0.1 | –0.2±0.3 | 7.5±1.3* | 1.3±0.4* | 8.5±1.7* |
Fig. 2.
Net 24h evapotranspiration (mmol m–2s–1) for young fully developed leaves of Aechmea ‘Maya’ under blue (A) and red (B) illumination of 10 μmol m–2 s–1. The dark period is indicated in grey. Curves are representative of three replicate runs with SE<15 %.
Metabolite dynamics
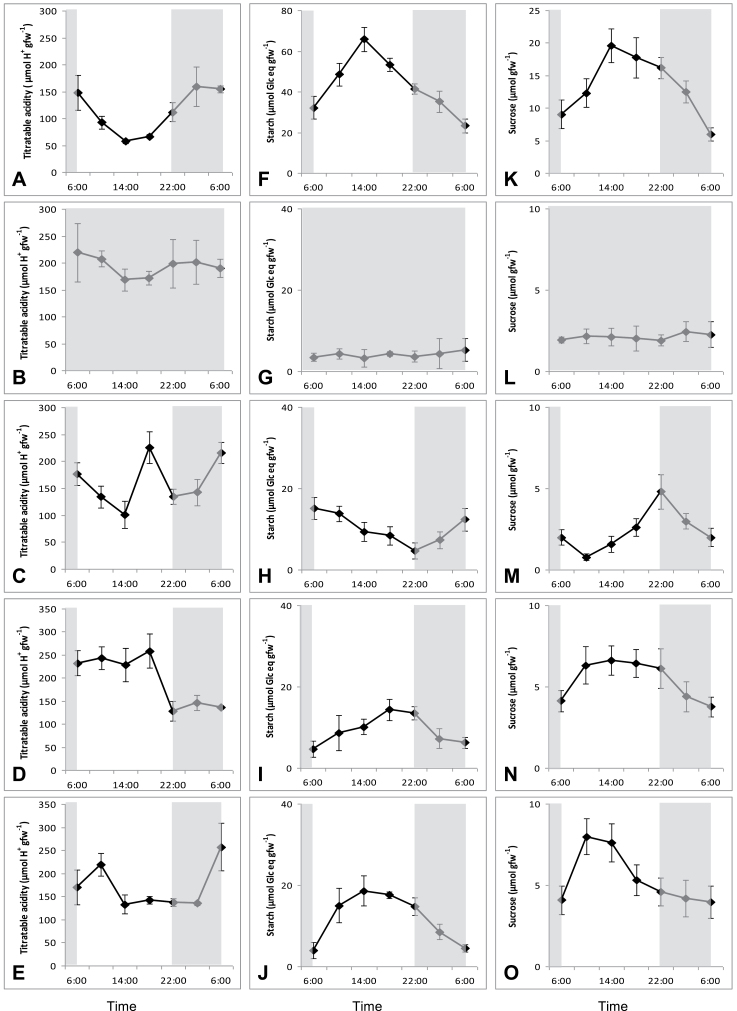
The typical CAM pattern of nocturnal accumulation and daytime degradation of organic acids was clearly present under white light/dark conditions (Fig. 3A). This diel cycle of organic acids was lost in continuous darkness where titratable acidities remained at maximal levels during the diel cycle (Fig. 3B). The monochromatic low-light treatments each provoked specific responses in the diel acidity cycle. Blue light induced degradation of organic acids (176±21 μmol H+ g–1 fw to 101±20 μmol H+ g–1 fw) at the start of the photoperiod in a comparable way to the plants under white light (Fig. 3C). However in the middle of the day (14.00h) titratable acidity suddenly increased to a maximal level of 223±32 μmol H+ g–1 fw followed by an immediate decline at 18.00h. During the night following blue light treatment, the organic acid content increased to levels comparable to that in the control light/dark treatment. However, although most acids accumulated over the first half of the dark period in the control white light plants, under blue light, most acid accumulation occurred in the latter half of the night. Plants subjected to red and green wavelengths showed a delay in acid breakdown at the start of the day, i.e. 4h for red and 12h for green after dawn (Fig. 3D, E). Maximal acidities in the white light control treatment noted at 02.00h corresponded with minimal values of titratable acidity at this time under green and red light Whilst there was no significant nocturnal accumulation of acids under green light (P>0.05), some 110±32 μmol H+ g–1 fw organic acids were accumulated overnight following red light illumination. As with the blue light treatment, nocturnal acid accumulation occurred over the latter half of the night after the red light treatment.
Fig. 3.
Diel patterns of titratable acidity (μmol H+ g–1fw; left panel), starch (μmol Glc eq g–1fw; middle panel) and sucrose (μmol g–1fw; right panel) for young fully developed leaves of Aechmea ‘Maya’ under control light-dark cycle (A, F, K; white light, 100 μmol m–2 s–1), continuous dark (B, G, L), and different monochromatic light-dark cycles (10 μmol m–2 s–1), i.e. blue (C, H, M), green (D, I, N), and red (E, J, O). The dark period is indicated in grey. Data are means±SE (n=5 plants).
Under white light/dark conditions, both starch and sucrose showed an inverse diel rhythm compared with titratable acidity with starch being the main carbohydrate degraded at night to sustain nocturnal CO2 uptake (diel turnover of 40±6 μmol Glc eq g–1 fw from starch versus 20±5 μmol Glc eq g–1 fw for sucrose) (Fig 3, middle and right panels). In line with the titratable acidity measurements, continuous darkness abolished any diel fluctuations in storage carbohydrates and minimal values of 4±1 μmol Glc eq g–1 fw and 3±1 μmol g–1 fw persisted in the leaves for starch and sucrose, respectively. Green and red light treatments resulted in nocturnal starch degradation of 12±3 μmol Glc eq g–1 fw, representing 33% of starch degradation in control plants. In contrast, exposure to blue light resulted in an inverse rhythm of starch turnover with starch degradation occurring during daytime followed by nocturnal accumulation. The diel turnover of sucrose under blue, green, or red light treatments was about 33% in comparison with control plants (8±2 μmol Glc eq g–1 fw). The diel pattern of sucrose turnover was generally less affected by light quality as compared with that of starch. The dissacharide accumulated during the day under all light treatments but a consistent delay in sucrose accumulation occurred under the blue light treatment.
Transcript abundance, protein content, and activities of PEPC and PEPCK
Under white light/dark conditions a diel rhythm in transcript abundance of ppc was observed with maximum transcript abundance noted over the latter part of the photoperiod (Fig. 4). This rhythm in ppc abundance was damped under the different light treatments and except for blue illumination, the timing of maximum ppc transcript abundance was also shifted relative to that noted under white light. Under green light, timing of max ppc abundance occurred at least 6h earlier than that under white light and under red light the diel rhythm of ppc transcript abundance with peaks in the early morning, was almost opposite to the one under white light. The transcript abundance of pepck also showed a diel rhythm with highest abundance during the day under all light treatments. The green light treatment resulted in a strong decline in pepck transcipts at night, compared with the other treatments.
Fig. 4.
Day-night changes in levels of transcripts for PEPC (ppc) and PEPCK (pepck) in young fully developed leaves of Aechmea ‘Maya’ under control light-dark cycle (LD, white light, 100 μmol m–2 s–1) and different monochromatic light-dark cycles (10 μmol m–2 s–1), i.e. blue, green, and red. The dark period was from 22.00–06.00h. Ubiquitin (ubq) served as control.
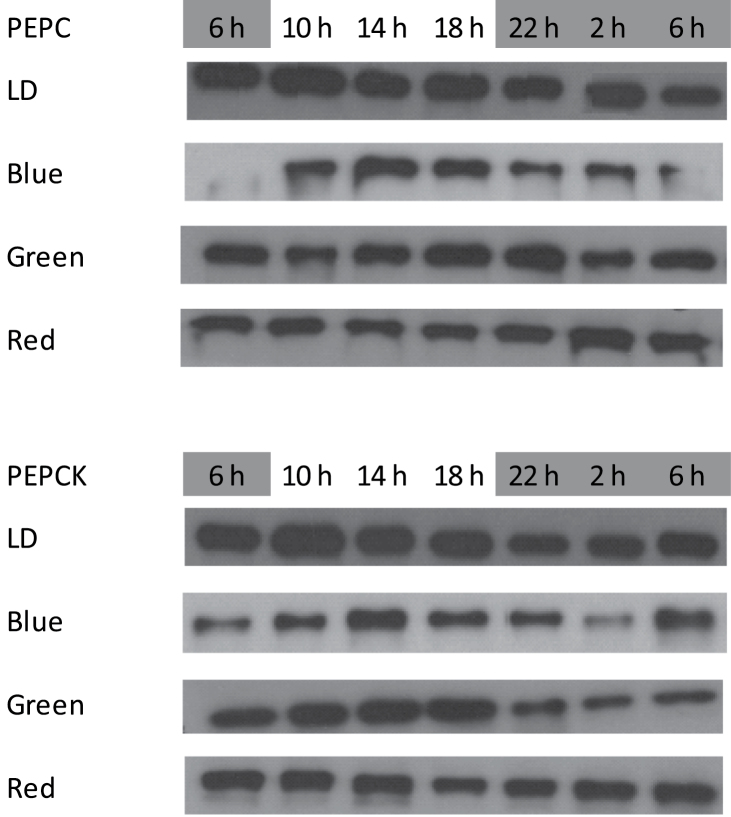
In terms of protein abundance, there was no obvious day/night change in PEPC protein under light/dark conditions (Fig. 5). However, under blue light, the amount of PEPC protein was reduced compared with that under white light and a diel pattern of abundance was apparent, with a decline in PEPC abundance over the night following blue light treatment. Red light also resulted in a decrease in PEPC protein. The abundance of PEPCK protein was highest under the white light treatment. Blue and red light resulted in a general decline in the amount of PEPCK protein. Under green light, the decline in PEPCK protein (compared with the white light control) was most apparent during the night period.
Fig. 5.
Western blots showing the abundance of PEPC (top) and PEPCK (bottom) protein in young fully developed leaves of Aechmea ‘Maya’ under control light-dark cycle (LD, white light, 100 μmol m–2 s–1) and different monochromatic light-dark cycles (10 μmol m–2 s–1), i.e. blue, green, and red. The dark period was from 22.00–06.00h. Similar amounts of soluble proteins were loaded for each treatment.
To quantify differences in enzyme capacities under the different light treatments the maximal activities of PEPC and PEPCK were measured with samples taken in the middle of the night (PEPC) and middle of the day (PEPCK) day (Table 2). There was no significant difference between PEPC activities in plants maintained under white (control) or blue light but red and green light resulted in a significant (P<0.05) decrease in PEPC activity compared with controls. The activities of PEPCK were comparable for all investigated wavelengths except for the green light treatment where PEPCK activity was twice as high (P<0.05) compared with the other treatments. Under continuous dark, both PEPC and PEPCK activities were significantly lower (P<0.05) in comparison with the light control and the monochromatic light treatments.
Table 2.
Activities of PEPC and PEPCK (μmol CO2 g–1fw h–1) extracted from young fully developed leaves of Aechmea ‘Maya’ in the middle of the night (PEPC) and midday (PEPCK) under control light-dark cycle (LD, white light, 100 μmol m–2s–1), continuous dark (DD) and different monochromatic light-dark cycles (10 μmol m–2s–1)
Data are means±SE (n=3) and those in each column followed by a different letter are significantly different by Tukey’s Studentized range test (P< 0.05).
| PEPC | PEPCK | |||
|---|---|---|---|---|
| LD | 52±14 | A | 33±4 | B |
| DD | 8±1 | C | 23±6 | C |
| Blue | 37±11 | A | 30±6 | B |
| Green | 23±2 | B | 60±4 | A |
| Red | 19±3 | B | 28±5 | B |
Discussion
Given the nature and importance of the circadian clock for the functioning of CAM (Boxall et al., 2005), the aim of the present study was to test the hypothesis that both red and blue light signalling processes are of pivotal importance for optimal synchronization of the diel phases of CAM. By building on the acknowledged influence of red light on the physiology and biochemistry of CAM plants (Wilkins, 1992), this study sought to get blue light responses ‘out of the dark’ in CAM research by comparing fundamental metabolic characteristics under diel light/dark cycles of different monochromatic wavelengths (i.e. blue, red, and green) in comparison with a control white light/dark treatment.
Leaf gas exchange
Under a low fluence rate of 10 μmol m–2 s–1, blue light sustained a typical CAM gas exchange pattern in leaves of A. `Maya` consisting of all four phases of CAM (Osmond, 1978). Although total net uptake of CO2 was only 33% of that measured in control plants (white light, 100 μmol m–2 s–1), no major differences in the timing of the different phases of gas exchange were apparent under blue or white light. These data indicate the need for a review of the previously proposed insensitivity of CAM stomata to blue light, inferred from studies with individual leaves or isolated stomata from facultative CAM plants (Lee and Assmann, 1992; Mawson and Zaugg, 1994; Tallman et al., 1997). The present study is the first in which whole CAM plants were monitored under full diel cycles. The occurrence of a complete Phase II under both white and low-fluence blue light, although absent under the other wavelengths (i.e. red and green), is consistent with the generally accepted view of blue-light induced early morning stomatal opening (Doi et al., 2004; Lawson, 2009). These observations are further strengthened by early morning (i.e. Phase II) patterns of leaf evapotranspiration for blue and red illuminated plants, which clearly illustrate a qualitative light effect on stomatal control. Phase IV CO2 assimilation was very similar for the white light control as well as for blue and red illuminated plants. Blue light might bring about higher energy dissipation, whereas more efficient energy capture could occur under red light. Further measurements of Phase IV photosynthetic rates under different intensities of blue and red light would be informative. In summary, the CAM phases seem to show some degree of plasticity in response to light quality but the observed phenomena clearly stress the importance of blue light inputs to mediate Phase II CO2 uptake and sustain a significant proportion of nocturnal carboxylation (i.e. Phase I).
Carboxylation and decarboxylation events
At the start of the photoperiod (06.00h) under low-fluence blue light, decarboxylation of malate was initiated and titratable acidities decreased to minimal values around the middle of the photoperiod (14.00h) comparable to the kinetics in control plants. In contrast, the higher wavelengths of the spectrum caused a consistent delay in breakdown of organic acids, which occurred ~4 and 12h into the photoperiod for red and green light, respectively. Comparing the maximum enzyme activity of PEPCK measured in vitro with measured rates of acid breakdown in vivo indicated that this delay in net acid breakdown was unlikely to be a consequence of insufficient intrinsic decarboxylating capacity but may have been due to postponement of malate efflux out of the vacuole. The maximum PEPCK activity measured in vitro was similar for all treatments except for plants exposed to green light, where a doubled activity was present that was not mirrored by changes in transcript or protein abundance. These observations suggest an influence at the post-translational level, implicating a higher degree of dephosphorylation brought about by green illumination, which could increase the maximum enzyme activity. PEPCK has been described previously to undergo reversible phosphorylation with the phosphorylated enzyme present at night when decarboxylation should be curtailed (Walker and Leegood, 1996).
Under low-fluence blue light during the latter part of the photoperiod, synchronization between carboxylation and decarboxylation events seemed to be uncoupled as premature PEPC activity was indicated by a significant net accumulation of titratable acids, followed by a 2h period of significant acid breakdown before entering the dark period. The diel oscillations of ppc and pepck transcript abundance noted in control plants were maintained under blue light but were damped in abundance. No difference was detected in maximum in vitro activities of PEPC and PEPCK under white or blue light. However, in vivo activity of PEPC is modulated by reversible phosphorylation by a dedicated kinase (PPCK), which is believed to be under circadian control at the transcript level (Hartwell et al., 1996, 1999; Nimmo, 2000). Moreover, it has been postulated that the same kinase could potentially also phosphorylate PEPCK in vivo and thereby curtail futile cycling between malate synthesis and breakdown (Dodd et al., 2002). Previous studies have shown that blue light (as compared with white or red light) is particularly important for stabilizing the clock protein ZTL, thereby allowing daytime degradation of TOC1, a core clock protein that normally accumulates during the day (Fujiwara et al., 2008; Harmer, 2009). Such blue light influences on core clock proteins might have profound influence on the transcript abundance of ppck, which is generally down regulated during the day (Taybi et al., 2004). However as ppck has not yet been identified in a CAM bromeliad (including Ananas comosus (pineapple) or A.’Maya’ (J Delahunty, J Ceusters and A Borland unpublished observations), this hypothesis remains to be tested.
Storage carbohydrate turnover
One of the key characteristics in CAM plants is the intimate reciprocal relationship between the diel cycling of organic acids and their storage carbohydrate counterparts, which can be either soluble sugars or starch (Borland and Taybi, 2004). In line with the retention of high backgrounds of titratable acidity, the diel cycles of accumulation/degradation of starch and sucrose, which sustain PEPC carboxylation in Bromelioideae (Antony et al., 2008; Ceusters et al., 2008a) were abolished under continuous darkness. Similar observations were previously made for Aechmea ‘Maya’ under low intensities of about 15 μmol m–2 s–1 white light (PPFD 0.46mol photons m–2 d–1) (Ceusters et al., 2011). However, low-fluence illumination of 10 μmol m–2 s–1 with specific wavelengths of light provoked different responses in the kinetics of storage carbohydrate turnover. Although damped in comparison with control plants, low-fluence red light assured appropriate diel cycling for both starch and sucrose, showing accumulation during the first part of the day (6–14h) followed by degradation during the second part of the day and night. As carbohydrate responses to green and red light were similar to those under white light, both red and green light might be regarded as positive regulators in inducing starch breakdown towards the end of the light period. Under blue light, sucrose accumulation was maintained until the end of the photoperiod, but a complete reversal of the starch cycle was observed with significant breakdown of starch during the day followed by nocturnal accumulation. Such a reversal of the diel starch cycle has not previously been reported in photosynthetic mesophyll cells. It remains to be established if the signals that orchestrate leaf starch turnover over the day/night cycle have diverged between the C3 and CAM pathways. However, blue-light-mediated degradation of starch (often accompanied by sucrose accumulation) typically resembles the response of guard cells in C3 plants such as Arabidopsis and Vicia when stomatal aperture increases following the onset of the light period (Tallman and Zeiger, 1988; Lasceve et al., 1997). The reversal of diel starch turnover invoked by blue light in leaves of the CAM species A. ‘maya’ is intriguing in light of previous suggestions that CAM may have evolved from C3 guard cell metabolism (Cockburn, 1981).
In conclusion, the results of this study indicate the importance of a combined input of different wavelengths of light to orchestrate and synchronize the diel phases of CAM. Although low-fluence blue light was a key determinant in inducing daytime decarboxylation and regulating stomatal responses, low-fluence red light was required to synchronize the diel cycles of storage carbohydrate (starch and sucrose) accumulation and degradation with acid accumulation. These novel findings open up several new avenues of research into how light quality and quantity interact to modulate the CAM phases. Further detailed experimentation is now possible and recommended, including combined inputs of both red and blue light as well as irradiance dependence curves for different light qualities to reveal more about the complex interplay of different signals that coordinate stomatal conductance with the metabolic processes that underpin CAM.
Acknowledgements
This research was supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen). Additional support was provided by the Office of Science (BER), U. S. Department of Energy and by FWO Vlaanderen. Deroose Plants NV is acknowledged for supplying plant material, Veerle Verdoodt (Leuven), and Sue Patterson (Newcastle) for assistance in the lab, and Johan Calcoen for assistance in assembling the different LED configurations. Oak Ridge National Laboratory is managed by UT-Battelle, LLC for the US Department of Energy under Contract Number DE–AC05–00OR22725.
References
- Antony E, Taybi T, Courbot M, Mugford S, Smith JAC, Borland AM. 2008. Cloning, localisation and expression analysis of vacuolar sugar transporters in the CAM plant Ananas comosus (pineapple). Journal of Experimental Botany 59, 1895–1908 [DOI] [PubMed] [Google Scholar]
- Barrow SR, Cockburn W. 1982. Effects of light quantity and quality on the decarboxylation of malic acid in crassulacean acid metabolism photosynthesis. Plant Physiology 69, 568–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing DH. 2000. Bromeliaceae-Profile of an adaptive radiation. Cambridge: Cambridge University Press [Google Scholar]
- Borland AM, Griffiths H. 1997. A comparative study on the regulation of C3 and C4 carboxylation processes in the constitutive crassulacean acid metabolism (CAM) plant Kalanchoë daigremontiana and the C3-CAM intermediate Clusia minor . Planta 201, 368–378 [DOI] [PubMed] [Google Scholar]
- Borland AM, Hartwell J, Jenkins GI, Wilkins MB, Nimmo HG. 1999. Metabolite control overrides circadian regulation of phosphoenolpyruvate carboxylase kinase and CO2 fixation in crassulacean acid metabolism. Plant Physiology 121, 889–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland AM, Taybi T. 2004. Synchronization of metabolic processes in plants with crassulacean acid metabolism. Journal of Experimental Botany 55, 1255–1265 [DOI] [PubMed] [Google Scholar]
- Borland AM, Griffiths H, Hartwell J, Smith JAC. 2009. Exploiting the potentials of plants with crassulacean acid metabolism for bioenergy production on marginal lands. Journal of Experimental Botany 60, 2879–2896 [DOI] [PubMed] [Google Scholar]
- Borland AM, Barrera Zambrano VA, Ceusters J, Shorrock K. 2011. The photosynthetic plasticity of crassulacean acid metabolism: an evolutionary innovation for sustainable productivity in a changing world. New Phytologist 191, 619–633 [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254 [DOI] [PubMed] [Google Scholar]
- Boxall SF, Foster JM, Bohnert HJ, Cushman JC, Nimmo HG, Hartwell J. 2005. Conservation and divergence of circadian clock operation in a stress-inducible crassulacean acid metabolism species reveals clock compensation against stress. Plant Physiology 137, 969–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brulfert J, Müller D, Kluge M, Queiroz O. 1982. Photoperiodism and crassulacean acid metabolism. I. Immunological and kinetic evidence for different patterns of phosphoenolpyruvate carboxylase isoforms in photoperiodically inducible and non-inducible crassulacean acid metabolism plants. Planta 164, 326–331 [DOI] [PubMed] [Google Scholar]
- Brulfert J, Kluge M, Güclü S, Queiroz O. 1988. Interaction of photoperiod and drought as CAM inducing factors in Kalanchoë blossfeldiana Poelln. Cv. Tom Thumb. Journal of Plant Physiology 133, 222–227 [Google Scholar]
- Ceusters J, Borland AM, Londers E, Verdoodt V, Godts C, De Proft MP. 2008a. Diel shifts in carboxylation pathway and metabolite dynamics in the CAM bromeliad Aechmea ‘Maya’ in response to elevated CO2 . Annals of Botany 102, 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceusters J, Londers E, Verdoodt V, Ceusters N, De Proft MP. 2008b. Seasonal impact on physiological leaf damage risk of Aechmea hybrid under greenhouse conditions. Scientia Horticulturae 118, 242–245 [Google Scholar]
- Ceusters J, Borland AM, De Proft MP. 2009a. Drought adaptation in plants with crassulacean acid metabolism involves the flexible use of different storage carbohydrate pools. Plant Signaling and Behavior 4, 212–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceusters J, Borland AM, Londers E, Verdoodt V, Godts C, De Proft MP. 2009b. Differential usage of storage carbohydrates in the CAM bromeliad Aechmea ‘Maya’ during acclimation to drought and recovery from dehydration. Physiologia Plantarum 135, 174–184 [DOI] [PubMed] [Google Scholar]
- Ceusters J, Londers E, Verdoodt V, Ceusters N, Godts C, De Proft MP. 2009c. Impact of developmental stage on CAM expression and growth in an Aechmea hybrid under greenhouse conditions. The Journal of Horticultural Science and Biotechnology 84, 393–398 [Google Scholar]
- Ceusters J, Borland AM, Ceusters N, Verdoodt V, Godts C, De Proft MP. 2010. Seasonal influences on carbohydrate metabolism in the CAM bromeliad Aechmea ‘Maya’: consequences for carbohydrate partitioning and growth. Annals of Botany 105, 301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceusters J, Borland AM, Godts C, Croonenborghs, Londers E, Van Goethem D, De Proft MP. 2011. Crassulacean acid metabolism under severe light limitation: a matter of plasticity in the shadows? Journal of Experimental Botany 62, 283–291 [DOI] [PubMed] [Google Scholar]
- Cockburn W. 1981. The evolutionary relationship between stomatal mechanism, crassulacean acid metabolism and C4 photosynthesis. Plant, Cell and Environment 4, 417–418 [Google Scholar]
- Cooper TG, Tchen TT, Wood HG, Benedict CR. 1968. The carboxylation of phosphoenolpyruvate and pyruvate. The active species of “CO2” utilized by phosphoenolpyruvate carboxykinase, carboxytransphosphorylase, and pyruvate carboxylase. Journal of Biological Chemistry 243, 3857–3863 [PubMed] [Google Scholar]
- Crayn DM, Winter K, Smith JAC. 2004. Multiple origins of crassulacean acid metabolism and the epiphytic habit in the neotropical family Bromeliaceae. Proceedings of the National Academy of Sciences, USA 101, 3703–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Proft MP, Ceusters J, Londers E. 2007. Leaf quality management of Aechmea cultivars throughout the supply chain. Acta Horticulturae 755, 39–43 [Google Scholar]
- Doi M, Shigenaga A, Emi T, Kinoshita T, Shimazaki K. 2004. A transgene encoding a blue light receptor, phot 1, restores blue light responses in the Arabidopsis phot1 phot2 double mutant. Journal of Experimental Botany 55, 517–523 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Borland AM, Haslam RP, Griffiths H, Maxwell K. 2002. Crassulacean acid metabolism: plastic fantastic. Journal of Experimental Botany 53, 569–580 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Griffiths H, Taybi T, Cushman JC, Borland AM. 2003. Integrating diel starch metabolism with the circadian and environmental regulation of crassulacean acid metabolism in Mesembryanthemum crystallinum . Planta 216, 789–797 [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Wang L, Han LQ, Suh SS, Salome PA, McClung CR, Somers DE. 2008. Post-translational regulation of the Arabidopsis circadian clock through selective proteolysis and phosphorylation of pseudo-response regulator proteins. Journal of Biological Chemistry 283, 23073–23083 [DOI] [PubMed] [Google Scholar]
- Grams TEE, Thiel S. 2002. High light-induced switch from C3-photosynthesis to crassulacean acid metabolism is mediated by UV-A/blue light. Journal of Experimental Botany 53, 1475–1483 [PubMed] [Google Scholar]
- Griffiths H, Lüttge U, Stimmel K.-H, Crook CE, Griffiths NM, Smith JAC. 1986. Comparative ecophysiology of CAM and C3 bromeliads. III. Environmental influences on CO2 assimilation and transpiration. Plant, Cell and Environment 9, 385–393 [Google Scholar]
- Harmer SL. 2009. The circadian system in higher plants. Annual Review of Plant Biology 60, 357–377 [DOI] [PubMed] [Google Scholar]
- Harris PJC, Wilkins MB. 1976. Light-induced changes in the period of the circadian rhythm of carbon dioxide output in Bryophyllum leaves. Planta 129, 253–258 [DOI] [PubMed] [Google Scholar]
- Harris PJC, Wilkins MB. 1978a. Evidence for phytochrome involvement in the entrainment of the circadian rhythm of CO2 metabolism in Bryophyllum . Planta 138, 271–272 [DOI] [PubMed] [Google Scholar]
- Harris PJC, Wilkins MB. 1978b. The circadian rhythm in Bryophyllum leaves: phase control by radiant energy. Planta 143, 323–328 [DOI] [PubMed] [Google Scholar]
- Hartwell J. 2005. The co-ordination of central plant metabolism by the circadian clock. Biochemical Society Transactions 33, 945–948 [DOI] [PubMed] [Google Scholar]
- Hartwell J, Smith LH, Wilkins MB, Jenkins GI, Nimmo HG. 1996. Higher plant phosphoenolpyruvate carboxylase kinase is regulated at the level of translatable mRNA in response to light or a circadian rhythm. Plant Journal 10, 1071–1078 [Google Scholar]
- Hartwell J, Gill A, Nimmo GA, Wilkins MB, Jenkins GI, Nimmo HG. 1999. Phosphoenolpyruvate carboxylase kinase is a novel protein kinase regulated at the level of expression. Plant Journal 20, 333–342 [DOI] [PubMed] [Google Scholar]
- Hatch MD. 1973. An essay for PEP carboxykinase in crude tissue extracts. Analytical Biochemistry 52, 280–285 [DOI] [PubMed] [Google Scholar]
- Hotta CT, Gardner MJ, Hubbard KE, Baek SJ, Dalchau N, Suhita D, Dodd AN, Webb AAR. 2007. Modulation of environmental responses of plants by circadian clocks. Plant, Cell and Environment 30, 333–349 [DOI] [PubMed] [Google Scholar]
- Lasceve G, Leymarie J, Vasasseur A. 1997. Alterations in light-induced stomatal opening in the starch deficient mutant of Arabidopsis thaliana L. deficient in chloroplast phosphoglucomutase activity. Plant, Cell and Environment 20, 350–358 [Google Scholar]
- Lawson T. 2009. Guard cell photosynthesis and stomatal function. New Phytologist 181, 13–34 [DOI] [PubMed] [Google Scholar]
- Lee DM, Assmann SM. 1992. Stomatal responses to light in the facultative crassulaceana cid metabolism species Portulacaria afra . Physiologia Plantarum 85, 35–42 [Google Scholar]
- Londers E, Ceusters J, Godts C, De Proft MP. 2009. Impact of fertiliser level on plant growth, plant shape, and physiological leaf damage in two cultivars of Aechmea characterised by crassulacean acid metabolism. The Journal of Horticultural Science and Biotechnology 84, 526–530 [Google Scholar]
- Lüttge U. 2004. Ecophysiology of crassulacean acid metabolism (CAM). Annals of Botany 93, 629–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawson BT, Zaugg MW. 1994. Modulation of light-dependent stomatal opening in isolated epidermis following induction of crassulacean acid metabolism in Mesembryanthemum crystallinum L. Journal of Plant Physiology 144, 740–746 [Google Scholar]
- Maxwell C, Griffiths H, Young AJ. 1994. Photosynthetic acclimation to light regime and water stress by the C3-CAM epiphyte Guzmania monostachia: gas exchange characteristics, photochemical efficiency and the xantophyll cycle. Functional Ecology 8, 746–754 [Google Scholar]
- Maxwell C, Griffiths H, Borland AM, Young AJ, Broadmeadow MSJ, Fordham MC. 1995. Short-term photosynthetic responses of the C3-CAM epiphyte Guzmania monostachia var. monostachia to tropical seasonal transitions under field conditions. Australian Journal of Plant Physiology 22, 771–781 [Google Scholar]
- Nimmo HG. 2000. The regulation of phosphoenolpyruvate carboxylase in CAM plants. Trends in Plant Science 5, 75–80 [DOI] [PubMed] [Google Scholar]
- Nobel PS, Hartsock TL. 1983. Relationships between photosynthetically active radiation, nocturnal acid accumulation, and CO2 uptake for a crassulacean acid metabolism plant, Opuntia ficus-indica . Plant Physiology 71, 71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond CB. 1978. Crassulacean acid metabolism: a curiosity in context. Annual review of Plant Physiology 29, 379–414 [Google Scholar]
- Tallman G, Zeiger E. 1988. Light quality and osmoregulation in Vicia fabia guard cells: evidence for the involvement of three metabolic pathways. Plant Physiology 88, 887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman G, Zhu J, Mawson BT, Amodeo G, Nouhi Z, Levy K, Zeiger E. 1997. Induction of CAM in Mesembryanthemum crystallinum abolishes the stomatal response to blue light and light-dependent zeaxanthin formation in guard cell chloroplasts. Plant Cell Physiology 38, 236–242 [Google Scholar]
- Taybi T, Cushman JC, Borland AM. 2002. Environmental, hormonal and circadian regulation of crassulacean acid metabolism expression. Functional Plant Biology 29, 669–678 [DOI] [PubMed] [Google Scholar]
- Taybi T, Patil S, Chollet R, Cushman JC. 2000. A minimal serine/threonine protein kinase circadianly regulates phosphoenolpyruvate carboxylase activity in crassulacean acid metabolism-induced leaves of the common ice plant. Plant Physiology 123, 1471–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taybi T, Nimmo HG, Borland AM. 2004. Expression of phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxylase kinase genes. Implications for genotypic cavacity and phenotypic plasticity in the expression of crassulacean acid metabolism. Plant Physiology 135, 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA, André M, Ganzin AM. 1987. Oxygen and carbon dioxide exchanges in crassulacean acid metabolism plants. II. Effects of CO2-concentration and irradiance. Plant Physiology and Biochemistry 25, 95–103 [Google Scholar]
- Walker RP, Leegood RC. 1995. Purification, and phosphorylation in vivo and in vitro of phosphoenolpyruvate carboxykinase from cucumber cotyledons. FEBS Letters 362, 70–74 [DOI] [PubMed] [Google Scholar]
- Walker RP, Leegood RC. 1996. Phosphorylation of phosphoenolpyruvate carboxykinase in plants. Studies in plants with C4 photosynthesis and crassulacean acid metabolism in germinating seeds. Biochemical Journal 317, 653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MB. 1992. Circadian rhythms: their origin and control. New Phytologist 121, 347–375 [DOI] [PubMed] [Google Scholar]